Abstract
BACKGROUND:
Although a variety of strategies have been employed for managing articular cartilage defects in the knee, overall outcomes have not been satisfactory. An alternative option may be autologous chondrocyte transplantation (ACT). However, as this method is still under investigation, here we assessed the efficacy of ACT for human knee defect cartilage repair.
METHODS:
In a randomized clinical trial study, eleven patients (mean age 31.09 years) were enrolled in the study with full thickness cartilage defects in the knee. Arthroscopically, healthy cartilage was obtained, chondrocytes expanded for 2-3 weeks and ACT performed. Clinical status was evaluated before ACT, 6 and 12 months after ACT using the Brittberg-Peterson functional assessment and modified Cincinnati rating score. Magnetic resonance imaging (MRI) findings were evaluated based on the scoring systems used by Sally Roberts and by Henderson.
RESULTS:
Modified Cincinnati rating indicated significant improvement of clinical score before ACT compared to 6 (p = 0.000) and 12 (p = 0.000) months after ACT (from 2.73 before ACT to 7.27, 8.36 and 9.5 at 6, 12, and 48 months after ACT, respectively). Brittberg-Peterson functional assessment indicated a decline from 79.27 to 25.82 and 19.27 at 6 and 12 months post ACT. Further, statistical test demonstrated significant differences 6, 12 and 48 months post ACT (p = 0.007). Evaluation of MRI revealed a score of 6.5 for Henderson criteria and a score of 2.5 for Robert criteria.
CONCLUSIONS:
Our study demonstrated that ACT of the knee provides an excellent treatment for full thickness cartilage defects with outstanding clinical and radiological outcomes.
KEYWORDS: Articular Cartilage, Full Thickness Cartilage Defect, Autologus Chondrocyte Transplantation, Knee
Articular cartilage defects present one of the most common disorders of the large articular joints and result in a wide spectrum of clinical manifestations. Indeed, the prevalence of articular cartilage defects, at the time of arthroscopy, was reported to be around 60%.1,2 However, it remains somewhat unclear that to which percentage these defects are symptomatic and require surgical intervention.3 Considering that the world's population not only continues to live longer but also a large proportion is more active into later life, new methods for cartilage repair and regeneration are needed.
While William Hunter reported in 1943 that “cartilage once destroyed never heals”,4 today we know that given the appropriate circumstances, there is potential for cartilage repair. In 1994, for the first time, Brittberg and colleagues established the technique of autologous chondrocyte implantation (ACI) reported for full-thickness chondral injuries.1 In this technique, a suspension of in vitro cultured autologous chondrocytes are injected into the cartilage defect beneath a tightly sealed periosteal flap.5 This procedure has been implemented in several joint defects such as the talus and ankle.6,7 As outcomes have been reported to be excellent, with regenerated cartilage comparable to that of the surrounding cartilage,8 today it is well accepted that autologous chondrocyte transplantation (ACT) produces mechanically and functionally stable cartilage in full-thickness cartilage defects.9 Therefore, although, in vitro culture techniques are costly,10 ACT seems to be the “gold standard” technique for cartilage defect repair.9 However, results of ACT in the knee are still discussed controversially and some studies do not recommend ACT for knee cartilage defect repair.11,12
We reported the use of ACT on human knee cartilage defect repair first in 2007. The aim of this study was a postoperative evaluation of this technique using Henderson's MR imaging score; modified Brittberg-Peterson functional assessment and Cincinnati rating score.13
Methods
Study design
This was a randomized clinical trial study that conducted in Alzahra hospital in Isfahan Between 2004-2007. Presented study consisted of 11 male patients with the mean age of 31.09 years (ranged 20 to 44 years); of which 90.9 % (10 patients) had the chondral lesion in the medial condyle and 9.1 % (one patient) had the chondral lesion in lateral condyle of the femur. Patients presented themselves with isolated symptomatic cartilage defects, a limited chondral lesion (mean surface area: 5 cm2) and without generalized osteoarthritis, genu valgum and/or genu varum of the knee. Patients under 40 years old with chief compliant of knee pain and up to three cartilaginous lesions between 2-9 cm2 were entered to study and patients lost to follow-up were excluded.
Knee arthroscopy was performed in Al-zahra hospital, Isfahan, Iran. Cartilage defect was inspected and a decision was made regarding the implementation of ACT. Mean duration of the symptoms before operation was 33.82 months. Follow-up of all patients was for at least 12 months after surgery, additionally follow-up for 4 patients continued for 48 months. The study was approved by the Scientific & Ethical Committee of Isfahan University of Medical Science (IUMS), Isfahan, Iran (No.186012).
Chondrocyte isolation and culture
After decision for performing ACT was taken, approximately, a 200-300 mg full thickness cartilage biopsy from a healthy area of the joint was obtained from the outer edge of the superior medial condyle, from a non-weight-bearing area (e.g., trochlear cartilage) and immediately transferred into a sterile transportation vessel containing calcium and magnesium free phosphate buffered saline solution at 4° C.
Biopsy specimens were transferred to Royan Institute, Isfahan Campus, for processing and culturing as previously described.14,15 Briefly, after enzymatic digestion of the tissue with 0.2% protease (Calbiochem, La Jolla, CA) for two hours in a 37°C shaking water bath, chondrocytes were seeded into a medium cell culture flask containing whole cell culture medium (including DMEM/Ham'sF12 (Dulbecco's), modified Eagle's medium containing human serum (10%), ascorbic acid (25 μg/ml), streptomycin (50 IU/ml) and penicillin (50 IU/ml)) all obtained from Seromed (Munich, Germany) and cultured until confluency was reached. Overall, chondrocytes were cultured for 2-3 weeks and Alcian blue staining performed to confirm maintenance of chondrogenic phenotype before re-implantation.
ACT procedure
For chondrocyte re-implantation, patients were readmitted to the hospital as previously described.14,15 To prepare for chondrocyte implantation, a medial or lateral para-patellar arthrotomy either in left or right (depended on the defect site) was performed. The osteochondral lesion was debrided with minimum bleeding. In two patients the defect was very deep, so an autogenous bone graft (from proximal of tibia) was placed in the depth of the defect before performing ACT.
In the next step, the periosteal flap was harvested from the proximal medial tibia through a small separate incision over the anteromedial tibia just distal to the insertion of the pes tendons and was fitted and sutured to the surrounding rim of the cartilage with PDS. The periosteal flap was sealed to the rim with fibrin glue except for one upper corner, within which the cultured chondrocytes were later injected into the defect. The flap was tested first with normal saline insufflations into the space between the periosteal flap and bone layers through a syringe-soft catheter setup to check that there was no leakage. The saline was then aspirated from under the periosteum. After chondrocyte injection beneath the periosteal flap, the remaining defect between the periosteal flap and rim was sutured with PDS and sealed with fibrin glue (Berlin Heart, GMIBH, Berlin, Germany).
Post-operative rehabilitation
The postoperative rehabilitation program was a critical part of the treatment, including active and passive movement, muscle training, and weight-bearing exercises. Patients began physiotherapy with 0°–30° angle of continuous passive motion beginning 6 hours after surgery. The range of motion was gradually increased until 12 weeks, culminating in full flexion. Each patient remained non-weight-bearing for the 1st-4th week, with partial weight-bearing exercise beginning after the 4th week. By the 12th week post ACT, patients had progressed to walking with full weight-bearing. Sports activity was gradually increased after 6 months; however, hard sporting activity was allowed only after 12 months.
Evaluation of ACT outcome
The clinical status of patients was evaluated before, 6 and 12 months after ACT, using the Brittberg-Peterson functional assessment (the best 0 to the worst 130), and modified Cincinnati rating score (the worst 2 to the best 10). Four patients were also followed for 48 month. Evaluation of MRI findings was performed based on the scoring systems used by Sally Roberts and by Henderson, by a blind skilled musculoskeletal radiologist.
Statistical analysis
Wilcoxon statistical test was used for data analysis in SPSS (version 18.0) and P-value < 0.05 was considered statistically significant.
Results
The outcome of the ACT was rated to be good or excellent by 82% of the patients after 12 months. In the 4 patients who were followed for 48 months rating increased to 100%, which is considered as excellent.
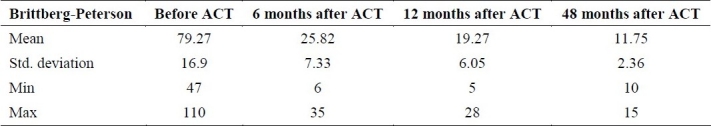
The results of Brittberg-Peterson functional assessment for all 11 patients were a mean of 79.27 ± 16.93 before ACT and declined to 25.82 ± 7.33 and 19.27 ± 6.05 at 6 and 12 months post ACT (Table 1). Statistical analysis revealed a significant difference between Brittberg-Peterson functional assessment scores before ACT compared to 6 months (p < 0.001) and 12 months (p < 0.001) post ACT. There was also a statistical significant difference between 6 and 12 months post ACT in Brittberg-Peterson functional assessment (p = 0.001). In the 4 patients that were followed for 48 month post ACT, Brittberg-Peterson functional assessment decreased from 86.25 ± 17.02 before ACT to 11.75 ± 2.36 48 months after ACT, and was statistical significant (p = 0.003 , p = 0.001 and p = 0.001 respectively). The results of Wilcoxon test demonstrated a statistical difference (p = 0.007) for the score before ACT to 6, 12 and 48 month post ACT.
Table 1.
Brittberg-Peterson functional assessment before, 6, 12 and 48 month after surgery
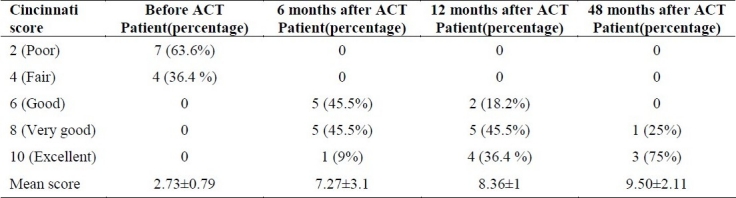
The modified Cincinnati rating score for all patients was 2.73 ±1.01 before ACT. It improved to 7.27 ± 1.35 and 8.36 ± 1.50 at 6 and 12 months post ACT (Table 2). Statistical analysis revealed a significant difference of modified Cincinnati rating score comparing patients before and after 6 months (p < 0.001) and 12 months (p < 0.001) post ACT. A statistically significant difference of the modified Cincinnati rating score was also found between 6 and month follow up post ACT (p = 0.007). In the 4 patients which were followed up to 48 month post ACT, modified Cincinnati rating score increased from 2.50 ± 1.0 before ACT to 9.50 ± 1 after 48 month post ACT, and was statistically significant (p = 0.001).
Table 2.
Modified Cincinnati rating score before, 6, 12 and 48 month after surgery
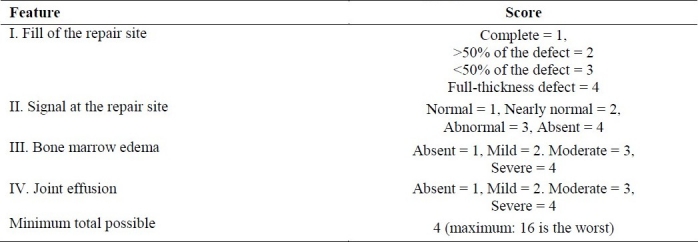
Para clinical evaluation by the MRI scoring system revealed a score of 2.5 for Robert criteria (Table 3) and a score of 6.5 for Henderson criteria (Table 4).
Table 3.
Robert's Magnetic resonance (MR) imaging score

Table 4.
Henderson's MR imaging score
Discussion
The aim of the presented clinical study was to evaluate the clinical outcome of ACT on circumscribed human knee cartilage defects after follow up of 6, 12 or 48 months. Excellent clinical outcome on all 11 patients after 6, 12 and 48 months follow up was obtained as defined by modified Cincinnati score, Brittberg-Peterson assessment and para-clinical MRI scoring by Henderson and Robert criteria.
The outcome of the ACT 12 months after treatment was rated as good or excellent by 82% of the patients. In 4 patients who were followed for 48 month this rate was 100%. In the presented study we compared the modified Cincinnati rating score and Brittberg-Peterson functional assessment in regard to their influence on ACT outcome rating. The findings reveal that the Cincinnati score increased after ACT treatment while the Brittberg-Peterson functional assessment score decreased after ACT, thus suggesting ACT improves functional capacity of knee including range of motion and decreases the symptoms of articular defects including pain while sitting, and/or during joint movement, locking, joint instability and running.
Para clinical evaluation by MRI scoring revealed a score of 6.5 out of 14 for Henderson criteria and a score of 2.5 out of 4 for Robert criteria. The results of Robert criteria were higher than in a previous study by Moriya et al.,16 while our Henderson score was lower than what had been reported by them, thus suggesting that our ACT outcome was better in some aspects of MRI scoring.
Our results concur with other studies that excellent results for graft integrity and short term follow up of ACT were observed.1,17,18 In a systematic review on ACT by Jobanputra et al. on 2600 patients, it was concluded that the outcome of the ACT two years after treatment was rated as good or excellent by 70% of the patients.18 Furthermore, Erggelet and colleagues determined ACT as “a safe and effective method for the treatment of large full thickness cartilage defects”.1 Clinical assessment of ACT by Bentley et al. also showed that 88% of patients had excellent or good results and 82% had excellent or good repairs in arthroscopy at one year post ACT.17 In a multicenter cohort study, it was further concluded that “ACT is a viable treatment option that may yield relatively long-term symptomatic relief and functional improvement”.19 In our study the modified Cincinnati rating score improved from 2.73 before ACT to 7.27, 8.36 and 9.5 at 6, 12 and 48 month post ACT. These results are similar to clinical outcome of a study by Scorrano et al., were the modified Cincinnati rating score improved from 2.2 before ACT to 7.6, 9, and 9.6 at 6, 12, and 24 months post ACT.20
However, although our results and results from the above mentioned studies were excellent, several studies also found contradictory findings after knee ACT. O’Driscoll et al.11 did not recommend ACT in general population and Gikas et al.21 even suggested that 80% of the patients have early pain relief but after 8 years 80% have osteoarthritis and 75% showed poor clinical results. Furthermore, Clar et al.22 concluded that there is insufficient evidence to suggest that ACT is cost-effective compared with microfracture or mosaicplasty and Horas et al.23 reported that the defects treated with ACT were primarily filled with fibrocartilage. A study by Knutsen et al.24 further suggested that microfracture is a less costly and less invasive procedure and should be preferred as the first-line cartilage repair procedure and that ACT may be preferred only as a second-line treatment. However, it is important to note that inclusion criteria of these studies were broad, and little consideration was paid to patients’ age or the size and location of the defects.
Mandelbaum et al.12 also report that the degree of improvement was not significantly different between patients who had a concurrent procedure with ACT than those who did not, however they did not take into consideration the history of failed marrow stimulation procedure in their exclusion criteria and 48% of their case had a history of failed marrow stimulation.
Conclusion
The findings that were obtained in this study provided clear evidence that indeed the Auto logous Chondrocyte Transplantation is an excellent method for full thickness cartilage repair of limited size and well-defined human articular knee cartilage defects as considered in this study.
Authors’ Contributions
KN has planned the study and finalized it. AS collect the data and did the statistical analysis and prepared the first version of manuscript. MF cultured the cartilage tissue in Rouyan Center. All authours read and approved the final manuscript.
Acknowledgments
The authors gratefully acknowledge Isfahan University of Medical Sciences and Royan Institute for their co-operation and financial supports.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Erggelet C, Steinwachs MR, Reichelt A. The operative treatment of full thickness cartilage defects in the knee joint with autologous chondrocyte transplantation. Saudi Med J. 2000;21(8):715–21. [PubMed] [Google Scholar]
- 2.Widuchowski W, Widuchowski J, Trzaska T. Articular cartilage defects: study of 25,124 knee arthroscopies. Knee. 2007;14(3):177–82. doi: 10.1016/j.knee.2007.02.001. [DOI] [PubMed] [Google Scholar]
- 3.Haasper C, Zeichen J, Meister R, Krettek C, Jagodzinski M. Tissue engineering of osteochondral constructs in vitro using bioreactors. Injury. 2008;39(Suppl 1):S66–S76. doi: 10.1016/j.injury.2008.01.037. [DOI] [PubMed] [Google Scholar]
- 4.Hunter W. Of the structure and disease of articulating cartilages. 1743. Clin Orthop Relat Res. 1995;(317):3–6. [PubMed] [Google Scholar]
- 5.Marlovits S, Zeller P, Singer P, Resinger C, Vecsei V. Cartilage repair: generations of autologous chondrocyte transplantation. Eur J Radiol. 2006;57(1):24–31. doi: 10.1016/j.ejrad.2005.08.009. [DOI] [PubMed] [Google Scholar]
- 6.Handl M, Trc T, Hanus M, Stastny E, Fricova-Poulova M, Neuwirth J, et al. Autologous chondrocyte implantation in the treatment of cartilage lesions of ankle joint. Acta Chir Orthop Traumatol Cech. 2007;74(1):29–36. [PubMed] [Google Scholar]
- 7.Thermann H, Driessen A, Becher C. [Autologous chondrocyte transplantation in the treatment of articular cartilage lesions of the talus] Orthopade. 2008;37(3):232–9. doi: 10.1007/s00132-008-1215-7. [DOI] [PubMed] [Google Scholar]
- 8.Henderson I, Lavigne P, Valenzuela H, Oakes B. Autologous chondrocyte implantation: superior biologic properties of hyaline cartilage repairs. Clin Orthop Relat Res. 2007;455:253–61. doi: 10.1097/01.blo.0000238829.42563.56. [DOI] [PubMed] [Google Scholar]
- 9.Peterson L, Minas T, Brittberg M, Nilsson A, Sjogren-Jansson E, Lindahl A. Two- to 9-year outcome after autologous chondrocyte transplantation of the knee. Clin Orthop Relat Res. 2000;(374):212–34. doi: 10.1097/00003086-200005000-00020. [DOI] [PubMed] [Google Scholar]
- 10.Lindahl A, Brittberg M, Peterson L. Health economics benefits following autologous chondrocyte transplantation for patients with focal chondral lesions of the knee. Knee Surg Sports Traumatol Arthrosc. 2001;9(6):358–63. doi: 10.1007/s001670100209. [DOI] [PubMed] [Google Scholar]
- 11.O’Driscoll SW, Keeley FW, Salter RB. The chondrogenic potential of free autogenous periosteal grafts for biological resurfacing of major full-thickness defects in joint surfaces under the influence of continuous passive motion. An experimental investigation in the rabbit. J Bone Joint Surg Am. 1986;68(7):1017–35. [PubMed] [Google Scholar]
- 12.Mandelbaum B, Browne JE, Fu F, Micheli LJ, Moseley JB, Jr, Erggelet C, et al. Treatment outcomes of autologous chondrocyte implantation for full-thickness articular cartilage defects of the trochlea. Am J Sports Med. 2007;35(6):915–21. doi: 10.1177/0363546507299528. [DOI] [PubMed] [Google Scholar]
- 13.Trattnig S, Millington SA, Szomolanyi P, Marlovits S. MR imaging of osteochondral grafts and autologous chondrocyte implantation. Eur Radiol. 2007;17(1):103–18. doi: 10.1007/s00330-006-0333-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Esfandiary E, Shakibaei M, Amirpour N, Fesharaki M, Nasr-esfahani MH, Moulavi F, et al. Study of human chondrocyte redifferentiation capacity in three-dimensional hydrogel culture. Iran J Basic Med Sci. 2008;11(3):152–8. [Google Scholar]
- 15.Esfandiary E, Amirpour N, Fesharaki M, Nasr MH, Molavi F, Molavi F, et al. Access to chondrocyte culture, with alginate, in Iran. Yakhte Med J. 2008;10(13):73–5. [Google Scholar]
- 16.Moriya T, Wada Y, Watanabe A, Sasho T, Nakagawa K, Mainil-Varlet P, et al. Evaluation of reparative cartilage after autologous chondrocyte implantation for osteochondritis dissecans: histology, biochemistry, and MR imaging. J Orthop Sci. 2007;12(3):265–73. doi: 10.1007/s00776-007-1111-8. [DOI] [PubMed] [Google Scholar]
- 17.Aston JE, Bentley G. Repair of articular surfaces by allografts of articular and growth-plate cartilage. J Bone Joint Surg Br. 1986;68(1):29–35. doi: 10.1302/0301-620X.68B1.3941138. [DOI] [PubMed] [Google Scholar]
- 18.Jobanputra P, Parry D, Fry-Smith A, Burls A. Effectiveness of autologous chondrocyte transplantation for hyaline cartilage defects in knees: a rapid and systematic review. Health Technol Assess. 2001;5(11):1–57. doi: 10.3310/hta5110. [DOI] [PubMed] [Google Scholar]
- 19.Browne JE, Anderson AF, Arciero R, Mandelbaum B, Moseley JB, Jr, Micheli LJ, et al. Clinical outcome of autologous chondrocyte implantation at 5 years in US subjects. Clin Orthop Relat Res. 2005;(436):237–45. doi: 10.1097/00003086-200507000-00036. [DOI] [PubMed] [Google Scholar]
- 20.Scorrano A. Autologous condrocyte implasntation for focal cartilage deffect in athletes histology and second look arthroscopy. J orthopaed traumatol. 2004;2:98–105. [Google Scholar]
- 21.Gikas PD, Aston WJ, Briggs TW. Autologous chondrocyte implantation: where do we stand now? J Orthop Sci. 2008;13(3):283–92. doi: 10.1007/s00776-007-1228-9. [DOI] [PubMed] [Google Scholar]
- 22.Clar C, Cummins E, Mclntyre L, Thomas S, Lamb J, Bain L, et al. Clinical and cost-effectiveness of autologous chondrocyte implantation for cartilage defects in knee joints: systematic review and economic evaluation. Health Technol Assess. 2005;9(47):iii–x. doi: 10.3310/hta9470. 1. [DOI] [PubMed] [Google Scholar]
- 23.Horas U, Pelinkovic D, Herr G, Aigner T, Schnettler R. Autologous chondrocyte implantation and osteochondral cylinder transplantation in cartilage repair of the knee joint. A prospective, comparative trial. J Bone Joint Surg Am. 2003;85-A(2):185–92. doi: 10.2106/00004623-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 24.Knutsen G, Drogset JO, Engebretsen L, Grontvedt T, Isaksen V, Ludvigsen TC, et al. A randomized trial comparing autologous chondrocyte implantation with microfracture. Findings at five years. J Bone Joint Surg Am. 2007;89(10):2105–12. doi: 10.2106/JBJS.G.00003. [DOI] [PubMed] [Google Scholar]


