Abstract
BACKGROUND:
The distinction between basal cell carcinoma (BCC) and trichoepithelioma (TE) may be very difficult in some cases because of the close similarities of these two lesions clinically and histopathologically. The purpose of this study is to investigate the usefulness of CD10 in distinguishing BCC and TE.
METHODS:
The immunohistochemical expression of CD10 was evaluated in an archived group of 30 BCCs and 12 TEs in a retrospective cross sectional study. The localization of anti-CD10 to the tumoral and/or stromal cells was determined in each case and was compared between these two tumors using Fisher's Exact Test.
RESULTS:
In BCC cases, the expression of CD10 was noted in tumoral cells in 26 cases (83.2%). Of these, 3 cases showed positivity of the stromal and basaloid cells, two cases demonstrated stromal expression alone and two BCCs were not immunoreactive. On the other hand, 10 out of 12 (83.3%) TEs showed positive stromal immunoreactivity. Of these, one case also showed positivity of the basaloid cells. One TE demonstrated epithelial expression alone and one TE was not immunoreactive. The pattern of staining of basaloid cells and stromal cells in BCC and trichoepithelioma was statistically different (p < 0.001).
CONCLUSIONS:
We conclude that CD10 is a useful marker in the differential diagnosis of BCC versus TE.
KEYWORDS: Basal Cell Carcinoma, Trichoepithelioma, CD10
Trichoepithelioma (TE) is a benign tumor derived from basal cells in the hair follicle. It may be sporadic or as the principal feature of a common genetic disorder called multiple familial trichoepithelioma characterized by the presence of many small tumors predominantly on face, inherited in an autosomal dominant pattern. Histologically, TE is characterized by a well circumscribed dermal tumor composed of islands, nests and cords of uniform basaloid cells in a cellular fibrous stroma. The tumor may be associated with epithelial structures resemble hair papillae or abortive hair follicle, small keratocysts (infundibular differentiation) lined by stratified squamous epithelium and foci of calcification. Retraction of stroma from adjacent dermis and few mitotic figures are two characteristic features of this tumor. The tumor in some instances may take on a pattern, resembling basal cell carcinoma (BCC), so the differential diagnosis of BCC versus TE can be problematic based on clinical presentation and routine hematoxylin and eosin stained sections.1
CD10 is a cell-surface zinc metalloproteinase of 100 KD that is also known as common acute lymphoblastic leukemia antigen (CALLA).2 It was originally found to be expressed on the cell surface of most cases of acute lymphoblastic leukemia,3,4 and was soon found in many other types of neoplasms.5,6 CD10 expression has been shown in tumors of follicular differentiation, including trichoepithelioma, pilomatricoma, basaloid follicular hamartoma and BCC.7–9 The limited information pertaining to the pattern of this marker expression in different studies made us investigate more closely its differential pattern in these two tumors. A few studies have indicated its expression in BCC and TE but this marker has not been used routinely for differentiating BCC and TE because of the limited number of available studies.
Methods
The studied group included 30 cases of BCC and 12 cases of TE selected from histopathologic archive of Al-Zahra hospital, Isfahan University of Medical Sciences, Iran. The samples were selected by a simple sampling method. Paraffin-embedded tissue sections were obtained from archival tissue blocks of the hospital. Hematoxylin and eosin sections were reviewed to confirm diagnosis. Since there is no absolutely objective external validator of the rendered diagnosis, we selected the cases that their history and histologic pattern were typical. For immunohistochemical staining, 3μm-thick sections were prepared from formalin-fixed, paraffine-embedded tissues. The sections were collected on glass slides coated with poly-l-lysine. They were deparaffinized by immersion in xylene, and this was followed by immersion in alcohol and then immersion in citrate buffer, pH 9.0, for 15 minutes at 95°C for antigen retrieval. Next, the sections were incubated with 3% hydrogen peroxide for 10 minutes. The slides were then incubated with the primary antibody at room temperature for 60 minutes. After washing in Phosphate Buffer Saline (PBS), the sections were treated with polymer envision for 30 minutes. The sections were then incubated with Diaminobenzidine (DAB) in a chromogen solution for 5 minutes at room temperature. Finally, the sections were stained with hematoxylin and were mounted. Normal intestinal biopsy was used as positive control. CD10 stained the cytoplasm of the surface epithelial cells of small intestine. Negative control was performed by omitting the primary antibody step. Positive CD10 staining was identified as brown cytoplasmic staining with or without cell membrane staining. Localization of anti-CD10 to the stroma and/or tumor cells was determined in cases with immunoreactivity.
The data were collected and analyzed with chi-square test using SPSS software (version 16). BCC and TE were compared for proportion of CD10 expression in tumoral cells using Fisher's Exact Test and Odds ratio for tumor type was calculated. The proportions of CD10 expression in basaloid and stromal cells for these two tumors were compared.
Results
This study included 30 cases of BCC (13 solid type, 5 morphea type, 6 adenoid type and 6 pigmented type), and 12 cases of TE. The average age (± SD) of the BCC cases in this study was 59 ± 9 years, with a 44-77 years range. The BCC group included 17 males and 13 females. In TE cases (7 males and 5 females), the average age was 30 with a 20-45 years range. Two patients had a history of multiple TEs while the others had solitary lesions. CD10 was positive in 28 out of 30 BCCs (93.3%), most demonstrating strong and/or diffuse staining of basaloid cells (26/30, 76.6%) (Figure 1). Of these, 3 cases (10%) showed staining of the stromal cells too. In 2 cases (6.6%) just stromal cells were positive and 2 BCCs (6.6%) were not immunoreactive.
Figure 1.
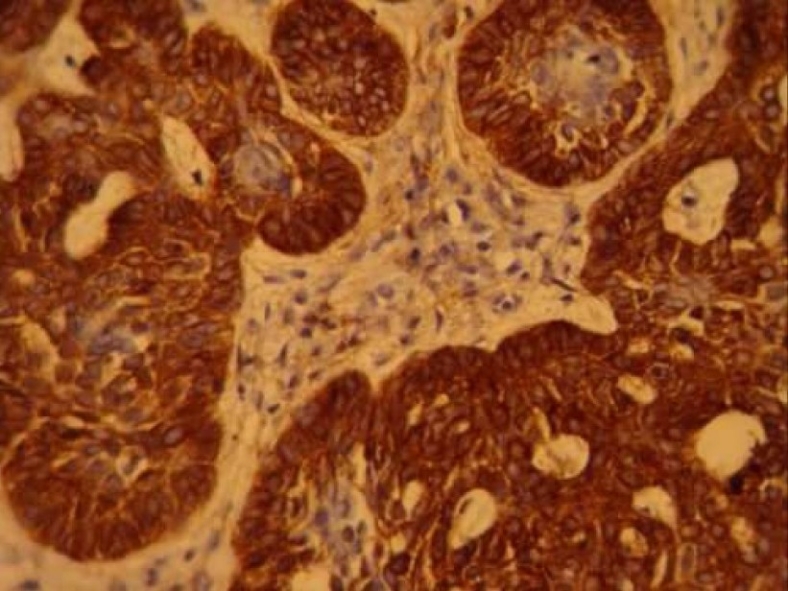
CD10 expression in basal cell carcinoma (× 400)
Eleven of twelve cases of TE were Immunoreactive. Ten cases (75%) showed strong CD10 staining of the stroma surrounding nests of basaloid cells (Figure 2). Of these, one case (8.3%) also showed staining of the basaloid cells. one TE (8.3%) demonstrated basaloid cell staining alone and one case (8.3%) was not immunoreactive. The pattern of staining of basaloid cells and stromal cells in BCC and trichoepithelioma was statistically different; more basaloid cells were stained in BCC and more stromal cells were stained in trichoepithelioma (p < 0.001). Accordingly, CD10 expression in stromal cells around basaloid nests was useful for differentiating TE from BCC. In contrast, CD10-positive basaloid cells and negative stromal cells were diagnostic for BCC (Figure 3). Odds ratio for tumor type was 32.50.
Figure 2-A.
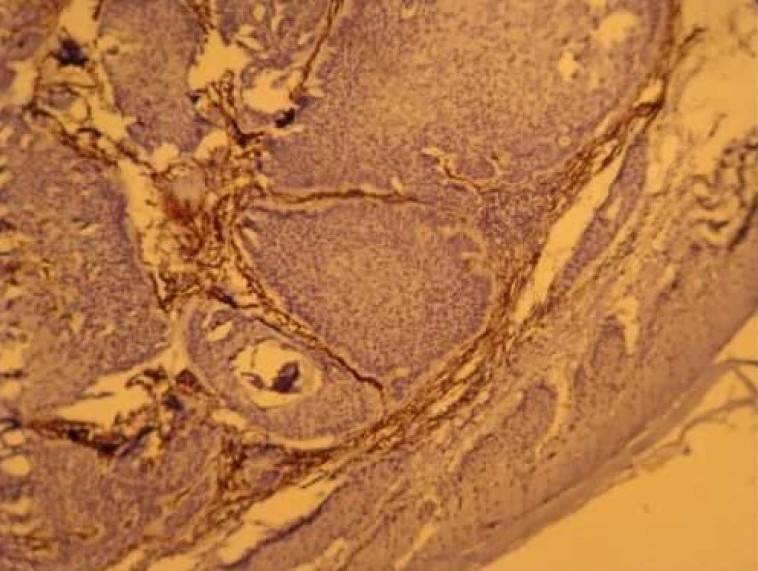
CD10 expression in trichoepithelioma (× 100)
Figure 3.
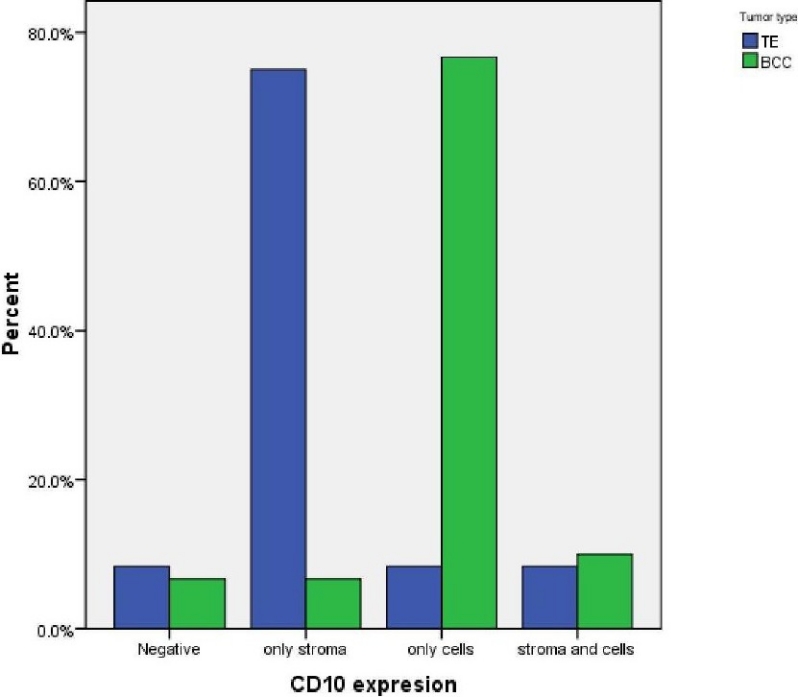
The comparison of CD10 expression in stromal and basaloid cells related to tumor type
Figure 2-B.
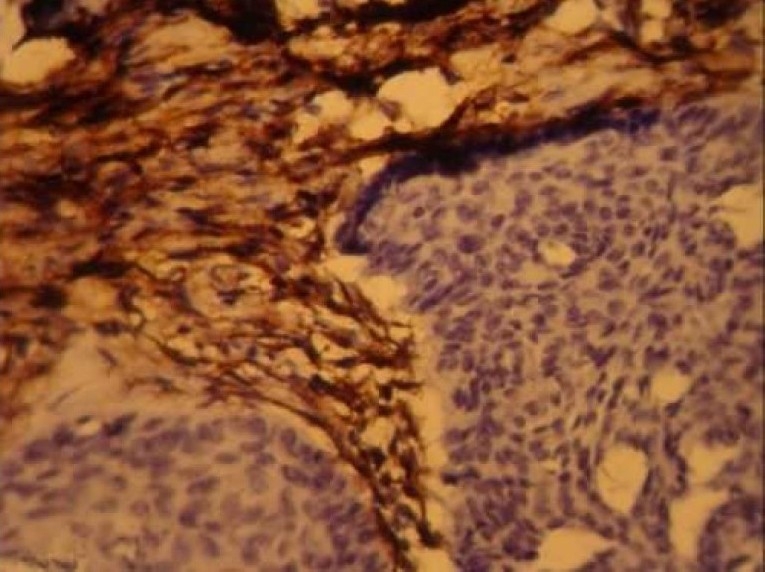
CD10 expression in trichoepithelioma (× 400)
Discussion
The results of the present study indicate that CD10 is a useful marker in the immunohistochemical evaluation of cutaneous neoplasms including TE and BCC. Different staining patterns of CD10 staining in these tumors, that is basaloid cells staining in BCC and stromal staining in TE, may be useful in resolving the existing problem in clinicohistological differentiation of these two entities.
Trichoepithelioma is a benign skin tumor with follicular differentiation, whose distinction from basal cell carcinoma is sometimes difficult, clinically and histologically. Both tumors are composed of nests of basaloid cells with follicular differentiation. Sometimes it may be impossible to make a histopathologic differentiation on the basis of routine hematoxylin and eosin staining. Such distinction is clinically important because of the differences in prognosis and treatment of these tumors.1 Therefore, several laboratory techniques have been investigated as an aid in this differentiation. In these instances, immunohistochemical examinations may provide further information.
In the past, several antibodies were used to differentiate between BCC and TE,10–20 although most were not specific for each of these tumors. A study in 2008 by Costache M,10 showed that CK20 and androgen receptor were helpful in differentiation between BCC and TE, but interpretation was difficult in some cases. In the same study, there was not any difference in staining with Bcl-2 and CD34 between BCC and TE. It was in contrast with Kirchmann et al.11 and Illueca et al.12 studies that showed the usefulness of CD34 by showing the lack of CD34 expression by tumor stroma in BCC, but positive in TE. In another study by Katona et al.13, the usefulness of CK20 and androgen receptor in differentiation of these tumors was further confirmed. They showed that the AR-, CK20+ immunophenotype was sensitive (87%) and specific (100%) for TE. But the AR+, CK20-immunophenotype was specific (100%) and moderately sensitive (61%) for BCC. In another study, Choi et al.14 showed that elastic fiber staining and cytokeratin 15 expression pattern may help in the differentiation of TE from BCC. Carvalho et al.15 investigated the expression of CD23 in desmoplastic trichoepithelioma and morpheaform BCC and found no statistically significant difference in expression of this marker in these tumors. In addition, there are other immunohistochemical markers which may be helpful in differential diagnosis of TE and BCC, including Bcl-2, TGF-β and Ber-EP4.
Bcl-2 is an oncogene associated with apoptosis, and can be overexpressed in some malignancies. There are some studies which stated that Bcl-2 diffusely stains the tumor nests in BCC while it stains the outermost cell layers in trichoepithelioma.16,17 In contrast, there exist a number of other studies which question the reliability of Bcl-2 in distinguishing TE from BCC.21–24
Recently increasing evidence has suggested that androgen receptor and transforming growth factor-β (TGF-β) may be useful in differentiating TE from BCC. Verhaegh et al.16 showed a diffuse cytoplasmic TGF-β staining in TE tumor cells, whereas negative staining was observed in BCCs. Izikson et al.25 found positive androgen receptor immunostaining in BCC cells compared with negative staining in TE. Ber-EP4 is a monoclonal directed against two glycopolypeptides found in most human epithelial cells. Krahl et al.26 did not find any consistent difference in the staining pattern of Ber-EP4 in BCC and TE.
The results of other previous studies were consistent with the results of our study.7,8 Moreover, Cordoba et al.27 reported the same pattern of CD10 expression in different forms of BCC. Based on these results CD10 can be used routinely for differentiating BCC and TE.
Authors’ Contributions
MH and PR participated in the design of the study and examined histologic sections. FS prepared and processed the specimens and retrieved data from the archive. All authors read and approved the final manuscript.
Acknowledgments
This study was performed as a thesis funded by Isfahan University of Medical Sciences.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Ahmed TS, Priore JD, Seykora JT. Tumors of the epidermal appendages. In: Elder DE, Elenitsas R, Johnson BL, Murphy GF, Xu X, editors. Lever's histopathology of the skin. 10th ed. Philadelphia: Lippincott Williams & Wilkins; 2008. pp. 858–60. [Google Scholar]
- 2.McIntosh GG, Lodge AJ, Watson P, Hall AG, Wood K, Anderson JJ, et al. NCL-CD10-270: a new monoclonal antibody recognizing CD10 in paraffin-embedded tissue. Am J Pathol. 1999;154(1):77–82. doi: 10.1016/S0002-9440(10)65253-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Greaves MF, Brown G, Rapson NT, Lister TA. Antisera to acute lymphoblastic leukemia cells. Clin Immunol Immunopathol. 1975;4(1):67–84. doi: 10.1016/0090-1229(75)90041-0. [DOI] [PubMed] [Google Scholar]
- 4.Greaves MF, Hariri G, Newman RA, Sutherland DR, Ritter MA, Ritz J. Selective expression of the common acute lymphoblastic leukemia (gp 100) antigen on immature lymphoid cells and their malignant counterparts. Blood. 1983;61(4):628–39. [PubMed] [Google Scholar]
- 5.Arber DA, Weiss LM. CD10, a review. Appl Immunohistochem. 1997;5:125–40. [Google Scholar]
- 6.Chu PG, Arber DA, Weiss LM, Chang KL. Utility of CD10 in distinguishing between endometrial stromal sarcoma and uterine smooth muscle tumors: an immunohistochemical comparison of 34 cases. Mod Pathol. 2001;14(5):465–71. doi: 10.1038/modpathol.3880335. [DOI] [PubMed] [Google Scholar]
- 7.Pham TT, Selim MA, Burchette JL, Jr, Madden J, Turner J, Herman C. CD10 expression in trichoepithelioma and basal cell carcinoma. J Cutan Pathol. 2006;33(2):123–8. doi: 10.1111/j.0303-6987.2006.00283.x. [DOI] [PubMed] [Google Scholar]
- 8.Yada K, Kashima K, Daa T, Kitano S, Fujiwara S, Yokoyama S. Expression of CD10 in basal cell carcinoma. Am J Dermatopathol. 2004;26(6):463–71. doi: 10.1097/00000372-200412000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Ramos-Ceballos FI, Pashaei S, Kincannon JM, Morgan MB, Smoller BR. Bcl-2, CD34 and CD10 expression in basaloid follicular hamartoma, vellus hair hamartoma and neurofollicular hamartoma demonstrate full follicular differentiation. J Cutan Pathol. 2008;35(5):477–83. doi: 10.1111/j.1600-0560.2007.00832.x. [DOI] [PubMed] [Google Scholar]
- 10.Costache M, Bresch M, Boer A. Desmoplastic trichoepithelioma versus morphoeic basal cell carcinoma: a critical reappraisal of histomorphological and immunohistochemical criteria for differentiation. Histopathology. 2008;52(7):865–76. doi: 10.1111/j.1365-2559.2008.03036.x. [DOI] [PubMed] [Google Scholar]
- 11.Kirchmann TT, Prieto VG, Smoller BR. CD34 staining pattern distinguishes basal cell carcinoma from trichoepithelioma. Arch Dermatol. 1994;130(5):589–92. [PubMed] [Google Scholar]
- 12.Illueca C, Monteagudo C, Revert A, Llombart-Bosch A. Diagnostic value of CD34 immunostaining in desmoplastic trichilemmoma. J Cutan Pathol. 1998;25(8):435–9. doi: 10.1111/j.1600-0560.1998.tb01770.x. [DOI] [PubMed] [Google Scholar]
- 13.Katona TM, Perkins SM, Billings SD. Does the panel of cytokeratin 20 and androgen receptor antibodies differentiate desmoplastic trichoepithelioma from morpheaform/infiltrative basal cell carcinoma? J Cutan Pathol. 2008;35(2):174–9. doi: 10.1111/j.1600-0560.2007.00783.x. [DOI] [PubMed] [Google Scholar]
- 14.Choi CW, Park HS, Kim YK, Lee SH, Cho KH. Elastic fiber staining and cytokeratin 15 expression pattern in trichoepithelioma and basal cell carcinoma. J Dermatol. 2008;35(8):499–502. doi: 10.1111/j.1346-8138.2008.00510.x. [DOI] [PubMed] [Google Scholar]
- 15.Carvalho J, Fullen D, Lowe L, Su L, Ma L. The expression of CD23 in cutaneous non-lymphoid neoplasms. J Cutan Pathol. 2007;34(9):693–8. doi: 10.1111/j.1600-0560.2006.00685.x. [DOI] [PubMed] [Google Scholar]
- 16.Verhaegh ME, Arends JW, Majoie IM, Hoekzema R, Neumann HA. Transforming growth factor-beta and bcl-2 distribution patterns distinguish trichoepithelioma from basal cell carcinoma. Dermatol Surg. 1997;23(8):695–700. doi: 10.1111/j.1524-4725.1997.tb00392.x. [DOI] [PubMed] [Google Scholar]
- 17.Smoller BR, Van de RM, Lebrun D, Warnke RA. Bcl-2 expression reliably distinguishes trichoepitheliomas from basal cell carcinomas. Br J Dermatol. 1994;131(1):28–31. doi: 10.1111/j.1365-2133.1994.tb08453.x. [DOI] [PubMed] [Google Scholar]
- 18.Merritt BG, Snow SN, Longley BJ. Desmoplastic trichoepithelioma, infiltrative/morpheaform BCC, and microcystic adnexal carcinoma: differentiation by immunohistochemistry and determining the need for Mohs micrographic surgery. Cutis. 2010;85(5):254–8. [PubMed] [Google Scholar]
- 19.Fernandez-Flores A. Advanced differentiation in trichoepithelioma and basal cell carcinoma investigated by immunohistochemistry against neurofilaments. Folia Histochem Cytobiol. 2009;47(1):61–4. doi: 10.2478/v10042-009-0011-5. [DOI] [PubMed] [Google Scholar]
- 20.Mitcov M, Scrivener Y, Cribier B. Desmoplastic trichoepithelioma: a clinicopathological study, including a comparison with morpheiform basal cell carcinoma. Ann Dermatol Venereol. 2009;136(6-7):501–7. doi: 10.1016/j.annder.2009.03.020. [DOI] [PubMed] [Google Scholar]
- 21.Basarab T, Orchard G, Russell-Jones R. The use of immunostaining for bcl-2 and CD34 and the lectin peanut agglutinin in differentiating between basal cell carcinomas and trichoepitheliomas. Am J Dermatopathol. 1998;20(5):448–52. doi: 10.1097/00000372-199810000-00003. [DOI] [PubMed] [Google Scholar]
- 22.Poniecka AW, Alexis JB. An immunohistochemical study of basal cell carcinoma and trichoepithelioma. Am J Dermatopathol. 1999;21(4):332–6. doi: 10.1097/00000372-199908000-00004. [DOI] [PubMed] [Google Scholar]
- 23.Abdelsayed RA, Guijarro-Rojas M, Ibrahim NA, Sangueza OP. Immunohistochemical evaluation of basal cell carcinoma and trichepithelioma using Bcl-2, Ki67, PCNA and P53. J Cutan Pathol. 2000;27(4):169–75. doi: 10.1034/j.1600-0560.2000.027004169.x. [DOI] [PubMed] [Google Scholar]
- 24.Swanson PE, Fitzpatrick MM, Ritter JH, Glusac EJ, Wick MR. Immunohistologic differential diagnosis of basal cell carcinoma, squamous cell carcinoma, and trichoepithelioma in small cutaneous biopsy specimens. J Cutan Pathol. 1998;25(3):153–9. doi: 10.1111/j.1600-0560.1998.tb01708.x. [DOI] [PubMed] [Google Scholar]
- 25.Izikson L, Bhan A, Zembowicz A. Androgen receptor expression helps to differentiate basal cell carcinoma from benign trichoblastic tumors. Am J Dermatopathol. 2005;27(2):91–5. doi: 10.1097/01.dad.0000154392.92099.aa. [DOI] [PubMed] [Google Scholar]
- 26.Krahl D, Sellheyer K. Monoclonal antibody Ber-EP4 reliably discriminates between microcystic adnexal carcinoma and basal cell carcinoma. J Cutan Pathol. 2007;34(10):782–7. doi: 10.1111/j.1600-0560.2006.00710.x. [DOI] [PubMed] [Google Scholar]
- 27.Cordoba A, Guerrero D, Larrinaga B, Iglesias ME, Arrechea MA, Yanguas JI. Bcl-2 and CD10 expression in the differential diagnosis of trichoblastoma, basal cell carcinoma, and basal cell carcinoma with follicular differentiation. Int J Dermatol. 2009;48(7):713–7. doi: 10.1111/j.1365-4632.2009.04076.x. [DOI] [PubMed] [Google Scholar]


