Abstract
BACKGROUND:
Evidence exists for reciprocal effects of insulin and desacyl-ghrelin (DAG) concentration, but the association between different fatty acid saturation in high fat diet (HFD) and these hormones remain to be established. To evaluate the impact of different sources of dietary fat and the level of fatty acid saturation on plasma insulin and DAG levels and also the association of DAG with insulin action this study was carried out.
METHODS:
Male weaning Wistar rats were randomly divided into four groups of HFDs, high fat butter (HF-B), high fat soy (HF-S), high fat olive (HF-O), high fat fish (HF-F), and a group of standard diet (SD). Blood samples were collected after 8 weeks and after they were fasted for 24 h. Body weight, food intake, plasma glucose, insulin, DAG and insulin resistance (HOMA-IR) were measured.
RESULTS:
Plasma insulin levels at fed and fasted status, were significantly higher in rats on HF-B compared to those on SD, HF-F and HF-O diets (P<0.05). Insulin concentration in rats on HF-S was also higher than those on SD, HF-F and HF-O diets (P<0.05), in the feeding status. Insulin resistance was significantly higher in rats on HF-B, compared to those on SD, HF-F and HF-O (P<0.05). Rats that were fed with HF-B diet had lower fasting plasma DAG levels than the SD, HF-F and HF-O groups (P<0.05); furthermore, the HF-F group had significantly higher DAG level than the HF-S groups (P<0.05).
CONCLUSIONS:
Fish and olive oils may hence contribute to lower insulin level and HOMA-IR by increasing DAG concentration and may have more health benefits than other fat sources in diets.
KEYWORDS: Fish oil, olive oil, desacyl-ghrelin, insulin resistance
Composition of the diet, in terms of quality and quantity of fat, plays an important role in glucose homeostasis and insulin sensitivity in both animals and humans.1 Some previous studies have revealed that high fat diets (HFDs) induce hyperglycemia and whole body insulin resistance2 and FFAs with different degrees of saturation could also affect fat-induced insulin resistance.3 It appears that variation in fatty acid composition or types of fat may independently affect insulin action and alter insulin sensitivity.4 For example, fat intake ranging from ~ 40 to 75% of total ingested calories, usually in the form of saturated fat (SFA) or ω-6 polyunsaturated fatty acid (ω-6 PUFA) like safflower and corn oil, have been reported to reduce whole body insulin-stimulated glucose uptake.5 On the other hand, the substitution of small percentage (6-7%) of ω-3 long chain fatty acids from fish oil with other types of lipids in high fat feeding prevents the development of insulin resistance.6–7 However, previous studies have shown contradictory effects of monounsaturated fatty acid (MUFA) on insulin resistance.1,8
Meanwhile, it has become increasingly evident that the gastrointestinal tract plays a key role in the association of fat contents of the meal and the subsequent metabolic and hormonal responses.9–10 Over the past several years, the gastrointestinal tract has been found to release important peptide hormones into the systemic circulation in response to the contents of the meal.9,11 These gastrointestinal peptides, such as glucose-dependent insulinotropic polypeptide (GIP), glucagon-like peptide-1 (GLP-1), peptide YY3-36 (PYY3-36) and ghrelin, have several important metabolic actions, including mediating pancreatic insulin secretion.10,12–13 Among these peptides, ghrelin and insulin levels, fluctuate reciprocally before and after fed status.14 Intravenous administration of ghrelin stimulates insulin secretion in free-feeding rats15 and in vitro studies have also shown that ghrelin increases insulin secretion from isolated rat pancreatic islets.16–17 In contrast, peripheral ghrelin administration suppresses insulin secretion in men18–19 and mice.20 Acylated (or n-octanoylated) and unacylated (or desoctanoylated or des-acylated) are 2 circulating forms of ghrelin.21 Acylghrelin plays important roles in the negative regulation of insulin secretion, insulin sensitivity, and glucose metabolism. On the other hand, excess of endogenous desacyl-ghrelin (DAG) improves insulin sensitivity suggesting that DAG might be implicated in the modulation of insulin release.17,22–23 However, the metabolic roles of DAG remain to be defined.24 In this study, the effect of different sources of dietary fat and the level of fatty acid saturation on plasma insulin and DAG levels and also, the association of DAG with insulin action following 8 weeks of HFD consumption have been investigated in growing rats.
Methods
Animals and experimental protocol
In an experimental study, fifty male weaning Wistar rats (Pasture Institute, Iran), 21 days of age on arrival were housed individually in wire bar-floor cages. Body weight (BW) in grams was recorded on arrival and weekly thereafter. Food intake was also monitored twice a week. The animals (weight: 35.59 ± 0.39) were allowed 1 week of acclimatization in a standard environment at 22°C, 50% humidity and 12-h light/dark cycles with free access to food and water. During the first week, all animals were fed a standard laboratory chow (Pasture Institute, Iran) and afterwards, they were randomly assigned to five groups. Diet composition and energy value are given in Table 1. The composition of standard diet (SD) was completely the same as the commercial AIN-93G. Diet treatments included high fat diets (HFD) with soy oil (HF-S), butter (HF-B), fish oil (HF-F) or olive oil (HF-O). High fat diets were prepared to provide equal vitamins and minerals per calorie, and contained equal percentages by weight of fiber. After an 8-week feeding period, blood samples were collected from the retro-orbital veins into polypropylene tubes containing sodium EDTA (1-mg/mL blood) and aprotinin (500-unit/mL blood, Bayer) in the non-fasted and 24 h-fasted state for measurement of serum parameters and were then centrifuged for 15 min at 3000x g. Plasma samples were stored at -80°C. Animals were anesthesized with CO2 to minimize the potential impact of anesthesia on hormone levels.
Table 1.
Composition of Diets
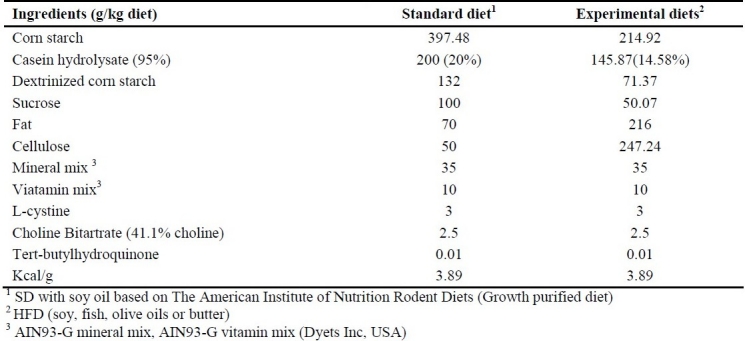
Experimental diets
The experimental HFDs were almost isocaloric (Table 1), and composed of a fat-free basal diet based on American Institute of Nutrition Rodent Diets -Growth purified diet-(AIN93-G),25 containing carbohydrate (corn starch, dextrinized corn starch, Dyets company, USA), protein (98.5% casein hydrolisate and 1.5% L-cyctine, choline bitartrate, Dyets companay, USA), fiber (cellulose, Dyets company, USA), vitamin-mineral mix, and tertbutylhydroquinone (Dyets company, USA) corn starch. Dietray fats were also added in order to provide a balanced diet and included fish oil and olive oil which were a generous gift from Nooshdarooye Darya Institute, Iran, soy oil (purchased from Ladan Institute, Iran) and butter (obtained locally). The diets were prepared weekly and stored as vacuum packed (500 g) at –20°C. Packs taken for use were thawed in the refrigerator at 4°C. The food was offered daily at the beginning of the dark phase, and the remains were weighed and removed after an 48 hours.
All experiments were carried out in accordance with standards approved by the local ethics committee of the Research Institute for Endocrine Science of Shahid Behesti University of Medical Sciences. The ethics code was 291EC1388.12.11.
Laboratory measurements
Plasma glucose was determined by an enzymatic (Glucose Oxidase) colorimetric method (Pars Azmoun Co, Tehran, Iran). The assay sensitivity was 1 mg/dl, the intra- and interassay coefficients of variation were 1.2% and 1.8%, respectively. Plasma Insulin was determined by an ELISA method (Mercodia AB, Uppsala, Sweden). The assay sensitivity was 0.07 μg/L, the intra- and interassay coefficients of variations were 2.5% and 4.1%, respectively.
Blood samples were immediately transferred to chilled tubes containing Na2-EDTA (1 mg/ml) and aprotinin (500-unit/mL blood, Bayer) centrifuged in 2000 rpm for 15 min at 4°C. Hydrogen chloride was added to the samples at a final concentration of 0.1 N immediately after separation of the plasma. Unacylated form of ghrelin was measured using DAG ELISA kit according to the manufacturer's protocol (Mitsubishi Kagaku Iatron, Inc). The minimal detection limit of DAG in this assay system was 12.5 fmol/ml. The assay used to detect DAG has less than 0.1% of cross-reaction with acylated ghrelin. The intra- and interassay coefficients of variation were 3.7% and 8.1%, respectively.
Insulin resistance was estimated by homeostasis model assessment (HOMA), according to the formula: insulin resistance index = fasting insulin (μg/L) × fasting glucose (mg/dl)/405.26
Gas chromatography analyses were carried out on gas chromatography (Younglin Instrument, 6000 series, South Korea) equipped with a split-injector and flame ionization detector. Methyl-esters of fatty acids (FAME) were prepared using methanolic KOH, according to the standard method (ISO5509:2000). The fatty acid profile was determined by gas chromatographic separation of their methyl esters (ISO 5508: 1990) on a capillary column (J&W Scientific DB-23, 30 m × 0.25mm × 0.25 μm). Chromatography software (Unicam 4880 chromatography data system) was used for data collection and processing. Data are presented in Table 2.
Table 2.
Fatty acid composition (%) of the diets analyzed by gas-liquid chromatography.
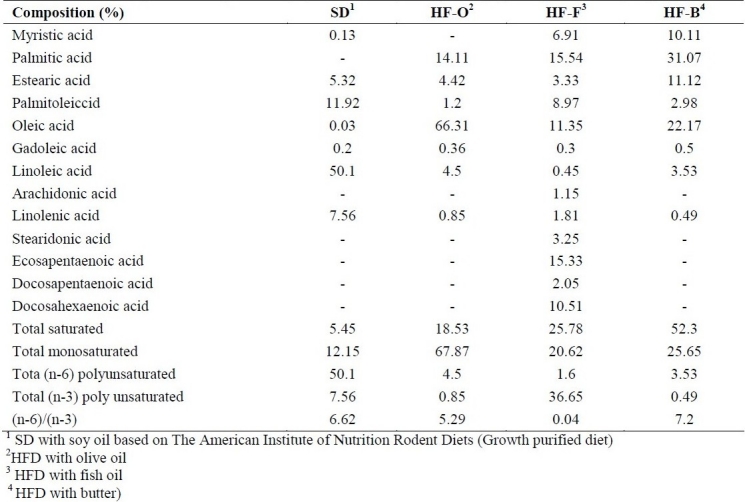
Statistical analysis
Statistical analyses were performed using SPSS 16.0 software (Chicago, IL, USA). The Kolmogorov–Smirnov test was applied to determine the normality of the distribution of the data to be used in the parametric test . One-way ANOVA, paired t test and Tukey test were used to compare the diet groups and p value<0.05 was considered statistically significant. Nonparametric tests (Kruskal-Wallis, Wilcoxon Signed-Rank and Mann-Whitney U) were also employed for insulin level and HOMA-IR variables and p value<0.05 was considered significant. The Spearman's rank correlation coefficient was also used to determine whether a significant relationship existed between insulin and DAG concentrations.
Results
Dietary intake, energy intake and body weight
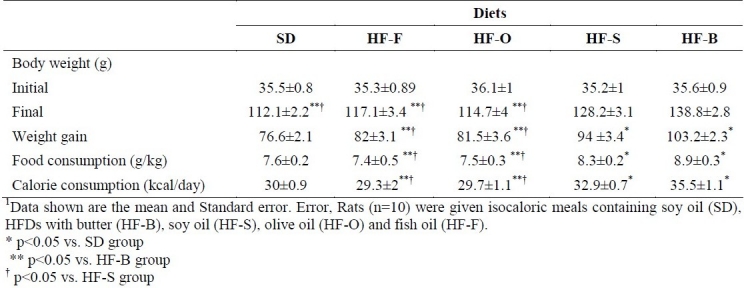
Food, calorie intakes and body weights of the groups are shown in Table 3. Food intake was significantly different among the groups fed with different dietary fats. High fat butter and HF-S groups had significantly higher food and calorie intake in comparison with SD, HF-F and HF-O groups (F=6.38, p=0.00). Initial body weights were not different among dietary groups but the weight gains in the HF-B and HF-S were 26.6 and 17.74 g higher, respectively, than the weight gain of SD group animals (p=0.01 and p=0.04, respectively). High fat butter had also 21.2 and 21.7 g higher weight gain compared with the HF-F and HF-O groups, respectively (p=0.00 and p=0.00, respectively). Weight gain of HF-F and HF-O groups were 12.3 and 12.8 g higher than the weight gain of HF-S group, respectively (p=0.04 and p=0.01, respectively). Final body weight in HF-S and HF-B groups was higher than that of DS, HF-F and HF-O groups (p=0.04).
Table 3.
Body weight, food and calorie intake of rats consuming high fat diets (HFDs) for 8 weeks1
Plasma desacyl-ghrelin, insulin and glucose levels
The plasma DAG concentration was significantly decreased in SD, HF-F, HF-O and HF-S diets in the ad libitum fed status, compared with fasting condition (p=0.00, Figure 1A). Plasma DAG levels at fed state in HF-B and HF-S diet were higher than those of SD, HF-F and HF-O diets; however, these findings were not significant.
Figure 1.
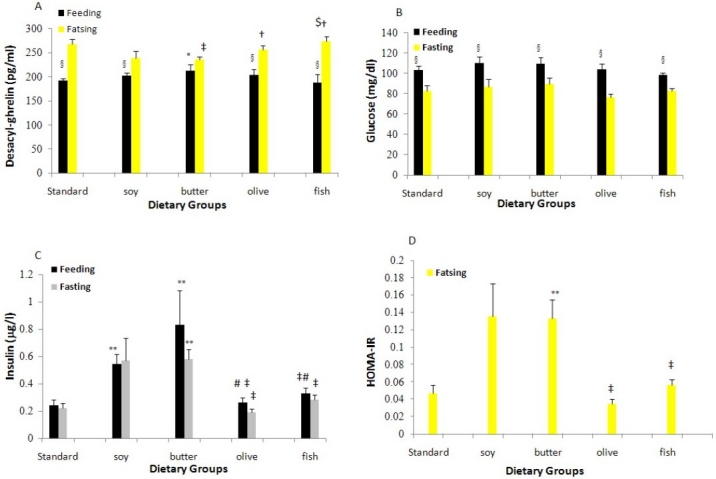
Plasma Desacyl-ghrelin (1A), Glucose (1B) and Insulin (1C) levels and insulin resistance (HOMA-IR) (1D) in rats consuming high dietary fats (HFDs) for 8 weeks (n=10 in each group).
High fat fish, SD and HF-O diet rats showed 47.3, 42.7 and 38.6 ρg/ml higher DAG concentrations than the HF-B diet rats at fasted state, respectively (p=0.00); meanwhile, plasma DAG level in the HF-S diet rats was 16.4 ρg/ml lower than the HF-F diet rats (p=0.00).
Ad libitum fed plasma insulin concentration in the HF-B diet was significantly higher than that of the SD, HF-F and HF-O diets (p=0.00) and the increase in the HF-S diet group was 0.35, 0.37 and 0.31 μ g/l higher than that of the SD, HF-O and HF-F diets, respectively (p=0.01, Figure 1B). Furthermore, plasma insulin levels were decreased by fasting in all diet groups, although the decreases were not significant. Rats on the SD, HF-F and HF-O diets had significantly lower fasting insulin plasma levels than those on HF-B (p=0.04).
Plasma glucose levels were significantly decreased by fasting in all of diet groups, compared with fed state (p=0.00, Figure 1C). However, plasma glucose levels were not significantly different among dietary groups.
Insulin resistance levels (HOMA-IR)
After 24 h fasting, HF-B diet had significantly higher HOMA-IR in comparison to the HF-F, HF-O and SD diets (p=0.05, Figure 1D).
There was no significant correlation between changes in insulin and changes in DAG levels in the feeding (Spearman correlation=0.16, p=0.3) and 24 hour fasting status (Spearman correlation=-0.34, p=0.06).
Discussion
Our findings showed that insulin concentration at fed state was significantly lower in the HF-F and HF-O groups, in comparison to the HF-B and HF-S diets, although in fasting state, insulin level differences were similar. Insulin resistance (HOMA-IR) was also higher in the HF-B diet, in comparison to the SD, HF-F and HF-O groups. On the other hand, food, energy intake and the following weight gain in HF-F and HF-O diets were lower than those of the HF-B and HF-S groups. Although, the effect of different fat sources on appetite is controversial and limited, but previous studies have reported that each energy unit from fat had weaker satiety effects compared to carbohydrate and protein; on the other hand, high fat foods usually have higher energy density, so these foods can lead to more food consumption and weight gain and obesity.27 Alfenas and Mattes have been shown that saturated fatty acid (SFA) absorption is less effective than unsaturated fatty acids in rats and they assumed that polyunsaturated fatty acid (PUFA) has more satiety effect than monosaturated fatty acid (MUFA) and MUFA more than SFA.28 Therefore, higher insulin levels or insulin resistance in rats receiving HF-B (containing SFA) or HF-S (containing ω-6 PUFA) groups, probably, is related to higher weight or appetite.29–30 So, in this study the satiety effect of PUFAs was not found in ω-6 PUFA. Body weight and food intake were also the same as those of SFA group. This finding probably was related to the scientific fact that high proportion of ω-6 PUFA to ω-6 PUFA in the diet shifts the physiological state in the tissues toward the pathogenesis of many diseases like obesity.31–32
Latown and co-workers also reported that food containing SFA, compared to PUFA, was accompanied by higher food and energy intake33 and may be, because of this, most previous studies suggest that fish oil high in polyunsaturated ω-3 fatty acids normalizes the insulin action and prevents insulin resistance induced by a HFD in rats3,34–36 or can lead to better glucose response and obesity parameters in mice.37 Furthermore, animal and human studies have demonstrated that SFA increases insulin resistance38–39 and animal studies also showed that ω-6 PUFA in comparison to ω-3 PUFA could decrease insulin sensitivity.6,35 However, other investigators have suggested that higher intakes of SFA and ω-6 PUFA do not adversely affect insulin secretion40–41 and HOMA-IR.34 In addition, some studies have demonstrated that HFD with MUFA does not improve insulin secretion and sensitivity in rats.2–3,42 It should be noticed that the majority of these studies have used adult rats (125–300 g body weight), fed more total calories than controls,2,43 while the present study utilized weaning, prepubertal rats fed an ad libitum diet. Furthermore, duration of the study and composition of basal diet, especially fiber content, could affect insulin sensitivity and basal glucose metabolism.44–45 These differences may explain the apparent contradictions among different researches.
The present study has shown that the decrease in fasting plasma insulin and insulin resistance in the fish oil and MUFA groups was associated with an increase in fasting plasma DAG, suggesting that the increased DAG levels observed in these rats may be due to decreased insulin secretion. Although, the mechanisms responsible for the differential effects of fatty acids on insulin action and glucose homeostasis have not been fully elucidated,46 ghrelin levels were found to be reciprocal to those of glucose and insulin14,47 and previous data available suggested a negative association between systemic ghrelin and insulin levels, with ghrelin inhibiting insulin secretion both in vitro and in vivo and in most human or animal studies.48
It should be pointed out that ghrelin and DAG are two separate peptides but they can modify the actions of each other on glucose handling.16 In this study, it is reasonable to postulate that the low fasting DAG levels in HF-B fed rats partially relieve its inhibition on insulin production. The insulin level therefore, is increased to compensate the peripheral insulin resistance caused by high SFA consumption as previously reported.39 Nevertheless, this study could not show any significant correlations between insulin and DAG concentrations. On one hand, this may be related to different metabolic status in growing rats and on the other hand, previous studies have measured insulin concentration in the portal circulation following intravenous injection of DAG, which may affect the findings.24,49–50 Gauna et al. have hypothesized that assessment of insulin concentration in the portal vein might be more informative than that in the systemic circulation.24
As we said above, calorie intake, food intake and weight gain were higher in HF-B and HF-S diets in comparison to SD, HF-F and HF-O groups and on the other hand, HF-F and HF-O had higher DAG and lower insulin and insulin resistance in comparison to the HF-S and HF-B diets. As, documented, ghrelin and insulin are two gut hormones playing an affective role in body weight regulation;6 DAG could hence decrease food intake and gastric emptying in mice and rats.51 Food intake modulator actions in the central nervous system have been suggested for insulin.52–53 According to the findings of this study, food intake, calorie intake and weight gain were lower in groups with higher DAG, which is consistent with recent report that DAG induces a negative energy balance by decreasing food intake and delaying gastric emptying.51 On the other hand, in groups with higher insulin concentration and insulin resistance, food intake, calorie intake and weight gain also were higher. These findings are comparable with the Buettner (and coworkers’) study in which they have shown that groups with higher weight gain, had higher plasma glucose levels and less effective insulin-induced glucose disposal.2
Conclusion
Rats fed with HFDs containing MUFA and ω-3 PUFA had significantly lower weight gain, food and calorie intake and these changes were associated with increased fasting plasma DAG concentrations, concomitant with lower insulin concentration and insulin resistance. Results of this study suggest that HFDs containing fish oil and olive oil can increase the DAG which may play a role in weight, appetite control and insulin resistance improvement in young rats.
Authors’ Contributions
AS carried out the design of study, coordinated, carried out all the experiments and also writing the manuscript. MK, SZ carried out the design, writing the manuscript and also supervised the project. MV carried out the design and coordinated the study, participated in most of the experiments and prepared the manuscript. AG, MSD and MZ participated in data collection and provided comments on the laboratory analysis of samples. AA coordinated in statistical analysis. All authors have read and approved the content of the manuscript.
Acknowledgments
This study was funded by Research Institute for Food and Nutrition Sciences, Shahid Beheshti University of Medical Sciences, the Interdisciplinary Research Program, Endocrine Research Center, Research Institute for Endocrine Sciences, Shahid Beheshti University of Medical Sciences. The research project number of this study was 358, dated 1358.5.5. Special thanks are extended to Dr. Mehdi Hedayati for advice on the laboratory procedures.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Tierney AC, Roche HM. The potential role of olive oil-derived MUFA in insulin sensitivity. Mol Nutr Food Res. 2007;51(10):1235–48. doi: 10.1002/mnfr.200700143. [DOI] [PubMed] [Google Scholar]
- 2.Buettner R, Parhofer KG, Woenckhaus M, Wrede CE, Kunz-Schughart LA, Scholmerich J, et al. Defining high-fat-diet rat models: metabolic and molecular effects of different fat types. J Mol Endocrinol. 2006;36(3):485–501. doi: 10.1677/jme.1.01909. [DOI] [PubMed] [Google Scholar]
- 3.Han P, Zhang YY, Lu Y, He B, Zhang W, Xia F. Effects of different free fatty acids on insulin resistance in rats. Hepatobiliary Pancreat Dis Int. 2008;7(1):91–6. [PubMed] [Google Scholar]
- 4.Lovejoy JC. The influence of dietary fat on insulin resistance. Curr Diab Rep. 2002;2(5):435–40. doi: 10.1007/s11892-002-0098-y. [DOI] [PubMed] [Google Scholar]
- 5.Wilkes JJ, Bonen A, Bell RC. A modified high-fat diet induces insulin resistance in rat skeletal muscle but not adipocytes. Am J Physiol. 1998;275(4 Pt 1):E679–86. doi: 10.1152/ajpendo.1998.275.4.E679. [DOI] [PubMed] [Google Scholar]
- 6.Jucker BM, Cline GW, Barucci N, Shulman GI. Differential effects of safflower oil versus fish oil feeding on insulin-stimulated glycogen synthesis, glycolysis, and pyruvate dehydrogenase flux in skeletal muscle: a 13C nuclear magnetic resonance study. Diabetes. 1999;48(1):134–40. doi: 10.2337/diabetes.48.1.134. [DOI] [PubMed] [Google Scholar]
- 7.Holness MJ, Smith ND, Greenwood GK, Sugden MC. Acute omega-3 fatty acid enrichment selectively reverses high-saturated fat feeding-induced insulin hypersecretion but does not improve peripheral insulin resistance. Diabetes. 2004;53(Suppl 1):S166–71. doi: 10.2337/diabetes.53.2007.s166. [DOI] [PubMed] [Google Scholar]
- 8.Storlien LH, Kriketos AD, Jenkins AB, Baur LA, Pan DA, Tapsell LC, et al. Does dietary fat influence insulin action? Ann N Y Acad Sci. 1997;827:287–301. doi: 10.1111/j.1749-6632.1997.tb51842.x. [DOI] [PubMed] [Google Scholar]
- 9.Elliott RM, Morgan LM, Tredger JA, Deacon S, Wright J, Marks V. Glucagon-like peptide-1 (7-36)amide and glucose-dependent insulinotropic polypeptide secretion in response to nutrient ingestion in man: acute post-prandial and 24-h secretion patterns. J Endocrinol. 1993;138(1):159–66. doi: 10.1677/joe.0.1380159. [DOI] [PubMed] [Google Scholar]
- 10.Schirra J, Katschinski M, Weidmann C, Schafer T, Wank U, Arnold R, et al. Gastric emptying and release of incretin hormones after glucose ingestion in humans. J Clin Invest. 1996;97(1):92–103. doi: 10.1172/JCI118411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Knuth ND, Shrivastava CR, Horowitz JF. Reducing dietary fat from a meal increases the bioavailability of exogenous carbohydrate without altering plasma glucose concentration. J Appl Physiol. 2009;106(1):122–9. doi: 10.1152/japplphysiol.90404.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Batterham RL, Cowley MA, Small CJ, Herzog H, Cohen MA, Dakin CL, et al. Gut hormone PYY(3-36) physiologically inhibits food intake. Nature. 2002;418(6898):650–4. doi: 10.1038/nature00887. [DOI] [PubMed] [Google Scholar]
- 13.Poykko SM, Kellokoski E, Horkko S, Kauma H, Kesaniemi YA, Ukkola O. Low plasma ghrelin is associated with insulin resistance, hypertension, and the prevalence of type 2 diabetes. Diabetes. 2003;52(10):2546–53. doi: 10.2337/diabetes.52.10.2546. [DOI] [PubMed] [Google Scholar]
- 14.Cummings DE, Purnell JQ, Frayo RS, Schmidova K, Wisse BE, Weigle DS. A preprandial rise in plasma ghrelin levels suggests a role in meal initiation in humans. Diabetes. 2001;50(8):1714–9. doi: 10.2337/diabetes.50.8.1714. [DOI] [PubMed] [Google Scholar]
- 15.Lee HM, Wang G, Englander EW, Kojima M, Greeley GH., Jr Ghrelin, a new gastrointestinal endocrine peptide that stimulates insulin secretion: enteric distribution, ontogeny, influence of endocrine, and dietary manipulations. Endocrinology. 2002;143(1):185–90. doi: 10.1210/endo.143.1.8602. [DOI] [PubMed] [Google Scholar]
- 16.Adeghate E, Ponery AS. Ghrelin stimulates insulin secretion from the pancreas of normal and diabetic rats. J Neuroendocrinol. 2002;14(7):555–60. doi: 10.1046/j.1365-2826.2002.00811.x. [DOI] [PubMed] [Google Scholar]
- 17.Date Y, Nakazato M, Hashiguchi S, Dezaki K, Mondal MS, Hosoda H, et al. Ghrelin is present in pancreatic alpha-cells of humans and rats and stimulates insulin secretion. Diabetes. 2002;51(1):124–9. doi: 10.2337/diabetes.51.1.124. [DOI] [PubMed] [Google Scholar]
- 18.Broglio F, Arvat E, Benso A, Gottero C, Muccioli G, Papotti M, et al. Ghrelin, a natural GH secretagogue produced by the stomach, induces hyperglycemia and reduces insulin secretion in humans. J Clin Endocrinol Metab. 2001;86(10):5083–6. doi: 10.1210/jcem.86.10.8098. [DOI] [PubMed] [Google Scholar]
- 19.Arosio M, Ronchi CL, Gebbia C, Cappiello V, Beck-Peccoz P, Peracchi M. Stimulatory effects of ghrelin on circulating somatostatin and pancreatic polypeptide levels. J Clin Endocrinol Metab. 2003;88(2):701–4. doi: 10.1210/jc.2002-021161. [DOI] [PubMed] [Google Scholar]
- 20.Reimer MK, Pacini G, Ahren B. Dose-dependent inhibition by ghrelin of insulin secretion in the mouse. Endocrinology. 2003;144(3):916–21. doi: 10.1210/en.2002-220819. [DOI] [PubMed] [Google Scholar]
- 21.Kojima M, Hosoda H, Date Y, Nakazato M, Matsuo H, Kangawa K. Ghrelin is a growth-hormone-releasing acylated peptide from stomach. Nature. 1999;402(6762):656–60. doi: 10.1038/45230. [DOI] [PubMed] [Google Scholar]
- 22.Gnanapavan S, Kola B, Bustin SA, Morris DG, McGee P, Fairclough P, et al. The tissue distribution of the mRNA of ghrelin and subtypes of its receptor, GHS-R, in humans. J Clin Endocrinol Metab. 2002;87(6):2988. doi: 10.1210/jcem.87.6.8739. [DOI] [PubMed] [Google Scholar]
- 23.Volante M, Allia E, Gugliotta P, Funaro A, Broglio F, Deghenghi R, et al. Expression of ghrelin and of the GH secretagogue receptor by pancreatic islet cells and related endocrine tumors. J Clin Endocrinol Metab. 2002;87(3):1300–8. doi: 10.1210/jcem.87.3.8279. [DOI] [PubMed] [Google Scholar]
- 24.Gauna C, Kiewiet RM, Janssen JA, van de ZB, Delhanty PJ, Ghigo E, et al. Unacylated ghrelin acts as a potent insulin secretagogue in glucose-stimulated conditions. Am J Physiol Endocrinol Metab. 2007;293(3):E697–704. doi: 10.1152/ajpendo.00219.2007. [DOI] [PubMed] [Google Scholar]
- 25.Reeves PG. Components of the AIN-93 diets as improvements in the AIN-76A diet. J Nutr. 1997;127(5 Suppl):838S–41S. doi: 10.1093/jn/127.5.838S. [DOI] [PubMed] [Google Scholar]
- 26.Skrha J, Haas T, Sindelka G, Prazny M, Widimsky J, Cibula D, et al. Comparison of the insulin action parameters from hyperinsulinemic clamps with homeostasis model assessment and QUICKI indexes in subjects with different endocrine disorders. J Clin Endocrinol Metab. 2004;89(1):135–41. doi: 10.1210/jc.2002-030024. [DOI] [PubMed] [Google Scholar]
- 27.MacIntosh CG, Holt SH, Brand-Miller JC. The degree of fat saturation does not alter glycemic, insulinemic or satiety responses to a starchy staple in healthy men. J Nutr. 2003;133(8):2577–80. doi: 10.1093/jn/133.8.2577. [DOI] [PubMed] [Google Scholar]
- 28.Alfenas RC, Mattes RD. Effect of fat sources on satiety. Obes Res. 2003;11(2):183–7. doi: 10.1038/oby.2003.29. [DOI] [PubMed] [Google Scholar]
- 29.Kahn SE, Hull RL, Utzschneider KM. Mechanisms linking obesity to insulin resistance and type 2 diabetes. Nature. 2006;444(7121):840–6. doi: 10.1038/nature05482. [DOI] [PubMed] [Google Scholar]
- 30.Anthony K, Reed LJ, Dunn JT, Bingham E, Hopkins D, Marsden PK, et al. Attenuation of insulin-evoked responses in brain networks controlling appetite and reward in insulin resistance: the cerebral basis for impaired control of food intake in metabolic syndrome? Diabetes. 2006;55(11):2986–92. doi: 10.2337/db06-0376. [DOI] [PubMed] [Google Scholar]
- 31.Simopoulos AP. Evolutionary aspects of diet, the omega-6/omega-3 ratio and genetic variation: nutritional implications for chronic diseases. Biomed Pharmacother. 2006;60(9):502–7. doi: 10.1016/j.biopha.2006.07.080. [DOI] [PubMed] [Google Scholar]
- 32.Calder PC. n-3 polyunsaturated fatty acids, inflammation, and inflammatory diseases. Am J Clin Nutr. 2006;83(6 Suppl):1505S–19S. doi: 10.1093/ajcn/83.6.1505S. [DOI] [PubMed] [Google Scholar]
- 33.Lawton CL, Delargy HJ, Brockman J, Smith FC, Blundell JE. The degree of saturation of fatty acids influences post-ingestive satiety. Br J Nutr. 2000;83(5):473–82. [PubMed] [Google Scholar]
- 34.Storlien LH, Jenkins AB, Chisholm DJ, Pascoe WS, Khouri S, Kraegen EW. Influence of dietary fat composition on development of insulin resistance in rats. Relationship to muscle triglyceride and omega-3 fatty acids in muscle phospholipid. Diabetes. 1991;40(2):280–9. doi: 10.2337/diab.40.2.280. [DOI] [PubMed] [Google Scholar]
- 35.Storlien LH, Kraegen EW, Chisholm DJ, Ford GL, Bruce DG, Pascoe WS. Fish oil prevents insulin resistance induced by high-fat feeding in rats. Science. 1987;237(4817):885–8. doi: 10.1126/science.3303333. [DOI] [PubMed] [Google Scholar]
- 36.Castellano CA, Audet I, Laforest JP, Chouinard Y, Matte JJ. Fish oil diets do not improve insulin sensitivity and secretion in healthy adult male pigs. Br J Nutr. 2010;103(2):189–96. doi: 10.1017/S0007114509991590. [DOI] [PubMed] [Google Scholar]
- 37.Ikemoto S, Takahashi M, Tsunoda N, Maruyama K, Itakura H, Ezaki O. High-fat diet-induced hyperglycemia and obesity in mice: differential effects of dietary oils. Metabolism. 1996;45(12):1539–46. doi: 10.1016/s0026-0495(96)90185-7. [DOI] [PubMed] [Google Scholar]
- 38.Hunnicutt JW, Hardy RW, Williford J, McDonald JM. Saturated fatty acid-induced insulin resistance in rat adipocytes. Diabetes. 1994;43(4):540–5. doi: 10.2337/diab.43.4.540. [DOI] [PubMed] [Google Scholar]
- 39.Luo J, Rizkalla SW, Boillot J, Alamowitch C, Chaib H, Bruzzo F, et al. Dietary (n-3) polyunsaturated fatty acids improve adipocyte insulin action and glucose metabolism in insulin-resistant rats: relation to membrane fatty acids. J Nutr. 1996;126(8):1951–8. doi: 10.1093/jn/126.8.1951. [DOI] [PubMed] [Google Scholar]
- 40.Lopez S, Bermudez B, Pacheco YM, Villar J, Abia R, Muriana FJ. Distinctive postprandial modulation of beta cell function and insulin sensitivity by dietary fats: monounsaturated compared with saturated fatty acids. Am J Clin Nutr. 2008;88(3):638–44. doi: 10.1093/ajcn/88.3.638. [DOI] [PubMed] [Google Scholar]
- 41.Dobbins RL, Szczepaniak LS, Myhill J, Tamura Y, Uchino H, Giacca A, et al. The composition of dietary fat directly influences glucose-stimulated insulin secretion in rats. Diabetes. 2002;51(6):1825–33. doi: 10.2337/diabetes.51.6.1825. [DOI] [PubMed] [Google Scholar]
- 42.Prieto PG, Cancelas J, Moreno P, Villanueva-Penacarrillo ML, Malaisse WJ, Valverde I. Effects of diet supplementation with olive oil and guar upon fructose-induced insulin resistance in normal rats. Endocrine. 2007;31(3):294–9. doi: 10.1007/s12020-007-0038-3. [DOI] [PubMed] [Google Scholar]
- 43.Chicco A, D’Alessandro ME, Karabatas L, Gutman R, Lombardo YB. Effect of moderate levels of dietary fish oil on insulin secretion and sensitivity, and pancreas insulin content in normal rats. Ann Nutr Metab. 1996;40(2):61–70. doi: 10.1159/000177897. [DOI] [PubMed] [Google Scholar]
- 44.Stein DT, Esser V, Stevenson BE, Lane KE, Whiteside JH, Daniels MB, et al. Essentiality of circulating fatty acids for glucose-stimulated insulin secretion in the fasted rat. J Clin Invest. 1996;97(12):2728–35. doi: 10.1172/JCI118727. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Brown JL, Spicer MT, Spicer LJ. Effect of high-fat diet on body composition and hormone responses to glucose tolerance tests. Endocrine. 2002;19(3):327–32. doi: 10.1385/ENDO:19:3:327. [DOI] [PubMed] [Google Scholar]
- 46.Stefan N, Wahl HG, Fritsche A, Haring H, Stumvoll M. Effect of the pattern of elevated free fatty acids on insulin sensitivity and insulin secretion in healthy humans. Horm Metab Res. 2001;33(7):432–8. doi: 10.1055/s-2001-16231. [DOI] [PubMed] [Google Scholar]
- 47.Gauna C, Meyler FM, Janssen JA, Delhanty PJ, Abribat T, van Koetsveld P, et al. Administration of acylated ghrelin reduces insulin sensitivity, whereas the combination of acylated plus unacylated ghrelin strongly improves insulin sensitivity. J Clin Endocrinol Metab. 2004;89(10):5035–42. doi: 10.1210/jc.2004-0363. [DOI] [PubMed] [Google Scholar]
- 48.Broglio F, Gottero C, Benso A, Prodam F, Destefanis S, Gauna C, et al. Effects of ghrelin on the insulin and glycemic responses to glucose, arginine, or free fatty acids load in humans. J Clin Endocrinol Metab. 2003;88(9):4268–72. doi: 10.1210/jc.2002-021940. [DOI] [PubMed] [Google Scholar]
- 49.Ariyasu H, Takaya K, Iwakura H, Hosoda H, Akamizu T, Arai Y, et al. Transgenic mice overexpressing des-acyl ghrelin show small phenotype. Endocrinology. 2005;146(1):355–64. doi: 10.1210/en.2004-0629. [DOI] [PubMed] [Google Scholar]
- 50.Sangiao-Alvarellos S, Cordido F. Effect of ghrelin on glucose-insulin homeostasis: therapeutic implications. Int J Pept. 2010;2010 doi: 10.1155/2010/234709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Gil-Campos M, Aguilera CM, Canete R, Gil A. Ghrelin: a hormone regulating food intake and energy homeostasis. Br J Nutr. 2006;96(2):201–26. doi: 10.1079/bjn20061787. [DOI] [PubMed] [Google Scholar]
- 52.McGowan MK, Andrews KM, Grossman SP. Chronic intrahypothalamic infusions of insulin or insulin antibodies alter body weight and food intake in the rat. Physiol Behav. 1992;51(4):753–66. doi: 10.1016/0031-9384(92)90112-f. [DOI] [PubMed] [Google Scholar]
- 53.Maffeis C, Bonadonna RC, Consolaro A, Vettor R, Banzato C, Silvagni D, et al. Ghrelin, insulin sensitivity and postprandial glucose disposal in overweight and obese children. Eur J Endocrinol. 2006;154(1):61–8. doi: 10.1530/eje.1.02055. [DOI] [PubMed] [Google Scholar]


