Abstract
A 56-year-old man with diabetes, hypertension, and chronic kidney disease presented to the emergency room with a complaint of pain in his right foot. He was found to have tremors. Gabapentin toxicity was suspected and the patient was found to have high gabapentin level (6.3 mcg/ml). Patient was commenced on continuous venovenous hemodiafiltration (CVVHD) and the pharmacokinetics of gabapentin was studied. The patient improved symptomatically and his tremors subsided. In this case report, we describe the successful management of gabapentin toxicity with continuous renal replacement therapy and calculate the clearance of gabapentin which will enable future treatment of gabapentin toxicity by CVVHD.
Keywords: Clearance, dialysis, gabapentin, toxicity
Introduction
Gabapentin is a commonly used antiepileptic agent that has also been used for controlling neuropathic pain. The prevalence of neuropathic pain in diabetic patients is up to 36%.[1] Gabapentin is the first-line treatment for painful diabetic polyneuropathy.[2] Gabapentin is primarily excreted from the kidneys and the clearance of gabapentin is altered in patients with deranged renal function.[3] Gabapentin toxicity can be manifested with symptoms such as myoclonus,[4] hypoglycemia,[5] and altered sensorium.[6] Modalities for treatment of cases of gabapentin toxicity have not been extensively investigated, but there has been a report of effective treatment and recovery by hemodialysis[7] in a patient with suspected gabapentin toxicity in which the clearance kinetics were not described. In this case report, we describe the kinetics of gabapentin clearance by continuous renal replacement therapy (CRRT) and provide, for the first time, clearance estimates of gabapentin. This information will be useful in prescribing adequate renal replacement therapy in patients with gabapentin toxicity.
Case Report
The patient was a 57 year old man with HTN, DM-2, and stage 4 CKD (baseline creatinine of 3.6 mg/dl, eGFR by MDRD = 23 ml/min). He presented to the emergency room with a complaint of pain in his right foot. Patient was compliant with his home medications which were tamsulosin, finasteride, diltiazem, aspirin, docusate, ferrous sulfate, gabapentin, sevelamer, lantus, and lactulose. His initial blood chemistry showed blood urea nitrogen (BUN) of 81 mg/dl, serum creatinine of 6.3 mg/dl, blood glucose of 33 mg/dl, blood pH of 7.24 mg/dl, bicarbonate of 13 mg/ dl, anion gap of 14 mEq/l, and a lactate of 0.5 mEq/l. On the medical floor, the patient was assessed by the Nephrology consult service which recommended that gabapentin be substituted as it may cause toxicity in acute on chronic renal failure. Suggested dose in renal impairment for adults with creatinine clearance (CrCl), 60 ml/min or greater is 900 to 3600 mg/day given in three divided doses; CrCl, 30 to 59 ml/min, 400 to 1400 mg/day given in two divided doses; CrCl, 15 to 29 ml/min, 200 to 700 mg/day given once daily.[8] Suggested dose for adult patients on hemodialysis with CrCl, 15 ml/min is 100 to 300 mg/day given once daily; CrCl less than 15 ml/min, reduced to daily dose in proportion to CrCl (e.g., patients with a CrCl of 7.5 ml/min should receive one-half the daily dose that patients with a CrCl of 15 ml/min receive).[8] The patient was receiving a dose of 900 mg daily which was twice that of the suggested dose for patients with renal impairment. He was noted to become increasingly drowsy on the medical floor and was transferred to the ICU on account of his electrolyte abnormalities and altered sensorium. He was noted to have diffuse muscle twitches and emergent dialysis was planned for the patient. Blood chemistry in the ICU showed a BUN of 106 mg/dl, serum creatinine of 9.8 mg/dl, blood glucose of 167 mg/dl, blood pH of 7.31 mg/dl, bicarbonate of 17 mg/dl, anion gap of 16 mEq/l, and a lactate of 0.7 mEq/l. The urine analysis was positive for 3+ blood and 3+ protein. The FeNa was 3.7%. The likely cause of the acute worsening of the patient's renal function was unclear, although he was suspected of having acute tubular necrosis of unclear etiology. With 80% of gabapentin clearance resulting from excretion by the kidneys unchanged, an acute worsening of renal function served to further decrease the ability of the patient to excrete the drug in the urine, thereby further exacerbating the supratherapeutic drug concentrations.
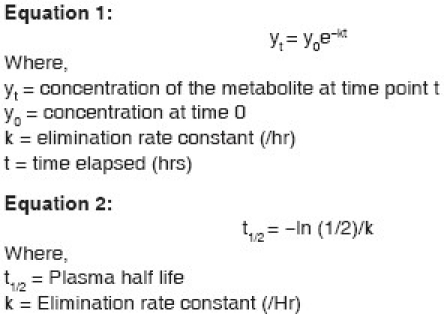
The patient remained anuric and his serum gabapentin level was 6.3 mcg/ml. The patient was commenced on CRRT for his metabolic abnormalities, acute on chronic renal failure, and for gabapentin toxicity. Gabapentin levels were drawn prior to the start of renal replacement therapy and, subsequently, levels were drawn pre- and post-filter 60, 360, and 720 minutes after being started on continuous venovenous hemodiafiltration (CVVHDF) using the Gambro Prismaflex® system. CRRT was continued approximately for 3 days and gabapentin levels were measured at multiple time points [Figure 1]. The patient started to produce urine and the CRRT was discontinued. During this period, the patient's symptoms improved significantly and his tremor completely resolved. The patient's renal function improved with his BUN decreasing to 27 and creatinine returning to 2.6. The mean hemofilter clearance of urea, creatinine, and gabapentin were 30.9 ml/min, 36.2 ml/min, and 41.1 ml/ min, respectively. The mean gabapentin clearance was 86% of the mean urea clearance and 75% of the mean CrCl. A plot of gabapentin concentration over time during CVVH showed an exponential decline [Figure 1]. By using an elimination rate constant (k) of 6.3 × 10–4/ hr and the mean gabapentin clearance (K) of 30.9 ml/ min in equation 1, the apparent volume of distribution (Vd) of gabapentin in our patient was 48.8 l. Using the elimination rate constant of gabapentin in equation 2 (detailed in Appendix), the calculated plasma half-life of gabapentin during CVVHDF was 18.23 hours.
Figure 1.
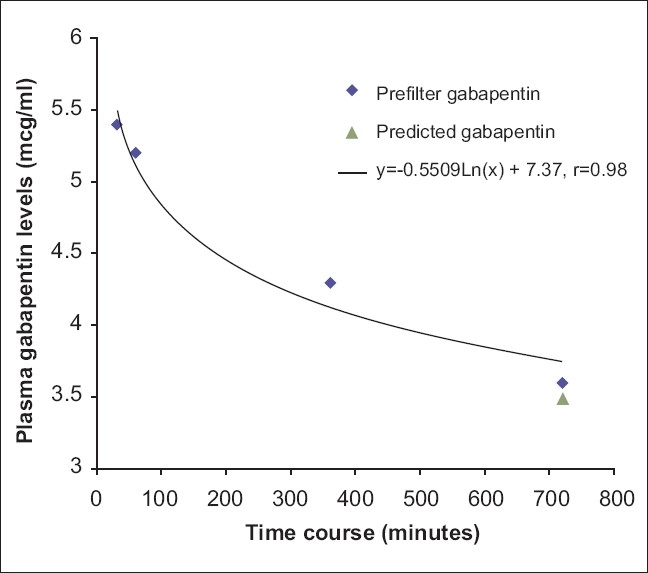
Plasma gabapentin levels vs time. Serum gabapentin levels are plotted as a function of their concentration (mcg/ml) with respect to duration of dialysis
Appendix.
Discussion
Agents that are likely to be cleared by extracorporeal techniques are those which are hydrophilic, smaller sized, polar, non-protein binding, and with a small volume of distribution. Gabapentin is a water soluble analogue of γ-aminobutyric acid. It has a low protein binding of about 3% and has a volume of distribution of 58 l in volunteers with normal renal function. Gabapentin is primarily excreted by the kidneys unchanged and its clearance linearly correlates with CrCl.[3] There is little evidence that the neurotoxicity is dose dependent, suggesting that the neurotoxicity results from a threshold effect rather than from a linear increase in concentration. Review of the patient's medications did not identify any potential drug interactions. Gabapentin-related myoclonus has even been reported to occur due to a single dose of gabapentin.[9] An increased risk of myoclonus has been associated with its use in patients with ESRD[10] and a patient with renal failure was noted to have coma attributed to gabapentin toxicity.[11] Toxins that are water soluble are easily removed by hemodialysis and tend to have a smaller volume of distribution. Lipid-soluble toxins are more difficult to remove by hemodialysis as the equilibration between the extravascular and intravascular is slower and their levels tend to rebound after cessation of hemodialysis. The volume of distribution as calculated in this case report was lesser than what was reported in normal human subjects. This is likely related to the advanced renal insufficiency in this patient. The molecular weight of gabapentin is 171.24, which is comparable with that of glucose (MW: 180). The low molecular weight, low protein binding, and resultant low volume of distribution suggest that the toxicity resulting from supratherapeutic levels can easily be corrected with the use of dialysis. This case report demonstrates and underlines the roles of dialysis in the management of gabapentin-induced neurotoxicity in chronic renal failure patients.
Acknowledgments
The authors thank Dr. Claude Neptune, MD, for assistance with measuring the gabapentin concentration.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
References
- 1.Davies M, Brophy S, Williams R, Taylor A. The prevalence, severity, and impact of painful diabetic peripheral neuropathy in type 2 diabetes. Diabetes Care. 2006;29:1518–22. doi: 10.2337/dc05-2228. [DOI] [PubMed] [Google Scholar]
- 2.Jensen TS, Backonja MM, Hernández Jiménez S, Tesfaye S, Valensi P, Ziegler D. New perspectives on the management of diabetic peripheral neuropathic pain. Diab Vasc Dis Res. 2006;3:108–19. doi: 10.3132/dvdr.2006.013. [DOI] [PubMed] [Google Scholar]
- 3.Blum RA, Comstock TJ, Sica DA, Schultz RW, Keller E, Reetze P, et al. Pharmacokinetics of gabapentin in subjects with various degrees of renal function. Clin Pharmacol Ther. 1994;56:154–9. doi: 10.1038/clpt.1994.118. [DOI] [PubMed] [Google Scholar]
- 4.Asconape J, Diedrich A, DellaBadia J. Myoclonus associated with the use of gabapentin. Epilepsia. 2000;41:479–81. doi: 10.1111/j.1528-1157.2000.tb00192.x. [DOI] [PubMed] [Google Scholar]
- 5.Penumalee S, Kissner PZ, Migdal SD. Gabapentin-induced hypoglycemia in a long-term peritoneal dialysis patient. Am J Kidney Dis. 2003;42:E3–5. doi: 10.1053/j.ajkd.2003.08.033. [DOI] [PubMed] [Google Scholar]
- 6.Sechi G, Murgia B, Sau G, Peddone L, Tirotto A, Barrocu M, et al. Asterixis and toxic encephalopathy induced by gabapentin. Prog Neuropsychopharmacol Biol Psychiatry. 2004;28:195–9. doi: 10.1016/S0278-5846(03)00166-0. [DOI] [PubMed] [Google Scholar]
- 7.Hung TY, Seow VK, Chong CF, Wang TL, Chen CC. Gabapentin toxicity: An important cause of altered consciousness in patients with uraemia. Emerg Med J. 2008;25:178–9. doi: 10.1136/emj.2007.053470. [DOI] [PubMed] [Google Scholar]
- 8.Backonja M, Glanzman RL. Gabapentin dosing for neuropathic pain: Evidence from randomized, placebo-controlled clinical trials. Clin Ther. 2003;25:81–104. doi: 10.1016/s0149-2918(03)90011-7. [DOI] [PubMed] [Google Scholar]
- 9.Holtkamp M, Halle A, Meierkord H, Masuhr F. Gabapentin-induced severe myoclonus in a patient with impaired renal function. J Neurol. 2006;253:382–3. doi: 10.1007/s00415-005-0970-1. [DOI] [PubMed] [Google Scholar]
- 10.Zhang C, Glenn DG, Bell WL, O’Donovan CA. Gabapentin-induced myoclonus in end-stage renal disease. Epilepsia. 2005;46:156–8. doi: 10.1111/j.0013-9580.2005.20804.x. [DOI] [PubMed] [Google Scholar]
- 11.Dogukan A, Aygen B, Berilgen MS, Dag S, Bektas S, Gunal AI. Gabapentin-induced coma in a patient with renal failure. Hemodial Int. 2006;10:168–9. doi: 10.1111/j.1542-4758.2006.00089.x. [DOI] [PubMed] [Google Scholar]


