Abstract
Background:
The clustering of cardiovascular risk factors is termed the metabolic syndrome (MS), which strongly predicts the risk of diabetes and cardiovascular disease (CVD). Adipokines may contribute to the development of obesity and insulin resistance and may be a causal link between MS, diabetes and CVD. Hence, we studied the adipokines – adiponectin and plasminogen activator inhibitor-1 (PAI-1) – in subjects with MS.
Materials and Methods:
We studied 50 subjects with MS diagnosed by International Diabetes Federation (IDF) criteria and 24 healthy age- and sex-matched controls. Clinical evaluation included anthropometry, body fat analysis by bioimpedance, highly sensitive C-reactive protein, insulin, adiponectin, and PAI-1 measurement.
Results:
Subjects with MS had lower adiponectin (4.01 ± 2.24 vs. 8.7 ± 1.77 μg/ml; P < 0.0001) and higher PAI-1 (53.85 ± 16.45 vs. 17.35 ± 4.45 ng/ml; P < 0.0001) levels than controls. Both were related with the number of metabolic abnormalities. Adiponectin was negatively and PAI-1 was positively associated with body mass index, waist hip ratio (WHR), body fat mass, percent body fat, and all the parameters of MS, except HDL where the pattern reversed. WHR and triglycerides were independent predictors of adipokines in multiple regression analysis. Receiver operating characteristic curve analysis showed that adiponectin (6.7 μg/ml) and PAI-1 (25.0 ng/ml) levels predicted the MS with high sensitivity, specificity and accuracy in Indian population.
Conclusions:
Subjects with MS have lower adiponectin and higher PAI-1 levels compared to healthy controls. Lifestyle measures have been shown to improve the various components of MS, and hence there is an urgent need for public health measures to prevent the ongoing epidemic of diabetes and CVD.
Keywords: Adiponectin, hsCRP, insulin resistance, metabolic syndrome, plasminogen activator inhibitor-1
INTRODUCTION
The clustering of cardiovascular risk factors that includes central adiposity, hypertension, hyperglycemia, and high triglycerides (TG) with low high density lipoprotein cholesterol (HDL-C) levels is termed the metabolic syndrome (MS). MS is known to strongly predict the long-term risk of diabetes and cardiovascular disease (CVD).[1] Obesity can be said to be the predominant driving force behind the MS.[2] Adipose tissue products are reported to affect systemic metabolism; among these are adiponectin, leptin, plasminogen activator inhibitor-1 (PAI-1), resistin, inflammatory cytokines, and angiotensinogen.[3] Adiponectin has recently attracted much attention with particular emphasis on the role of adiponectin in the regulation of insulin sensitivity and the development of insulin resistance.[4] Plasma levels of adiponectin have also been reported to be significantly reduced in obese humans.[5] Moreover, plasma adiponectin levels have been shown to be decreased in patients with type-2 diabetes mellitus (T2DM), CVD, hypertension, and MS.[6–9]
Adiponectin activates adenosine mono-phosphate activated protein kinase and peroxisome proliferator activated receptor-ϒ in the liver and skeletal muscle, thereby stimulating phosphorylation of acetyl-CoA carboxylase, fatty acid oxidation, thus decreasing tissue triglyceride (TG) content in muscle and liver. These alterations increase insulin sensitivity in vivo.[3,4] The expression levels of AdipoR1/R2 are also decreased in obesity, which reduces adiponectin sensitivity and finally leads to insulin resistance, the so-called “vicious cycle.”[10] Adiponectin was demonstrated to strongly inhibit the expression of adhesion molecules, including intracellular adhesion molecule-1, vascular cellular adhesion molecule-1, and E-selectin. Thus, various studies have highlighted the potential antidiabetic, antiatherosclerotic and anti-inflammatory properties of adiponectin. Decreased levels of plasma adiponectin are associated with increased body mass index (BMI), decreased insulin sensitivity, less favorable plasma lipid profiles, increased levels of inflammatory markers and increased risk for the development of CVD. Therefore, adiponectin levels hold great promise for use in clinical application, serving as a potent indicator of underlying metabolic complications.[9]
The mature adipocyte is an important source of circulating PAI-1 in humans. Plasma PAI-1 levels are elevated in obesity and insulin resistance, are positively correlated with features of the MS, and predict future risk for type 2 diabetes and CVD. Thus, PAI-1 may contribute to the development of obesity and insulin resistance and may be a causal link between obesity and CVD.[11–13] There are few studies which have evaluated adipokines in Indian population.[14–17] Hence, we studied adiponectin and PAI-1 levels and their correlation with various parameters in subjects with MS.
MATERIAL AND METHODS
This study was carried out at the Department of Endocrinology of a tertiary care center and details are provided in our previous publication in this journal.[18] Subjects with age ≥30 years and ≤50 years (to exclude all postmenopausal women) were screened for the presence of MS according to International Diabetes Federation (IDF)[19] criteria as follows: central obesity (waist circumference – males >90 cm, females >80 cm) plus any two of the following: raised triglycerides (>150 mg/dl), reduced HDL-C (<40 mg/dl in men and <50 mg/dl in women), raised blood pressure (systolic ≥130 mm Hg or diastolic ≥85 mm Hg), raised fasting plasma glucose (FPG) (≥100 mg/dl). Age- and sex-matched healthy subjects were screened for the absence of MS. Only those cases with waist circumference below the IDF criteria and absence of at least three of the four parameters were included.
A total of 50 drug-naïve subjects with MS (25 males and 25 females) and 24 controls (12 males and 12 females) were included in the study. All the subjects underwent clinical examination. Subjects with hepatic disease, renal disease, other endocrine diseases, alcoholism, infectious diseases or receiving any medications were excluded from the study.
BMI was calculated as weight in kilogram divided by square of height in meters. Body fat measurement was done using InBODY composition analyser-biospacer manufactured by M/S Biodex Medical Systems Inc. (New York, NY, USA). It measured waist hip ratio (WHR), body fat mass (BFM), percent body fat (PBF) and basal metabolic rate (BMR). Fasting blood samples were drawn for the estimation of FPG, renal and hepatic parameters, A1C, lipid profile, highly sensitive C-reactive protein (hsCRP) and fibrinogen. One aliquot of the sample was frozen at –80°C for the measurement of plasma insulin, adiponectin and PAI-1. Urine spot samples were collected for the measurement of urine microalbumin. Adiponectin was measured with AviBion Human Adiponectin (Acrp30) ELISA commercial kits supplied by Orgenium Laboratories Business Unit, Finland, as per manufacturer's instructions. It had an assay range of 0.185–15 ng/ml and a sensitivity of <0.3 ng/ml. Intra-assay precision was ≤10% and inter-assay precision was ≤12%. PAI-1 was measured with Human PAI-1 ELISA (EA-0207) supplied by Signosis BioSignal Capture (Sunnyvale, CA, USA), as per manufacturer's instructions. It had an assay range of 0.3–20 ng/ml and a sensitivity of <0.79 ng/ml. Intra-assay and inter-assay precision was ≤15%. The HOMA model was used to calculate insulin resistance and insulin insensitivity. The formulae are as follows:
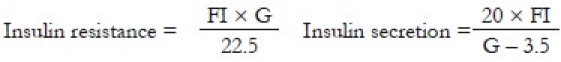
where FI = fasting insulin in μIU/ml and G = fasting glucose in mmol/l.
The study was approved by the ethics committee of Army Hospital (Research and Referral), Delhi Cantt, and all subjects gave written informed consent.
Statistical analysis was carried out using EPI INFO 3.5.3 (CDC; Atlanta; USA). Data were presented as mean ± SD or number (%) unless specified. All parametric data were analyzed by Student's t-test. If Barlett's chi-square test for equality of population variances was <0.05, then Kruskal–Wallis test was applied. All nonparametric data were analyzed by Chi-square test. Multiple regression analysis was done to ascertain the association between various parameters. A P value of <0.05 was considered statistically significant.
RESULTS
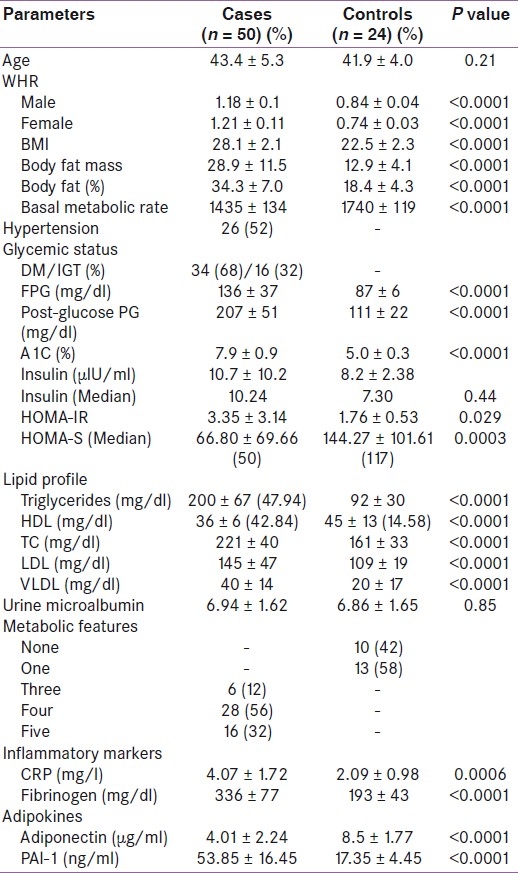
This study was carried out in 50 cases of MS and 24 normal healthy controls. Sex ratio was equal in both the groups. Basal characteristics of the cases and controls are shown in Table 1 (also given in our previous publication).[18] Mean ages of cases and controls were comparable. All cases had significantly higher WHR than controls in both the sexes. BMI¸ BFM and PBF were significantly higher in cases than controls. However, cases had significantly lower BMR than controls. There were 37 (74%) cases with T2DM and 13 (26%) cases with impaired glucose tolerance. Hypertension was present in 26 cases (52%). TG¸ total cholesterol (TC)¸ low density lipoprotein (LDL)¸ and very low density lipoprotein (VLDL) were significantly higher and HDL was significantly lower among cases than in controls. However¸ 14 controls (58%) also had low HDL. Most of the cases (28.56%) had four features of MS, followed by cases with all features (16.32%), and 6 (12%) cases had three features of MS [Table 1].
Table 1.
Basic characteristics of cases and controls
Adiponectin
Subjects with MS had significantly lower adiponectin levels than controls (4.01 ± 2.24 vs. 8.7 ± 1.77 μg/ml; P < 0.0001) [Table 1]. Adiponectin levels decreased with increasing number of metabolic abnormalities [Figure 1]. There was no difference in adiponectin levels between sexes (5.23 ± 3.12 vs. 5.71 ± 2.65 μg/ml; P = 0.49).
Figure 1.
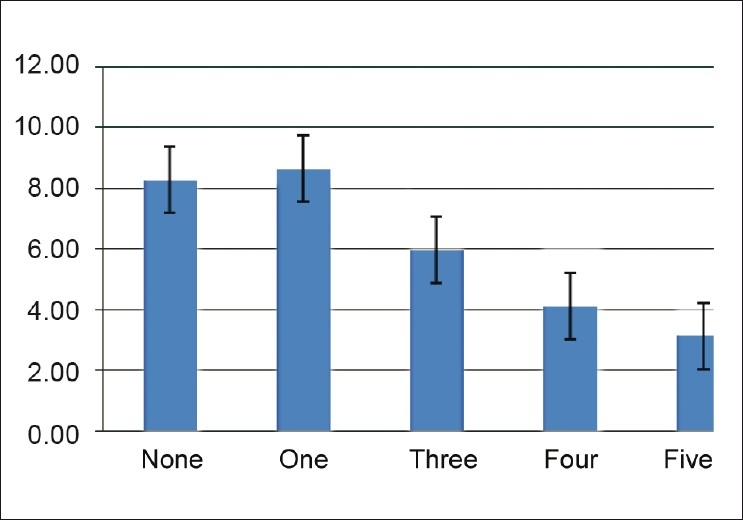
Adiponectin levels according to the number of metabolic abnormalities
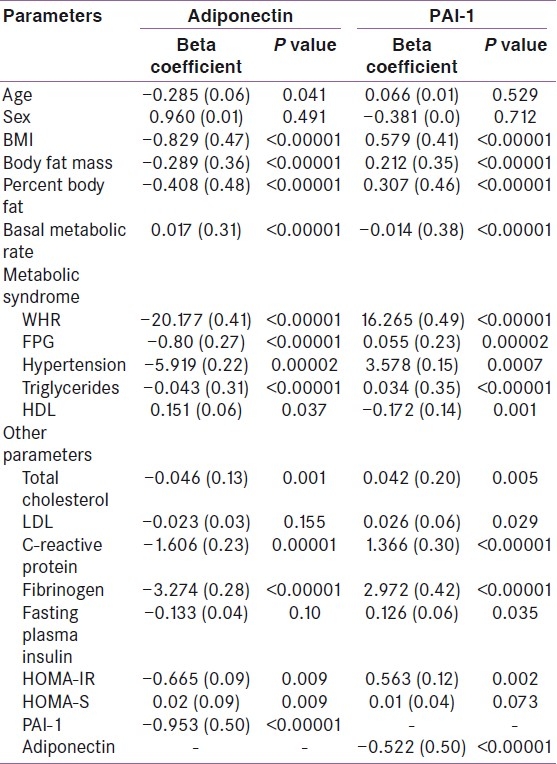
In univariate regression analysis, adiponectin was negatively associated with age, BMI, BFM and PBF, and positively associated with BMR. Among the various parameters of MS, adiponectin was negatively associated with all parameters, except HDL-C with which it was positively associated. There was no association between adiponectin and LDL-C. Adiponectin showed strong negative association with inflammatory markers (CRP, fibrinogen) and procoagulant marker (PAI-1). Adiponectin was positively associated with insulin secretion (HOMA-S) and negatively associated with insulin resistance (HOMA-IR) [Table 2].
Table 2.
Univariate regression analysis of adipokines among all subjects
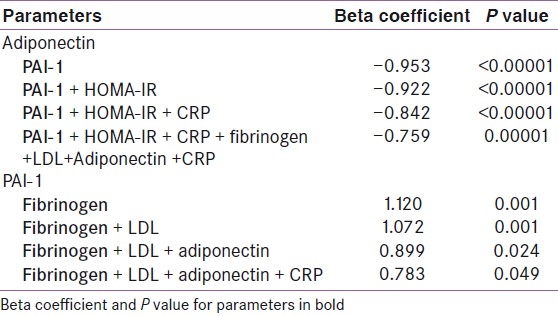
Multiple regression analysis showed that among the metabolic parameters, WHR, FPG and TG maintained negative association with adiponectin even after adjustment for all metabolic variables. However, HDL and hypertension lost significance during multiple regression analysis [Table 3]. Only BMI remained negatively associated with adiponectin when adjusted for anthropometric parameters, e.g. BMR, BFM, and percent body fat, in multiple regression analysis [Table 4]. BMR showed positive association when adjusted with BMI, but lost significance when BFM was added. Similarly, PAI-1 levels showed strong negative association with adiponectin [Figure 2] even after adjustment with other parameters like HOMA-IR, CRP, and fibrinogen [Table 5].
Table 3.
Multivariate regression analysis of adipokines with metabolic parameters among all subjects
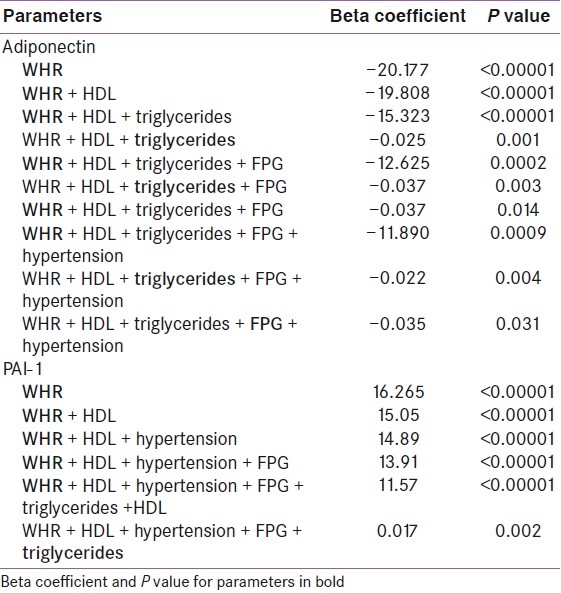
Table 4.
Multivariate regression analysis of adipokines with anthropometric parameters among all subjects
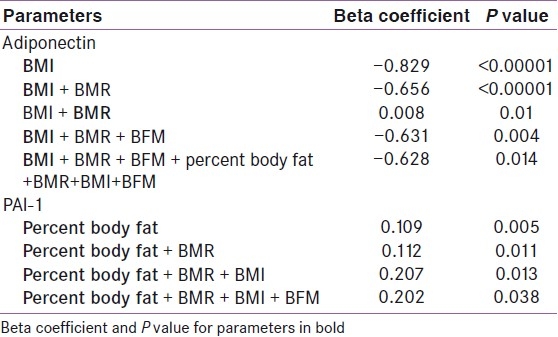
Figure 2.
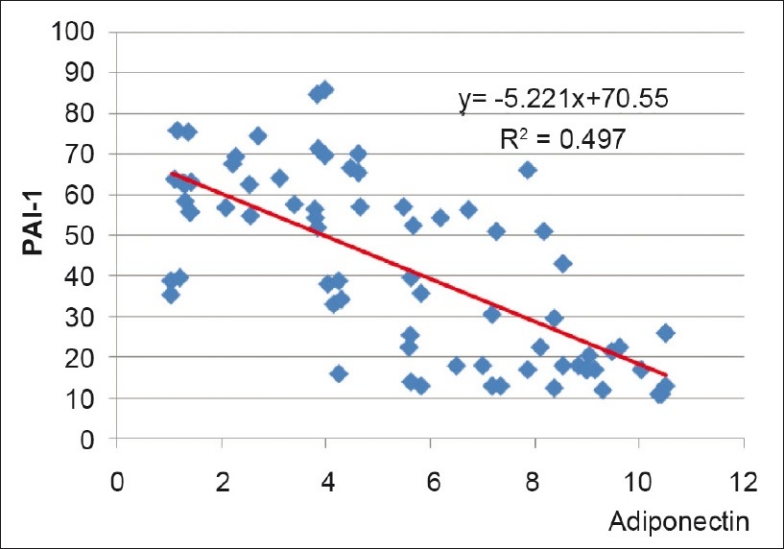
Correlation between adiponectin and PAI-1 among all subjects
Table 5.
Multivariate regression analysis of adipokines with other parameters among all subjects
Receiver operating characteristic (ROC) curve analysis revealed best prediction value of adiponectin at 6.7 μg/ml with a sensitivity of 83%, specificity of 86%, accuracy of 85%, positive predictive value of 74%, negative predictive value of 84% and positive and negative likelihood ratios of 5.95 and 0.19, respectively (ROC 0.934, SE 0.0277, 95% CI = 0.879–0.988).
Plasminogen activator inhibitor-1
Subjects with MS had significantly higher PAI-1 levels than controls (53.85 ± 16.45 vs. 17.35 ± 4.45 ng/ml; P < 0.0001) [Table 1]. PAI-1 levels increased with increasing number of metabolic abnormalities [Figure 3]. There was no difference in PAI-1 levels between sexes (8.59 ± 4.42 vs. 8.21 ± 4.44 ng/ml; P = 0.71).
Figure 3.
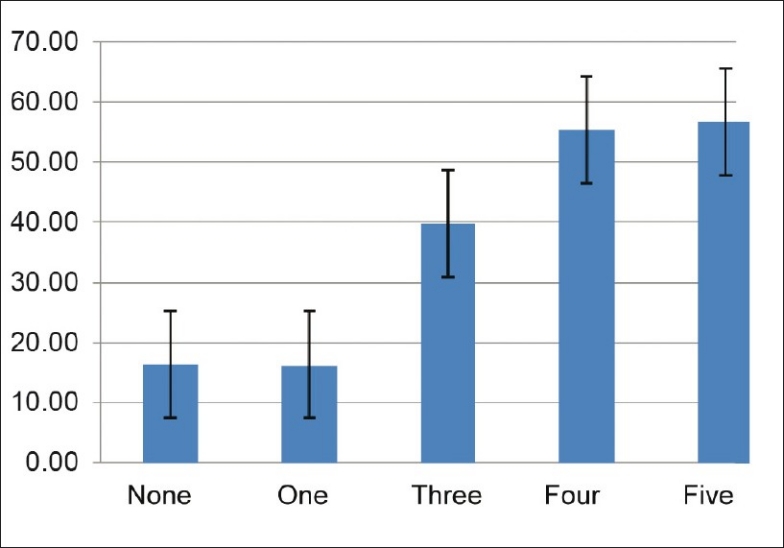
PAI-1 according to the number of metabolic abnormalities
In univariate regression analysis, PAI-1 was positively associated with BMI, BFM and PBF, and negatively associated with BMR. Among the various parameters of MS, PAI-1 was positively associated with all parameters of MS, except HDL with which it was negatively associated. PAI-1 showed strong positive association with inflammatory markers (CRP, fibrinogen), insulin resistance (fasting plasma insulin and HOMA-IR) and total and LDL cholesterol, but had strong negative association with adiponectin [Table 2].
Multiple regression analysis of various metabolic parameters showed that WHR and TG maintained positive association with PAI-1 even after adjustment for all metabolic variables. However, HDL, FPG and hypertension lost significance during multiple regression analysis [Table 3]. Only PBF remained positively associated with PAI-1 when adjusted for anthropometric parameters, e.g. BMR, BFM, and BMI, in multiple regression analysis [Table 4]. Similarly, PAI-1 levels showed strong positive association with fibrinogen even after adjustment with other parameters like HOMA-IR, CRP, and fibrinogen [Table 5].
ROC curve analysis revealed best prediction value of PAI-1 at 25.0 ng/ml, with a sensitivity of 92%, specificity of 96%, accuracy of 95%, positive predictive value of 92%, negative predictive value of 96% and positive and negative likelihood ratios of 22.9 and 0.08, respectively (ROC 0.975, SE 0.0178, 95% CI = 0.942–1.009).
DISCUSSION
MS is known to strongly predict long-term risk of diabetes and CVD, and has also been reported to be associated with increased morbidity and mortality.[1] It is becoming increasingly common in the United States and worldwide, and is emerging as the dominant risk factor for CVD in Asia.[2,11,20] The MS represents a combination of risk determinants, which include atherogenic dyslipidemia, elevated blood pressure, elevated glucose, a prothrombotic state, and a proinflammatory state.[1] In obese persons, adipose tissue products are reported to affect systemic metabolism; among these are adiponectin, leptin, inflammatory cytokines, PAI-1, resistin, and angiotensinogen.[3]
In this study, we evaluated 50 subjects with MS (25 males and 25 females) and 24 controls (12 males and 12 females). Different definitions have been proposed for MS.[2] We have used here IDF criteria as it provides ethnic-specific criteria for central obesity. All cases had significantly higher WHR, BMI¸ BFM and PBF than controls in both the sexes, which is similar to Asian Indian obesity phenotype.[20]
Subjects with MS had significantly lower adiponectin levels than controls. Low adiponectin levels have been reported in most of the studies on MS among people of various age groups, ethnicity and gender.[14,15,21–27] However, one study reported higher adiponectin levels in MS[28] and another found low adiponectin level which was statistically not significant.[29] There was negative graded response of adiponectin levels with metabolic abnormalities [Figure 1]. Similar observations have been reported by other studies.[23,27,30] There was no difference in adiponectin levels between sexes. However, others[15,16,30] have reported higher adiponectin levels in females. Adiponectin was negatively related with age, BMI, BFM and PBF, WHR, FPG, hypertension, TG, TC, hsCRP, fibrinogen, HOMA-IR and PAI-1, and positively related with BMR, HDL and HOMA-S in the present study. Similar associations have been reported in the literature.[17,26,31–35] However, certain differences were observed between the present study and other studies. In this study, adiponectin showed no relation with fasting insulin levels, as also observed by one study from South India,[16] whereas others have reported negative correlation.[26,31,36] We have observed negative correlation of cholesterol with adiponectin, similar to that reported by Bilgili et al.[15,26] But others[16,31,37] found no relation of adiponectin with TC. Vikram et al.,[17] did not observe any relation between adiponectin and hsCRP in post-pubertal Asian Indian adolescents, as seen by us. Multiple regression analysis of the metabolic parameters showed WHR, FPG and TG to have an independent negative association with adiponectin even after adjustment for all metabolic variables. Yang et al.,[31] observed positive association of HDL with adiponectin in multiple regression analysis. However, in our study, HDL and hypertension lost significance during multiple regression analysis. Ku et al.,[36] reported correlation between serum adiponectin and BMI, triglyceride, HDL-C and fasting glucose in multiple regression analysis. In the present study, only BMI remained negatively associated with adiponectin when adjusted for anthropometric parameters, e.g. BMR, BFM, and PBF, in multiple regression analysis. Jung et al.,[38] showed significant negative correlation of adiponectin levels with percent body fat, lipid profile and HOMA-IR. BMR showed positive association when adjusted with BMI, but lost significance when BFM was added. Similarly, PAI-1 levels showed strong negative association with adiponectin even after adjustment with other parameters like HOMA-IR, CRP, and fibrinogen. Bilgili et al.,[26] also found an inverse relationship between plasma adiponectin and PAI-1 (r = –0.653, P < 0.001). ROC curve analysis revealed best prediction value at 6.7 μg/ml, with a sensitivity of 83%, specificity of 86%, and accuracy of 85%. Mohan et al.,[15] also reported similar adiponectin levels (5.0 μg/ml for males and 6.5 μg/ml for females) in a study from South India. However, studies from Japan have suggested lower cut-off point (4 μg/ml) of serum adiponectin level for the diagnosis of MS.[29,35] There is wide geographic variation in the adiponectin levels reported in literature. Seino et al.,[32] also suggested that measuring adiponectin may be effective for evaluating the presence of MS in nondiabetic subjects through analysis of area under the curve of ROC curves.
Subjects with MS had significantly higher PAI-1 levels than controls, which is similar to that reported in many studies in the literature.[13,26,39–45] However, PAI-1 level was higher in the present study than that reported from other ethnic populations. Indian population has higher PAI-1 compared to African population, which is explained by the increased frequency of PAI-1 4G allele.[42] PAI-1 levels increased with increasing number of metabolic abnormalities. Similar observations have been made in other studies.[27,39,43,46] In univariate regression analysis, PAI-1 was positively associated with BMI, BFM and PBF, and negatively associated with BMR. Among the various parameters of MS, PAI-1 was positively associated with all parameters of MS, except HDL with which it was negatively associated. PAI-1 showed a strong positive association with inflammatory markers (hsCRP, fibrinogen), insulin resistance (fasting plasma insulin, HOMA-IR) and total and LDL cholesterol, but had strong negative association with adiponectin. Others have also reported significant correlation between PAI-1 and all obesity and dyslipidemia variables.[27,47] TG and WHR were independently associated with PAI-1 even after adjustment for all metabolic variables in multiple regression analysis. Greyling et al.,[40] also found waist circumference as the strongest independent predictor of PAI-1. However, HDL, FPG and hypertension lost significance during multiple regression analysis. Only PBF remained positively associated with PAI-1 when adjusted for anthropometric parameters, e.g. BMR, BFM, and BMI, in multiple regression analysis. Similarly, PAI-1 levels showed a strong positive association with fibrinogen even after adjustment with other parameters like HOMA-IR, CRP, and adiponectin. ROC curve analysis revealed the best prediction value at 25.0 ng/ml, with a sensitivity of 92%, specificity of 96%, accuracy of 95%, and positive predictive value of 92%. PAI-1 had higher predictive value compared to adiponectin in the present study. Other studies have also made similar observation that PAI-1 is an independent determinant of MS in multivariate logistic regression analysis.[39,41,43,47] Surprisingly, we have found higher cut-off value for adiponectin and lower cut-off value for PAI-1 for diagnosis of MS. This can possibly be explained by lower BMI and BFM in our cases.
The primary limitation of the present study is its cross-sectional design and the inherent possibility that genetic and/or lifestyle factors may have influenced the results of our group comparisons. However, in an effort to minimize the influence of lifestyle behaviors, we studied subjects of similar age who were non-smokers, who were not currently taking medication that could influence inflammatory and oxidative markers (i.e. statins), and who did not differ in habitual physical activity. In addition, we used strict inclusion criteria to eliminate the confounding effects of underlying cardiovascular and metabolic disease.
CONCLUSION
In the present study, subjects with MS had higher BMI, BFM and percent body fat, which is reflected in lower level of adiponectin and higher level of PAI-1. Also, they had increased insulin resistance and decreased insulin secretion compared to healthy controls. Subjects with MS were associated with higher hsCRP level, emphasizing low-grade systemic inflammatory state. All these factors were interdependent and associated with other cardiovascular risk factors. Although the knowledge on pathogenesis and its association with metabolic risk factors such as obesity, hypertension, and cardiovascular risk is increasing day by day, the challenge is to translate research findings into substantial clinical improvements for patients. Demographic and epidemiological evidence indicates that unless an effective preventive strategy is developed, there will be a sharp increase in the global prevalence of cardiometabolic risk factors including diabetes and MS. Unfortunately, clinical therapies to treat underlying obesity often are unsuccessful.[48] Lifestyle measures have been shown to improve insulin resistance, insulin secretion and various components and effects of MS,[1,49–51] and hence there is an urgent need for public health measures to prevent the ongoing epidemic of diabetes and CVD.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Misra A, Misra R, Wijesuriya M, Banerjee D. The metabolic syndrome in South Asians: Continuing escalation and possible solutions. Indian J Med Res. 2007;125:345–54. [PubMed] [Google Scholar]
- 2.Chen G, Liu C, Yao J, Jiang Q, Chen N, Huang H, et al. Overweight, obesity, and their associations with insulin resistance and -cell function among Chinese: A cross-sectional study in China. Metabolism. 2010;59:1823–32. doi: 10.1016/j.metabol.2010.06.009. [DOI] [PubMed] [Google Scholar]
- 3.Scherer PE. Adipose tissue: From lipid storage compartment to endocrine organ. Diabetes. 2006;55:1537–45. doi: 10.2337/db06-0263. [DOI] [PubMed] [Google Scholar]
- 4.Kadowaki T, Yamauchi T. Adiponectin and Adiponectin Receptors. Endocr Rev. 2005;26:439–51. doi: 10.1210/er.2005-0005. [DOI] [PubMed] [Google Scholar]
- 5.Arita Y, Kihara S, Ouchi N, Takahashi M, Maeda K, Miyagawa J, et al. Paradoxical decrease of an adipose-specific protein, adiponectin, in obesity. Biochem Biophys Res Commun. 1999;257:79–83. doi: 10.1006/bbrc.1999.0255. [DOI] [PubMed] [Google Scholar]
- 6.Hotta K, Funahashi T, Arita Y, Takahashi M, Matsuda M, Okamoto Y, et al. Plasma concentrations of a novel, adipose-specific protein, adiponectin, in type 2 diabetic patients. Arterioscler Thromb Vasc Biol. 2000;20:1595–9. doi: 10.1161/01.atv.20.6.1595. [DOI] [PubMed] [Google Scholar]
- 7.Kumada M, Kihara S, Sumitsuji S, Kawamoto T, Matsumoto S, Ouchi N, et al. Osaka CAD Study Group. Association of hypoadiponectinemia with coronary artery disease in men. Arterioscler Thromb Vasc Biol. 2003;23:85–89. doi: 10.1161/01.atv.0000048856.22331.50. [DOI] [PubMed] [Google Scholar]
- 8.Ouchi N, Ohishi M, Kihara S, Funahashi T, Nakamura T, Nagaretani H, et al. Association of hypoadiponectinemia with impaired vasoreactivity. Hypertension. 2003;42:231–4. doi: 10.1161/01.HYP.0000083488.67550.B8. [DOI] [PubMed] [Google Scholar]
- 9.Trujillo ME, Scherer PE. Adiponectin: Journey from an adipocyte secretory protein to biomarker of the metabolic syndrome. J Intern Med. 2005;257:167–75. doi: 10.1111/j.1365-2796.2004.01426.x. [DOI] [PubMed] [Google Scholar]
- 10.Tsuchida A, Yamauchi T, Ito Y, Hada Y, Maki T, Takekawa S, et al. Insulin/Foxo1 pathway regulates expression levels of adiponectin receptors and adiponectin sensitivity. J Biol Chem. 2004;279:30817–22. doi: 10.1074/jbc.M402367200. [DOI] [PubMed] [Google Scholar]
- 11.Alessi MC, Juhan-Vague I. PAI-1 and the metabolic syndrome: links, causes, and consequences. Arterioscler Thromb Vasc Biol. 2006;26:2200–7. doi: 10.1161/01.ATV.0000242905.41404.68. [DOI] [PubMed] [Google Scholar]
- 12.De Maat MP, Bladbjerg EM, Drivsholm T, Borch-Johnsen K, Moller L, Jespersen J. Inflammation, thrombosis and atherosclerosis: Results of the Glostrup study. J Thromb Haemost. 2003;1:950–7. doi: 10.1046/j.1538-7836.2003.00213.x. [DOI] [PubMed] [Google Scholar]
- 13.Vague P, Juhan-Vague I, Aillaud MF, Badier C, Viard R, Alessi MC, et al. Correlation between blood fibrinolytic activity, plasminogen activator inhibitor level, plasma insulin level, and relative body weight in normal and obese subjects. Metabolism. 1986;35:250–3. doi: 10.1016/0026-0495(86)90209-x. [DOI] [PubMed] [Google Scholar]
- 14.Mahadik SR, Deo SS, Mehtalia SD. Association of adiposity, inflammation and atherosclerosis: The role of adipocytokines and CRP in Asian Indian subjects. Metab Syndr Relat Disord. 2008;6:121–8. doi: 10.1089/met.2007.0034. [DOI] [PubMed] [Google Scholar]
- 15.Mohan V, Deepa R, Pradeepa R, Vimaleswaran KS, Mohan A, Velmurugan K, et al. Association of low adiponectin levels with the metabolic syndrome--The Chennai Urban Rural Epidemiology Study (CURES-4) Metabolism. 2005;54:476–81. doi: 10.1016/j.metabol.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 16.Snehalatha C, Yamuna A, Ramachandran A. Plasma adiponectin does not correlate with insulin resistance and cardiometabolic variables in nondiabetic Asian Indian teenagers. Diabetes Care. 2008;31:2374–9. doi: 10.2337/dc08-1083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vikram NK, Misra A, Pandey RM, Dwivedi M, Luthra K. Adiponectin, insulin resistance, and C-reactive protein in postpubertal Asian Indian adolescents. Metabolism. 2004;53:1336–41. doi: 10.1016/j.metabol.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 18.Garg MK, Dutta MK, Mahalle N. Study of beta-cell function (by HOMA model) in metabolic syndrome. Indian J Endocrinol Metab. 2011;15:S44–9. doi: 10.4103/2230-8210.83059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Alberti KG, Zimmet P, Shaw J. IDF epidemiology task force consensus group. Lancet. 2005;66:1059–62. doi: 10.1016/S0140-6736(05)67402-8. [DOI] [PubMed] [Google Scholar]
- 20.Bhatnagar D, Anand IS, Durrington PN, Patel DJ, Wander GS, Mackness MI, et al. Coronary risk factors in people from the Indian Subcontinent living in west London and their siblings in India. Lancet. 1995;345:405–9. doi: 10.1016/s0140-6736(95)90398-4. [DOI] [PubMed] [Google Scholar]
- 21.Banerji MA, Faridi N, Atluri R, Chaiken R, Lebovitz HE. Body composition, visceral fat, leptin, and insulin resistance in Asian Indian men. J Clin Endocrinol Metab. 1999;84:137–44. doi: 10.1210/jcem.84.1.5371. [DOI] [PubMed] [Google Scholar]
- 22.Tajtákova M, Petrásova D, Petrovicová J, Pytliak M, Semanová Z. Adiponectin as a biomarker of clinical manifestation of metabolic syndrome. Endocr Regul. 2006;40:15–9. [PubMed] [Google Scholar]
- 23.Santaniemi M, Kesäniemi YA, Ukkola O. Low plasma adiponectin concentration is an indicator of the metabolic syndrome. Eur J Endocrinol. 2006;155:745–50. doi: 10.1530/eje.1.02287. [DOI] [PubMed] [Google Scholar]
- 24.Choi KM, Ryu OH, Lee KW, Kim HY, Seo JA, Kim SG, et al. Serum adiponectin, interleukin-10 levels and inflammatory markers in the metabolic syndrome. Diabetes Res Clin Pract. 2007;75:235–40. doi: 10.1016/j.diabres.2006.06.019. [DOI] [PubMed] [Google Scholar]
- 25.Restituto P, Colina I, Varo JJ, Varo N. Adiponectin diminishes platelet aggregation and sCD40L release.Potential role in the metabolic syndrome. Am J Physiol Endocrinol Metab. 2010;298:E1072–7. doi: 10.1152/ajpendo.00728.2009. [DOI] [PubMed] [Google Scholar]
- 26.Bilgili S, Celebiler AC, Dogan A, Karaca B. Inverse relationship between adiponectin and plasminogen activator inhibitor-1 in metabolic syndrome patients. Endocr Regul. 2008;42:63–8. [PubMed] [Google Scholar]
- 27.You T, Nicklas BJ, Ding J, Penninx BW, Goodpaster BH, Bauer DC, et al. The metabolic syndrome is associated with circulating adipokines in older adults across a wide range of adiposity. J Gerontol A Biol Sci Med Sci. 2008;63:414–9. doi: 10.1093/gerona/63.4.414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Sepulveda KK, Huffman FG, Mclean M, Zarini GG. Increased adiponectin among Cuban American type 2 diabetics with metabolic syndrome. FASEB J. 2009;23:547–8. [Google Scholar]
- 29.Mori Y, Hoshino K, Yokota K, Itoh Y, Tajima N. Role of hypoadiponectinemia in the metabolic syndrome and its association with post-glucose challenge hyper-free fatty acidemia: A study in prediabetic Japanese males. Endocrine. 2006;29:357–61. doi: 10.1385/ENDO:29:2:357. [DOI] [PubMed] [Google Scholar]
- 30.Patel DA, Srinivasan SR, Xu JH, Chen W, Berenson GS. Adiponectin and its correlates of cardiovascular risk in young adults: The Bogalusa Heart Study. Metabolism. 2006;55:1551–7. doi: 10.1016/j.metabol.2006.06.028. [DOI] [PubMed] [Google Scholar]
- 31.Yang WS, Lee WJ, Funahashi T, Tanaka S, Matsuzawa Y, Chao CL, et al. Plasma adiponectin levels in overweight and obese Asians. Obes Res. 2002;10:1104–10. doi: 10.1038/oby.2002.150. [DOI] [PubMed] [Google Scholar]
- 32.Seino Y, Hirose H, Saito I, Itoh H. High molecular weight multimer form of adiponectin as a useful marker to evaluate insulin resistance and metabolic syndrome in Japanese men. Metabolism. 2007;56:1493–9. doi: 10.1016/j.metabol.2007.06.015. [DOI] [PubMed] [Google Scholar]
- 33.Hung J, McQuillan BM, Thompson PL, Beilby JP. Circulating adiponectin levels associate with inflammatory markers, insulin resistance and metabolic syndrome independent of obesity. Int J Obes (Lond) 2008;32:772–9. doi: 10.1038/sj.ijo.0803793. [DOI] [PubMed] [Google Scholar]
- 34.Tanianskiĭ DA, Firova EM, Shatilina LV, Denisenko AD. Adiponectin: Lowering in metabolic syndrome and independent relation to hypertriglyceridemia. Kardiologiia. 2008;48:20–5. [PubMed] [Google Scholar]
- 35.Ryu HK, Yu SY, Park JS, Choi YJ, Huh KB, Park JE, et al. Hypoadiponectinemia is strongly associated with metabolic syndrome in Korean type 2 diabetes patients. J Am Coll Nutr. 2010;29:171–8. doi: 10.1080/07315724.2010.10719831. [DOI] [PubMed] [Google Scholar]
- 36.Ku BJ, Kim SY, Lee TY, Park KS. Serum ferritin is inversely correlated with serum adiponectin level: Population-based cross-sectional study. Dis Markers. 2009;27:303–10. doi: 10.3233/DMA-2009-0676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lewandowski KC, Szosland K, O’Callaghan C, Tan BK, Randeva HS, Lewinski A. Adiponectin and resistin serum levels in women with polycystic ovary syndrome during oral glucose tolerance test: A significant reciprocal correlation between adiponectin and resistin independent of insulin resistance indices. Mol Genet Metab. 2005;85:61–9. doi: 10.1016/j.ymgme.2004.12.014. [DOI] [PubMed] [Google Scholar]
- 38.Jung CH, Rhee EJ, Choi JH, Bae JC, Yoo SH, Kim WJ, et al. The relationship of adiponectin/leptin ratio with homeostasis model assessment insulin resistance index and metabolic syndrome in apparently healthy korean male adults. Korean Diabetes J. 2010;34:237–43. doi: 10.4093/kdj.2010.34.4.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Mertens I, Verrijken A, Michiels JJ, Van der Planken M, Ruige JB, Van Gaal LF. Among inflammation and coagulation markers, PAI-1 is a true component of the metabolic syndrome. Int J Obes (Lond) 2006;30:1308–14. doi: 10.1038/sj.ijo.0803189. [DOI] [PubMed] [Google Scholar]
- 40.Greyling A, Pieters M, Hoekstra T, Oosthuizen W, Schutte AE. Differences in the association of PAI-1 activity with the metabolic syndrome between African and Caucasian women. Nutr Metab Cardiovasc Dis. 2007;17:499–507. doi: 10.1016/j.numecd.2006.04.006. [DOI] [PubMed] [Google Scholar]
- 41.Ajjan R, Carter AM, Somani R, Kain K, Grant PJ. Ethnic differences in cardiovascular risk factors in healthy Caucasian and South Asian individuals with the metabolic syndrome. J Thromb Haemost. 2007;5:754–60. doi: 10.1111/j.1538-7836.2007.02434.x. [DOI] [PubMed] [Google Scholar]
- 42.Naran NH, Chetty N, Crowther NJ. The influence of metabolic syndrome components on plasma PAI-1 concentrations is modified by the PAI-1 4G/5G genotype and ethnicity. Atherosclerosis. 2008;196:155–63. doi: 10.1016/j.atherosclerosis.2007.03.024. [DOI] [PubMed] [Google Scholar]
- 43.Chou YY, Sheu WH, Tang YJ, Chen YM, Liao SC, Chuang YW, et al. Plasminogen activator inhibitor type 1 (PAI-1) is a valuable biomarker for predicting the metabolic syndrome (MS) in institutionalized elderly residents in Taiwan. Arch Gerontol Geriatr. 2009;49(Suppl 2):S41–5. doi: 10.1016/S0167-4943(09)70012-3. [DOI] [PubMed] [Google Scholar]
- 44.Kressel G, Trunz B, Bub A, Hülsmann O, Wolters M, Lichtinghagen R, et al. Systemic and vascular markers of inflammation in relation to metabolic syndrome and insulin resistance in adults with elevated atherosclerosis risk. Atherosclerosis. 2009;202:263–71. doi: 10.1016/j.atherosclerosis.2008.04.012. [DOI] [PubMed] [Google Scholar]
- 45.Guilder GP, Hoetzer GL, Greiner JJ, Stauffer BL, Desouza CA. Influence of metabolic syndrome on biomarkers of oxidative stress and inflammation in obese adults. Obesity. 2006;14:2127–31. doi: 10.1038/oby.2006.248. [DOI] [PubMed] [Google Scholar]
- 46.Juhan-Vague I, Alessi MC, Mavri A, Morange PE. Plasminogen activator inhibitor-1, inflammation, obesity, insulin resistance and vascular risk. J Thromb Haemost. 2003;1:1575–9. doi: 10.1046/j.1538-7836.2003.00279.x. [DOI] [PubMed] [Google Scholar]
- 47.Kraja AT, Province MA, Arnett D, Wagenknecht L, Tang W, Hopkins PN, et al. Do inflammation and procoagulation biomarkers contribute to the metabolic syndrome cluster? Nutr Metab (Lond) 2007;4:28. doi: 10.1186/1743-7075-4-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Wooley SC, Garnern DM. Obesity treatment: The high cost of false hope. J Am Diet Assoc. 1991;191:1248–51. [PubMed] [Google Scholar]
- 49.Singhal N, Misra A, Shah P, Gulati S, Bhatt S, Sharma S, et al. Impact of intensive school-based nutrition education and lifestyle interventions on insulin resistance, β-cell function, disposition index, and subclinical inflammation among Asian Indian adolescents: A controlled intervention study. Metab Syndr Relat Disord. 2011;9:143–50. doi: 10.1089/met.2010.0094. [DOI] [PubMed] [Google Scholar]
- 50.Esposito K, Pontillo A, Di Palo C, Giugliano G, Masella M, Marfella R, et al. Effect of weight loss and lifestyle changes on vascular inflammatory markers in obese women: A randomized trial. JAMA. 2003;289:1799–804. doi: 10.1001/jama.289.14.1799. [DOI] [PubMed] [Google Scholar]
- 51.Balagopal P, George D, Patton N, Yarandi H, Roberts WL, Bayne E, et al. Lifestyle-only intervention attenuates the inflammatory state associated with obesity: A randomized controlled study in adolescents. J Pediatr. 2005;146:342–8. doi: 10.1016/j.jpeds.2004.11.033. [DOI] [PubMed] [Google Scholar]


