Abstract
BACKGROUND:
In vitro and in vivo antileishmanial activities of crude hydroalcoholic extract of peganum harmala seeds were investigated against Leishmania major.
METHODS:
The extract of aerial parts of P harmala was obtained by maceration. The in vitro experiments were performed on promastigotes to assess antileishmanial activity of the extract using amphotericin B as a reference. The in vivo studies were carried out on cutaneous leishmaniasis in outbred mice to evaluate the effects of topical application of the ointment-based extract.
RESULTS:
The in vitro experiments showed a concentration-dependent decrease of parasites number caused by the extract with an IC50 value of 59.4 μg/ml. In vivo studies demonstrated a significant post-treatment decrease in the lesion size and parasite count in infected animals, compared to placebo and control groups. High performance liquid chromatography (HPLC) of the crude extract demonstrated the existence of harmaline and harmine as beta-carboline alkaloids.
CONCLUSIONS:
P harmala seeds extract showed significant in vitro and in vivo antileishmanial activities. Most biological activity of the extract could be attributed to its beta-carboline content. However, another alkaloid of P harmala seeds extract, peganine, has also been reported to have antileishmanial activity. These beneficial effects can be attributed to the cumulative effects of various biologically active components present in it.
KEYWORDS: Cutaneous leishmaniasis, Leishmania major, Peganum harmala, outbred mice
Cutaneous leishmaniasis (CL), an endemic disease in many tropical and subtropical areas, including central and southern parts of Iran, continues to present serious treatment problems. The disease, although usually self-limiting, can cause considerable morbidity and may result in severe disfigurement. The manifestations can be greatly variable depending on the strain of the infecting organism, the host's immunological status and the probable secondary infection. Pentavalent antimonials such as sodium stibogluconate, have been the mainstay for therapy in the endemic regions because of its efficacy and cost effectiveness.1,2 The disadvantages of the antimonials are their requirement for intramuscular or intravenous injection each day for 20-28 days, their toxicity and the growing incidence of resistance in endemic and non-endemic regions.2,3 The second line drugs such as amphotericin B, paromomycin and miltefosine all suffer from limitations of either cost, specific toxicities or need for parenteral administration.2,4 Other therapeutic modalities include pentamidine and azole antifungals.1 Recent investigations focused on plants have shown an alternative way to get a potentially rich source of drug candidates against leishmaniasis, in which effective alkaloids have been found.2 Moreover, topical treatment of CL is attractive compared with the systemic treatment because of the easy application, particularly in remote areas.
Peganum harmala (Zygophylaceae) is a medicinal herb with a long history of folkloristic use in Iran. Antimicrobial,5–7 antifungal,8 antiprotozoal7 and anticancer9,10 effects of P harmala extract have been reported. Also, the inhibitory effects of P harmala seeds extract and its beta-carboline alkaloids on human DNA topoisomerase I have been previously shown.11 The current study is an attempt to develop a topical treatment for CL. The objectives were to assess the in vitro and in vivo antileishmanial potentials of the P harmala seeds extract on Leishmania major. In vitro antileishmanial activity of P harmala seeds extract against L major promastigotes has been previously reported;12,13 however, there is some inconsistency between their results and those achieved in our experiments that will be discussed later.
Methods
Plant material
Aerial parts of P. harmala were collected from around Tehran province. The plant was verified by a taxonomist, and a voucher specimen (6520 THE) was deposited at the Herbarium of Department of Pharmacognosy, Faculty of Pharmacy, Tehran University of Medical Sciences, Tehran, Iran. The seeds were air dried at room temperature and kept in a dark-amber-colored bottle until processed.
Preparation of extract of P harmala seeds
The crude extract was obtained by maceration of 100 g dried seeds in 200 ml solvent (55 ml double distilled water, 45 ml ethyl acetate and 100 ml 95% ethanol) in a dark place at room temperature. After one week, the fresh extract was filtered. The solvent was evaporated in vacuo. Dried extract was stored in a dark amber-colored bottle. All the concentrations of the extract were based on dry weight of the extract.
High performance liquid chromatography (HPLC) of the extract
Based on the method developed by Kartal et al.,14 HPLC method was performed with a LC system consisting of a Young Lin model SP-930D pump and a UV-730D Young Lin UV/VIS detector. Separation was carried out using an EC 250/4.6 Nucleosil 100-10 C18 Macherey-Nagel column. All the calculations concerning the quantitative analysis were carried out with external standardization by measurement of peak areas. HPLC analysis was performed by isocratic elution with a flow rate of 1 ml/min. The mobile phase composition was acetonitrile-water-trifluoroacetic acid (TFA) (25:75:0.1) (v/v/v) and pH was adjusted to 8.4 with triethylamine. All solvents were filtered through a 0.22 μM milipore filter before use and degassed in an ultrasonic bath. 10 μl of sample was injected into the column. Quantification was affected by measuring at 254 nm. The chromatographic run time was 15 minutes.
Parasite preparation
The strain of L. major, obtained from Iran Pasteur Institute, had been isolated from CL cases in Isfahan, one of the central provinces of Iran in which CL is endemic. The parasites were kept in BALB/c mice or in Novy-Macneal-Nicolle (NNN) medium and subpassages in NNN medium were used for the experiments. The culture was incubated at 25°C and used within 2 weeks of cultivation.
In vitro experiments
Leishmania major promastigotes in late logphase were incubated in RPMI medium supplemented with 12% fetal calf serum, at an average of 104 parasites/ml. Parasites were incubated, in duplicate cultures, with ascending concentrations of the extract solubilized in dimethyl sulphoxide (DMSO). The highest DMSO concentration used was 1.0% (v/v), which was not hazardous to the parasites. After 24 hours incubation period at 26°Q the surviving promastigotes were counted in a Neubauer's chamber. Half maximal (50%) inhibitory concentration (IC50) and maximal (100%) inhibitory concentration (IC100) were determined as the concentration of the extract necessary to inhibit 50% and 100%, respectively, of parasite growth. Negative controls treated by solvent (DMSO) and positive controls containing amphotericin B (1 μg/ml) were added to each set of experiments.
In vivo experiments
Animals
Outbred albino mice, 8 weeks old, were used for in vivo studies. The protocol of in vivo experiments was approved by the Institutional Ethical Committee (letter # P387 of Iran University of Medical Sciences).
Effect of the extract on L. major development
The effect of the extract on in vivo development of L major was monitored in mice by following the development of a local dermal lesion caused by the parasite. The mice were inoculated in the base of the tail with 1-2 × 106 infective promastigotes. The development of lesion was followed macroscopically, and the presence of parasites in biopsy material was monitored microscopically in smears. Material aspirated with a fine syringe through a small incision made at the margin of the lesion with sterile surgical blade, was stained with Giemsa. The results were expressed as lesion size and parasite count before (week 0), just after (beginning of week 4) and 6 weeks after completion of (beginning of week 10) the treatment. These parameters were compared with those of placebo and control groups. Control group did not receive any treatment and placebo group received the ointment base without the extract.
Topical formulation and ointment regimen design
A dried extract was prepared in the ointment base with the following formulation: P. harmala extract 5% and 20%, lanolin 10% and DMSO 12%, all incorporated in white soft paraffin. No preservative was added and the ointments were kept at 4°C and tested within 1 week after preparation. Once a well-developed lesion was observed, the ointment was applied twice daily (early in the morning and late afternoon) for a period of 20 days. To determine the amount of the ointment applied to the lesion, the ointment was weighted before application. It was found that a 200-mg amount of ointment was used per mouse per day.
Statistical analysis
The data are expressed as mean ± SEM (standard error of mean) of n animals. Mean values were analyzed with a two way analysis of variance (ANOVA). Differences between mean values were accepted as significant when p < 0.05. All statistical analyses were done using SPSS software, version 11.5.
Results
HPLC of the extract of Peganum harmala
Using known standards of harman alkaloids,15 i.e., harmaline and harmine, the HPLC analysis of the crude extract showed the existence of these two beta-carboline alkaloids (Figure 1). Harmaline and harmine made up 3% and 1.7% of total weight of Peganum harmala seeds, respectively.
Figure 1.
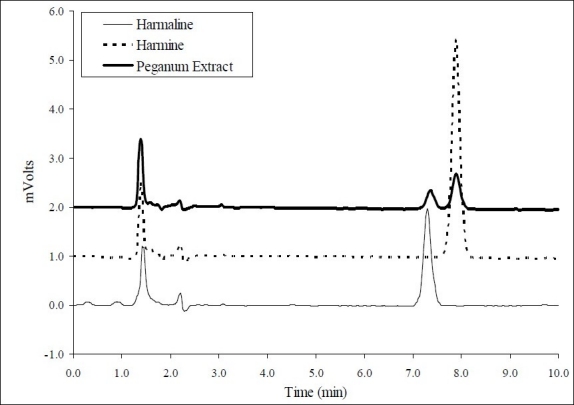
HPLC chromatograms of harmaline (retention time = 7.33 min) and harmine (retention time = 7.75 min) standards and the hydroalcoholic extract of Peganum harmalaseeds.
In vitro effects on parasites
As shown in Figure 2, the extract of P. harmala seeds showed activity against extracellular flagellated promastigotes in a concentration-dependent manner. The extract exhibited moderate cytotoxic activity with IC50 = 59.4 μg/ml and IC100 = 350 μg/ml.
Figure 2.
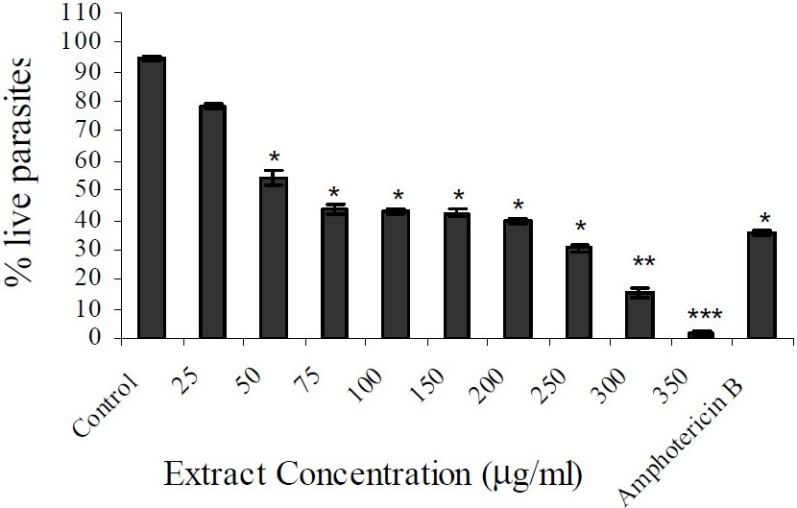
In vitro effects of the extract of Peganum harmala seeds on Leishmania major promastigotes. Control: 1% DMSO; 1μg/ml amphotericin B was used as the reference. The values represent the mean ± SEM. *p<0.05, **p<0.005, ***p<0.001
In vivo experiments
Development of cutaneous leishmaniasis in outbred mice
A nodule developed 2 to 3 weeks after the inoculation of 1-2 × 106 promastigotes of L major into the base of the tail of a mouse. Two weeks later, the nodule transformed into an ulcer that was increased in size. The internal organs, i.e., spleen and liver, did not become parasitized in outbred mice. This was confirmed in random autopsies of control mice. Therefore, the infected animal could be regarded as a suitable model for studying the topical effect of drugs in CL. No spontaneous healing or recovery from infection was observed in control mice.
Antileishmanial activity of P. harmala extract
After 20 days of treatment, twice daily, with the ointment base of P. harmala seed extract, the parasite in 2 out of 10 animals in each test group (5% and 20% ointments) disappeared from the lesion area, and the open lesion completely healed in a period of 6 weeks, i.e., up to the completion of the study. In other animals, the lesion size in the two test groups (5% and 20% extract) were significantly decreased in comparison with placebo and control groups (Figure 3). The parasite count in both test groups was also diminished significantly in comparison with placebo and control groups (Figure 4).
Figure 3.
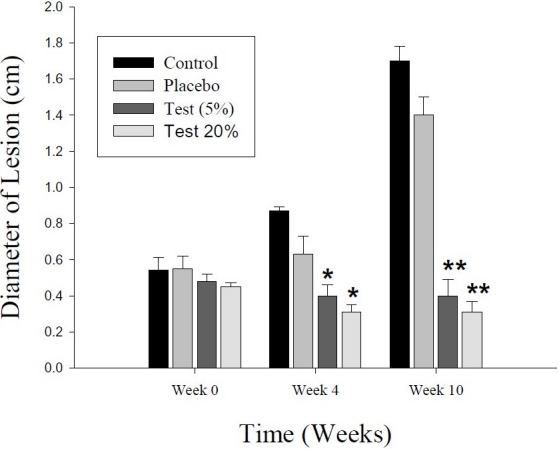
Effect of a 20-day period of topical treatment with ointment-based extract (5% and 20%) of Peganum harmala seeds on cutaneous leishmaniasis in outbred mice infected with Leishmania major. Lesion size in each test group is compared with those of control and placebo groups at the beginning of (week 0), just after (week 4) and 6 weeks after completion of (week 10) the treatment. The values represent the mean ± SEM. *p < 0.05; **p < 0.001
Figure 4.
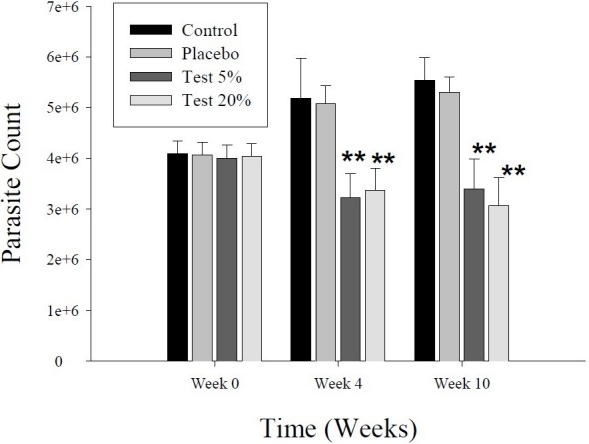
Effect of a 20-day period of topical treatment with ointment-based extract (5% and 20%) of Peganum harmala seeds on cutaneous leishmaniasis in outbred mice infected with Leishmania major. Parasite count in each test group is compared with those of control and placebo groups at the beginning of (week 0), just after (week 4) and 6 weeks after completion of (week 10) the treatment. The values represent the mean ± SEM. **p< 0.001
Discussion
L major is a causative agent of CL in many central and southern parts of Iran. Since antileishmanial chemotherapy depends upon a small group of compounds,1,3,16 development of new drugs is of great importance. In this regard, medicinal plants seem to be promising candidates for drug discovery against leishmaniasis.2
The present study was an attempt to develop a topical treatment for CL. An effective anti-leishmanial activity for the extract of P. harmala seeds was demonstrated in vitro and in vivo. The extract exhibited moderate in vitro cytotoxic activity, compared with amphotericin B (1 μg/ml), with IC50 = 59.4 μg/ml against extracellular flagellated promastigotes. Moreover, the results of in vivo study showed that the lesion size and parasite count decreased dramatically, in comparison with control and placebo groups, after a 20-day topical treatment of 5% and 20% ointments of P. harmala seed extract. Discontinuation of treatment did not lead to significant increase in the lesion size or parasite count during a 6-week period. In each test groups (i.e., 5% and 20%), the lesions in 2 animals, out of 10, disappeared completely. The results did not differ significantly in the two test groups.
An in vivo study was performed on outbred albino mice. During the experiments, L. major did not produce visceral infection in the animals so they seemed to be a suitable animal model for the present study. The fact that a spontaneous healing was not demonstrated in the control mice indicates that healing, to any extent, was due to topical treatment alone.
To develop a suitable semisolid antileishmanial preparation, an ointment base of the extract was prepared. White soft paraffin (petrolatum) was selected as a typical oleaginous ointment base in view of its widespread use for many pharmaceutical ointments and lanolin was added to increase the hydrophillicity of the vehicle. DMSO is a well known penetration enhancer16,17 and was used to increase percutaneous absorption of the drug.
In vitro antileishmanial activity of P. harmala seeds extract against L. major promastigotes has been previously reported;12,13 however, we found IC50 values of 59.4 μg/ml as compared to results from Mirzaie et al. in which IC50 values of the extract against L. major promastigotes demonstrated to be about 1832 μg/ml. This might arise from the differences due to plant collection parameters (locality, season, etc.), protocols of extract preparation and/or protocols of in vitro experiments.
It has been shown that P. harmala seed extract inhibited human DNA topoisomerase I and it appears that the biological activity of the extract can be explained by its beta-carboline content.11,19 P. harmala, especially in its seeds and roots, contains several alkaloids. The alkaloid content of the plant includes beta-carbolines (harmaline, harmine, harmalol and harmane) and quinazoline derivatives (vasicine and vasicinone).20 In the present study, using the HPLC method, harmaline was found to be present in the highest concentration in the extract followed by harmine. This is consistent with the results reported by others.7,11 Beta-carboline derivatives have been demonstrated to have antiparasitic properties. Evans and Croft21 showed that harmaline exerted in vitro and in vivo antileishmanial activity. In another study, in vitro antileishmanial activity of other beta-carboline alkaloids, i.e., harmine and harmane, against L. infantum was compared to that of harmaline.22 Harmine and harmane displayed a moderate antiproliferative activity toward human monocytes and exerted a weak antileishmanial activity toward both the promastigote and the amastigote forms of the parasite. On the contrary, harmaline was devoid of toxicity toward human cells and leishmania promastigotes; however, it exerted a strong antileishmanial activity toward the intracellular amastigote form of the parasite. The results observed in the latter study suggested that inhibition of promastigote internalization within macrophages could result from inhibition of protein kinase C activity by harmaline. In another study, harmine was tested for its anti-leishmanial properties against L. donovani both in vitro and in vivo. In vitro antileishmanial activity of harmine was encouraging. Harmine was also found to reduce spleen parasite load in hamster model and toxicity of the drug was reduced using vesicular forms such as liposomes, niosomes and nanoparticles.23
In addition to beta-carboline alkaloids, fractionation of P. harmala seed extract has led to isolation and identification of an alkaloid named peganine. In vitro antileishmanial activity of the compound was assessed against L. donovanis. The results indicated that, besides being safe, it could induce apoptosis in the parasite via loss of mitochondrial transmembrane potential. The compound also inhibited L. donovani topoisomerase I.24 In another study, peganine exhibited in vitro activity against both extracellular promastigotes as well as intracellular amastigotes residing within murine macrophages in L. donovani. Furthermore, oral administration of the compound also exerted in-vivo antileishmanial activity against established visceral leishmaniasis in hamsters.25 It should be mentioned that a case of intoxication after the ingestion of self-made P. harmala preparation has been reported.26 it seems that harmine and harmaline inhibit mono-amino oxidase type A (MAO-A) enzyme which leads to psychological disorders such as hallucination.27 This means that a caution should be practiced in systemic use of this drug although in topical application, e.g., as an antileishmanial drug, the incidence of the adverse reactions may be reduced.
In conclusion, the beneficial effect of P. harmala seeds extract can be attributed to the cumulative effect of various biologically active components present in it.28,29 Future comparative studies are needed to reveal which fraction(s) shows more antileishmanial activity and less toxicity.
Authors’ Contributions
PRM and MSh designed the study and served as supervisors. MSe, MK and GHH did the experiments and collected the data. Data from HPLC method was analyzed under SAE guidance. MHA provided parasites from Iran Pasture Institute and instructed the student how to make dermal lesion in animals. HO as the Head of Department of Parasitology and MM as the Head of Department of Pharmacology provided some facilities. MM also proposed the general subject of the study. PRM and MSh wrote the manuscript. All authors have read and approved the content of the manuscript.
Acknowledgments
This study was supported by a grant from Tehran University of Medical Sciences and a grant from Razi Institute for Drug Research (Grant no. 56-MT).
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Markle WH, Makhoul K. Cutaneous leishmaniasis: recognition and treatment. Am Fam Physician. 2004;69(6):1455–60. [PubMed] [Google Scholar]
- 2.Mishra BB, Kale RR, Singh RK, Tiwari VK. Alkaloids: future prospective to combat leishmaniasis. Fitoterapia. 2009;80(2):81–90. doi: 10.1016/j.fitote.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 3.Berman J. Current treatment approaches to leishmaniasis. Curr Opin Infect Dis. 2003;16(5):397–401. doi: 10.1097/00001432-200310000-00005. [DOI] [PubMed] [Google Scholar]
- 4.Croft SL, Seifert K, Yardley V. Current scenario of drug development for leishmaniasis. Indian J Med Res. 2006;123(3):399–410. [PubMed] [Google Scholar]
- 5.Harsh ML, Nag TN. Antimicrobial principles from in vitro tissue culture of Peganum harmala. J Nat Prod. 1984;47(2):365–7. doi: 10.1021/np50032a022. [DOI] [PubMed] [Google Scholar]
- 6.Al-Shamma A, Drake S, Flynn DL, Mitscher LA, Park YH, Rao GS, et al. Antimicrobial agents from higher plants.Antimicrobial agents from Peganum harmala seeds. J Nat Prod. 1981;44(6):745–7. doi: 10.1021/np50018a025. [DOI] [PubMed] [Google Scholar]
- 7.Arshad N, Zitterl-Eglseer K, Hasnain S, Hess M. Effect of Peganum harmala or its beta-carboline alkaloids on certain antibiotic resistant strains of bacteria and protozoa from poultry. Phytother Res. 2008;22(11):1533–8. doi: 10.1002/ptr.2528. [DOI] [PubMed] [Google Scholar]
- 8.el Bahri L, Chemli R. Peganum harmala L: a poisonous plant of North Africa. Vet Hum Toxicol. 1991;33(3):276–7. [PubMed] [Google Scholar]
- 9.al-Allaf TA, Khuzaie RF, Rashan LJ, Halaseh WF. Cytotoxic activity against a series of tumour cell lines of dimethyltin dichloride complexes with various donor ligands. Boll Chim Farm. 1999;138(6):267–71. [PubMed] [Google Scholar]
- 10.Lamchouri F, Settaf A, Cherrah Y, Zemzami M, Lyoussi B, Zaid A, et al. Antitumour principles from Peganum harmala seeds. Therapie. 1999;54(6):753–8. [PubMed] [Google Scholar]
- 11.Sobhani AM, Ebrahimi SA, Mahmoudian M. An in vitro evaluation of human DNA topoisomerase I inhibition by Peganum harmala L.seeds extract and its beta-carboline alkaloids. J Pharm Pharm Sci. 2002;5(1):19–23. [PubMed] [Google Scholar]
- 12.Mirzaie M, Nosratabadi SJ, Derakhshanfar A, Sharifi I. Antileishmanial activity of Peganum harmala extract on the in vitro growth of Leishmania major promastigotes in comparison to a trivalent antimony drug. Vet Arhiv. 2007;77(4):365–75. [Google Scholar]
- 13.Yousefi R, Ghaffarifar F, Dalimi-Asl A. The effect of Alkanna tincturia and Peganum harmala extracts on Leishmania major (MRHO/IR/75/ER) in vitro. Iranian J Parasitol. 2009;4(1):40–7. [Google Scholar]
- 14.Kartal M, Altun ML, Kurucu S. HPLC method for the analysis of harmol, harmalol, harmine and harmaline in the seeds of Peganum harmala L. J Pharm Biomed Anal. 2003;31(2):263–9. doi: 10.1016/s0731-7085(02)00568-x. [DOI] [PubMed] [Google Scholar]
- 15.Bukhari N, Choi JH, Jeon CW, Park HW, Kim WH, Khan MA, et al. Phytochemical studies of the alkaloids from Peganum harmala. Applied Chamistry. 2008;12(1):101–4. [Google Scholar]
- 16.Enk CD, Gardlo K, Hochberg M, Ingber A, Ruzicka T. [Cutaneous leishmaniasis] Hautarzt. 2003;54(6):506–12. doi: 10.1007/s00105-003-0530-5. [DOI] [PubMed] [Google Scholar]
- 17.Idson B. Percutaneous absorption. J Pharm Sci. 1975;64(6):901–24. doi: 10.1002/jps.2600640604. [DOI] [PubMed] [Google Scholar]
- 18.Wood DC, Wood J. Pharmacologic and biochemical considerations of dimethyl sulfoxide. Ann N Y Acad Sci. 1975;243:7–19. doi: 10.1111/j.1749-6632.1975.tb25339.x. [DOI] [PubMed] [Google Scholar]
- 19.Nafisi S, Bonsaii M, Maali P, Khalilzadeh MA, Manouchehri F. Beta-Carboline alkaloids bind DNA. J Photochem Photobiol B Biol. 2010;100(2):84–91. doi: 10.1016/j.jphotobiol.2010.05.005. [DOI] [PubMed] [Google Scholar]
- 20.Kamel SH, Ibrahim TM, Afifi AA, Hamza SM. Major alkaloidal constituents of the Egyptian plant, Peganum harmala. UAR J Vet Sci. 1970;7(1):71–85. [Google Scholar]
- 21.Evans AT, Croft SL. Antileishmanial activity of harmaline and other tryptamine derivatives. Phytother Res. 1987;1(1):25–7. [Google Scholar]
- 22.Di Giorgio C, Delmas F, Ollivier E, Elias R, Balansard G, Timon-David P. In vitro activity of the beta-carboline alkaloids harmane, harmine, and harmaline toward parasites of the species Leishmania infantum. Exp Parasitol. 2004;106(3-4):67–74. doi: 10.1016/j.exppara.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 23.Lala S, Pramanick S, Mukhopadhyay S, Bandyopadhyay S, Basu MK. Harmine: evaluation of its antileishmanial properties in various vesicular delivery systems. J Drug Target. 2004;12(3):165–75. doi: 10.1080/10611860410001712696. [DOI] [PubMed] [Google Scholar]
- 24.Misra P, Khaliq T, Dixit A, SenGupta S, Samant M, Kumari S, et al. Antileishmanial activity mediated by apoptosis and structure-based target study of peganine hydrochloride dihydrate: an approach for rational drug design. J Antimicrob Chemother. 2008;62(5):998–1002. doi: 10.1093/jac/dkn319. [DOI] [PubMed] [Google Scholar]
- 25.Khaliq T, Misra P, Gupta S, Reddy KP, Kant R, Maulik PR, et al. Peganine hydrochloride dihydrate an orally active antileishmanial agent. Bioorg Med Chem Lett. 2009;19(9):2585–6. doi: 10.1016/j.bmcl.2009.03.039. [DOI] [PubMed] [Google Scholar]
- 26.Frison G, Favretto D, Zancanaro F, Fazzin G, Ferrara SD. A case of β-carboline alkaloid intoxication following ingestion of Peganum harmala seed extract. Forens Sci Int. 2008;179(2):e37–43. doi: 10.1016/j.forsciint.2008.05.003. [DOI] [PubMed] [Google Scholar]
- 27.McKenna DJ, Towers GHN, Abbott F. Monoamino oxidase inhibitors in South American hallucinogenic plants: tryptamine and beta carboline constituents of Ayahuasca. J Ethnopharmacol. 1984;10(2):195–223. doi: 10.1016/0378-8741(84)90003-5. [DOI] [PubMed] [Google Scholar]
- 28.Mishra BB, Kale RR, Singh RK, Tiwari VK. Alkaloids: Future prospective to combat leishmaniasis. Fitoterapia. 2009;80(2):81–90. doi: 10.1016/j.fitote.2008.10.009. [DOI] [PubMed] [Google Scholar]
- 29.Richard JV, Werbovetz KA. New antileishmanial candidates and lead compounds. Curr Opin Chem Biol. 2010;14(4):447–55. doi: 10.1016/j.cbpa.2010.03.023. [DOI] [PubMed] [Google Scholar]


