Abstract
BACKGROUND:
It is a well-known fact that there is increased oxidative stress and decreased serum antioxidant levels in smokers than in non-smokers. In this study, the aim was to compare the serum levels of malondialdehyde (MDA), a lipid peroxidation product and vitamin C, an antioxidant, between non- smokers (Group A) and chronic smokers (Group B) and also between chronic smokers (Group B) and chronic smokers with acute myocardial infarction (AMI) (Group C).
METHODS:
Thirty six non-smokers and 36 chronic smokers appropriately matched with AMI patients were selected. Thirty six smokers with AMI were selected from Hanagal Kumareshwara hospital, Bagalkot, Karnataka, India. Fasting blood sample was collected in group A and group B. In AMI patients, blood sample was collected before any intervention. Serum levels of MDA and vitamin C were estimated. Statistical analysis was done by t test using SPSS version 11. The p< 0.05 was considered statistically significant. All the results were expressed as mean ± SD.
RESULTS:
The MDA and vitamin C were compared between Group A and Group B and also between Group B and Group C. There was a significant rise in MDA (p<0.0001) and significant decrease in vitamin C (p<0.01) in Group B compared to Group A. There was a significant rise in MDA (p<0.0001) and significant decrease in vitamin C (p<0.001) in Group C compared to Group B.
CONCLUSIONS:
The increase in serum MDA level and decrease in vitamin C was found in chronic smokers compared to non-smokers. It was also found that there is increase in serum MDA and decrease in vitamin C in smokers with AMI compared with smokers without AMI, and the reason for this inter-subject variability of MDA and vitamin C levels may be due to gene-environmental factors.
KEYWORDS: Chronic smokers, Myocardial infarction, MDA, Vitamin C
Smoking is a potent but modifiable risk factor of cancer, chronic obstructive pulmonary disease (COPD) and cardiovascular diseases. It acts substantially via reactive species to initiate and promote pathology and is consistently associated with elevated oxidative damage to DNA, lipids and proteins.1,2 It has been suggested that cigarette smoking done regularly more than 10 per day, constitute a major risk for coronary heart disease (CHD).3
Cigarette smoke is a complex mixture of chemicals containing more than 4000 different constituents. Some of the compounds identified include pyridine alkaloids such as nicotine, ammonia, acrolein, phenols, acetaldehyde N-nitrosamine, polycyclic aromatic hydrocarbons such as benzopyrine, combustion gases such as carbon monoxide, nitrogen oxides, hydrogen cyanide, and trace metals, α-emitter radioactive elements such as polonium, radium and thorium.1
Two major phases were identified in cigarette smoke: A tar phase and a gas phase. The tar phase or particulate phase is defined as the material that is trapped when the smoke stream is passed through the Cambridge glass – fiber filter that retains 99.9% of all particulate materials with a size >0.1 μm. The gas phase is the material that passes through the filter. The radicals associated with the tar phase are long lived (hours to months), whereas the radicals associated with gas phase have a shorter life span (seconds). It was estimated that a single cigarette puff contains approximately 1014 free radicals in tar phase and 1015 radicals in the gas phase, which are capable of causing an in-crease in the generation of various reactive oxygen species (ROS) like superoxide (O¯2), hydrogen peroxide (H2O2), hydroxyl (OH•) and peroxide (ROO•) radicals. These reactive oxygen species in turn are capable of initiating and promoting oxidative damage in the form of lipid peroxidation.1,4
Lipid peroxidation may increase endothelial permeability, resulting in haemoconcentration and elevated hematocrit, triglycerides and consequently, may increase blood viscosity, possibly through oxidative damage to erythrocytes. Lipid peroxidation may promote acute-phase reactions by increasing endothelial/monocyte interactions and release of cytokines such as IL-6, which are key mediators of inflammatory reactions including elevation of white cell count, fibrinogen and C - reactive protein.5
Cigarette smokers have an increased risk of cardiovascular diseases (CVD), possibly mediated by elevated levels of oxidized macromolecules owing to heightened ROS production. Smokers are exposed to significant quantities of ROS in both gas and tar phase. Further ROS production mediated through inflammatory processes may exacerbate those produced through direct exposure.6 Blood of cigarette smokers routinely displays decreased antioxidant capacity and increased oxidized lipids compared to non-smokers.6
Vitamin C is a potent water soluble antioxidant because, by donating its electrons, it prevents other compounds from being oxidized. The species formed after the loss of one electron is a free radical, semidehydroascorbic acid or ascorbonyl radical, a reactive and possibly harmful free radical. Many studies have demonstrated low plasma concentration of vitamin C in smokers and acute myocardial infarction (AMI) patients.1,6,7
Many studies have been done to compare the level of lipid peroxidation and antioxidants among smokers and non-smokers, and the non-smokers and smokers with myocardial infarction.5,6,8–12 None of the studies which we have reviewed, have compared chronic smokers without myocardial infarction and chronic smokers with myocardial infarction. Therefore, the focus of this study was to find any other component that could have a role to play in lipid peroxidation and antioxidants among chronic smokers. In this context, the present study was undertaken to compare the serum levels of lipid peroxidation product malondialdehyde (MDA) and antioxidant vitamin C in non-smokers (Group A), apparently healthy chronic smokers (Group B) and chronic smokers with acute myocardial infarction (Group C).
Methods
The present study was conducted in Hanagal Shri Kumareshwara (HSK) hospital, Bagalkot. The study was approved by S.Nijalingappa Medical College ethical committee (Ref No: SNMC/09-10/602). Informed consent was obtained from all the subjects. The study was conducted from Jun 2009 to December 2010. Thirty six controls who were apparently healthy non-smokers were included after appropriate matching (for age and sex) and 36 apparently healthy chronic smokers were selected.3,13–15 Thirty six chronic smokers with diagnosed acute myocardial infarction were selected from HSK hospital. All of them were male subjects. Both chronic smokers and chronic smokers with myocardial infarction, who smoked more than 10 cigarettes per day for more than 10 years, were selected.
Those excluded from the study were persons abusing alcohol, ex-smokers, patients with diabetes mellitus, hypertension, renal diseases, hepatic impairment, endocrine disorders, obese individuals and patients on drugs like multivitamins.
Each subject was interviewed and information about demographic details and smoking was obtained. The demographic details included age, sex, body weight, and body mass index (BMI). Both systolic blood pressure (SBP) and diastolic blood pressure (DBP) were recorded. Smoking habit included smoking period and number of cigarettes smoked daily.
Fasting venous blood sample was collected in all subjects and care was taken to ensure that in AMI patients, blood sample was collected before thrombolysis. Serum was separated and analyzed for the following parameters: MDA was measured by thiobarbituric acid reactive substances assay (TBRAS) method16 and ascorbic acid (Vitamin C) measured by dinitrophenyl hydrazine (DNPH) method.17 The optical densities of MDA and ascorbic acid were measured at 532 nm and 520nm, respectively, using spectrophotometer. Statistical analysis was done by ‘t’ test using SPSS version 11. The p< 0.05 was considered statistically significant. All the results were expressed as mean ± SD.
Results
The demographic characteristics of all subjects are shown in Table 1. All of them were male. There was no significant difference in mean age, body weight and BMI among three Groups. Group B and Group C subjects were hypertensive, and smoked 13 or more cigarettes per day for 12 or more years (Table 1).
Table 1.
Demographic characters of Non-smokers, Chronic Smokers and Chronic Smokers with Acute Myocardial Infarction (AMI).
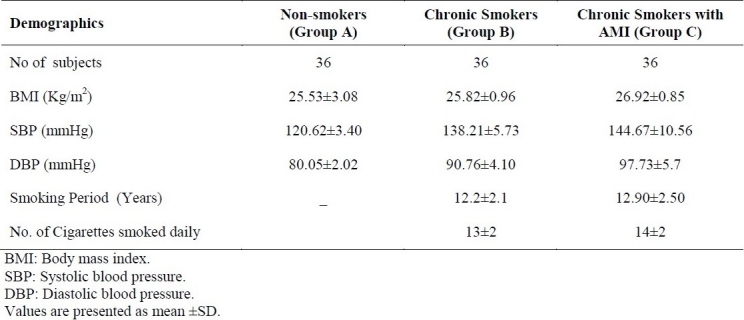
Table 2 shows the serum levels of MDA and vitamin C in non-smokers (Group A) and chronic smokers (Group B). The MDA of Group A was 236 ± 3.50 nmol/l and in Group B was 430 ± 8.90 nmol/l. The mean vitamin C in Group A was 1.22 ± 0.20 mg/dl and that of Group B was 0.97 ± 0.19 mg/dl. MDA was significantly higher (p<0.0001) in Group B compared to Group A. Vitamin C level was significantly lower (p<0.01) in Group B than in Group A.
Table 2.
Serum levels of MDA and Vitamin C in Non-smokers and chronic Smokers.
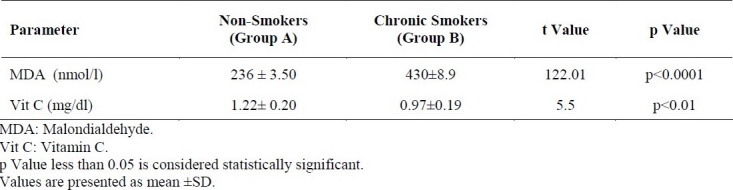
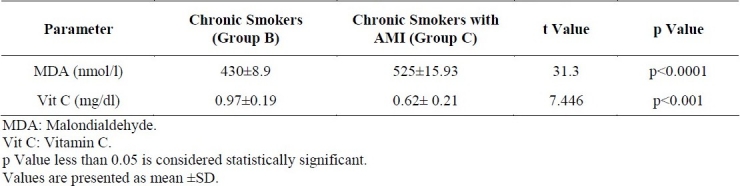
Table 3 shows the serum levels of MDA and vitamin C in chronic smokers (Group B) and chronic smokers with MI (Group C). The MDA of Group B was 430 ± 8.90 nmol/l and in Group C was 525 ± 15.93 nmol/l. The mean vitamin C in Group B was 0.97 ± 0.19 mg/dl and that of Group C was 0.62 ± 0.21mg/dl. MDA was significantly higher (p<0.0001) in Group C compared to Group B. Vitamin C level was significantly lower (p<0.001) in Group C than in Group B.
Table 3.
Serum levels of MDA and Vitamin C in chronic Smokers and Smokers with Acute Myocardial Infarction (AMI)
Discussion
The present study showed that there was a significant elevation of serum MDA (p<0.0001) and significant decrease in serum ascorbic acid levels (p<0.01) in chronic smokers compared to non-smokers.
Cigarette smoking leads to the uptake of many hazardous compounds or their metabolites which may be electrophilic and thereby, able to react with biological macromolecules, or they may give rise to oxidative stress by formation of reactive oxygen species (ROS) which are capable of initiating the self perpetuating chain reactions of lipid peroxidation in the membranes.18
The serum triglycerides and smaller denser LDL particles are substrates and are more susceptible to peroxidation.5 Many studies have shown that, in smokers, nicotine causes significant increase in the levels of serum cholesterol, triglycerides and LDL-C, but HDL-C is lowered in smokers than in non-smokers.3,10,12 Hence, there is an increased level of lipid peroxidation product of MDA in chronic smokers compared to the non-smokers.
Smoking of 10 cigarettes daily on an average, for 6-8 years was associated with enhanced lymphocyte DNA strand breaks as well as greater urinary excretion of 8- OHdG and MDA compared with non-smokers. Further-more, smoking was co-related with lower activities of principal antioxidants in the plasma.2
Epidemiologic studies showed that cigarette smokers consume fewer fruits, vegetables and vitamin supplementation than do nonsmokers and this leads to low serum P/S ratio (polyunsaturated fatty acids/saturated fatty acids),19 which in turn results in a status of increased oxidative stress and decreased antioxidant capacity,9 as observed in the present study.
It has been difficult to determine whether differences in the plasma antioxidants between smokers and non-smokers are actually due to the effect of cigarette smoke exposure or due to differences in dietary antioxidant intakes or in other covariates (e.g., BMI).5,19
In this study, we have also compared chronic smokers and chronic smokers with acute myocardial infarction. The serum MDA was significantly higher (p<0.0001) and serum ascorbic acid was significantly lower (p<0.001) in chronic smokers with AMI than in chronic smokers, even when both groups were matched for age, body weight, BMI and smoking habits. Although cigarette smoking is a well established risk factor for vascular diseases, the genetic mechanisms that link cigarette smoking to an increased incidence of cardiovascular diseases are not well understood.
Cigarette smoking depending on the number of years spent in smoking (pack year history of 3±4), may independently promote negative changes like lower antioxidant capacity and greater degree of lipid peroxidation, despite having similar dietary intake.6 While dietary intake, as well as other genetic and environmental factors, which were not determined in this investigation, may contribute to these results, it appears that smoking plays a major role in promoting these changes.
Several environmental factors are documented to influence redox metabolism, but relatively little is known about genetic effects. TAS, an indicator of redox homeostasis, is under strong genetic control, especially in smokers and this could lead to the difference in MDA and vitamin C levels in chronic smokers with MI than in chronic smokers without ML.20
The inter-subject variability in the atherosclerotic process in smokers may be partially mediated by genetic variants. Either CYP1A1 MSP polymorphism or certain endothelial NO synthase intorn 4 polymorphisms increased the susceptibility to cigarette smoke exposure –related atherosclerotic diseases including multi-vessel CHD and MI.4
C alleles, proinflammatory cytokines, CD14 and IL-6 polymorphism were associated with higher risk of carotid plaque formation and were found to act synergistic ally with other inflammatory single nucleotide polymorphisms (SNPs) to increase carotid intima media thickness (IMT), specifically among the smokers. Cigarette smoking may modulate stroke risk through a gene-environment interaction.21
However, at present, the importance of these genetic variants is unknown, as their prevalence in the entire population of cigarette smokers has not been documented.4
The limitations of the present study were as follows:
The sample size was small.
MDA is more accurate when it is applied for larger population during epidemiological sur-veys.
Diet history was not taken in depth.
Extent of smoking, i.e., amount of smoke exposed to lungs was not documented which is very difficult to measure.
All the subjects were male.
In conclusion, the present findings indicated that cigarette smokers had higher lipid peroxidation levels and lower antioxidant capacity compared to non-smokers. Inter-subject variability of both elevated serum MDA and reduced serum vitamin C levels in chronic smokers with AMI may be influenced by gene-environmental factors.
However, further study and long period follow up is needed to find whether Group B subjects end up with myocardial infarction in due course, to ascertain the gene-environment interaction in Group A and Group B subjects and to answer why all chronic smokers (13 or more cigarettes per day for a period of 12 or more years) do not experience myocardial infarction.
Authors’ Contributions
SVK and GSK carried out the design and coordinated the study, participated in most of the experiments and prepared the manuscript. PK and SVK provide assistance in the design of the study, coordinated and carried out all the experiments and participated in manuscript preparation. MR and JBI provided assistance for all experiments. All authors have read and approved the content of the manuscript.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Pasupathi P, Saravanan G, Farook J. Oxidative Stress Bio Markers And Antioxidant Status In Cigarette Smokers Compared To Nonsmokers. Journal of Pharmaceutical Sciences and Research. 2009;1(2):16–21. [Google Scholar]
- 2.Li N, Jia X, Chen CY, Blumberg JB, Song Y, Zhang W, et al. Almond consumption reduces oxidative DNA damage and lipid peroxidation in male smokers. J Nutr. 2007;137(12):2717–22. doi: 10.1093/jn/137.12.2717. [DOI] [PubMed] [Google Scholar]
- 3.Akbari ZA, Bhatti MS, Shakoor M. Lipid profile in smoking. JAMC. 2000;12(3):19–21. [Google Scholar]
- 4.Ambrose JA, Barua RS. The pathophysiology of cigarette smoking and cardiovascular disease: an update. J Am Coll Cardiol. 2004;43(10):1731–7. doi: 10.1016/j.jacc.2003.12.047. [DOI] [PubMed] [Google Scholar]
- 5.Rumley AG, Woodward M, Rumley A, Rumley J, Lowe GD. Plasma lipid peroxides: relationships to cardiovascular risk factors and prevalent cardiovascular disease. QJM. 2004;97(12):809–16. doi: 10.1093/qjmed/hch130. [DOI] [PubMed] [Google Scholar]
- 6.Bloomer RJ. Decreased blood antioxidant capacity and increased lipid peroxidation in young cigarette smokers compared to nonsmokers: Impact of dietary intake. Nutr J. 2007;6:39. doi: 10.1186/1475-2891-6-39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Padayatty S, Katz A, Wang Y, Eck P, Kwon O, Lee JH, et al. Vitamin C as an antioxidant: evaluation of its role in disease prevention. J Am Coll Nutr. 2003;22(1):18–35. doi: 10.1080/07315724.2003.10719272. [DOI] [PubMed] [Google Scholar]
- 8.Morrow JD, Frei B, Longmire AW, Gaziano JM, Lynch SM, Shyr Y, et al. Increase in circulating products of lipid peroxidation (F2-isoprostanes) in smokers.Smoking as a cause of oxidative damage. N Engl J Med. 1995;332(18):1198–203. doi: 10.1056/NEJM199505043321804. [DOI] [PubMed] [Google Scholar]
- 9.Miller ER, Appel LJ, Jiang L, Risby TH. Association between cigarette smoking and lipid peroxidation in a controlled feeding study. Circulation. 1997;96(4):1097–101. doi: 10.1161/01.cir.96.4.1097. [DOI] [PubMed] [Google Scholar]
- 10.Kharb S, Singh GP. Effect of smoking on lipid profile, lipid peroxidation and antioxidant status in normal subjects and in patients during and after acute myocardial infarction. Clin Chim Acta. 2000;302(1-2):213–9. doi: 10.1016/s0009-8981(00)00343-0. [DOI] [PubMed] [Google Scholar]
- 11.Al-Senaidy AM. Effects of Short-term Supplementation with Vitamin C on Lipid Peroxidation in Cigarette Smokers. Australian Journal of Basic and Applied Sciences. 2010;4(3):487–93. [Google Scholar]
- 12.Waqar A. Effect of tobacco smoking on the lipid profile of teenage male population in Lahore City. International Journal of Medicine and Medical Sciences. 2010;2(6):171–7. [Google Scholar]
- 13.Lang N, Hasan A, Sueske E, Paulus W, Nitsche MA. Cortical hypoexcitability in chronic smokers.A transcranial magnetic stimulation study? Neuropsychopharmacology. 2008;33(10):2517–23. doi: 10.1038/sj.npp.1301645. [DOI] [PubMed] [Google Scholar]
- 14.Heitzer T, Brockhoff C, Mayer B, Warnholtz A, Mollnau H, Henne S, et al. Tetrahydrobiopterin improves endothelium-dependent vasodilation in chronic smokers : evidence for a dysfunctional nitric oxide synthase. Circ Res. 2000;86(2):36–41. doi: 10.1161/01.res.86.2.e36. [DOI] [PubMed] [Google Scholar]
- 15.Rytila P, Rehn T, Ilumets H, Rouhos A, Sovijarvi A, Myllarniemi M, et al. Increased oxidative stress in asymptomatic current chronic smokers and GOLD stage 0 COPD. Respir Res. 2006;7:69. doi: 10.1186/1465-9921-7-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Draper HH, Hadley M. Malondialdehyde determination as index of lipid peroxidation. Methods Enzymol. 1990;186:421–31. doi: 10.1016/0076-6879(90)86135-i. [DOI] [PubMed] [Google Scholar]
- 17.Rifai N, Bachorik PS, Albers JJ. Lipids and Lipoprotein and apolipoprotein. In: Burtis CA, Ashwood ER, editors. Tietz Textbook of clinical chemistry. 3rd ed. Philadelphia: Saunders; 1999. pp. 809–61. [Google Scholar]
- 18.Pasupati P, Yegneshwar Rao Y, Farooq J, et al. Effect of cigarette smoking on lipids and oxidative stress biomarkers in patients with Acute myocardial infarction. Research Journal of Medicine and Medical Sciences. 2009;4(2):151–9. [Google Scholar]
- 19.Dietrich M, Block G, Norkus EP, Hudes M, Traber MG, Cross CE, et al. Smoking and exposure to environmental tobacco smoke decrease some plasma antioxidants and increase gamma-tocopherol in vivo after adjustment for dietary antioxidant intakes. Am J Clin Nutr. 2003;77(1):160–6. doi: 10.1093/ajcn/77.1.160. [DOI] [PubMed] [Google Scholar]
- 20.Wang XL, Rainwater DL, VandeBerg JF, Mitchell BD, Mahaney MC. Genetic contributions to plasma total antioxidant activity. Arterioscler Thromb Vasc Biol. 2001;21(7):1190–5. doi: 10.1161/hq0701.092146. [DOI] [PubMed] [Google Scholar]
- 21.Cole JW, Brown DW, Giles WH, Stine OC, O’Connell JR, Mitchell BD, et al. Ischemic stroke risk, smoking, and the genetics of inflammation in a biracial population: the stroke prevention in young women study. Thromb J. 2008;6(11):1–8. doi: 10.1186/1477-9560-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]


