Abstract
Acute kidney injury (AKI) is one of the most dreaded complications of severe malaria. We carried out prospective study in 2010, to describe clinical characteristics, laboratory parameters, prognostic factors, and outcome in 59 (44 males, 15 females) smear-positive malaria patients with AKI. The severity of illness was assessed using Acute Physiology and Chronic Health Evaluation (APACHE) II, Sequential Organ Failure Assessment (SOFA) score, Multiple Organ Dysfunction Score (MODS), and Glasgow Coma Scale (GCS) scores. All patients received artesunate and hemodialysis (HD). Mean age of patients was 33.63 ± 14 years. Plasmodium falciparum malaria was seen in 76.3% (n = 45), Plasmodium vivax in 16.9% (n = 10), and mixed infection in 6.8% (n = 4) patients. Presenting clinical features were fever (100%), nausea-vomiting (85%), oliguria (61%), abdominal pain/tenderness (50.8%), and jaundice (74.5%). Mean APACHE II, SOFA, MODS, and GCS scores were 18.1 ± 3, 10.16 ± 3.09, 9.71 ± 2.69, and 14.15 ± 1.67, respectively, all were higher among patients who died than among those who survived. APACHE II ≥20, SOFA and MODS scores ≥12 were associated with higher mortality (P < 0.05). 34% patients received blood component transfusion and exchange transfusion was done in 15%. Mean number of HD sessions required was 4.59 ± 3.03. Renal biopsies were performed in five patients (three with patchy cortical necrosis and two with acute tubular necrosis). 81.3% of patients had complete renal recovery and 11.8% succumbed to malaria. Prompt diagnosis, timely HD, and supportive therapy were associated with improved survival and recovery of kidney functions in malarial with AKI. Mortality was associated with higher APACHE II, SOFA, MODS, GCS scores, requirement of inotrope, and ventilator support.
Keywords: Acute kidney injury, hemodialysis, jaundice, malaria, outcome
Introduction
Malaria is a major public health problem in tropical countries. About 500 million people suffer from malaria, leading to death in 1 to 3 million cases. Acute kidney injury (AKI) is one of the most dreaded complications of severe malaria.[1] The overall prevalence of AKI in falciparum malaria varies between <1 and 60%, with the mortality rate up to 45%.[1–4] AKI occurs commonly in Plasmodium falciparum (PF) malaria and rarely in Plasmodium vivax (PV) malaria.[5–7] Malaria is an important cause of multiple organ failure; mortality rate is 6.4% when one or fewer organs fail, but increases to 48.8% with failure of two or more organs. However, outcomes are better than for similar degrees of organ failure in sepsis.[8] Prompt and accurate diagnosis of malaria is needed for implementation of appropriate treatment to reduce associated morbidity and mortality. The management of malaria-induced AKI includes appropriate antimalarials (parenteral artesunate or quinine), fluid electrolyte management, supportive therapy, avoidance of nephrotoxic drugs, and renal replacement therapy (RRT) at the earliest.[1,9] Hemodialysis (HD) is effective for malaria-associated AKI.[10,11]
We carried out prospective single-center study to describe the clinical characteristics, laboratory parameters, prognostic factors, and outcome in patients with malaria with AKI who underwent HD.
Materials and Methods
All 50 patients with malaria-associated AKI seen at our center in 2010 were included in the study. The diagnosis of malaria was confirmed by direct visualization of the parasite in Giemsa-stained peripheral blood smears. In addition, once a diagnosis has been established and treatment has been initiated, serial examinations permit monitoring of the parasitological response. Clinical history and assessment were recorded in all the study patients and all other known etiological causes of fever and jaundice were excluded by relevant investigations. Other causes of jaundice excluded by absence of evidence of exposure to hepatotoxic drugs and absence of clinical or serological evidence of infective etiology (like viral hepatitis, leptospirosis). All the patients were subjected to complete hemogram. Urine samples were examined for routine and microscopy examinations. Serum was tested for sodium, potassium, alkaline phosphatase, alanine aminotransaminase, bilirubin, lactic acid dehydrogenase, and glucose 6-phosphate dehydrogenase. Serological tests for Human Immunodeficiency Virus and hepatitis B and C were performed. Estimation of blood sugar, coagulation profile for disseminated intravascular coagulation (DIC), and arterial blood gas analysis were carried out when indicated. Chest x-ray and ultrasonography (USG) of abdomen were recorded in all the patients. AKI was defined as serum creatinine (SCr) of >3 mg/dl and urine output <400 ml/24 hours, with normal kidney size on USG as per World Health Organization (WHO) definition.[12] Jaundice was defined as icteric sclera and/ or total bilirubin levels >3 mg/dl. The severity of illness was assessed using APACHE II, SOFA, MODS, and GCS scores.[13–16] The patients were managed using antimalarial drugs, fluid replacement, and RRT. All patients received artesunate 2.4 mg/kg intravenously, followed by 2.4 mg/kg at 12 and 24 hours, followed by 2.4 mg/kg once daily for a total of 7 days and doxycycline (adults: 100 mg orally twice daily; children: 2.2 mg/kg [up to 100 mg] orally twice daily).[12] In absence of parasitological or clinical response to parenteral artesunate, patients received quinine 20 mg salt/kg body weight through intravenous (IV) infusion in 5 percent dextrose over 4 hours, then 10 mg/kg body weight every 8 hour for 5 to 7 days. IV 25% dextrose injections were administered to prevent and treatment of hypoglycemia. Broad-spectrum antibiotic treatment (IV ceftriaxone 1 g BD) was given initially until a bacterial infection (notably Salmonella) was excluded.[17–19] Supportive measures (e.g., antipyretics, oxygen, ventilatory support, cardiac monitoring, and pulse oximetry) were instituted as needed. RRT was initiated for fluid overload, hyperkalemia, clinical evidence of uremia, metabolic acidosis, rapidly increasing SCr level, blood urea nitrogen (BUN)>100 mg/dl, and SCr >4 to 5 mg/dl. RRT was initiated prior to the development of overt symptoms and signs of renal failure due to malaria.[1,3–11,17] The HD prescription included a polysulfone membrane dialyzer with surface area 1.3 to 1.8 m2, a blood flow rate of 200 to 300 ml/ min, and a dialysate flow rate of 500 ml/min against a 35 mEq/l bicarbonate bath. Dialysate potassium was adjusted individually. Heparin-free (in most of patients) intermittent HD was provided on alternate days through temporary femoral/jugular catheter for 4 hours and continuous renal replacement therapies (CRRT) were done in hemodynamically unstable patients. RRT was usually continued until the patient manifested evidence of recovery of kidney function. In oliguric patients, the primary manifestation of recovery of kidney function was an increase in urine output. In patients who were nonoliguric, recovery of kidney function was manifested by a progressive decline in SCr concentration after initial attainment of stable values (assessed daily during CRRT or predialysis in patients managed with intermittent HD), despite a constant dose of renal support. Fluid requirements were assessed individually and fluid resuscitation based on estimated deficit. Many patients with oliguria who were dehydrated on admission received fluid challenge. They received fluid up to 20 ml/kg of 0.9% saline infused over 60 minutes. To prevent fluid overload, auscultation of the lungs and jugular venous pressure measurements were done. Careful attention was given toward fluid and electrolyte requirements during diuretic phase. In addition, repeated estimation of Na, K, and bicarbonate was done. Patients with severe malaria with clinically significant DIC were given fresh whole blood transfusions, vitamin K, and fresh frozen plasma. Blood transfusion was reserved for patients with hemoglobin ≤7 g/dl.[8,12] 10 ml/kg of packed red blood cells or 20 ml/ kg of whole blood was transfused during HD. In addition, support of erythropoiesis in the form of replacement of folic acid and iron was instituted since many patients were malnourished. Exchange transfusion was done for patients with parasite density of >10 percent with end organ complications, serum bilirubin >25 mg/ dl.[3,17,20–22] Renal biopsy was performed when oliguria/heavy proteinuria (>3 g/day) or hematuria persisted for three weeks.[3]
Data entry and statistical analysis were performed using the SPSS 12.0 software. The continuous variables were presented as mean ± standard deviation and assessed by analysis of Mann–Whitney U-test to identify the differences between groups, with statistical significance set at P < 0.05. Univariate and multivariate analysis were performed using SPSS version-12. Multivariate analysis of variance (MANOVA) was performed using SPSS version-12. Analysis of each individual-dependent variable, using Bonferroni-adjusted alpha level, shows that there was no contribution of the dependent variables. We considered the values for the Wilks’ Lambda and Pillai's Trace for robustness of MANOVA. Levene's Test of Equality of Error Variances was performed.
Results
Baseline characteristics of the study patients are shown in Table 1. Comparison of patients who survived and died is shown in Table 2. The mean age of the 59 patients was 33.63 ± 14.6 years (range, 6-75 years); 44 (74.5 %) were males and 15(25.5%) were females. PF malaria was seen in 76.3% (n = 45), PV in 16.9% (n = 10), and mixed infection in 6.8% (n = 4) patients. Presenting clinical features were fever (100%), nausea-vomiting (85%), oliguria (61%), abdominal pain/tenderness (50.8%), jaundice (74.5 %), dyspnea (30.5%), diarrhea (15.2%), altered sensorium (10%), and convulsions (8.4%).The time between onset of symptoms and admission to the hospital for AKI ranged from 7 to 14 days (median, 10 days). Hyponatremia, usually asymptomatic, was observed in 71% (n = 42) of patients.
Table 1.
Baseline characteristics of the study patients of malaria and AKI
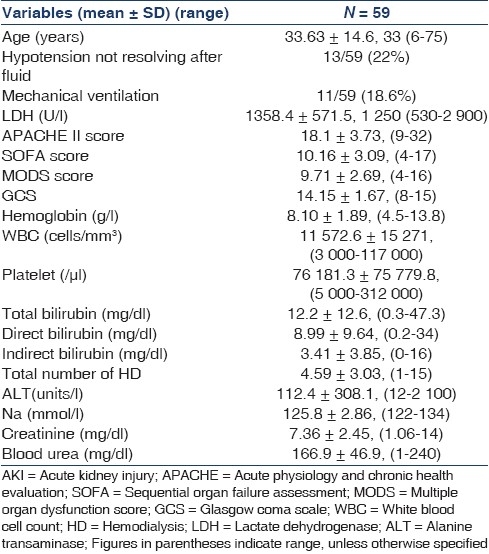
Table 2.
Univariate analysis of factors associated with outcome in survivors and nonsurvivors
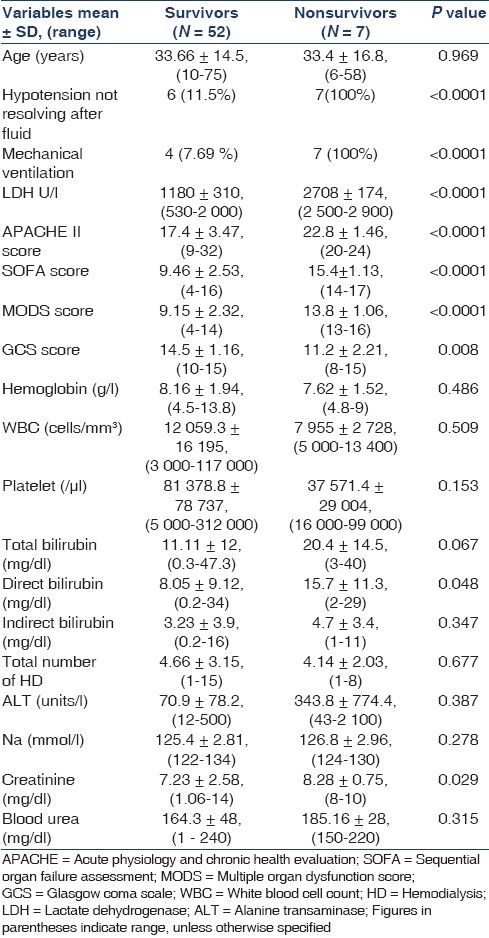
The mean APACHE II score was 18.1 ± 3, (9-32); the mean SOFA score was 10.16 ± 3.09, (4-17); the mean MODS score was 9.71 ± 2.69, (4-16); and the mean GCS score was 14.15 ± 1.67, (8-15), all were higher, indicating more severe abnormalities among the patients who died than among those who survived as shown in Tables 1 and 2. SOFA score <12 were associated with a low mortality rate (41 patients, 0 deaths), whereas score ≥12 showed poorer outcome (18 patients, 7 deaths, P < 0.0001; relative risk, 0.19; 95% confidence interval, 0.10-0.34). MODS score <12 were associated with a low mortality rate (43 patients, 0 deaths), whereas score ≥12 showed a worse outcome (16 patients, 7 deaths, P < 0.001; relative risk, 0.19; 95% confidence interval, 0.10–0.34). APACHE II score <20 were associated with a low mortality rate (42 patients, 0 deaths), whereas score ≥20 showed a worse outcome (17 patients, 7 deaths, P < 0.001; relative risk, 0.21; 95% confidence interval, 0.11–0.37). On multivariate analysis, statistically significant predictors of outcome were APACHE II score (P = 0.0004), SOFA score (P = 0.0003), MODS score (P = 0.002), GCS score (P = 0.00002), and Lactate dehydrogenase (LDH) level (P = 0.00001).
Cerebral malaria was observed in eight patients, and three of them survived. Approximately 34% (n = 20) patients received blood component transfusion. HD was performed in 57 patients and CRRT was performed in two patients. Additional peritoneal dialysis was initially performed in two pediatric patients. Mean number of HD sessions required was 4.59 ± 3.03 (range, 1-15). All patients received artesunate and additional quinine was required in 10 (16.9%), exchange transfusion was done in 15% (n = 9) patients and 10% (n = 6) of these survived. Renal biopsies were performed in 5 patients, of whom 60% (n = 3) had patchy cortical necrosis and 40% (n = 2) had acute tubular necrosis (ATN) with mesangial proliferative glomerulonephritis without any immune deposits. 81.3% (n = 48) of the patients had complete recovery with normal renal function (SCr <1.4 mg/ dl), 5% (n = 3) had incomplete recovery (SCr, 1.5-3 mg/dl) and remained on conservative management, 1.6% (n = 1) ended up as chronic renal failure and continued on maintenance HD, and 11.8% (n = 7) succumbed to malaria due to multiorgan dysfunction syndrome (MODS). The most common adverse effects associated with artesunate included nausea, vomiting, anorexia, and dizziness, although these may be due to malaria rather than drug adverse effects. Factors related to AKI in vivax malaria were heavy parasitemia, hyperbilirubinemia, volume depletion, hypercatabolic state, intravascular hemolysis, and sepsis.
Discussion
Our analysis of malaria patients with AKI reveals that renal failure was observed in adults and more common in males. This could be explained by more outdoor activities of males in Asian countries as compared with females. Most common presentation was jaundice with AKI (75%). AKI occurs commonly with jaundice, thrombocytopenia, and rarely with cerebral malaria. AKI was usually seen in early second week and is oliguric in 61% of cases. The etiology of AKI was usually multifactorial due to hyperbilirubinemia, intravascular hemolysis, hyperparasitemia, volume depletion, hypoxia, shock, pigment nephropathy, DIC, and sepsis.[1–9,23–27] The cascade of inflammatory cytokine activation leading specifically to renal damage remains enigmatic.[27] The overall mortality rate among those with kidney injury ranges from 15 to 50% in different series.[7,8,28,29] Low mortality (11.8%) in our study of malaria with AKI despite of having jaundice in 75% is due to prompt diagnosis, timely HD, exchange transfusion, and supportive therapy.[3]
In earlier reports, almost all cases of malarial AKI were observed in adults and more common in males.[30] AKI is associated with the jaundice in more than 75% patients.[23] Similar findings are noted in our study. The effect of associated AKI on mortality in cerebral malaria patients indicated that mortality was as high as 39.5% when associated with AKI, whereas it was only 13.9% when unassociated with AKI.[31]
Hyponatremia, usually asymptomatic, is observed in 71% (n = 42) of the patients.[32] Hyponatremia in adults with severe malaria is common and associated with preserved consciousness and decreased mortality. It likely reflects continued oral hypotonic fluid intake in the setting of hypovolemia and requires no therapy beyond dehydration.[33]
The incidence of jaundice in falciparum malaria has been reported to be between 2 and 57%.[30,34,35] AKI associated with jaundice had high mortality in comparison with nonjaundiced AKI patients.[36] Jaundice is probably associated with toxicity to tubular cells with more risk of development of acute tubular necrosis. In patients with falciparum malaria with jaundice, 40% succumbed to the disease.[37] Indirect hyperbilirubinemia from RBC destruction is the most common form of jaundice reported in malaria.[37,38] Some patients with falciparum malaria have direct hyperbilirubinemia and elevated hepatic enzymes.[39] In our study, 75% patients had jaundice as a presenting symptom, which is in accordance to the findings reported in other studies.
A number of investigators have recommended that exchange transfusion be considered solely for patients with high-grade parasitemia.[3,5,20–23] The rationale for exchange transfusion therapy is based on rapid reduction in the parasite load by direct removal, decreased risk of severe intravascular hemolysis and its consequences, improved rheology with transfused blood and reduced microcirculatory sludging, and improved oxygen-carrying capacity with transfused erythrocytes.[21] However, the WHO guidelines indicate that it is not possible to make any recommendations regarding the use of exchange transfusion based on the available evidence. The Centers for Disease Control (CDC) recommends consideration of exchange transfusion for patients with parasite density of >10% with end organ complications.[22]
APACHE II, SOFA, and MODS scores have been used in malaria for determining severity of malaria.[8,40–41] MODS score ≥16 was associated with significantly longer disease duration. Thus, this score might provide a predictive value for morbidity in PF malaria. The MODS is simple and easy to apply and needs a recording time of less than three minutes. Thus, this score might provide a quantitative approach for determining severity in PF malaria.[40] High APACHE II score, deep unconsciousness, AKI, and acidemia were identified as poor prognostic factors in cerebral malaria patients. With the cut-off point at a score of 24, the APACHE II score stratified the patients’ mortality outcome with 95.8% accuracy.[41] Increasing SOFA scores indicated worse outcomes in study of severe falciparum malaria. SOFA score <10 were associated with a low mortality rate, whereas scores >10 showed a significantly worse outcome.[8] APACHE II score ≥20, SOFA and MODS scores ≥12 were associated with higher mortality in our study. These score can be used for prognosis in malaria with AKI. These scores can help us for better stratification of patients for better and intensive managements to improve outcome. In our study, on univariate and multivariate analysis, statistically significant predictors of outcome were APACHE II, SOFA, MODS, GCS scores, and LDH level.
There is no direct pathogenic linkage between vivax malaria and AKI, but the associated conditions such as heavy parasitemia, hypercatabolic state, volume depletion, hyperbilirubinemia, intravascular hemolysis, sepsis, and DIC can contribute to AKI. Hence, despite the association, cause and effect relationships remain doubtful.[5,23,42] Hyperbilirubinemia may contribute to reduction in total peripheral vascular resistance and in renal blood flow due to left ventricular dysfunction.[43] More studies are required to address factors related to AKI in vivax malaria. Acute cortical necrosis (ACN) has rarely been reported in patients with AKI due to malaria.[44–47] The possible pathogenetic factors in patients with ACN were the renal damage through renal hypoperfusion or endothelial injury through release of various circulating substances (intravascular hemolysis, sepsis, and pancreatitis). It should be suspected in patients with AKI who have a prolonged phase of oligoanuria.[44–48]
These findings are of importance for clinicians in prognosticating their patients with AKI due to malaria. Future studies representing a large population with the full spectrum of malaria patients in different geographical areas will determine the usefulness of this scoring method for both clinicians and researchers and recommendation for adjunct exchange transfusion.
Conclusion
Malaria is an important cause of AKI in Asia and particularly in tropical areas. Prompt diagnosis, timely HD, and supportive therapy are associated with improved survival and recovery of kidney functions in malarial with AKI. Mortality was associated with higher APACHE II, SOFA, MODS, GCS scores, requirement of inotrope, and ventilator support.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Mishra SK, Das BS. Malaria and acute kidney injury. Semin Nephrol. 2008;28:395–408. doi: 10.1016/j.semnephrol.2008.04.007. [DOI] [PubMed] [Google Scholar]
- 2.Sheehy TW, Reba RC. Complications of Falciparum malaria and their treatment. Ann Inter Med. 1967;66:807–9. doi: 10.7326/0003-4819-66-4-807. [DOI] [PubMed] [Google Scholar]
- 3.Kanodia KV, Shah PR, Vanikar AV, Kasat P, Gumber M, Trivedi HL. Malaria induced acute renal failure: A single center experience. Saudi J Kidney Dis Transpl. 2010;21:1088–91. [PubMed] [Google Scholar]
- 4.Eiam-Ong S, Sitprija V. Falciparum malaria and the kidney: A model of inflammation. AM J Kidney Dis. 1998;32:361–75. doi: 10.1053/ajkd.1998.v32.pm9740151. [DOI] [PubMed] [Google Scholar]
- 5.Prakash J, Singh AK, Kumar NS, Saxena RK. Acute renal failure in Plasmodium vivax malaria. J Assoc Physicians India. 2003;51:265–7. [PubMed] [Google Scholar]
- 6.J Prakash, AK Singh. Acute renal failure in Malaria: Changing trends. Indian J Nephrol. 2002;12:113–7. [Google Scholar]
- 7.Mehta KS, Halankar AR, Makwana PD, Torane PP, Satija PS, Shah VB. Severe acute renal failure in malaria. J Postgrad Med. 2001;47:24–6. [PubMed] [Google Scholar]
- 8.Krishnan A, Karnad DR. Severe Falciparum malaria: An important cause of multiple organ failure in Indian intensive care unit patients. Crit Care Med. 2003;31:2278–84. doi: 10.1097/01.CCM.0000079603.82822.69. [DOI] [PubMed] [Google Scholar]
- 9.Das BS. Renal failure in malaria. J Vector Borne Dis. 2008;45:83–97. Review. [PubMed] [Google Scholar]
- 10.Wilairatana P, Westerlund EK, Aursudkij B, Vannaphan S, Krudsood S, Viriyavejakul P, et al. Treatment of malarial acute renal failure by hemodialysis. Am J Trop Med Hyg. 1999;60:233–7. doi: 10.4269/ajtmh.1999.60.233. [DOI] [PubMed] [Google Scholar]
- 11.Trang TT, Phu NH, Vinh H, Hien TT, Cuong BM, Chau TT, et al. Acute renal failure in patients with severe Falciparum malaria. Clin Infect Dis. 1992;15:874–80. doi: 10.1093/clind/15.5.874. [DOI] [PubMed] [Google Scholar]
- 12. [Last cited on 2011 Jan 25]. WHO, WHO releases new malaria guidelines for treatment and procurement of medicines. Available from: http://ww.who.int/malaria/world_malaria_report_2010/en/index.html . [Google Scholar]
- 13.Knaus WA, Draper EA, Wagner DP, Zimmerman JE. APACHE II: A severity of disease classi-fication system. Crit Care Med. 1985;13:818–29. [PubMed] [Google Scholar]
- 14.Vincent JL, Mendonca A, Cantraine F, Moreno R, Takala J, Suter PM, et al. Use of the SOFA score to assess the incidence of organ dysfunction/failure in intensive care units: Results of a multicenter, prospective study. Crit Care Med. 1998;26:1793–800. doi: 10.1097/00003246-199811000-00016. [DOI] [PubMed] [Google Scholar]
- 15.Weiler Th, Baldering HJ, Heinrichs W, Schmitz JE. Quality assurance in intensive care medicine.Results of a multicenter study in Germany. Anästhesiol Intensivmed Schmerzther. 1997;32:372–5. doi: 10.1055/s-2007-995073. [DOI] [PubMed] [Google Scholar]
- 16.Helbok R, Dent W, Nacher M, Treeprasertsuk S, Krudsood S, Wilairatana P, et al. Use of the multi-organ dysfunction score as a tool to discriminate different levels of severity in uncomplicated Plasmodium Falciparum malaria. Am J Trop Med Hyg. 2003;68:372–5. [PubMed] [Google Scholar]
- 17.Talor TE, Daily JP, Baron Elinor L. Treatment of severe Falciparum malaria. [Last cited on 2011 Jan 25]. Available from http://www.uptodate.com .
- 18.Berkley J, Mwarumba S, Bramham K, Lowe B, Marsh K. Bacteraemia complicating severe malaria in children. Trans R Soc Trop Med Hyg. 1999;93:283–6. doi: 10.1016/s0035-9203(99)90024-x. [DOI] [PubMed] [Google Scholar]
- 19.Bronzan RN, Taylor TE, Mwenechanya J, Tembo M, Kayira K, Bwanaisa L, et al. Bacteremia in Malawian children with severe malaria: Prevalence, etiology, HIV coinfection, and outcome. J Infect Dis. 2007;195:895–904. doi: 10.1086/511437. [DOI] [PubMed] [Google Scholar]
- 20.Riddle MS, Jackson JL, Sanders JW, Blazes DL. Exchange transfusion as an adjunct therapy in severe Plasmodium Falciparum malaria: A meta-analysis. Clin Infect Dis. 2002;34:1192–8. doi: 10.1086/339810. [DOI] [PubMed] [Google Scholar]
- 21.Phillips P, Nantel S, Benny WB. Exchange transfusion as an adjunct to the treatment of severe Falciparum malaria: Case report and review. Rev Infect Dis. 1990;12:1100–8. doi: 10.1093/clinids/12.6.1100. [DOI] [PubMed] [Google Scholar]
- 22.Zucker JR, Campbel CC. Malaria. Principles of prevention and treatment. Infect Dis Clin North Am. 1993;7:547–67. [PubMed] [Google Scholar]
- 23.Jha V, Chugh KS. Community-acquired acute kidney injury in Asia. Semin Nephrol. 2008;28:330–47. doi: 10.1016/j.semnephrol.2008.04.002. [DOI] [PubMed] [Google Scholar]
- 24.Prakash J, Gupta A, Kumar O, Rout SB, Malhotra V, Srivastava PK. Acute renal failure in Falciparum malaria-increasing prevalence in some areas of India-a need for awareness. Nephrol Dial Transplant. 1996;11:241–6. doi: 10.1093/oxfordjournals.ndt.a027206. [DOI] [PubMed] [Google Scholar]
- 25.Rajapurkar MM. Renal involvement in malaria. J Postgrad Med. 1994;40:132–4. [PubMed] [Google Scholar]
- 26.Ehrich JH, Eke FU. Malaria-induced renal damage: Facts and myths. Pediatr Nephrol. 2007;22:626–37. doi: 10.1007/s00467-006-0332-y. [DOI] [PubMed] [Google Scholar]
- 27.Sakhuja V, Sud K. Acute renal failure in the tropics. Saudi J Kidney Dis Transpl. 1998;9:247–60. [PubMed] [Google Scholar]
- 28.WHO. Severe Falciparum malaria. Trans R Soc TropMed Hyg. 2000;1:s1–90. [PubMed] [Google Scholar]
- 29.Habte B. Acute renal failure due to Falciparum malaria. Ren Fail. 1990;12:15–9. doi: 10.3109/08860229009066960. [DOI] [PubMed] [Google Scholar]
- 30.Mohanty S, Mishra SK, Pati SS, Pattnaik J, Das BS. Complications and mortality patterns due to Plasmodium Falciparum malaria in hospitalized adults and children, Rourkela, Orissa, India. Trans R Soc Trop Med Hyg. 2003;97:69–70. doi: 10.1016/s0035-9203(03)90027-7. [DOI] [PubMed] [Google Scholar]
- 31.Mishra SK, Dietz K, Mohanty S, Pati SS. Influence of acute renal failure in patients with cerebral malaria: A hospitalbased study from India. Trop Doct. 2007;37:103–4. doi: 10.1177/004947550703700216. [DOI] [PubMed] [Google Scholar]
- 32.Miller LH, Makaranond P, Sitprija V, Suebsanguan C, Canfield CJ. Hyponatraemia in malaria. Ann Trop Med Parasitol. 1967;61:265–79. doi: 10.1080/00034983.1967.11686487. [DOI] [PubMed] [Google Scholar]
- 33.Hanson J, Hossain A, Charunwatthana P. Hyponatremia in severe malaria: Evidence for an appropriate anti-diuretic hormone response to hypovolemia. Am J Trop Med Hyg. 2009;80:141–5. doi: 10.4269/ajtmh.2009.08-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mehta SR. Falciparum malaria-210 cases. J Assoc Physicians India. 1986;34:119–20. [PubMed] [Google Scholar]
- 35.Nand N, Aggarwal H, Sharma M, Singh M. Systemic Manifestations of Malaria. J Indian Acad Clin Med. 2001;2:189–94. [Google Scholar]
- 36.Pati SS, Mishra SK, Mohanty S, Patnaik JK, Das BS. Influence of renal impairment on plasma concentrations of conjugated bilirubin in cases of Plasmodium Falciparum malaria. Ann Trop Med Parasitol. 2003;97:581–6. doi: 10.1179/000349803225001418. [DOI] [PubMed] [Google Scholar]
- 37.Ahsan T, Ali H, Bkaht SF, Ahmad N, Farooq MU, Shaheer A, et al. Jaundice in Falciparum malaria; Changing trends in clinical presentation-a need for awareness. J Pak Med Assoc. 2008;58:616–21. [PubMed] [Google Scholar]
- 38.World Health Organization: Severe and complicated malaria. Trans Royal Soc Trop Med Hyg. 1990;84:1–65. [PubMed] [Google Scholar]
- 39.Willairatana P, Looaresuwan S, Charoenlarp P. Liver profile changes and complications in jaundiced patients with Falciparum malaria. Trop Med Parasitol. 1994;45:298–302. [PubMed] [Google Scholar]
- 40.Helbok R, Dent W, Nacher M, Treeprasertsuk S, Krudsood S, Wilairatana P, et al. Use of the multi-organ dysfunction score as a tool to discriminate different levels of sev. Am J Trop Med Hyg. 2003;68:372–5. [PubMed] [Google Scholar]
- 41.Wilairatana P, Looareesuwan S. APACHE II scoring for predicting outcome in cerebral malaria. J Trop Med Hyg. 1995;98:256–60. [PubMed] [Google Scholar]
- 42.Chung BH, Lee SW, Lee SE, Hwang TJ, Shin HS. Predictors of Plasmodium vivax malaria-induced nephropathy in young Korean men. Nephron Clin Pract. 2008;110:c172–7. doi: 10.1159/000167023. [DOI] [PubMed] [Google Scholar]
- 43.Green J, Beyar R, Bomzon L, Finberg JP, Better OS. Jaundice, the circulation and the kidney. Nephron. 1984;37:145–52. doi: 10.1159/000183235. [DOI] [PubMed] [Google Scholar]
- 44.Chugh KS, Jha V, Sakhuja V, Joshi K. Acute renal cortical necrosis-A study of 113 patients. Ren Fail. 1994;16:37–47. doi: 10.3109/08860229409044846. [DOI] [PubMed] [Google Scholar]
- 45.Chugh KS, Singhal PC, Kher V, Gupta VK, Malik GH, Narayan G, et al. Spectrum of acute cortical necrosis in Indian patients. Am J Med Sci. 1983;286:10–20. doi: 10.1097/00000441-198307000-00002. [DOI] [PubMed] [Google Scholar]
- 46.Sakhuja V, Chugh KS. Renal cortical necrosis. Int J Artif Organs. 1986;9:145–6. [PubMed] [Google Scholar]
- 47.Baliga KV, Narula AS, Khanduja R. Acute cortical necrosis in Falciparum malaria: An unusual manifestation. Ren Fail. 2008;30:461–3. doi: 10.1080/08860220801964293. [DOI] [PubMed] [Google Scholar]
- 48.Singhal MK, Arora P, Kher V, Pandey R, Gulati S, Gupta A. Acute cortical necrosis in Falciparum malaria: An unusual cause of end-stage renal disease. Ren Fail. 1997;19:491–4. doi: 10.3109/08860229709047736. [DOI] [PubMed] [Google Scholar]


