Abstract
Metabolic syndrome is a complex disorder caused by a cluster of interrelated factors that increases the risk of cardiovascular diseases and type 2 diabetes. Obesity is the main precursor for metabolic syndrome that can be targeted in developing various therapies. With this view, several physical, psychological, pharmaceutical and dietary therapies have been proposed for the management of obesity. However, dietary strategies found more appropriate without any adverse health effects. Application of probiotics and prebiotics as biotherapeutics is the new emerging area in developing dietary strategies and many people are interested in learning the facts behind these health claims. Recent studies established the role of probiotics and prebiotics in weight management with possible mechanisms of improved microbial balance, decreased food intake, decreased abdominal adiposity and increased mucosal integrity with decreased inflammatory tone. Hence, the above “Pharmaco-nutritional” approach has been selected and extensively reviewed to gain thorough knowledge on putative mechanisms of probiotic and prebiotic action in order to develop dietary strategies for the management of metabolic syndrome.
Keywords: Gut microbiota, metabolic syndrome, prebiotics, probiotics
INTRODUCTION
Metabolic syndrome (MetS) is a constellation of overweight/obesity, hypertension, and disturbances of lipid and carbohydrate metabolism. Each component of MetS is a known risk factor for the development of type 2 diabetes, atherosclerosis, and coronary artery disease (CAD). This chronic disorder with serious health and social implications is one of the major contributors of disease prevalence due to its pathophysiological link to other cardiovascular risks. It is estimated that 750 million people worldwide are overweight, out of which 300 million are obese and accounts for 325,000 deaths each year (http://www.foodcrisis2010.com/world-obesity-statistics). Since, obesity is a precursor for MetS, treating obesity with physical activities (exercises), behavioral modifications (counseling), calorie-restricted diets, weight-losing drugs, and finally with weight losing surgery will be the crucial factors in the management and control of MetS. However, strategies like exercise, behavior modifications need strong mind control and are difficult to adopt. Calorie-restricted diets are also found to be less effective in case of obese children. Similarly the current pharmacological therapies suffer from drawbacks of adverse side-effects and high cost of treatment.[1] Hence, in the present scenario, the development of dietary strategies, i.e., designing natural food products with probiotics and prebiotics that modulate MetS will be a cost-effective approach without the fear of adverse side effects on health. Probiotics are defined as live micro-organisms with Gebnerally regarded as safe (GRAS) status, which when administered in adequate amounts confer a health benefit on the host.[2] The two key members of this group include lactobacilli and bifidobacteria. Prebiotics, on the other hand, are defined as specific indigestible substances such as inulin, oligofructose/galactose complex which selectively support the growth of probiotic bacteria and possibly other microorganisms in the intestine. Since, probiotics are now well recognized as powerful functional food and dietary ingredients with multiple health promoting functions along with their ability to fight specific diseases, they are currently the major focus of attention all over the world to be explored as potential biotherapeutics in the management of several inflammatory metabolic disorders. However, these specific physiological functions attributed to probiotics are highly strain specific and hence, selection of strain could be very crucial to demonstrate their functional efficacy.
Various dietary strategies based on probiotic and prebiotic interventions have been proposed by several investigators after establishing strong relationship between diet, gut microbiota, and pathophysiology of MetS. The scientific data reveal that the gut microbiota is one of the important environmental factors co-evolved with the host since birth and maintains dynamic interactions with host throughout the life. The metabolic role of the gut microbiota is also essential for the biochemical activities of the human body, resulting in salvage of energy, generation of absorbable compounds, and production of vitamins and other essential nutrients.[3]
The gut microbiota also regulates many aspects of innate and acquired immunity, protecting the host from pathogen invasion and chronic inflammation.[4,5] Recently, investigators related the imbalances in gut microbiota with susceptibility to infections, immune-based disorders and more importantly with obesity and insulin resistance.[6,7] These studies provided strong scientific evidence for using probiotics and prebiotics in formulation of dietary strategies in the management of MetS.[8–13]
The present review focuses on various risk factors involved in pathophysiology of MetS and metagenomic changes of gut microbiota in MetS to gain knowledge on relationship between gut microbiota and MetS. The dietary strategies based on probiotic and prebiotic formulations have also been discussed with unique mechanisms of improved gut microbial balance with conjugated linoleic acid (CLA) production, decreased food intake, decreased abdominal adiposity and total cholesterol, decreased inflammatory tone with improved mucosal integrity in the management of MetS like obesity and type 2 diabetes.
METABOLIC SYNDROME: PATHOPHYSIOLOGY
Metabolic Syndromes have a multi-factorial etiology, comprising complex interactions among factors such as genetic predisposition, life style, diet, and environmental including epigenetic changes during development.[14] The physiological risk factors like adipose tissue saturation, dyslipidaemia, lipotoxicity, insulin resistance, chronic inflammation, oxidative stress are inter-related and associated with pathogenesis and progression of metabolic abnormalities like obesity, type 2 diabetes, non-alcoholic fatty liver disease (NAFLD), hypertension, etc. These conditions often lead to pathophysiology of MetS, which increases the risk of cardiovascular diseases as shown in the following [Figure 1].
Figure 1.
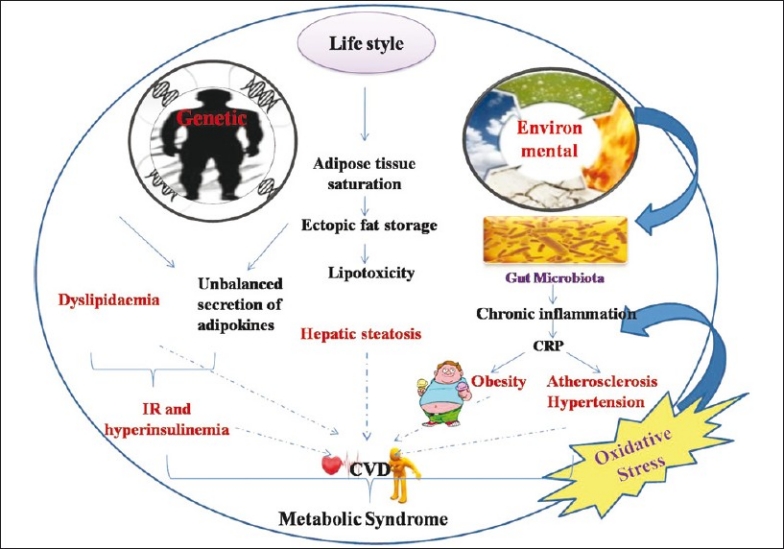
Pathophysiological risk factors of Metabolic Syndrome
METABOLIC SYNDROME: ALTERED GUT MICROBIOTA (METAGENOMIC STUDIES)
Human gastrointestinal (GI) tract contains a complex consortia of trillions of microorganisms (approximately 1 × 1013 to 1 × 1014), which includes thousands of bacterial phylotypes, methanogenic archaea with a collective genome (also termed microbiome), encoding a consortium of genes exceeding the human genome by a magnitude of 150.[15,16] Although, the exact composition of the gut microbiota is not known, advances in metagenomic technologies have recently begun to unravel the diversity of our microbial partners (human microbiome). It is estimated that each individual houses at least 160 such species from a consortium of 1000 to 1150 prevalent bacterial species.[17] Amongst these bacteria, 90% of the bacterial phylotypes are members of two phyla viz. Bacteroidetes and Firmicutes followed by Actinobacteria and Proteobacteria.[17–19] Importantly, due to the persistence of difficulties in collecting samples from the different regions of the intestine, most of the studies for investigating the ecology and activities of microbiota within the intestinal tract have been carried out using fecal matter of the host.[20]
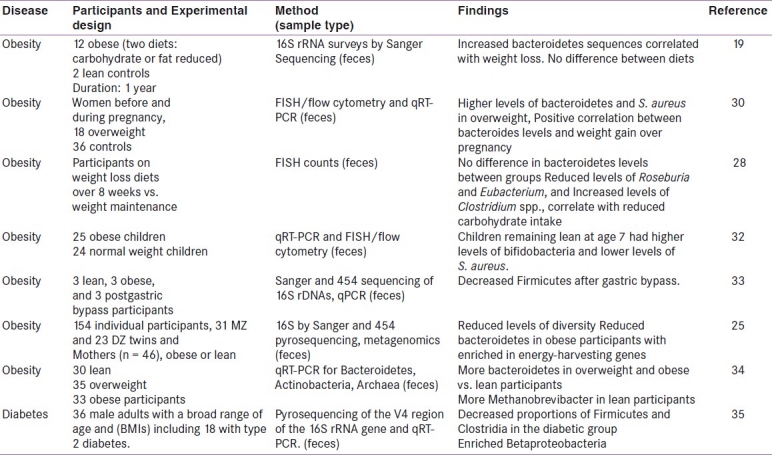
Recent studies based on large-scale 16S rRNA gene sequencing, quantitative real time PCR (qRT-PCR), fluorescent in situ hybridization (FISH), high-throughput technology of pyrosequencing and DNA barcodes have shown a relationship between the composition of the intestinal microbiota and metabolic diseases like obesity and diabetes. Researchers have demonstrated that obesity may lead to the composition shift of gut microbiota in both mice and humans. Lean experimental animals (mice, rats, and pigs) have a greater abundance of bacteroidetes compared with their obese counterparts where firmicutes predominate.[19,21–23] However, it should be noted that many of the above models involve high-fat feeding and thus the diet itself may affect the microbial composition.[23,24] Metagenomic analysis of the gut microbiota in obese mice supports the hypothesis that shifts in microbial ecology affect functional shifts in the microbiota that could contribute to the obese phenotype. Compared with lean wild type littermates, the metagenomes of obese mice are enriched in genes that encode the catabolism of complex polysaccharides, including glycoside hydrolases, which results in increased energy absorption from the gut.[25] Transplantation of the microbiota from obese mice to Germ Free (GF) wild-type C57Bl/6 recipient mice caused a greater increase in adiposity than that caused by transplantation of a microbiota from lean counterparts, which directly demonstrated that the gut microbiota could modulate obesity.[23,26] In humans, the picture is somewhat more unclear where bacteroidetes-related taxa have been reported to increase, remain neutral, or decrease after weight loss.[27–30] It should be noted that rather than using 16S rRNA for enumeration of metagenomic techniques, these studies assessed specific taxa using probes, which raises the question of how much impact the differences in methodology/probe can have on the patterns observed. In another study, using full cloning and full length sequencing of 18,348 16S rRNA clones obtained from 12 obese subjects who were randomly assigned to either carbohydrate restricted or fat-restricted diets, it was observed that bacteroidetes bacteria positively correlated with reductions in host weight.[31] A subsequent larger human study examining the microbiome (the complement of genes encoded by the microbiota) associated with obesity in twins concordant for either leanness or obesity confirmed that obesity was associated with reduced levels of bacteroidetes, reduced bacterial density with enrichment in carbohydrate and lipid utilizing genes in the microbiome.[25] Thus, this study supports that similar mechanisms affect obesity in both mice and humans and may involve increased microbial metabolism of carbohydrates and lipids. The outcome of few of the metagenomic studies with regard to MetS particularly obesity have been given in Table 1.
Table 1.
Human metagenomic studies of gut microbial ecology in relation to metabolic syndrome
Dietary strategies for the prevention and treatment of metabolic syndrome
As already indicated, there is an increasing demand for alternative therapies particularly diet-based interventions. Recently, there has been a surmounting interest in the use of food supplements containing probiotics and prebiotics for their suggestive role in the control and management of MetS including obesity. The putative mechanisms involved during these dietary interventions [Figure 2] were studied by several groups of investigators and are reviewed as follows.
Figure 2.
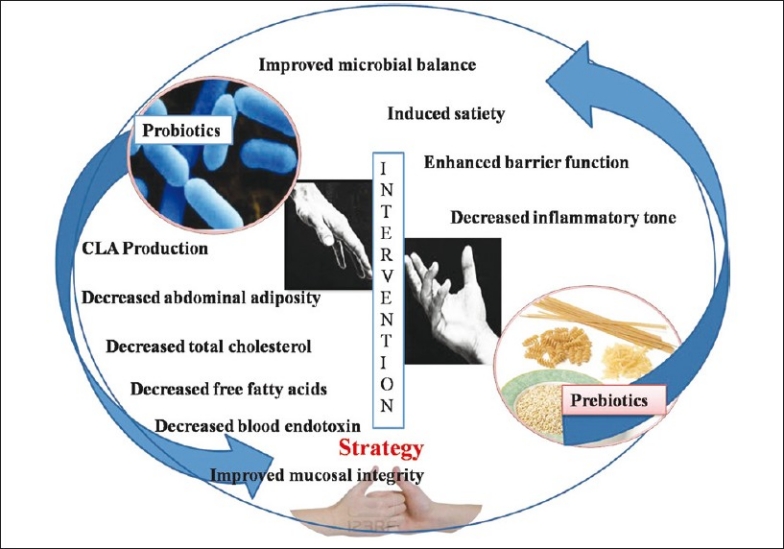
Probiotic and prebiotic intervention strategy for the management of metabolic syndrome
PROBIOTIC INTERVENTIONS
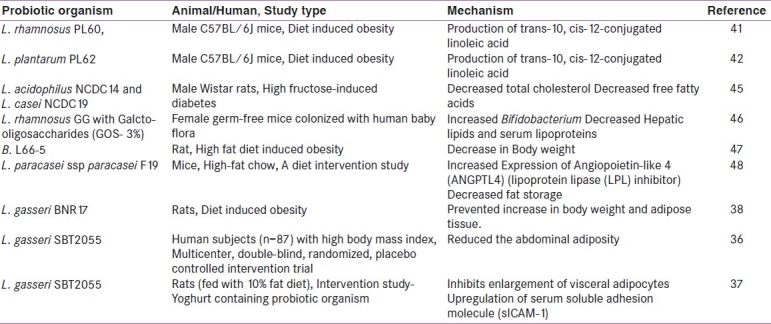
The application of probiotics as prospective biotherapies in the management of metabolic disorders including obesity and diabetes has been explored in some studies conducted by some workers. A new study by Danisco indicates that probiotic strain Bifidobacterium (B.) animalis subsp. lactis 420 (B420) could significantly improve the MetS by counteracting the adverse effects of a high-fat diet (http://www.danisco.com/wps/wcm/connec). The outcome of this study revealed that, the probiotic treatment led to significant reduction in tissue inflammation and metabolic endotoxaemia. A different but related multicenter, double-blind, randomized placebo-controlled intervention trial was conducted on 87 subjects with high body mass index who were randomly assigned to receive Lactobacillus (L.) gasseri SBT 2055 (LG2055).[36] In this study, the probiotic LG2055 was provided as an adjunct culture in yoghurt that had been fermented using conventional yoghurt cultures, Streptococcus thermophilus and L. delbrueckii ssp. bulgaricus; yoghurt without LG2055 was used as placebo. The outcome of this study concludes that the probiotic strain significantly reduced the abdominal adiposity, body weight and other measures suggesting its beneficial influence on metabolic disorders. In a subsequent study, the same research workers used visceral adiposity as a measure of obesity, and the level of soluble intercellular adhesion molecule-1 (sICAM-1) in the blood as an inflammatory marker that is elevated in obesity.[37] The results of the study showed that the probiotic strain inhibited the enlargement of visceral adipocytes and prevented up regulation of sICAM-1. In another study, oral administration of L. gasseri BNR17 prevented increases in body weight and adipose tissue in diet-induced overweight rats.[38] The supplementation of probiotic B. breve strain B-3 in a mouse model with obesity induced by high fat diet suppressed the accumulation of body weight and epididymal fat.[39] In a previous study, the association of CLA with decreased body fat and increased lean body mass was also been established.[40] In this context, the anti-obesity activity of human derived L. rhamnosus PL60, L. plantarum PL62 producing trans-10, cis-12-CLA was also investigated.[41,42] After 8 weeks of feeding, L. rhamnosus PL60 reduced body weight without reducing energy intake and caused a significant, specific reduction of white adipose tissue. Thus, the amount of CLA produced by L. rhamnosus PL60 was adequate to produce an anti-obesity effect.[41] Recombinant L. paracasei NFBC 338 (Lb338) with CLA expressing CLA isomerase from Propionibacterium acnes showed 4-fold increase in trans-10, cis-12 CLA in adipose tissues of the mice when compared with mice that received the isogenic non-CLA-producing strain.[43] These data demonstrated that a single gene (encoding CLA isomerase) expressed in an intestinal microbe can improve the lipid profile of the host. The supplementation of polyphenols with low amounts of probiotics in diet has also been proposed recently for weight loss .[44] These dietary strategies with probiotics, CLAs, and polyphenols could have relevant implication in planning a successful dietary regimen and/or neutraceutical/pharmaceutical preparations for achieving and maintaining a normal body weight in obese individuals [Table 2].
Table 2.
Assessing functional efficiency of probiotic intervention in the management of metabolic disorder in animal and human subjects
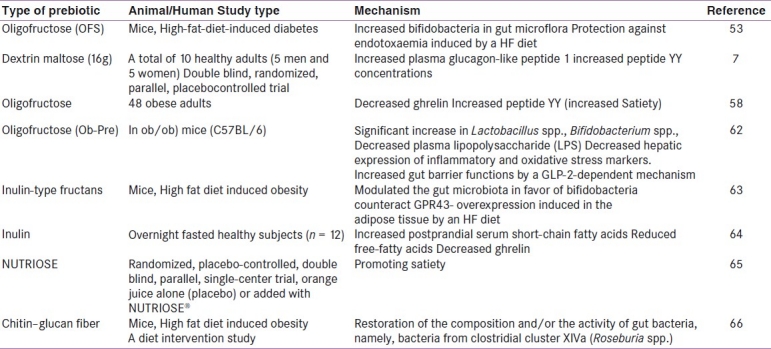
PREBIOTIC INTERVENTION
The putative role of prebiotics in the management of metabolic disorders has also been studied by different investigators. The scientific literature documents several favorable putative effects of prebiotics on food intake, body weight, glucose homeostasis, plasma lipid profile, and associated risk factors for cardiovascular disease.[49] To support this hypothesis several mechanisms have been proposed: First, the modulation of gut flora microbiota supporting beneficial organisms.[20,31] Second, induce enteroendocrine L cell proliferation and modulate gut peptide production and secretion (i.e., glucagon-like peptide-1 [GLP-1], peptide-YY, and ghrelin).[7,49,50] Third, modulate inflammation in obese individuals.[5]
Modulation of gut microbiota
This hypothesis was supported by increase in bifidobacterial counts after dietary supplementation of prebiotic dietary fibres (oligofructose, OFS) in high-fat fed mice. The increase in number of Bifidobacterium spp. was significantly and positively correlated with improved glucose-tolerance, glucose-induced insulin-secretion, and normalized low-grade inflammation (decreased endotoxemia, plasma, and adipose tissue proinflammatory cytokines).[51–53] Recently, the ability of chitin–glucan (CG) from a fungal source to modulate both the gut microbiota and glucose and lipid metabolism in high-fat (HF) diet-induced obese mice have been studied. The supplementation of the HF diet with fungal CG (10% w/w) restored the number of bacteria from clostridial cluster XIVa including Roseburia spp., which were decreased due to HF feeding. Furthermore, CG treatment significantly decreased HF induced body weight gain, fat mass development, fasting hyperglycemia, glucose intolerance, hepatic triglyceride accumulation and hypercholesterolemia, independently of the caloric intake.[54]
Enteroendocrine L cell proliferation and modulation of gut peptide production
Recent studies have demonstrated that the distributions of free fatty acid receptor 2 (FFA2) positive, glucagon-like peptide-1 (GLP-1) containing enteroendocrine L-cells in human and rats were almost consistent. The supplementation of fructo-oligosaccharides (FOS) increased the densities of FFA2 – positive enteroendocrine L-cells over control in rats.[50] This involves the fermentation of prebiotics by selective bacterial strains that increases the production of short-chain fatty acids in the gut lumen.[55] These short-chain fatty acids stimulate intestinal proglucagon (precursor for GLP-1) mRNA expression and peptide-YY (PYY) secretion in rats.[56,57] The short chain fatty acids (SCFAs) have recently been demonstrated to act as ligands for several G-protein-coupled receptors (GPCRs): FFA2 and FFA3 of enteroendocrine L cells and increases both the FFA2-positive enteroendocrine L-cells proliferation with FFA2 activation, which might be an important trigger to produce and release GLP-1 and PYY by enteroendocrine cells in the lower intestine.[58,50] The released GLP-1 and PYY cross the blood–brain barrier and associated with neural activation in areas of the hypothalamus and prefrontal cortex that are involved in the regulation of feeding behavior.[59]
Modulate low grade inflammation
Recent data suggest the role of other important gut peptide, glucagon-like peptide-2(GLP-2) in the regulation of gut permeability which could affect the plasma levels of microbial components that increase the inflammatory tone. The increased endogenous GLP-2 production was associated with improved mucosal barrier function via the restoration of tight junction protein expression and distribution. The role of GLP-2 in the protective effects of prebiotics was established recently.[7] Pharmacological inhibition of GLP-2 signaling receptor abolished the effects of prebiotics, thus, established a direct link between the GLP-2 and gut permeability.[7] Hence, without a functional GLP-2 receptor, the prebiotic treatment failed to reduce metabolic endotoxaemia, hepatic inflammation, and oxidative stress markers. Collectively, these data support the concept that specific changes in the gut microbiota improve gut permeability and inflammatory tone via a GLP-2-dependent mechanism. Amongst the potential mechanisms involved, the gut microbial compositional change by prebiotics controls and increases endogenous production of the intestinotrophic proglucagon derived peptide GLP-2 not only in the colon but also in the jejunum and consequently improves gut barrier functions.[7] In addition, SCFA from prebiotic fermentation down regulates inflammatory markers of insulin resistance by modulating gut hormone secretion was also studied [Table 3].[60,61]
Table 3.
Assessing functional efficiency of prebiotic intervention in the management of metabolic disorder in animal and human subjects
CONCLUSION
Combined data established the interrelationship between diet, gut microbiota, and metabolic syndrome. Unraveled mechanisms of probiotic and prebiotics action provided strong scientific base for developing dietary intervention strategies. However, more in depth studies related to their efficacy and effectiveness are required to be carried in human subjects to make these therapies more competitive in global functional food market.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Hays NP, Galassetti PR, Coker RH. Prevention and treatment of type 2 diabetes: Current role of lifestyle, natural product, and pharmacological interventions. Pharmacol Ther. 2008;118:181–91. doi: 10.1016/j.pharmthera.2008.02.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.FAO/WHO. Working Group Report on Drafting Guidelines for the Evaluation of Probiotics in Food London, Ontario, Canada. 2002 [Google Scholar]
- 3.Reid G, Sanders ME, Gaskins HR, Gibson GR, Mercenier A, Rastall A, et al. New scientific paradigms for probiotics and prebiotics. J Clin Gastroenterol. 2003;37:105–18. doi: 10.1097/00004836-200308000-00004. [DOI] [PubMed] [Google Scholar]
- 4.Sanz Y, Nadal I, Sanchez E. Probiotics as drugs against human gastrointestinal infections. Recent Pat Antiinfect Drug Discov. 2007;2:148–56. doi: 10.2174/157489107780832596. [DOI] [PubMed] [Google Scholar]
- 5.Furet JP, Kong LC, Tap J, Poitou C, Basdevant A, Bouillot JL, et al. Differential adaptation of human gut microbiota to bariatric surgery-induced weight loss: Links with metabolic and low-grade inflammation markers. Diabetes. 2010;59:3049–57. doi: 10.2337/db10-0253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Cani PD, Bibiloni R, Knauf C, Waget A, Neyrinck AM, Delzenne NM, et al. Changes in gut microbiota control metabolic endotoxemia-induced inflammation in high-fat diet-induced obesity and diabetes in mice. Diabetes. 2008;57:1470–81. doi: 10.2337/db07-1403. [DOI] [PubMed] [Google Scholar]
- 7.Cani PD, Lecourt E, Dewulf EM, Sohet FM, Pachikian BD, Naslain D, et al. Gut microbiota fermentation of prebiotics increases satietogenic and incretin gut peptide production with consequences for appetite sensation and glucose response after a meal. Am J Clin Nutr. 2009;90:1236–43. doi: 10.3945/ajcn.2009.28095. [DOI] [PubMed] [Google Scholar]
- 8.Delzenne NM, Cani PD, Neyrinck AM. Modulation of glucagon-like peptide 1 and energy metabolism by inulin and oligofructose: Experimental data. J Nutr. 2007;137:2547S–51S. doi: 10.1093/jn/137.11.2547S. [DOI] [PubMed] [Google Scholar]
- 9.Cani PD, Dewever C, Delzenne NM. Inulin-type fructans modulate gastrointestinal peptides involved in appetite regulation (glucagon-like peptide-1 and ghrelin) in rats. Br J Nutr. 2004;92:521–5. doi: 10.1079/bjn20041225. [DOI] [PubMed] [Google Scholar]
- 10.Cani PD, Daubioul CA, Reusens B, Remacle C, Catillon G, Delzenne NM. Involvement of endogenous glucagon-like peptide-1(7–36) amide on glycaemia-lowering effect of oligofructose in streptozotocin-treated rats. J Endocrinol. 2005;185:457–65. doi: 10.1677/joe.1.06100. [DOI] [PubMed] [Google Scholar]
- 11.Cani PD, Neyrinck AM, Maton N, Delzenne NM. Oligofructose promotes satiety in rats fed a high-fat diet: Involvement of glucagon-like Peptide-1. Obes Res. 2005;13:1000–7. doi: 10.1038/oby.2005.117. [DOI] [PubMed] [Google Scholar]
- 12.Cani PD, Knauf C, Iglesias MA, Drucker DJ, Delzenne NM, Burcelin R. Improvement of glucose tolerance and hepatic insulin sensitivity by oligofructose requires a functional glucagon-like peptide-1 receptor. Diabetes. 2006;55:1484–90. doi: 10.2337/db05-1360. [DOI] [PubMed] [Google Scholar]
- 13.Cani PD, Joly E, Horsmans Y, Delzenne NM. Oligofructose promotes satiety in healthy human: A pilot study. Eur J Clin Nutr. 2006;60:567–72. doi: 10.1038/sj.ejcn.1602350. [DOI] [PubMed] [Google Scholar]
- 14.Burdge GC, Lillycrop KA, Phillips ES, Slater-Jefferies JL, Jackson AA, Hanson MA. Folic acid supplementation during the juvenile-pubertal period in rats modifies the phenotype and epigenotype induced by prenatal nutrition. J Nutr. 2009;139:1054–60. doi: 10.3945/jn.109.104653. [DOI] [PubMed] [Google Scholar]
- 15.Nicholson JK, Holmes E, Wilson ID. Gut microorganisms, mammalian metabolism and personalized healthcare. Nat Rev Microbiol. 2005;3:431–8. doi: 10.1038/nrmicro1152. [DOI] [PubMed] [Google Scholar]
- 16.Neish AS. Microbes in gastrointestinal health and disease. Gastroenterology. 2009;136:65–80. doi: 10.1053/j.gastro.2008.10.080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Qin J, Li R, Raes J, Arumugam M, Burgdorf KS, Manichanh C, et al. A human gut microbial gene catalogue established by metagenomic sequencing. Nature. 2010;464:59–65. doi: 10.1038/nature08821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Eckburg PB, Bik EM, Bernstein CN, Purdom E, Dethlefsen L, Sargent M, et al. Diversity of the human intestinal microbial flora. Sci. 2005;308:1635–8. doi: 10.1126/science.1110591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ley RE, Backhed F, Turnbaugh P, Lozupone CA, Knight RD, Gordon JI. Obesity alters gut microbial ecology. Proc Natl Acad Sci USA. 2005;102:11070–5. doi: 10.1073/pnas.0504978102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Roberfroid M, Gibson GR, Hoyles L, McCartney AL, Rastall R, Rowland I. Prebiotic effects: Metabolic and health benefits. Br J Nutr. 2010;104:S1–63. doi: 10.1017/S0007114510003363. [DOI] [PubMed] [Google Scholar]
- 21.Mozes S, Bujnakova D, Sefcikova Z, Kmet V. Developmental changes of gut microflora and enzyme activity in rat pups exposed to fat-rich diet. Obesity. 2008;16:2610–5. doi: 10.1038/oby.2008.435. [DOI] [PubMed] [Google Scholar]
- 22.Mozes S, Bujnakova D, Sefcikova Z, Kmet V. Intestinal microflora and obesity in rats. Folia Microbiologica. 2008;53:225–8. doi: 10.1007/s12223-008-0031-0. [DOI] [PubMed] [Google Scholar]
- 23.Turnbaugh PJ, Backhed F, Fulton L, Gordon JI. Diet-induced obesity is linked to marked but reversible alterations in the mouse distal gut microbiome. Cell Host Microbe. 2008;3:213–23. doi: 10.1016/j.chom.2008.02.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Hildebrandt MA, Hoffman C, Sherrill-Mix SA, Keilbaugh SA, Hamady M, Chen YY, et al. High-Fat diet determines the composition of the Murine gut microbiome Independently of obesity. Gastroenterology. 2009;312:1355–9. doi: 10.1053/j.gastro.2009.08.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Turnbaugh PJ, Hamady M, Yatsunenko T, Cantarel BL, Duncan A, Ley RE, et al. A core gut microbiome in obese and lean twins. Nature. 2009;457:480–4. doi: 10.1038/nature07540. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Turnbaugh PJ, Ley RE, Mahowald MA, Magrini V, Mardis ER, Gordon JI. An obesity-associated gut microbiome with increased capacity for energy harvest. Nature. 2006;444:1027–31. doi: 10.1038/nature05414. [DOI] [PubMed] [Google Scholar]
- 27.Nadal I, Santacruz A, Marcos A, Warnberg J, Garagorri M, Moreno L, et al. Shifts in clostridia, bacteroides and immunoglobulin-coating fecal bacteria associated with weight loss in obese adolescents. Int J Obesity. 2009;33:758–67. doi: 10.1038/ijo.2008.260. [DOI] [PubMed] [Google Scholar]
- 28.Duncan SH, Lobley GE, Holtrop G, Ince J, Johnstone AM, Louis P, et al. Human colonic microbiota associated with diet, obesity and weight loss. Int J Obesity. 2008;32:1720–4. doi: 10.1038/ijo.2008.155. [DOI] [PubMed] [Google Scholar]
- 29.Santacruz A, Marcos A, Warnberg J, Marti A, Martin-Matillas M, Campoy C, et al. Interplay between weight loss and gut microbiota composition in overweight adolescents. Obesity. 2009;17:1906–15. doi: 10.1038/oby.2009.112. [DOI] [PubMed] [Google Scholar]
- 30.Collado MC, Isolauri E, Laitinen K, Salminen S. Distinct composition of gut microbiota during pregnancy in overweight and normal-weight women. Am J Clin Nutr. 2008;88:894–9. doi: 10.1093/ajcn/88.4.894. [DOI] [PubMed] [Google Scholar]
- 31.Ley RE, Turnbaugh PJ, Klein S, Gordon JI. Microbial ecology: Human gut microbes associated with obesity. Nature. 2006;444:1022–3. doi: 10.1038/4441022a. [DOI] [PubMed] [Google Scholar]
- 32.Kalliomaki M, Collado C, Salminen S, Isolauri E. Early differences in fecal microbiota composition in children may predict overweight. Am J Clin Nutr. 2008;87:534–8. doi: 10.1093/ajcn/87.3.534. [DOI] [PubMed] [Google Scholar]
- 33.Zhang H, DiBaise J, Zuccolo A, Kudrna D, Braidotti M, Yu Y, et al. Human gut microbiota in obesity and after gastric bypass. Proc Natl Acad Sci USA. 2009;106:2365–70. doi: 10.1073/pnas.0812600106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schwiertz A, Taras D, Schafer K, Beijer S, Bos NA, Donus C, et al. Microbiota and SCFA in lean and overweight healthy subjects. Obesity (Silver Spring) 2010;18:190–5. doi: 10.1038/oby.2009.167. [DOI] [PubMed] [Google Scholar]
- 35.Larsen N, Vogensen FK, van den Berg FW, Nielsen DS, Andreasen AS, Pedersen BK, et al. Gut microbiota in human adults with type 2 diabetes differs from non-diabetic adults. PLoS One. 2010;5:e9085. doi: 10.1371/journal.pone.0009085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Kadooka Y, Sato M, Imaizumi K, Ogawa A, Ikuyama K, Akai Y, et al. Regulation of abdominal adiposity by probiotics (Lactobacillus gasseri SBT2055) in adults with obese tendencies in a randomized controlled trial. Eur J Clin Nutr. 2010;64:636–43. doi: 10.1038/ejcn.2010.19. [DOI] [PubMed] [Google Scholar]
- 37.Kadooka Y, Ogawa A, Ikuyama K, Akai Y, Sato M. The probiotic Lactobacillus gasseri SBT2055 inhibits enlargement of visceral adipocytes and upregulation of serum soluble adhesion molecule (sICAM-1) in rats. Int Dairy J. 2011;30:1–5. [Google Scholar]
- 38.Kang JH, Yun SI, Park HO. Effects of Lactobacillus gasseri BNR17 on body weight and adipose tissue mass in diet-induced overweight rats. J Microbiol. 2010;48:712–4. doi: 10.1007/s12275-010-0363-8. [DOI] [PubMed] [Google Scholar]
- 39.Kondo S, Xiao JZ, Satoh T, Odamaki T, Takahashi S, Sugahara H, et al. Antiobesity effects of Bifidobacterium breve strain B-3 supplementation in a mouse model with high-fat diet-induced obesity. Biosci Biotechnol Biochem. 2010;74:1656–61. doi: 10.1271/bbb.100267. [DOI] [PubMed] [Google Scholar]
- 40.Park Y, Storkson JM, Albright KJ, Liu W, Pariza MW. Evidence that the trans-10, cis-12 isomer of conjugated linoleic acid induces body composition changes in mice. Lipids. 1999;34:235–41. doi: 10.1007/s11745-999-0358-8. [DOI] [PubMed] [Google Scholar]
- 41.Lee HY, Park JH, Seok SH, Baek MW, Kim DJ, Lee KE, et al. Human originated bacteria, Lactobacillus rhamnosus PL60, produce conjugated linoleic acid and show anti-obesity effects in diet-induced obese mice. Biochim Biophys Acta. 2006;1761:736–44. doi: 10.1016/j.bbalip.2006.05.007. [DOI] [PubMed] [Google Scholar]
- 42.Lee K, Paek K, Lee HY, Park JH, Lee Y. Antiobestity effect of trans-10, cis-12-conjugated linoleic acid-producing Lactobacillus plantarum PL62 on diet-induced obese mice. J Appl Microbiol. 2007;103:1140–6. doi: 10.1111/j.1365-2672.2007.03336.x. [DOI] [PubMed] [Google Scholar]
- 43.Rosberg-Cody E, Stanton C, O’Mahony L, Wall R, Shanahan F, Quigley E, et al. Recombinant lactobacilli expressing linoleic acid isomerase can modulate the fatty acid composition of host adipose tissue in mice. Microbiology. 2011;157:609–15. doi: 10.1099/mic.0.043406-0. [DOI] [PubMed] [Google Scholar]
- 44.Rastmaneh R. High polyphenol, low probiotic diet for weight loss because of intestinal microbiota interaction. Chem Biol Interact. 2011;189:1–8. doi: 10.1016/j.cbi.2010.10.002. [DOI] [PubMed] [Google Scholar]
- 45.Yadav H, Jain S, Sinha PR. The effect of probiotic Dahi Containing Lactobacillus acidophilus and Lactobacillus casei on gastropathic consequences in diabetic rats. J Med Food. 2008;11:62–8. doi: 10.1089/jmf.2006.136. [DOI] [PubMed] [Google Scholar]
- 46.Martin FP, Wang Y, Sprenger N, Yap IK, Lundstedt T, Lek P, et al. Probiotic modulation of symbiotic gut microbial-host metabolic interactions in a humanized microbiome mouse model. Mol Syst Biol. 2008;4:157. doi: 10.1038/msb4100190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Yin YN, Yu QF, Fu N, Liu XW, Lu FG. Effects of four Bifidobacteria on obesity in high-fat diet induced rats. World J Gastroenterol. 2010;16:3394–401. doi: 10.3748/wjg.v16.i27.3394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Aronsson L, Huang Y, Parini P, Korach-Andre M, Hakansson J, Gustafsson JA, et al. Decreased fat storage by Lactobacillus paracasei is associatedwith increased levels of angiopoietin-like 4 protein (ANGPTL4) PLoS One. 2010;5:e13087. doi: 10.1371/journal.pone.0013087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Piche T, des Varannes SB, Sacher-Huvelin S, Holst JJ, Cuber JC, Galmiche JP. Colonic fermentation influences lower esophageal sphincter function in gastroesophageal reflux disease. Gastroenterology. 2003;124:894–902. doi: 10.1053/gast.2003.50159. [DOI] [PubMed] [Google Scholar]
- 50.Kaji I, Karaki S, Tanaka R, Kuwahara A. Density distribution of free fatty acid receptor 2 (FFA2)- expressing and GLP-1-producing enteroendocrine L cells in human and rat lower intestine, and increased cell numbers after ingestion of fructo-oligosaccharide. J Mol Hist. 2011;42:27–38. doi: 10.1007/s10735-010-9304-4. [DOI] [PubMed] [Google Scholar]
- 51.Cani PD, Amar J, Iglesias MA, Poggi M, Knauf C, Bastelica D, et al. Metabolic endotoxemia initiates obesity and insulin resistance. Diabetes. 2007;56:1761–72. doi: 10.2337/db06-1491. [DOI] [PubMed] [Google Scholar]
- 52.Cani PD, Hoste S, Guiot Y, Delzenne NM. Dietary non-digestible carbohydrates promote L-cell differentiation in the proximal colon of rats. Br J Nutr. 2007;98:32–37. doi: 10.1017/S0007114507691648. [DOI] [PubMed] [Google Scholar]
- 53.Cani PD, Neyrinck AM, Fava F, Knauf C, Burcelin RG, Tuohy KM, et al. Selective increases of bifidobacteria in gut microflora improve high-fat-diet-induced diabetes in mice through a mechanism associated with endotoxaemia. Diabetologia. 2007;50:2374–83. doi: 10.1007/s00125-007-0791-0. [DOI] [PubMed] [Google Scholar]
- 54.Neyrincka AM, Possemiersb S, Verstraeteb W, Backera FD, Cani PD, Delzennea NM. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin–glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2010.10.008. in press. [DOI] [PubMed] [Google Scholar]
- 55.Pylkas AM, Juneja LR, Slavin JL. Comparison of different fibers for in vitro production of short chain fatty acids by intestinal microflora. J Med Food. 2005;8:113–6. doi: 10.1089/jmf.2005.8.113. [DOI] [PubMed] [Google Scholar]
- 56.Reimer RA, McBurney MI. Dietary fiber modulates intestinal proglucagon messenger ribonucleic acid and postprandial secretion of glucagon- like peptide-1 and insulin in rats. Endocrinology. 1996;137:3948–56. doi: 10.1210/endo.137.9.8756571. [DOI] [PubMed] [Google Scholar]
- 57.Dumoulin V, Moro F, Barcelo A, Dakka T, Cuber JC, Peptide YY. Glucagon-like peptide-1, and neurotensin responses to luminal factors in the isolated vascularly perfused rat ileum. Endocrinology. 1998;139:3780–6. doi: 10.1210/endo.139.9.6202. [DOI] [PubMed] [Google Scholar]
- 58.Parnell JA, Reimer RA. Weight loss during oligofructose supplementation is associated with decreased ghrelin and increased peptide YY in overweight and obese adults. Am J Clin Nutr. 2009;89:1751–9. doi: 10.3945/ajcn.2009.27465. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Kastin A, Akerstrom V, Pan W. Interactions of glucagon-like peptide-1(GLP-1) with the blood-brain barrier. J Mol Neurosci. 2002;18:7–14. doi: 10.1385/JMN:18:1-2:07. [DOI] [PubMed] [Google Scholar]
- 60.Freeland KR, Wolever TM. Acute effects of intravenous and rectal acetate on glucagon-like peptide-1, peptide YY, ghrelin, adiponectin and tumour necrosis factor-alpha. Br J Nutr. 2010;103:460–6. doi: 10.1017/S0007114509991863. [DOI] [PubMed] [Google Scholar]
- 61.Freeland KR, Wilson C, Wolever TM. Adaptation of colonic fermentation and glucagon-like peptide-1 secretion with increased wheat fibre intake for 1 year in hyperinsulinaemic human subjects. Br J Nutr. 2010;103:82–90. doi: 10.1017/S0007114509991462. [DOI] [PubMed] [Google Scholar]
- 62.Cani PD, Possemiers S, van de WT, Guiot Y, Everard A, Rottier O, et al. Changes in gut microbiota control inflammation in obesemice through amechanism involving GLP-2-driven improvement of gut permeability. Gut. 2009;58:1091–03. doi: 10.1136/gut.2008.165886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Dewulf EM, Cani PD, Neyrinck AM, Possemiers S, Van Holle A, Muccioli GG, et al. Inulin-type fructans with prebiotic properties counteract GPR43 overexpression and PPARgamma-related adipogenesis in the white adipose tissue of high-fat diet-fed mice. J Nutr Biochem. 2011;22:712–22. doi: 10.1016/j.jnutbio.2010.05.009. [DOI] [PubMed] [Google Scholar]
- 64.Tarini J, Wolever TM. The fermentable fibre inulin increases postprandial serum short-chain fatty acids and reduces free-fatty acids and ghrelin in healthy subjects. Appl Physiol Nutr Metab. 2010;35:9–16. doi: 10.1139/H09-119. [DOI] [PubMed] [Google Scholar]
- 65.Lefranc-Millot C, Guérin-Deremaux L, Wils D, Pochat M, Li S. Effects of a soluble dietary fiber supplementation with NUTRIOSE® on risk factors of the metabolic syndrome in Chinese male adults. Obesity Rev. 2010;11:438a.s. [Google Scholar]
- 66.Neyrincka AM, Possemiersb S, Verstraeteb W, Backera FD, Cani PD, Delzennea NM. Dietary modulation of clostridial cluster XIVa gut bacteria (Roseburia spp.) by chitin–glucan fiber improves host metabolic alterations induced by high-fat diet in mice. J Nutr Biochem. 2011 doi: 10.1016/j.jnutbio.2010.10.008. [In Press] [DOI] [PubMed] [Google Scholar]


