Abstract
BACKGROUND:
Cancer is one of the leading causes of mortality and morbidity worldwide. The incidence of cancer has increased markedly in recent decades in most countries. Studies have shown that diseases such as cancer affect the individuals’ quality of life.
METHODS:
The sample of study consisted of 384 patients selected through non-random convenient sampling procedure from three general hospitals and outpatient clinics in Isfahan and Tehran. The measures used in the study included a demographic questionnaire, the Iranian version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30), the Cancer Coping Questionnaire, and the Religious Attitude Questionnaire.
RESULTS:
The results revealed significant correlation between patients’ scores on the total scale of the Cancer Coping Questionnaire and their scores on the Global health status/Quality of Life. Significant correlations were also found between patients’ scores on the Religious Attitude Questionnaire and various scales of the Quality of Life Questionnaire. However, no significant correlations were found between Cancer Coping and Religious Attitude measures in any type of cancer except for the prostate cancer.
CONCLUSIONS:
Religious attitude was a significant and important factor in coping with cancer. In addition, patients’ quality of life correlated significantly with religious attitude as well as cancer coping measures. However, the results did not show any significant relationship between religious attitude and cancer coping measures except in patients with prostate cancer. The findings of this study are consistent with other studies that have shown significant correlations between religiosity and spirituality and quality of life in patients with life threatening diseases.
KEYWORDS: Cancer, Coping, Quality of Life, Religion, Attitude
Cancer is one of the leading causes of mortality and morbidity worldwide. The incidence of cancer has increased markedly in recent decades in most countries. However, there have been only limited attempts to control these costs by implementing primary prevention programs. The cost of cancer care has substantially increased over the years. Aside from the direct cost of patient care,1 millions of dollars are spent each year on finding more effective treatment methods. In addition, billions of dollars are lost due to work related problems. Patients with cancer experience symptoms and signs including pains and various physical and mental distresses. Immediately after being diagnosed with cancer, the patients may experience anxiety and mood disturbances, which may fluctuate over time in response to treatment procedures, remission, and recurrence of the symptoms.2
Researchers have studied the role and importance of the quality of life in various medical conditions, such as cancer,3–18 multiple sclerosis,19–21 Parkinson's diseases,22 post organ transplantation,23–24 serious injuries,25 kidney diseases,26–29 inflammatory bowel disease,30 advanced heart failure,31 systemic lupus erythematosus,32–33 strokes,34 dementia35 and chronic obstructive pulmonary disease.36 Despite medical advances in the treatment of cancer which has resulted in better patient care, increased life expectancy, and improved quality of life; however, almost all of the patients experience hopelessness and helplessness. Cancer causes extreme fear in patients.37
Undoubtedly, diagnosis of a life threatening disease such as cancer can affect patients’ quality of life. Cancer is not just an event with certain end, but due its nature, treatment outcome, and related psychological issues, it is an ambiguous and continuing condition characterized by delayed and at times unpredictable consequences.38
For many patients who face life-threatening diseases, religion and religious coping are among the most important determinant factors of their quality of life. Strategies that patients use to cope with challenging diseases can be an important predictor of their quality of life. Religious/spiritual resources may be particularly relevant when dealing with life threatening situations.39 A number of studies have found the religion to be a major source of support and hope for patients in coping with such diseases.40–42
In numerous studies, the majority of participants with different types of cancer have reported, often spontaneously, religiousness to be an important source of support in dealing with their illness.43 Sherman and Simonton43 found that religious activities usually rank among the most frequent coping responses reported by cancer patients. Religious resources may also play a significant role in long-term adjustment to cancer, such as relieving stress, retaining a sense of control, maintaining self-esteem, providing emotional comfort and hope, as well as a sense of meaning and purpose in life.39
The locus of control concept developed by Rotter,44 refers to the individuals’ belief regarding the control they have over their lives. Control orientation, which describes the extent to which one's actions are instrumental to goal attainment, was first measured in Rotter's Internal–External Scale (I–E). Individuals with high internal scores are more likely to exert efforts to control their environment and to take responsibility for their actions than those with high external scores. An external locus of control orientation indicates that goal attainment attributed to external factors outside the control of the individual. The external orientation has been divided into “powerful others” and “chance”.45 According to the concept of “external locus of control”, patients will ask medical specialists for help, and may trust in a helping God.46
Despite several studies on the subject,47–53 the protective effect of religious belief against cancer remains unclear. In many religious communities, it is expected that religious belief and practices have beneficial effects on health including decreased risks of cancer.47–49 In fact, the results of several epidemiological studies have shown that compared to the general population, members of certain religious communities are at lower risk for some types of cancer,47–49 have lower mortality rates,50–51 and live longer.52
This study examined the relationships between quality of life, coping strategies, and religious attitude in patients with cancer in Iran.
Methods
This was a cross-sectional study. The sample consisted of 384 patients selected through non-random convenient sampling procedure. The hospitalized patients and those attending the outpatient clinics at three general hospitals in Isfahan and Tehran, who met the inclusion criteria, were asked to participate in the study. The inclusion criteria were as follows; 18 years or older, at least 8 years of education, absence of any concomitant diseases, and lack of any type of communication problems.
After obtaining their verbal consent and informing them that participation in the study was voluntary and that they were free to discontinue their participation at any time without jeopardizing their ongoing treatment, the patients completed the research questionnaires. The measures used in the study included a demographic questionnaire, the Iranian version of the European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30),54 the Cancer Coping Questionnaire,55 and the Religious Attitude Questionnaire.56
The Cancer Coping Questionnaire,55 was translated into Farsi through back translation process and edited five times by authors. Then in a pilot study, 30 patients with cancer completed the Persian (Farsi) version of the questionnaire. The internal consistency and factor structure of the questionnaire were assessed through Chronbach's alpha. The Chronbach's alpha coefficient was 0.92.
The results of confirmatory factor analysis confirmed that the factors of the Persian version were compatible to the original version. This questionnaire consists of 21 items rated on a 4-point scale ranging from one (not at all) to four (very often). It is divided into two sections: Total Individual Scale (Items 1-14), which includes coping (Items 2, 6, 7, 11, 12), positive Focus (Items 1, 9, 14), diversion (Items 3, 4, 8) and planning (Items 5, 10, 13); and Interpersonal Scale (Items 15–21).
The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30)54 includes functional scales comprises of physical functioning, role functioning, emotional functioning, cognitive functioning and social functioning; and global health status/Quality of Life (QoL) which is composed of symptom scales / items such as fatigue, nausea and vomiting, pain, dyspnoea, insomnia, appetite loss, constipation, diarrhea, financial difficulties.
The Religious Attitude Questionnaire is a Persian instrument developed by Shirshahi56 for assessing Iranians’ religious attitudes. It consists of 31 items to which the participants are asked to respond using a 5-point rating scale ranging from 0 (Never) to 4 (Always).The author reported a test-retest reliability coefficient of 0.82 and a Chronbach's alpha coefficient of 0.83.
The data were analyzed using SPSS 15. The level of significance was set at p < 0.05 for all analyses. Descriptive statistics were used to describe the characteristics of the sample. Kolmogorov-Smirnov was employed for normal distribution testing. Chi-square test, Student t-test, ANOVA, correlation test, and stepwise regression analysis were used to analyze data. Non-parametric tests such as Kruskal-Wallis Test and Mann-Whitney Test were used for non-normally distributed variables.
Results
The mean (SD) age of the sample was 48.9 (14.3) years, and more than half of them (55.5%) were men. Being married was in 86.9% of participants, and more than two-thirds had high school education or lower. More than one-third of participants were homemakers. In terms of the cancer type, 20.6% had breast cancer, 17.2% had lung cancer, 20.9% suffered Gastrointestinal (GI) tract cancer, 22.5% had leukemia, and 18.8% had prostate cancer. Most of the participants had undergone chemotherapy. The mean (SD) period between the initial diagnosis of cancer and participation in the project was 23.8 (26.4) months.
Table 1 shows the results of Iranian version of Quality of Life Questionnaire (EORTC QLQ-C30). Table 2 shows the results of Cancer Coping Questionnaire and the Religious Attitude Questionnaire.
Table 1.
Mean and standard deviation of the patients’ scores on the Quality of Life (QoL) Questionnaire
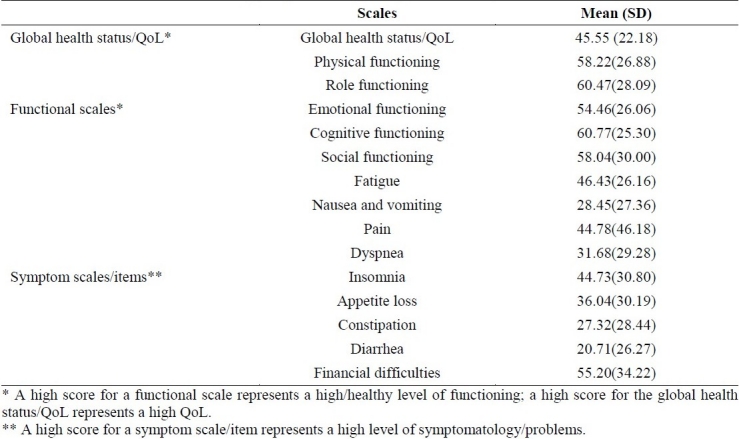
Table 2.
Mean and standard deviation of patients’ scores of the Cancer Coping and the Religious Attitude Questionnaires

The results of correlation analyses revealed significant correlation between patients’ scores on the Total scale of the Cancer Coping Questionnaire and their scores on the Global health status/QoL (r = 0.11, p = 0.032). The correlations between the total scale of the Cancer Coping Questionnaire and the subscales of the Quality of Life Questionnaire were as follows: with physical functioning (r = 0.28, p < 0.0001), with role functioning (r = 0.16, p = 0.003), with emotional functioning (r = 0.30, p < 0.0001), with cognitive functioning (r = 0.23, p < 0.0001), and with social functioning (r = 0.18, p = 0.001). Also, the scores on the total scale of the Cancer Coping Questionnaire correlated negatively with fatigue (r = -0.27, p < 0.001), pain (r = -0.18, p < 0.0001), insomnia (r = -0.11, p = 0.031), correlated with diarrhea (r = 0.18, p = 0.001) and financial difficulties (r = -0.24, p < 0.0001).
The correlation coefficients between scores on the Religious Attitude Questionnaire and the Quality of Life Questionnaire were as follows: 0.323 (p < 0.0001) for global health status/QoL, 0.14 (p = 0.005) for cognitive functioning and 0.11 (p = 0.033) for fatigue. However, negative correlations were found between religious attitude scores and nausea and vomiting (r = -0.14, p = 0.005), dyspnea (r = -0.17, p = 0.001), constipation (r = -0.11, p = 0.032), diarrhea (r = -0.19, p < 0.0001) and correlated with financial difficulties (r = 0.14, p = 0.005).
No significant correlations were found between cancer coping (Total Individual Scale and Interpersonal Scale) and religious attitude measures in all types of cancer except for prostate cancer (r = 0.224, p = 0.041 and r = 0.242, p = 0.041, respectively). In addition, in patients older than 60, religious attitude measure significantly correlated with the Total and Interpersonal scales of cancer coping measures (r = 0.272, p = 0.012 and r = 0.345, p = 0.001, respectively).
Statistically significant correlations were found between types of cancer and age (p < 0.0001), cancer coping, the Total and Interpersonal scales (p = 0.044 and p = 0.009, respectively). No significant correlations were found between types of cancer and Religious attitude (p > 0.05).
In addition, the results revealed significant correlations between type of cancer and subscales of the Iranian version of EORTC QLQ-C30, i.e., physical functioning (p = 0.006), role functioning (p = 0.007), cognitive functioning (p = 0.012), social functioning (p = 0.009), fatigue (p = 0.004), nausea and vomiting (p = 0.011), pain (p = 0.002), dyspnea (p = 0.001), insomnia (p = 0.03), appetite loss (p = 0.027), and diarrhea (p = 0.023).
Statistically significant correlations were also found between types of treatment and age (p < 0.0001), Cancer Coping Questionnaire Total Scale Score and Total Individual Scale score (p = 0.015 and p = 0.006, respectively), and Religious attitude measure (p < 0.0001).
Statistically significant correlations were found between gender and cancer coping, Total and Interpersonal scales scores (p = 0.029 and p = 0.015, respectively). However, no significant relationship was observed between gender and Religious attitude measure (p > 0.05).
Statistically significant relationships were found between age groups and cancer coping, Total scale, Total Individual and Interpersonal Scales scores (p = 0.001, p < 0.0001 and p = 0.006, respectively).
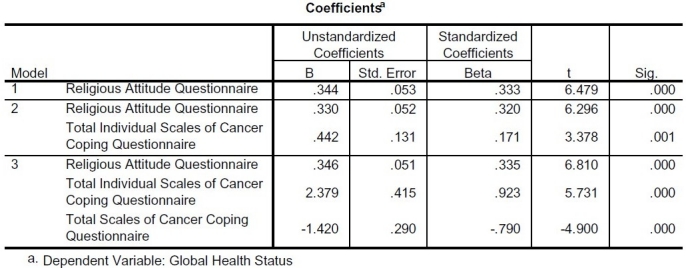
In a stepwise regression analysis, the authors examined relationships between Global Health Status as dependent variable and religious attitude, cancer coping and demographic characteristics as independent variables. Table 3 shows the result of this analysis.
Table 3.
Stepwise regression analysis of Global Health Status as dependent variable and Religious Attitude, Cancer Coping and demographic characteristics as independent variables
Other stepwise regression analyses examined relationships between functional scales of EORTC QLQ-C30 as dependent variables and religious attitude and cancer coping and demographic characteristics as independent variables.
Stepwise regression analysis found significant relationship between physical functioning and Total Individual Scales of Cancer Coping Questionnaire (β = 0.367, p < 0.0001), age (β = -0.159, p = 0.003), type of cancer (β = 0.148, p = 0.009), job of patients (β = -0.136, p = 0.02) and Total Interpersonal Scales of Cancer Coping Questionnaire (β = -0.138, p = 0.045).
Stepwise regression analysis found significant relationship between role functioning and Total Individual Scales of Cancer Coping Questionnaire (β = 0.321, p < 0.0001), Total Interpersonal Scales of Cancer Coping Questionnaire (β = -0.247, p < 0.0001), job of patients (β = -0.306, p < 0.0001), sex of patients (β = -0.201, p = 0.011) and age (β = -0.123, p = 0.02).
Stepwise regression analysis found significant relationship between emotional functioning and Total Individual Scales of Cancer Coping Questionnaire (β = 0.569, p < 0.0001), Total Interpersonal Scales of Cancer Coping Questionnaire (β = -0.304, p < 0.0001) and type of treatment (β = -0.15, p = 0.002).
Stepwise regression analysis found significant relationship between cognitive functioning and Total Individual Scales of Cancer Coping Questionnaire (β = 0.346, p < 0.0001), Religious Attitude Questionnaire (β = 0.134, p = 0.01) and Total Interpersonal Scales of Cancer Coping Questionnaire (β = -0.135, p = 0.048).
Stepwise regression analysis of Social Functioning found significant relationship between Total Individual Scales of Cancer Coping Questionnaire (β = 1.084, p < 0.0001), Total Scale of Cancer Coping Questionnaire (β = -0.897, p < 0.0001), job of patients (β = -0.272, p = 0.001) and sex of patients (β = -0.198, p = 0.011).
Another stepwise regression analyses examined relationships between Symptom scales / items of EORTC QLQ-C30 as dependent variables and religious attitude, cancer coping and demographic characteristics as independent variables.
Stepwise regression analysis found significant relationship between fatigue and Total Individual Scales of Cancer Coping Questionnaire (β = -1.08, p < 0.0001), Total Scales of Cancer Coping Questionnaire (β = 0.781, p < 0.0001) and type of treatment (β = 0.208, p < 0.0001).
Stepwise regression analysis found significant relationship between nausea and vomiting and Total Interpersonal Scales of Cancer Coping Questionnaire (β = 0.345, p < 0.0001), Total Individual Scales of Cancer Coping Questionnaire (β = -0.236, p < 0.0001), Religious Attitude Questionnaire (β = -0.164, p = 0.002) and type of cancer (β = -0.132, p = 0.01).
Stepwise regression analysis found significant relationship between pain and Total Individual Scales of Cancer Coping Questionnaire (β = -0.2, p < 0.0001) and type of treatment (β = 0.132, p = 0.013).
Stepwise regression analysis found significant relationship between dyspnea and Total Individual Scales of Cancer Coping Questionnaire (β = -0.663, p < 0.0001), Total Scales of Cancer Coping Questionnaire (β = 0.542, p = 0.002) and Religious Attitude Questionnaire (β = -0.153, p < 0.004).
Stepwise regression analysis found significant relationship between insomnia and Total Individual Scales of Cancer Coping Questionnaire (β = -0.382, p < 0.0001) and Total Interpersonal Scales of Cancer Coping Questionnaire (β = 0.28, p < 0.0001).
Stepwise regression analysis found significant relationship between appetite loss and age of patients (β = 0.155, p < 0.004) and type of cancer (β = -0.113, p < 0.036). Stepwise regression analysis found significant relationship between constipation and Religious Attitude Questionnaire (β = -0.137, p < 0.011).
Stepwise regression analysis found significant relationship between Diarrhea and Total Interpersonal Scales of Cancer Coping Questionnaire (β = 0.212, p < 0.0001), Religious Attitude Questionnaire (β = -0.199, p < 0.0001) and type of cancer (β = -0.131, p < 0.012).
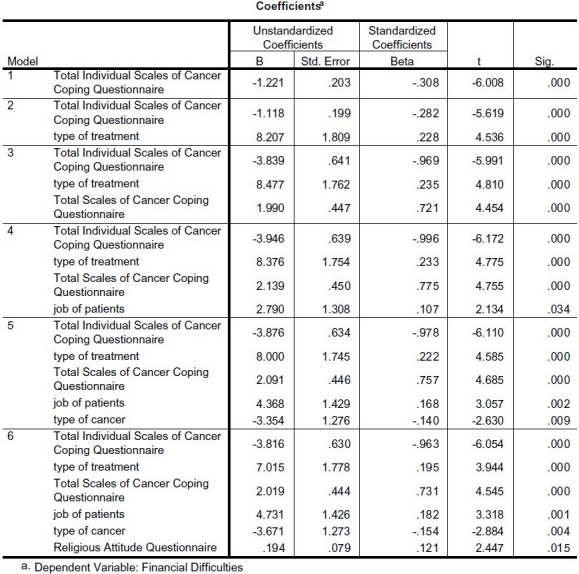
Table 4 shows the result of stepwise regression analysis of financial difficulties as dependent variable and religious attitude, cancer coping and demographic characteristics as independent variables.
Table 4.
Stepwise regression analysis of Financial Difficulties as dependent variable and Religious Attitude, Cancer Coping and demographic characteristics as independent variables
Discussion
The present study examined the relationships between quality of life, coping strategies, and religious attitude in a sample of Iranian patients with cancer. The results showed that religious attitude was a significant and important factor in coping with cancer.
Consistent with the stated hypothesis, patients’ quality of life correlated significantly with religious attitude as well as cancer coping measures. However, the results revealed no relationship between religious attitude and cancer coping measures except in patients with prostate cancer.
Several longitudinal studies reported correlation between spirituality and quality of life.57 Furthermore, the results of various qualitative studies have indicated that many individuals have found that their religiosity has often in creased or deepened following the traumatic experience.
Our findings regarding the relationships between quality of life and cancer coping, as well as religious attitude are consistent with studies that have shown significant correlations between religiosity/spirituality and quality of life measures.39–41,58,59
In particular, several studies have identified prayer as a significant coping strategy among physically ill individuals. For example, it was found that patients with both acute and chronic conditions, such as sickle cell disease, arthritis, acute cystitis, human immunodeficiency virus/acquired immune deficiency syndrome (HIV/AIDS), and renal disease often use prayer to help them cope with their illness.60
In addition, the literature indicates that spiritual issues significantly affect the quality of life and health of cancer patients, and that spirituality facilitates cancer coping. Consequently, it has been suggested that spirituality should be considered as an integral part of the models addressing the quality of life in patients with life threatening diseases, and that spirituality measures should be included in studies examining the quality of life of such patients. The spiritual needs of cancer patients often include finding meaning and hope, having access to spiritual resources and drawing meaning from their suffering.61
As showed above, the regression analysis confirmed the relationships between QOL, Cancer coping and religious attitude and some demographic characteristics.
Limitations of this study include the potential influence of selection bias, statistically significant age and sex differences between patients with different types of cancer, and age difference between patients receiving different types of treatment. Finally, the cross-sectional nature of the study limits the interpretation of the relationship between quality of life, cancer coping and religious attitude.
Authors’ Contributions
All the authors have carried out the study, participated in the design of the study and acquisition of data performed the statistical analysis and wrote the manuscript. All authors read and approved the final manuscript.
Acknowledgments
This project was carried out with grants from the following organizations:
-
1-
Social Harm and Psychological Research Center (Parva)
-
2-
Research Institute for Islamic and Complementary Medicine
We thank the Research Institute for Islamic and Complementary Medicine for providing financial support for this project. We are grateful to the Office of Research and Development of Isfahan University. We also wish to thank the directors of Hazrat Rassoul (in Tehran), Shahid Hashemi-Nejad (in Tehran), and Ha-zrat Seyed Al-Shohada (in Isfahan) hospitals for their support. We also thank Ms. Behjatpanah of Parva for her assistance throughout the study and typing the manuscripts.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Micheli A, Coebergh JW, Mugno E, Massimiliani E, Sant M, Oberaigner W, et al. European health systems and cancer care. Ann Oncol. 2003;14(Suppl 5):v41–60. doi: 10.1093/annonc/mdg753. [DOI] [PubMed] [Google Scholar]
- 2.Deng G, Cassileth BR. Integrative oncology: complementary therapies for pain, anxiety, and mood disturbance. CA Cancer J Clin. 2005;55(2):109–16. doi: 10.3322/canjclin.55.2.109. [DOI] [PubMed] [Google Scholar]
- 3.Lemieux J, Goodwin PJ, Bordeleau LJ, Lauzier S, Theberge V. Quality-of-life measurement in randomized clinical trials in breast cancer: an updated systematic review (2001-2009) J Natl Cancer Inst. 2011;103(3):178–231. doi: 10.1093/jnci/djq508. [DOI] [PubMed] [Google Scholar]
- 4.Pizzo E, Pezzoli A, Stockbrugger R, Bracci E, Vagnoni E, Gullini S. Screenee perception and health-related quality of life in colorectal cancer screening: a review. Value Health. 2011;14(1):152–9. doi: 10.1016/j.jval.2010.10.018. [DOI] [PubMed] [Google Scholar]
- 5.Siddiqui F, Konski AA, Movsas B. Quality-of-life concerns in lung cancer patients. Expert Rev Pharmacoecon Outcomes Res. 2010;10(6):667–76. doi: 10.1586/erp.10.81. [DOI] [PubMed] [Google Scholar]
- 6.Chen CM, Cano SJ, Klassen AF, King T, McCarthy C, Cordeiro PG, et al. Measuring quality of life in oncologic breast surgery: a systematic review of patient-reported outcome measures. Breast J. 2010;16(6):587–97. doi: 10.1111/j.1524-4741.2010.00983.x. [DOI] [PubMed] [Google Scholar]
- 7.Reimer T, Gerber B. Quality-of-life considerations in the treatment of early-stage breast cancer in the elderly. Drugs Aging. 2010;27(10):791–800. doi: 10.2165/11584700-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 8.Chase DM, Watanabe T, Monk BJ. Assessment and significance of quality of life in women with gynecologic cancer. Future Oncol. 2010;6(8):1279–87. doi: 10.2217/fon.10.96. [DOI] [PubMed] [Google Scholar]
- 9.Elliott S, Latini DM, Walker LM, Wassersug R, Robinson JW. Androgen deprivation therapy for prostate cancer: recommendations to improve patient and partner quality of life. J Sex Med. 2010;7(9):2996–3010. doi: 10.1111/j.1743-6109.2010.01902.x. [DOI] [PubMed] [Google Scholar]
- 10.Taphoorn MJ, Sizoo EM, Bottomley A. Review on quality of life issues in patients with primary brain tumors. Oncologist. 2010;15(6):618–26. doi: 10.1634/theoncologist.2009-0291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Jansen L, Koch L, Brenner H, Arndt V. Quality of life among long-term (>/=5 years) colorectal cancer survivors--systematic review. Eur J Cancer. 2010;46(16):2879–88. doi: 10.1016/j.ejca.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 12.Luckett T, King M, Butow P, Friedlander M, Paris T. Assessing health-related quality of life in gynecologic oncology: a systematic review of questionnaires and their ability to detect clinically important differences and change. Int J Gynecol Cancer. 2010;20(4):664–84. doi: 10.1111/IGC.0b013e3181dad379. [DOI] [PubMed] [Google Scholar]
- 13.Singh J, Trabulsi EJ, Gomella LG. The quality-of-life impact of prostate cancer treatments. Curr Urol Rep. 2010;11(3):139–46. doi: 10.1007/s11934-010-0103-y. [DOI] [PubMed] [Google Scholar]
- 14.Sherman AC, Simonton S. Advances in quality of life research among head and neck cancer patients. Curr Oncol Rep. 2010;12(3):208–15. doi: 10.1007/s11912-010-0092-5. [DOI] [PubMed] [Google Scholar]
- 15.Chambers A, Routledge T, Pilling J, Scarci M. In elderly patients with lung cancer is resection justified in terms of morbidity, mortality and residual quality of life? Interact Cardiovasc Thorac Surg. 2010;10(6):1015–21. doi: 10.1510/icvts.2010.233189. [DOI] [PubMed] [Google Scholar]
- 16.Rogers SN. Quality of life perspectives in patients with oral cancer. Oral Oncol. 2010;46(6):445–7. doi: 10.1016/j.oraloncology.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 17.Fan SY, Eiser C, Ho MC. Health-related quality of life in patients with hepatocellular carcinoma: a systematic review. Clin Gastroenterol Hepatol. 2010;8(7):559–64. doi: 10.1016/j.cgh.2010.03.008. [DOI] [PubMed] [Google Scholar]
- 18.Al-Batran SE, Ajani JA. Impact of chemotherapy on quality of life in patients with metastatic esophagogastric cancer. Cancer. 2010;116(11):2511–8. doi: 10.1002/cncr.25064. [DOI] [PubMed] [Google Scholar]
- 19.Opara JA, Jaracz K, Brola W. Quality of life in multiple sclerosis. J Med Life. 2010;3(4):352–8. [PMC free article] [PubMed] [Google Scholar]
- 20.Goretti B, Portaccio E, Zipoli V, Razzolini L, Amato MP. Coping strategies, cognitive impairment, psychological variables and their relationship with quality of life in multiple sclerosis. Neurol Sci. 2010;31(Suppl 2):S227–30. doi: 10.1007/s10072-010-0372-8. [DOI] [PubMed] [Google Scholar]
- 21.Miller DM, Allen R. Quality of life in multiple sclerosis: determinants, measurement, and use in clinical practice. Curr Neurol Neurosci Rep. 2010;10(5):397–406. doi: 10.1007/s11910-010-0132-4. [DOI] [PubMed] [Google Scholar]
- 22.Chen JJ. Parkinson's disease: health-related quality of life, economic cost, and implications of early treatment. Am J Manag Care. 2010;16(Suppl Implications):S87–93. [PubMed] [Google Scholar]
- 23.Taylor RM, Wray J, Gibson F. Measuring quality of life in children and young people after transplantation: methodological considerations. Pediatr Transplant. 2010;14(4):445–58. doi: 10.1111/j.1399-3046.2010.01316.x. [DOI] [PubMed] [Google Scholar]
- 24.Landreneau K, Lee K, Landreneau MD. Quality of life in patients undergoing hemodialysis and renal transplantation--a meta-analytic review. Nephrol Nurs J. 2010;37(1):37–44. [PubMed] [Google Scholar]
- 25.Polinder S, Haagsma JA, Belt E, Lyons RA, Erasmus V, Lund J, et al. A systematic review of studies measuring health-related quality of life of general injury populations. BMC Public Health. 2010;10:783. doi: 10.1186/1471-2458-10-783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Brown EA. Can quality of life be improved for the increasing numbers of older patients with end-stage kidney disease? Expert Rev Pharmacoecon Outcomes Res. 2010;10(6):661–6. doi: 10.1586/erp.10.78. [DOI] [PubMed] [Google Scholar]
- 27.Danquah FV, Wasserman J, Meininger J, Bergstrom N. Quality of life measures for patients on hemodialysis: a review of psychometric properties. Nephrol Nurs J. 2010;37(3):255–69. [PubMed] [Google Scholar]
- 28.Soni RK, Porter AC, Lash JP, Unruh ML. Health-related quality of life in hypertension, chronic kidney disease, and coexistent chronic health conditions. Adv Chronic Kidney Dis. 2010;17(4):e17–26. doi: 10.1053/j.ackd.2010.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Porter AC, Vijil JC, Jr, Unruh M, Lora C, Lash JP. Health-related quality of life in Hispanics with chronic kidney disease. Transl Res. 2010;155(4):157–63. doi: 10.1016/j.trsl.2009.10.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Umanskiy K, Fichera A. Health related quality of life in inflammatory bowel disease: the impact of surgical therapy. World J Gastroenterol. 2010;16(40):5024–34. doi: 10.3748/wjg.v16.i40.5024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Jaarsma T, Johansson P, Agren S, Stromberg A. Quality of life and symptoms of depression in advanced heart failure patients and their partners. Curr Opin Support Palliat Care. 2010;4(4):233–7. doi: 10.1097/SPC.0b013e328340744d. [DOI] [PubMed] [Google Scholar]
- 32.Thumboo J, Fong KY. Health-related quality of life of patients with systemic lupus erythematosus in Asia: how can this be improved? Lupus. 2010;19(12):1430–5. doi: 10.1177/0961203310374309. [DOI] [PubMed] [Google Scholar]
- 33.Toloza SM, Jolly M, Alarcon GS. Quality-of-life measurements in multiethnic patients with systemic lupus erythematosus: cross-cultural issues. Curr Rheumatol Rep. 2010;12(4):237–49. doi: 10.1007/s11926-010-0110-5. [DOI] [PubMed] [Google Scholar]
- 34.Opara JA, Jaracz K. Quality of life of post-stroke patients and their caregivers. J Med Life. 2010;3(3):216–20. [PMC free article] [PubMed] [Google Scholar]
- 35.Venturato L. Dignity, dining and dialogue: reviewing the literature on quality of life for people with dementia. Int J Older People Nurs. 2010;5(3):228–34. doi: 10.1111/j.1748-3743.2010.00236.x. [DOI] [PubMed] [Google Scholar]
- 36.Antoniu SA. Effects of inhaled therapies on health-related quality of life in stable chronic obstructive pulmonary disease. Expert Rev Pharmacoecon Outcomes Res. 2010;10(2):155–62. doi: 10.1586/erp.10.11. [DOI] [PubMed] [Google Scholar]
- 37.Lee A, Wu HY. Diagnosis disclosure in cancer patients--when the family says “no!”. Singapore Med J. 2002;43(10):533–8. [PubMed] [Google Scholar]
- 38.Zebrack BJ. Cancer survivor identity and quality of life. Cancer Pract. 2000;8(5):238–42. doi: 10.1046/j.1523-5394.2000.85004.x. [DOI] [PubMed] [Google Scholar]
- 39.Thune-Boyle IC, Stygall JA, Keshtgar MR, Newman SP. Do religious/spiritual coping strategies affect illness adjustment in patients with cancer? A systematic review of the literature. Soc Sci Med. 2006;63(1):151–64. doi: 10.1016/j.socscimed.2005.11.055. [DOI] [PubMed] [Google Scholar]
- 40.Tarakeshwar N, Vanderwerker LC, Paulk E, Pearce MJ, Kasl SV, Prigerson HG. Religious coping is associated with the quality of life of patients with advanced cancer. J Palliat Med. 2006;9(3):646–57. doi: 10.1089/jpm.2006.9.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Balboni TA, Vanderwerker LC, Block SD, Paulk ME, Lathan CS, Peteet JR, et al. Religiousness and spiritual support among advanced cancer patients and associations with end-of-life treatment preferences and quality of life. J Clin Oncol. 2007;25(5):555–60. doi: 10.1200/JCO.2006.07.9046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Bussing A, Fischer J, Ostermann T, Matthiessen PF. Reliance on God's help, depression and fatigue in female cancer patients. Int J Psychiatry Med. 2008;38(3):357–72. doi: 10.2190/PM.38.3.j. [DOI] [PubMed] [Google Scholar]
- 43.Zwingmann C, Muller C, Korber J, Murken S. Religious commitment, religious coping and anxiety: a study in German patients with breast cancer. Eur J Cancer Care (Engl) 2008;17(4):361–70. doi: 10.1111/j.1365-2354.2007.00867.x. [DOI] [PubMed] [Google Scholar]
- 44.Rotter JB. Generalized expectancies for internal versus external control of reinforcement. Psychol Monogr. 1966;80(1):1–28. [PubMed] [Google Scholar]
- 45.Norman P, Bennet P. Health Locus of Control. In: Conner M, Norman P, editors. Predicting Health Behaviour. Buckingham: Open University Press; 1996. pp. 62–94. [Google Scholar]
- 46.Bussing A, Ostermann T, Matthiessen PF. Role of religion and spirituality in medical patients: confirmatory results with the SpREUK questionnaire. Health Qual Life Outcomes. 2005;3:10. doi: 10.1186/1477-7525-3-10. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Jensen OM. Cancer risk among Danish male Seventh-Day Adventists and other temperance society members. J Natl Cancer Inst. 1983;70(6):1011–4. [PubMed] [Google Scholar]
- 48.Jussawalla DJ, Yeole BB, Natekar MV. Cancer incidence in Indian Christians. Br J Cancer. 1985;51(6):883–91. doi: 10.1038/bjc.1985.134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Lyon JL, Gardner JW, West DW. Cancer incidence in Mormons and non-Mormons in Utah during 1967--75. J Natl Cancer Inst. 1980;65(5):1055–61. [PubMed] [Google Scholar]
- 50.McEvoy L, Land G. Life-style and death patterns of the Missouri RLDS church members. Am J Public Health. 1981;71(12):1350–7. doi: 10.2105/ajph.71.12.1350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Phillips RL, Kuzma JW, Beeson WL, Lotz T. Influence of selection versus lifestyle on risk of fatal cancer and cardiovascular disease among Seventh-day Adventists. Am J Epidemiol. 1980;112(2):296–314. doi: 10.1093/oxfordjournals.aje.a112996. [DOI] [PubMed] [Google Scholar]
- 52.Berkel J, de WF. Mortality pattern and life expectancy of Seventh-Day Adventists in the Netherlands. Int J Epidemiol. 1983;12(4):455–9. doi: 10.1093/ije/12.4.455. [DOI] [PubMed] [Google Scholar]
- 53.Hoff A, Johannessen-Henry CT, Ross L, Hvidt NC, Johansen C. Religion and reduced cancer risk: what is the explanation? A review. Eur J Cancer. 2008;44(17):2573–9. doi: 10.1016/j.ejca.2008.08.001. [DOI] [PubMed] [Google Scholar]
- 54.Montazeri A, Harirchi I, Vahdani M, Khaleghi F, Jarvandi S, Ebrahimi M, et al. The European Organization for Research and Treatment of Cancer Quality of Life Questionnaire (EORTC QLQ-C30): translation and validation study of the Iranian version. Support Care Cancer. 1999;7(6):400–6. doi: 10.1007/s005200050300. [DOI] [PubMed] [Google Scholar]
- 55.Moorey S, Frampton M, Greer S. The Cancer Coping Questionnaire: a self-rating scale for measuring the impact of adjuvant psychological therapy on coping behaviour. Psychooncology. 2003;12(4):331–44. doi: 10.1002/pon.646. [DOI] [PubMed] [Google Scholar]
- 56.Shahshiri Z. [Thesis] Tehran: The University of Tehran; 1999. Investigating Junior High School Students’ Monitoring of Values. [Google Scholar]
- 57.Tate DG, Forchheimer M. Quality of life, life satisfaction, and spirituality: comparing outcomes between rehabilitation and cancer patients. Am J Phys Med Rehabil. 2002;81(6):400–10. doi: 10.1097/00002060-200206000-00002. [DOI] [PubMed] [Google Scholar]
- 58.Leak A, Hu J, King CR. Symptom distress, spirituality, and quality of life in African American breast cancer survivors. Cancer Nurs. 2008;31(1):E15–21. doi: 10.1097/01.NCC.0000305681.06143.70. [DOI] [PubMed] [Google Scholar]
- 59.Lauver DR, Connolly-Nelson K, Vang P. Stressors and coping strategies among female cancer survivors after treatments. Cancer Nurs. 2007;30(2):101–11. doi: 10.1097/01.NCC.0000265003.56817.2c. [DOI] [PubMed] [Google Scholar]
- 60.Taylor EJ, Outlaw FH. Use of prayer among persons with cancer. Holist Nurs Pract. 2002;16(3):46–60. doi: 10.1097/00004650-200204000-00010. [DOI] [PubMed] [Google Scholar]
- 61.Schulz E, Holt CL, Caplan L, Blake V, Southward P, Buckner A, et al. Role of spirituality in cancer coping among African Americans: a qualitative examination. J Cancer Surviv. 2008;2(2):104–15. doi: 10.1007/s11764-008-0050-5. [DOI] [PMC free article] [PubMed] [Google Scholar]


