Abstract
BACKGROUND:
There are controversial evidences on the association between fingerprint traits and schizophrenia. We compared fingerprint traits of patients with schizophrenia and normal individuals in Iranian population.
METHODS:
Finger tip dermal ridge of 290 patients with schizophrenia and 290 normal subjects were studied for four dermal traits. Data was analyzed with Pearson correlation and student's tests.
RESULTS:
Finger print patterns and secondary creases were not significantly different between the two groups (p > 0.05). Although mean ridge counts of left and right index fingers of the case group were greater than the control group (p < 0.05), these differences were not significant in females.
CONCLUSIONS:
Probably the left index ridge counts and fluctuating asymmetry in schizophrenic patients are different from those of the normal population. This difference may serve as a diagnostic biological marker for screening people susceptible to schizophrenia. Further studies are needed to determine predictive value of fingerprint trait as a biomarker for the schizophrenia.
KEYWORDS: Schizophrenia, Fingerprint Pattern, Biomarker, Dermatoglyphics, Fluctuating Asymmetry, Ridge Counts
The relation between somatic and psychic characteristics has been addressed for many centuries. The oldest classification of psychic characteristics is based on somatic characteristics referred by Hippocrates and Claudius. They attributed several personality characters to each of the sputum.1 The development of biological and psychological sciences has suggested the idea that by scientific methods we can understand the relation between psychic and somatic characteristics. Today, considerable progression has been made in understanding the association between dermatoglyphics and various medical disorders. Dermatoglyphics analysis has been investigated as a useful diagnostic and research tool in medicine and provides valuable insight on the inheritance and/ or embryologie formation of many known clinical disorders.2 For example, ridge count is increased in Turner's syndrome and decreased in Klinefelter's and chromosome 5p deletion syndromes,3,4 betathalasemia,5 and rheumatoid patients.6
So far, dermatoglyphics and social behavior have been studied.7,8 Significant dermatoglyphic differences were found among the patients suffering schizophrenia with or without a positive family history of the disease, suggesting a strong “genetic loading” in familial cases of schizophrenia.10 Genetics plus encountering environmental stressors at the second-trimester are proposed to be involved for the etiology of familial cases of schizophrenia. It has been suggested that probably during the second prenatal trimester, an environmental stressor (such as anatomical insults) differentially affects the twins and only one of them will show a tendency towards developing schizophrenia.9
In a bilaterally symmetrical organism such as man, each half of the body tends to develop as a mirror image of the other. One exception that may occur is called “fluctuating asymmetry”. Fluctuating asymmetry (FA) is a non-directional, random asymmetry that may occur for any measurable bilateral feature of an organism such as length of arms or size of feet. It therefore differs from those directional asymmetries found in all members of a species such as number of lobes in the right or left lung in man. FA occurs when environmental factors interfere with the ability of an organism to execute its developmental program the same way in both sides. Some individuals are better able than others to buffer these environmental interferences during development. Although schizophrenia has been shown to have a familial basis, the exact nature of its transmission has yet to be elucidated.1–10,11
Markow and Wandler12 studied a sample of 81 subjects using the palmar ridge count and fingerprint patterns. For both traits, the FA was significantly more frequent in patients with schizophrenia than in controls. This finding shows the organism's capacity to buffer the adverse effects of factors that could disturb its developmental process. Dermatoglyphics have been recently used in case finding of developmental disorders. Markow and Gottesman subsequently found that twins who showed concordance for schizophrenia had higher levels of finger ridge count FA than discordant twin pairs.13 On the basis of the period of dermatoglyphics development (14th-22nd gestational week), the relatively high rate of minor physical anomalies observed in schizophrenia can be considered to reflect at least second trimester maldevelopment.14,15
A study in Ireland compared finger and thumb prints in 46 schizophrenic patients with 43 normal controls. Seven prints had very high density creases in the case group.16 Due to high variability of results obtained from different researches, more studies are needed to clarify the differences between patients with schizophrenia and normal people.
Most previous studies had focused on epidemiological analysis of quantitative dermatoglyphic traits as a marker of prenatal disturbance during the second trimester in schizophrenic patients.17,18 Although more recent studies are conducted for other diseases controversies still exist.5–7 We conducted this study to find out the difference between the fingerprint traits (not only quantitative items such as ridge count, secondary creases but also qualitative ones i.e. pattern and FA) of patients with schizophrenia and normal individuals, as a marker regardless of genetic or environmental etiology of them.
Methods
This study examined four dermatoglyphic traits of left and right index fingerprints of 290 patients with schizophrenia (case group) and 290 normal subjects (control group). Cases were randomly selected from outpatient and inpatient psychiatric departments of Noor hospital, Isfahan, Iran. All subjects were diagnosed as schizophrenic based on the DSM-IV diagnostic criteria. The controls were selected randomly by researchers from the staff of the same three hospitals who had no psychiatric disorders. Fingerprints were obtained with black ink spread on glass method, in which fingertips were rolled on ink and then on a white paper to produce a black print.2–4
Dermatoglyphic traits were selected for detection of bilateral asymmetry because of the ease at which permanent arc prints were obtained and also due to the fact that they do not change after birth. The four dermatoglyphic traits consisted of fingerprint patterns, finger ridge counts, FA of finger ridge counts and secondary creases density.
· FA: This investigation consisted of a new analysis, i.e. the estimation of the FA of dermatoglyphic material that Mellor et al. had previously described.4,17 Random differences in size between supposedly identical right-sided and left-sided structures are believed to be an indicator of developmental stability. A high level of FA signifies that the organism has a low capacity for buffering adverse environmental effects that could deflect the course of its genetically determined program of development.1–4
· Finger ridge counts: All ridges along a straight line which connected the triradial point (a triradius is formed by the confluence of the three ridge systems) to the closest point of the core were counted.3,15,16
· Fingerprint patterns: As shown in Figure 1, the main patterns are simple arch, tented arch, whorl and loop pattern which have several accessory patterns.3,15,16
Figure 1.
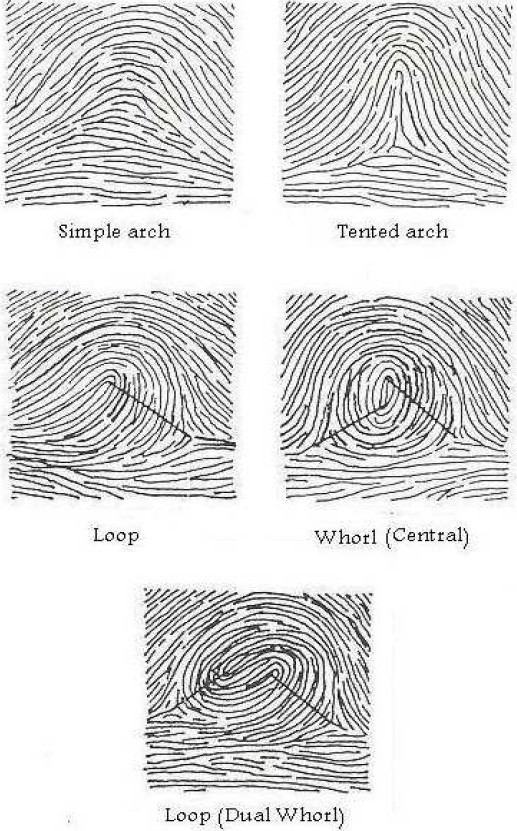
Ridge count of different finger patterns.
· Secondary creases: They are very little grooves that present and cut skin ridges and are observed as white lines (Figure 2).3,15,16
Figure 2.

A fingerprint that has high density secondary creases covering the main pattern.
Moreover, the correlation coefficient (r) of the ridge counts of the patterns on index fingers of right and left hands were measured separately. The square of the correlation coefficient (r2) of the two variables (right and left finger ridge counts of identical patterns) is a measure of their common variance and 1- r2, sometimes known as the coefficient of indetermination (Sokal and Rohlf, 1981), is an estimate of their unshared variance and thus of FA.19
Data was analyzed by chi-square, running Pearson correlation and t-student tests using SPSS software version 11.5 (SPSS Inc. Chicago, IL). A p-value less than 0.05 was considered statistically significant.
Results
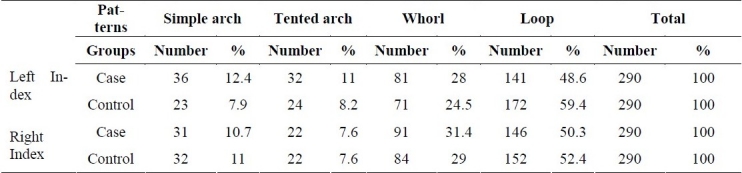
Male to female ratio was equal in both groups (203 males vs. 87 females) so there was no difference between the two groups in terms of gender. Table 1 displays frequencies of left and right index fingerprint patterns in the case and control groups. Chi-square test did not show any significant difference between index patterns of the case and control groups (p = 0.062).
Table 1.
Frequency distribution of index fingerprint patterns in the two groups
As shown in Table 2, the mean of both index finger ridge counts was 15.5 ± 4.3 and 13.6 ± 6.3 for the case and control groups, respectively.The t-student test analysis showed a significant difference between left and right index ridge counts between the case and control groups (p < 0.001).
Table 2.
Average and fluctuating asymmetry of both index ridge counts in the two groups (Mean ± standard deviation).
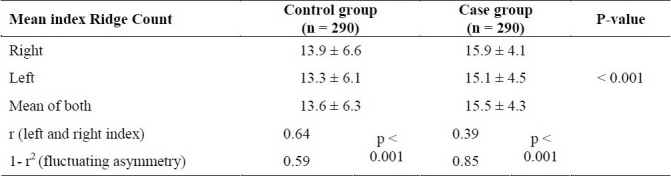
Correlation coefficient (r) between right and left index ridge counts was 0.64 and 0.39 in control and case group respectively (p < 0.0001). In the other word the level of Fluctuating Asymmetry (1- r2) was 0.59 in the control group and 0.85 in the patients with schizophrenia (Table 2).
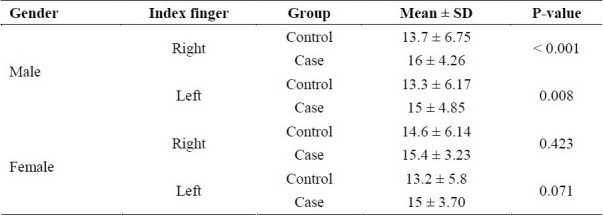
However, significant differences were found between control and case groups in terms of the mean ridge count of their both index fingers, but as shown in Table 3, these differences were contributed to males, not females. In other words men with schizophrenia had higher mean ridge count for both index fingers than normal men (p < 0.05) while for women this difference was not significant (p > 0.05).
Table 3.
Right and left mean ridge count in each sex of both groups (Mean ± standard deviation).
On the other hand, while paired t-test analysis between right and left mean ridge counts of index fingers in the case group did not show any significant differences (15.9 ± 3.8 vs. 15.3 ± 6.2, respectively; p = 0.126), in the control group right index fingers had significantly higher ridge counts than left ones (14.2 ± 6.6 vs. 13.4 ± 6.2; p = 0.037; Figure 3).
Figure 3.
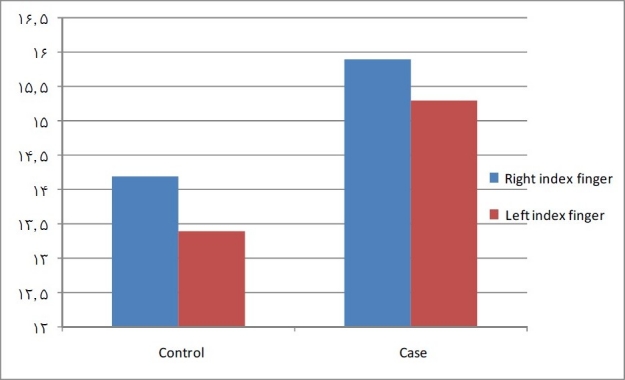
Comparison between right and left index fingers’ mean ridge counts in each group.
Finally, secondary creases in each group were divided to low density (< 5 lines in each finger) and high density (≥5 lines) and these creases had no significant difference between the case and the control groups according to student-t test (p > 0.05).
Discussion
A high level of FA signifies that the organism has a low capacity for buffering adverse environmental effects that could deflect the course of its genetically determined program of development (Van Valen, 1962). An association between FA and a particular disorder probably suggests that the same multiple alleles play a part in the etiology of both.20
Our results revealed that there are some differences between patients with schizophrenia and normal population in their fingerprint findings. In order to investigate the proposed hypothesis, right index fingerprint patterns of case group were compared with those of the control group, but no differences were found. Findings from both index fingers of the case and the control groups show that none of the left and right index fingerprint patterns have statistically significant different frequencies between two studied groups but their differences in the left index finger patterns were near the acceptable level of significance. These findings suggest that left index finger print patterns of patients with schizophrenia are different from normal controls which is consistent with the report of higher frequency of arch pattern and lower frequency of whorl pattern than others in patients with schizophrenia.19 In this study, the observed frequency of loop pattern was more common and some differences were found between index fingers of the two groups.
Some previous studies suggest that the density of secondary creases shows differences between patients with schizophrenia and normal persons.16 In our study, secondary creases of the patients’ right and left index fingers had no differences. Similarly, secondary creases of right and left index fingers of low and high density categories were same between the two groups. The second hypothesis of the study, however, was not confirmed. This finding is not consistent with the reported higher frequency of higher density secondary creases in some patients with schizophrenia by Canon and colleagues.16 The difference in the results of the two studies may be due to different numbers of subjects (they investigated 89 persons whereas 580 persons were involved in this study).
The third hypothesis of this study proposing different finger ridge counts between schizophrenic patients and normal persons was confirmed. Case and control groups showed a statistically significant difference in terms of their both index finger ridge counts and this item was higher in patients with schizophrenia (p < 0.001). This finding was consistent with some previous dermatology reports.9 Use of finger ridge count as a biological marker in screening of persons susceptible to schizophrenia may provide a new approach.
The same amount of FA in both case and control groups was reported by Mellor and colleagues.4 Although there are nine methods of measuring FA, the one including 1- r2 is the least sensitive. In our study, measurement of FA showed a greater degree of FA in schizophrenic patients. Thus, the fourth hypothesis was that higher mean ridge counts were only found among males and that the difference was not statistically significant among women (Table 3). Differences in the pattern and ridge count of left finger between the case and control groups may demonstrate the role of second-trimester insults in expressing genetic predisposition towards schizophrenia. Considering the increase of the subject number and the limitation of information sources in this study, first, third and fourth hypotheses can provide valuable information about etiological factors and their probable timing in the development of schizophrenia. Moreover, these findings are important in screening by providing a biological marker which may help in prediction and diagnosis. Dermatoglyphics is a promising method in studying schizophrenia. FA may also help in assessing the relationship between prenatal environmental influences and structural brain changes. Finally, it may help to explain the somehow perplexing findings in schizophrenia when conventional methods of dermatoglyphics analysis are used. The existing different patterns of fingerprints can address the effect of genetics. However, we could not really exclude the role of environmental factors. It is important to note that our results encourage further studies to explain the exact role of both genetics and environment. In addition, they could answer the questions about weather or not we can use FA as a biomarker criterion for screening schizophrenia, and how much its predictive value is.
Authors’ Contributions
Two first authors participated in the design of the study and acquisition of data. First and third authors performed the statistical analysis and wrote the manuscript.
Acknowledgments
The authors would like to thank Dr. Nasrollah Bashardost, Dr. Mohammad Reza Maracy, Dr. Ali Gholamrezaei, Dr. Ali Amini and Mrs. Fariba Noori at Isfahan University of Medical Sciences and the School of Medicine.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Siasy A. Tehran: AmirKabir; 1976. Types of Personalities the Typologies of Kretschmer and Sheldon. [Google Scholar]
- 2.Schaumann BA, Opitz JM. Clinical aspects of dermatoglyphics. Birth Defects Orig Artic Ser. 1991;27(2):193–228. [PubMed] [Google Scholar]
- 3.Mavalwala J, Mavalwala P, Kamali SM. Issues of sampling and of methodologies in dermatoglyphics. Birth Defects Orig Artic Ser. 1991;27(2):291–303. [PubMed] [Google Scholar]
- 4.Mellor CS. Dermatoglyphic evidence of fluctuating asymmetry in schizophrenia. Br J Psychiatry. 1992;160:467–72. doi: 10.1192/bjp.160.4.467. [DOI] [PubMed] [Google Scholar]
- 5.Solhi H, Hashemieh M, Nejad ML, Vishteh HR, Nejad MR. Diagnostic value of fingerprint patterns: an explorative study on beta-thalassemia diagnosis. Bangladesh Med Res Counc Bull. 2010;36(1):27–31. doi: 10.3329/bmrcb.v36i1.4631. [DOI] [PubMed] [Google Scholar]
- 6.Elsaadany HM, Kassem E, El-Sergany M, Sheta A. Can dermatoglyphics be used as an anatomical marker in Egyptian rheumatoid patients? Journal of American Science. 2010;6(11):457–66. [Google Scholar]
- 7.Gustavson KH, Modrzewska K, Sjoquist KE. Dermatoglyphics in individuals with asocial behaviour. Ups J Med Sci. 1994;99(1):63–7. doi: 10.3109/03009739409179351. [DOI] [PubMed] [Google Scholar]
- 8.Balgir RS, Murthy RS, Wig NN. Genetic loadings in schizophrenia: a dermatoglyphic study. Isr J Med Sci. 1993;29(5):265–8. [PubMed] [Google Scholar]
- 9.Bracha HS, Torrey EF, Gottesman II, Bigelow LB, Cunniff C. Second-trimester markers of fetal size in schizophrenia: a study of monozygotic twins. Am J Psychiatry. 1992;149(10):1355–61. doi: 10.1176/ajp.149.10.1355. [DOI] [PubMed] [Google Scholar]
- 10.Gottesman II, Shields J. New York: Cambridge University Press; 1982. Schizophrenia: Ti7e Epigeneric, Puzzle. [Google Scholar]
- 11.Matthysse SW, Kidd KK. Estimating the genetic contribution to schizophrenia. Am J Psychiatry. 1976;133(2):185–91. doi: 10.1176/ajp.133.2.185. [DOI] [PubMed] [Google Scholar]
- 12.Markow TA, Wandler K. Fluctuating dermatoglyphic asymmetry and the genetics of liability to schizophrenia. Psychiatry Res. 1986;19(4):323–8. doi: 10.1016/0165-1781(86)90125-3. [DOI] [PubMed] [Google Scholar]
- 13.Markow TA, Gottesman II. Fluctuating dermatoglyphic asymmetry in psychotic twins. Psychiatry Res. 1989;29(1):37–43. doi: 10.1016/0165-1781(89)90185-6. [DOI] [PubMed] [Google Scholar]
- 14.Green MF, Bracha HS, Satz P, Christenson CD. Preliminary evidence for an association between minor physical anomalies and second trimester neurodevelopment in schizophrenia. Psychiatry Res. 1994;53(2):119–27. doi: 10.1016/0165-1781(94)90103-1. [DOI] [PubMed] [Google Scholar]
- 15.Fearon P, Lane A, Airie M, Scannell J, McGowan A, Byrne M, et al. Is reduced dermatoglyphic a-b ridge count a reliable marker of developmental impairment in schizophrenia? Schizophr Res. 2001;50(3):151–7. doi: 10.1016/s0920-9964(00)00089-x. [DOI] [PubMed] [Google Scholar]
- 16.Cannon M, Byrne M, Cotter D, Sham P, Larkin C, O’Callaghan E. Further evidence for anomalies in the hand-prints of patients with schizophrenia: a study of secondary creases. Schizophr Res. 1994;13(2):179–84. doi: 10.1016/0920-9964(94)90100-7. [DOI] [PubMed] [Google Scholar]
- 17.Fananas L, van Os J, Hoyos C, McGrath J, Mellor CS, Murray R. Dermatoglyphic a-b ridge count as a possible marker for developmental disturbance in schizophrenia: replication in two samples. Schizophr Res. 1996;20(3):307–14. doi: 10.1016/0920-9964(95)00013-5. [DOI] [PubMed] [Google Scholar]
- 18.Rosa A, Fananas L, Bracha HS, Torrey EF, van Os J. Congenital dermatoglyphic malformations and psychosis: a twin study. Am J Psychiatry. 2000;157(9):1511–3. doi: 10.1176/appi.ajp.157.9.1511. [DOI] [PubMed] [Google Scholar]
- 19.Sokal RR, Rohlf FJ. 2nd ed. New York: W.H.Freeman and Company; 1981. Biometry. [Google Scholar]
- 20.Van Valen L. A Study of fluctuating asymmetry. Evolution. 1962;16(2):125–42. [Google Scholar]


