Abstract
We describe a case of intracranial malignant fibrous histiocytoma which had infiltrated pons, cerebellum and basal surface of left temporal lobe without any visible mass. The patient presented with a sudden loss of consciousness and vomiting. Clinical findings, laboratory tests, imaging and examination of the cerebrospinal fluid tended to establish the diagnosis of an infectious condition than a malignancy. Without any response to the antibiotics and with a progressive deterioration of neurologic and mental condition, the patient died after 20 days. In the autopsy, histological and immunohistochemical study of the brain revealed the diagnosis of malignant fibrous histiocytoma (MFH).
KEYWORDS: Malignant Fibrous Histiocytoma, Central Nervous System, Immunohistochemistry, Sarcoma, Autopsy
The term malignant fibrous histiocytoma (MFH) is defined as a group of pleomorphic sarcomas which shows no line of differentiation.1 Intracranial MFH is an extremely rare tumor. It can originate in the central nervous system or arise as a metastasis from a primary extracranial tumor. The authors describe a case of intracranial MFH which had infiltrated pons, cerebellum and basal surface of left temporal lobe without any visible mass.
Case report
A 40-year-old man was admitted to our hospital (Alzahra hospital, Isfahan University of medical sciences) with a sudden loss of consciousness and vomiting in early February 2010. His wife mentioned that he had a two-month history of slowly progressive confusion and right side paresthesia. At the time of admission, physical examination only revealed agitation, high blood pressure (160/70 mmHg) and nuchal rigidity. Neurological examination was not completely achievable because of the patient's agitation. Laboratory studies included hemoglobin (16 mg/dL) and leukocyte count (17700/mcL; the normal range is between 4000 and 11000/mcL). Brain computed tomography (CT) showed no abnormality. Brain magnetic resonance imaging (MRI) showed hyperintense areas in anterior and peripheral aspects of the pons, cerebral peduncles and left cerebellar hemisphere (Figure 1).
Figure 1.
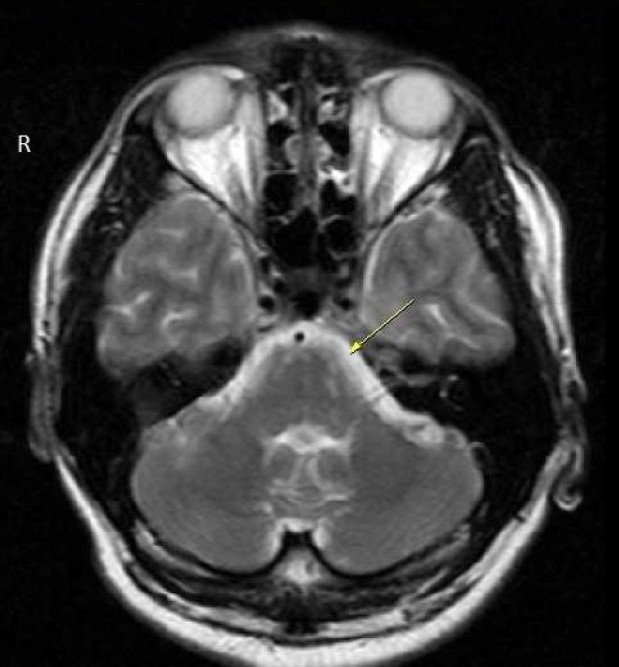
Magnetic resonance imaging (MRI) showing small and oval shaped lesions in anterior and peripheral aspects of pons and left cerebellar hemisphere as hypersignal intensities in dual image
Lumbar puncture (LP) study showed glucose 86 mg/dL (normal ≥ 45 mg/dL) and protein 353 mg/dL (normal ≤ 45 mg/dL). The culture and smear of the cerebrospinal fluid (CSF) were negative. Polymerase chain reaction (PCR) assay for Herpes simplex virus was also negative. Considering nuchal rigidity, peripheral blood leukocytosis and lumbar puncture findings, the patient was diagnosed with meningoencephalitis and antibiotic therapy (antifungal and then anti-tuberculosis regimens) was started.
During the hospitalization, he presented a progressive deterioration of neurologic and mental condition including vertigo, delirium, hallucination and the manifestations of cranial nerve palsies. Without any response to the treatment, he died after 20 days.
In the autopsy, serial slides of brain parenchyma revealed opacifications in the pons, cerebellum and basal surface of the left temporal lobe. Histopathological study of the brain showed focal involvement of the subarachnoid layer by neoplastic cell aggregation.
Hematoxylin and Eosin (H&E) stained slides revealed anaplastic proliferation of spindle-shaped cells admixed with atypical giant cells arranged in fascicular and storiform patterns (Fig. 2, 3, 4). These cells contained a typical hyper-chrome nuclei with moderate cytoplasms. Mitotic figures were also easily identified. For further investigation, immunohistochemistry panels were performed. The immunophenotyping studies suggested a strong positive reaction for vimentin and focally positive staining for CD68 (Fig. 5). However, tumor cells had negative reactions to cytokeratin, desmin, Melan A, leukocyte common antigen (LCA), S-100 protein, chromogranin, glial fibrillary acidic protein (GFAP), epithelial membrane antigen (EMA), CD31 and CD99. Based on these findings, the histological diagnosis was MFH. Despite making several cuts, no considerable finding was discovered in the other parts of the body.
Figure 2.
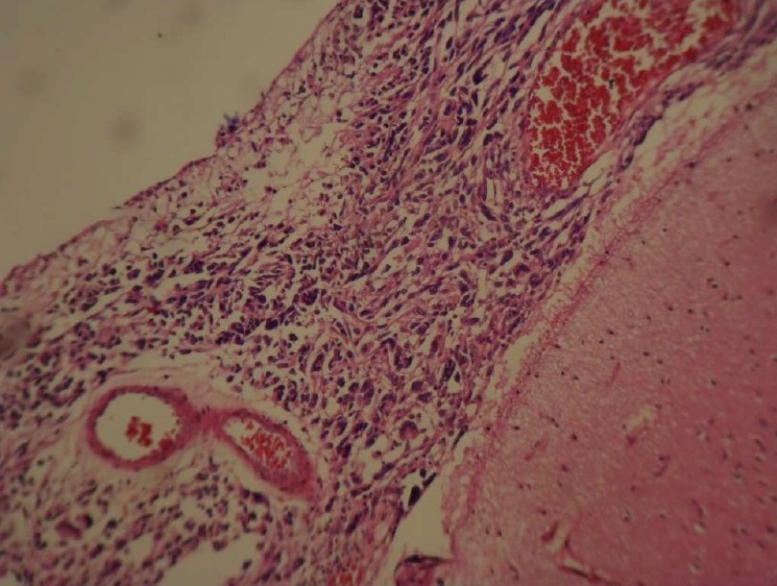
Photomicrograph revealed neoplastic spindle-shaped cells admixed with giant cells in an H&E stained slide (100×)
Figure 3.
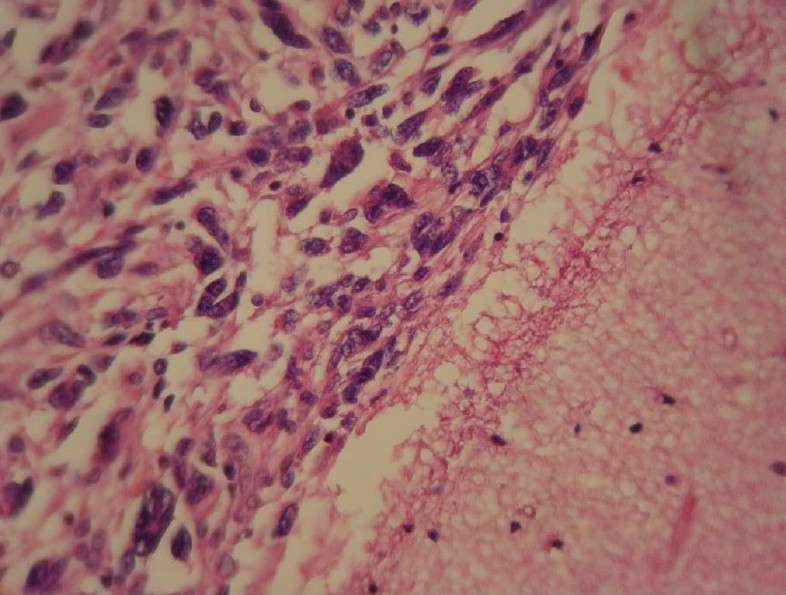
Photomicrograph revealed atypical spindle-shaped cells admixed with giant cells in an H&E stained slide (200×)
Figure 4.
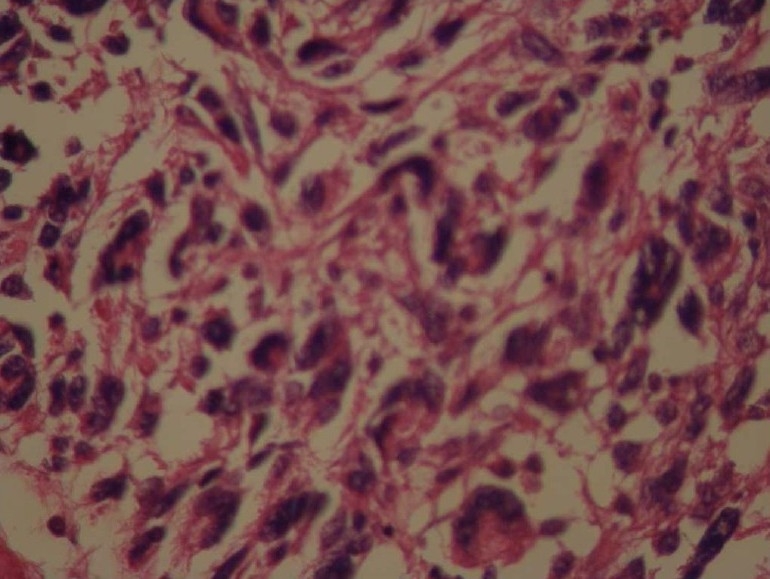
Photomicrograph revealed marked cellular atypia and pleomorphism in an H&E stained slide (400×)
Figure 5.
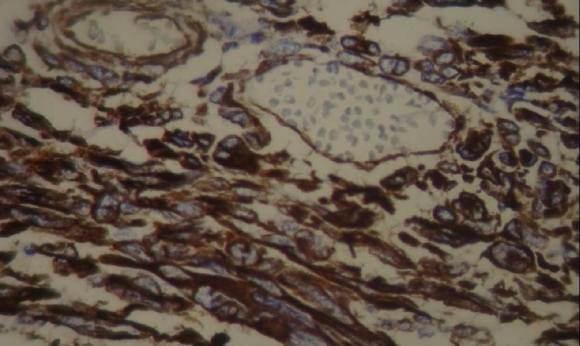
Photomicrograph revealed immunoreactive cells for vimentin
Discussion
In this report, we present a case in which the clinical findings, laboratory tests, imaging and examination of the CSF led to a diagnosis of an infectious condition than a malignancy. However, the autopsy findings indicated a rare intracranial sarcoma.
Sarcomas are malignant mesenchymal tumors that account for a low percentage of all brain tumors.2 The term MFH is defined as “a group of pleomorphic sarcomas which shows no line of differentiation”.1 Intracranial MFH is an extremely rare tumor. It can originate in the central nervous system or arise as a metastasis from a primary extracranial tumor.3–6
Microscopically, MFH is composed of collagen-forming spindle cell proliferation arranged in a storiform pattern. Commonly benign and malignant giant cells are seen.7,8
Clinical manifestations of intracranial MFH are various and mainly depend on the location of the tumor. Our patient presented the signs of increased intracranial pressure (such as vomiting and loss of consciousness) and involvement of cranial nerves. These manifestations might be due to infiltration of neoplastic cells in the subarachnoid layer of brainstem and basal surface of the brain.
The diagnosis of MFH is based primarily upon excellent sampling in conjunction with a targeted panel of immunohistochemistry. The reduced number of glial and meningeal cells is helpful in separating MFH from other meningeal non-sarcoma neoplasms.8 However, differentiating MFH from malignant tumors with a similar degree of cellular pleomorphism such as anaplastic carcinoma and pleomorphic sarcoma is difficult.7 Immunohistochemistry markers have been used as a means to exclude these similar pleomorphic tumors.
The tumor in the present case was diagnosed as MFH since the pathology showed undifferentiated proliferation of spindle shaped cells admixed with atypical giant cells arranged in the fascicular and storiform patterns. In addition, immunophenotype results suggested strongly positive staining for vimentin and focally positive reactivity for CD68.
Increased protein level in the CSF and lack of response to antibiotic therapy could be diagnostic signals to detect a malignant condition. However the course of disease in this patient was very rapid and virulent. Furthermore, with nonspecific signs in MRI and LP, initiating an empirical antibiotic therapy seemed reasonable.
In our case, any attempt to find an extracranial primary MFH failed. Even brain metastasis from an extracranial undifferentiated sarcoma is rare with few reported cases in literature.9 Like the first reported case by Gonzalez-Vitale,10 intracranial MFH in the present case had infiltrated the base of the brain. Contrasting to most reported cases, no mass was observed.11 Although patients with primary intracranial MFH often die within 12 months, 23% survive for 2 years.11
Conclusion
This case may emphasize the importance of putting the malignancies in the differential diagnosis for patients with the obscure neurological deficits. Rarity of MFHs does not eliminate their importance as they have clinical resemblance to other intracranial tumors and they are also associated with a very poor prognosis.
Authors’ Contributions
AHS participated in the preparation of manuscript. MS assisted in the pathologic diagnosis and participated in preparation of the manuscript. NAM provided assistance in the immunohistochemical diagnosis and prepared the manuscript. MI assisted in pathologic diagnosis and participated in preparation of the manuscript. MS provided assistance in management of the patient and prepared the manuscript. All authors have read and approved the content of the manuscript.
Acknowledgments
We are thankful to technicians of Legal Medicine Center of Isfahan for their assistance in the process of autopsy and preparation of samples for pathology and immunohistochemistry examinations.
Footnotes
Conflict of Interests Authors have no conflict of interests.
References
- 1.Lehnhardt M, Daigeler A, Homann HH, Schwaiberger V, Goertz O, Kuhnen C, et al. MFH revisited: outcome after surgical treatment of undifferentiated pleomorphic or not otherwise specified (NOS) sarcomas of the extremities --an analysis of 140 patients. Langenbecks Arch Surg. 2009;394(2):313–20. doi: 10.1007/s00423-008-0368-5. [DOI] [PubMed] [Google Scholar]
- 2.Maruno M, Ghulam Muhammad AK, Taguchi J, Suzuki T, Wada K, Isaka T, et al. Giant cell type of primary intracranial malignant fibrous histiocytoma: a case report. Brain Tumor Pathol. 2006;23(1):65–70. doi: 10.1007/s10014-006-0200-2. [DOI] [PubMed] [Google Scholar]
- 3.Baehring JM, Alemohammed S, Croul SE. Malignant fibrous histiocytoma presenting as an intraventricular mass five years after incidental detection of a mass lesion. J Neurooncol. 2001;52(2):157–60. doi: 10.1023/a:1010685020995. [DOI] [PubMed] [Google Scholar]
- 4.Tsutsumi M, Kawano T, Kawaguchi T, Kaneko Y, Ooigawa H, Yoshida T. Intracranial meningeal malignant fibrous histiocytoma mimicking parasagittal meningioma--case report. Neurol Med Chir (Tokyo) 2001;41(2):90–3. doi: 10.2176/nmc.41.90. [DOI] [PubMed] [Google Scholar]
- 5.Mitsuhashi T, Watanabe M, Ohara Y, Hatashita S, Ueno H. Multifocal primary intracerebral malignant fibrous histiocytoma--case report. Neurol Med Chir (Tokyo) 2004;44(5):249–54. doi: 10.2176/nmc.44.249. [DOI] [PubMed] [Google Scholar]
- 6.Yoshikai T, Shimokawa S, Uchino A, Kato A, Takase Y, Abe M, et al. Thallium-201 SPECT of adjacent intracranial tumours: a contrast in thallium kinetics. Neuroradiology. 1999;41(9):646–9. doi: 10.1007/s002340050817. [DOI] [PubMed] [Google Scholar]
- 7.Weiss SH, Goldblum JR. Malignant fibrous histiocytoma. Enzinger & Weiss's soft tissue tumors. New York: Mosby; 2008. pp. 406–14. [Google Scholar]
- 8.Stenberg SS, Mills SE, Carter D. Stenberg's diagnostic surgical pathology. Philadelphia: Lippinocott Williams & Wilkins; 2010. pp. 131–422. [Google Scholar]
- 9.Tanaka H, Sasayama T, Nishihara M, Arai A, Kawamura A, Kanomata N, et al. Brain metastasis of undifferentiated sarcoma and response to temozolomide treatment. Case report. Neurol Med Chir (Tokyo) 2010;50(8):689–93. doi: 10.2176/nmc.50.689. [DOI] [PubMed] [Google Scholar]
- 10.Gonzalez-Vitale JC, Slavin RE, McQueen JD. Radiation-induced intracranial malignant fibrous histiocytoma. Cancer. 1976;37(6):2960–3. doi: 10.1002/1097-0142(197606)37:6<2960::aid-cncr2820370653>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- 11.Akimoto J, Takeda Y, Hasue M, Ito H, Kiguchi E. Primary meningeal malignant fibrous histiocytoma with cerebrospinal dissemination and pulmonary metastasis. Acta Neurochir (Wien) 1998;140(11):1191–6. doi: 10.1007/s007010050236. [DOI] [PubMed] [Google Scholar]


