Abstract
Aim:
The present study was aimed to assess the early effects of ischemia/reperfusion injury on the oxidants and anti-oxidant defense status in rat testicular tissue by measuring MDA, glucose-6-phosphte dehydrogenase activity and reduced glutathione levels in a designated time frame sequel to reperfusion. Animals were divided randomly into six groups (12 animals per group) in the following order: Group I: Sham-operated control group (Cso) without the application of the torsion. Group 2: Torsion-induced ischemia group (T30 m): Ischemia was induced through the torsion of spermatic cord for a period of 30 min. Group 3: One hour reperfusion group after detorsion (T30 mR1 h). Group 4: Twenty-four hour reperfusion group after detorsion (T30 mR24 h). Group 5: Forty-eight hours reperfusion group after detorsion (T30mR48h). Group 6: One week reperfusion group after detorsion (T30mR1wk).
Results and Discussion:
The oxidant-antioxidant system of the testicular tissue is altered during torsion as well as detorsion which results in the altered activities involved in the key enzyme of hexose monophosphate shunt pathway, glucose 6 phosphate dehydrogenase activity along with a reduction of glutathione (G.SH) content. The increase in G6PDH activity during torsion and followed by an increase in detorsion indicates the tissue's response to counter the oxidant stress caused by reduced blood supply. Continued exposure to such oxidant stressed physiological state of a tissue may lead to decreased capacity of the tissue to perform its physiological function such as testicular steroidogenesis and spermiogenesis shown in the present study.
Keywords: Torsion, detorsion, oxidant and antioxidant defense system
INTRODUCTION
The sperm cell membrane requires various testicular biosynthetic processes and passage through the epididymidis for its maturation. Both germ cell and spermatozoa undergoing epididymal transit have various enzymatic and nonenzymatic scavengers systems to prevent damage from lipid peroxidation. These include catalase, superoxide dismutase (SOD), glutathione-dependent oxidoreductases, glucose-6-phosphate dehydrogenase (G6PDH), which are present in different amounts depending upon the stage of development. GSH is one of the scavengers, which helps restore the cell membrane's physiological PUFA concentration. Albeit the antioxidant protection afforded to the testes in order to support its dual functions of steroidogenesis and sperm production, a wide variety of endogenous and exogenous factors are known to impair/perturb these defenses and give rise to a state of oxidative stress. According to one of the hypothesis, ischemia/reperfusion insult is implicated in the impairment of spermatogenesis which is considered to be caused by the accumulation of reactive oxygen species (ROS) in conditions such as cryptorchidism, testicular torsion, and varicocele. Testicular torsion is a relatively common, painful condition that must be treated rapidly if the testes are not to suffer permanent damage. Since both spermatogenesis[1] and Leydig cell steroidogenesis[2,3] are vulnerable to oxidative stress, notwithstanding the low oxygen tension due to poor vascularization that characterizes this tissue.[4] The testis remains vulnerable to oxidative stress mainly due to the abundance of highly unsaturated fatty acids, 20:4 and 22:6, in particular.
Not many investigations have been carried out during the early phase of torsion/detorsion and at the same time, the literature is sparse about the early molecular changes occurring in testicular torsion/detorsion-induced ischemia/reperfusion injury (IR). It was therefore worthwhile to assess the early effects of testicular torsion and detorsion on the oxidants and antioxidant status in an experimental animal model of rats. The investigations such as malondialdehyde content for lipid peroxidation activity, reduced glutathione, and G6PDH activity as a component of the antioxidant defense system were carried out in testicular tissue of experimental animals.
MATERIALS AND METHODS
Seventy-two male Sprague-Dawley rats were obtained from the Central Animal House of AL-Arab Medical University, Benghazi, Libya. All animals weighed between 250 and 270 g. The animals were housed in cages in groups of three in each colony. The colonies were maintained at room temperature and on a 12-h light/dark cycle. All the animals had a free access to food and water. Animals were divided randomly into six groups (12 animals per group) in the following order: Group I: Sham-operated control group (Cso) without the application of the torsion. Group 2: Torsion-induced ischemia group (T30 m): Ischemia was induced through the torsion of spermatic cord for a period of 30 min. Group 3: One hour reperfusion group after detorsion (T30 m R 1 h). Group 4: Twenty-four hour reperfusion group after detorsion (T30 m R24 h). Group 5: Forty-eight hours reperfusion group after detorsion (T30m R48h). Group 6: One week reperfusion group after detorsion (T30m R1wk).
Experimental testicular torsion
Adult male rats were anesthetized using 20 mg/ml of xylazine and 10 mg/ml of ketamine, and the testis was rotated as described.[5] Briefly, the testis was exteriorized through a low midline laparotomy, the gubernaculums was divided and the testis was freed from the epididymo-testicular membrane. The testis was torsioned (720°) and repositioned in the scrotum. The testis was kept wet using sterile normal saline soaked gauze. During the sham or torsion period, the testis remained in the abdomen with a closed incision. At the appropriate time the incision was reopened, the testis was counter-rotated to the natural position, the gubernaculum was rejoined, and the testis was reinserted into the scrotum via the inguinal canal. At the time of repair, testes were examined and scored for apparent degree of ischemia and of reperfusion, respectively. Testes were collected at appropriate specified time intervals under the experimental conditions as above after the repair of torsion on a glass plate resting over crushed ice. The testes were homogenised by taking 1 g of tissue in a volume made to 10 ml of ice-cold 0.15 M KCl (10% w/v; pH adjusted to 7.4) using a Teflon Potter Elvehjem homogenizer.
The homogenate was employed for the estimation of glutathione (GSH),[6] glucose-6-phosphate dehydrogenase (G6PDH);[7,8] lipid peroxidation by thiobarbituric acid reaction (TBAR) method;[9] and total protein.[10] After the surgery the animals were given a lethal dose of barbiturates.
Statistical analysis
Data were analyzed by a commercially available statistical package for social sciences (SPSS) program for windows software. P values <0.05 were regarded as statistically significant. The one-way analysis of variance (ANOVA) test was performed and post hoc multiple comparisons were done with least-squares differences (LSD).
RESULTS
The effects of ischemia/reperfusion on the levels of MDA, the lipid peroxides in testicular tissue are presented in Figure 1 and Table 1.
Figure 1.
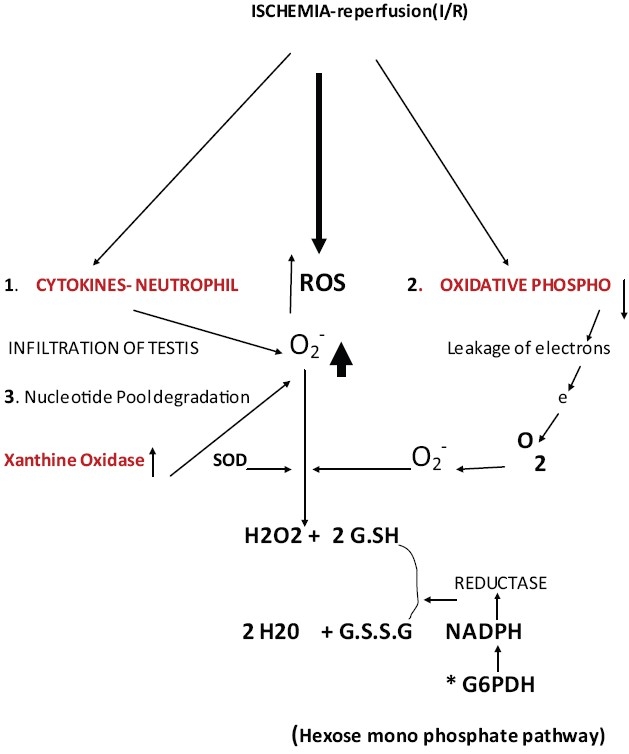
Testis-torsion and detorsion-biochemical changes, O-2 superoxide radical SOD- super oxide dismutase, ROS- reactive oxygen species, 1,2,3- processes that may contribute for an increase in O-2 *6PH-glucose 6 phosphate dehydrogenase
Table 1.
The effect of testicular torsion and detorsion on lipid peroxide concentration expressed as: (a) Micromoles of MDA per mg protein

When expressed on the basis of per milligram of tissue proteins, the data showed a remarkable increase in the level of lipid peroxides in torsed testis compared with the sham operated control group and continued to remain elevated throughout the reperfusion period of designated duration (P<0.5) When the 1 h reperfusion group was compared to the torsed testis group, lipid peroxidation activity showed a significant decline while 48 h and 1 week reperfusion groups exhibited significant increase in MDA content compared to control, torsed, and other reperfusion groups (P<0.05).
The data on the effects of the testicular torsion with or without reperfusion on the activity of glucose-6-phosphate dehydrogenase, when expressed as per milligram of proteins or per gram of fresh tissue, are exhibited in Table 2.
Table 2.
The effect of testicular torsion and detorsion on glucose-6-phosphate dehydrogenase activity (G6PDH) expressed as: (a) international unit per milligram protein

It is evident that ischemia without reperfusion increased the activity of G6PDH (P<0.05) compared to controls. Reperfusion for 1 h declined the activity compared to torsed testis. Upon further reperfusion for 24 h, 48 h and 1 week, G6PDH activity remained statistically unaltered compared to torsed testis except in the 1 week reperfusion group, a significant decline was showed, but the levels remained significantly higher compared to controls. The enzyme activity increased significantly in the trend in 24 h, 48 h, and 1 week reperfusion groups compared to the 1 h reperfusion group (P<0.05).
Table 3 summarize the observations on the effect of testicular torsion/detorsion on the level of reduced glutathione.
Table 3.
The effect of testicular torsion and detorsion on reduced glutathione (GSH) concentration expressed as: (a) milligram per milligram protein

An increase in reduced glutathione level was observed in testis of the torsion-induced ischemic group compared to sham-operated controls (P<0.05). Upon reperfusion for 1 h after a period of 30 min torsion, a decrease was observed in reduced glutathione compared to torsion-induced ischemia (P<0.05) and level returned to normal compared to the sham-operated controls.
When reperfusion extended further for 24 h, 48 h and a maximum period of 1 week, first a decrease (P<0.05) followed by an increase in reduced glutathione levels (P<0.05) were observed, respectively, compared to other reperfused, torsed, and sham-operated control groups. Reperfusion for 1 week produced a significant decline in the glutathione level (P<0.05).
DISCUSSION
Testicular torsion characterized by a twisting of the spermatic cord rendered the testis ischemic for a transient period of 30 min, owing to the obtrusion of blood supply carrying oxygen and the nutrients. A significant increase in oxidative stress was evident in the torsed testis on observing an elevation in the lipid peroxidation activity compared to sham-operated controls [Table 3]. Similar observations have been reported by previous investigators in experimental animals those underwent testicular torsion for extended periods.[11] The ischemic condition would impair the mitochondrial electron transport-oxidative phosphorylation resulting in a severe decline in cellular ATP levels[11] and leaving the mitochondrial carriers in a more fully reduced state. A higher increase of electron leakage from the respiratory chain that, in turn, would react with the residual molecular oxygen entrapped within the inner mitochondrial membrane, thus leading to the formation of superoxide radicals and thereby eventually raising oxidative stress status. The accumulation of hypoxanthine due to increased degradation of the nucleotide pool is yet another potential source of superoxide formation in ischemia/reperfusion (I/R).[12]
Ischemia also triggers the release of cytokines which attract neutrophils infiltration into the testes and may represent yet another good source of uncontrolled free radical generation, ROS for mediating the pathophysiological consequences of temporary testicular ischemia.[13]
The intracellular reduction-oxidation (redox) potential plays an important role in cell survival. The principal intracellular reductant NADPH is mainly produced by the pentose phosphate pathway by G6PDH, the rate-limiting enzyme.[14]
In this study, G6PDH enzyme activity was found to be increased in the ischemic phase of testicular torsion compared to the sham-operated control group [Table 2]. The over expression of G6PDH increased resistance to H2O2 -induced cell death while G6PDH inhibitors potentiated H2O2 -induced cell death.[14]
Ischemia results in the release and increase of cytokines. There is infiltration of neutrophils into the testicular tissue resulting in increased macrophage activity and increased production of super oxide radicals (O2-•).[11]
Ischemia is insufficient blood flow to provide adequate oxygenation. This, in turn, leads to tissue hypoxia (reduced oxygen) or anoxia (absence of oxygen). As blood flow is reduced to an organ, oxygen extraction increases. When the tissue is unable to extract adequate oxygen, the partial pressure of oxygen within the tissue falls (hypoxia) leading to a reduction in mitochondrial respiration and oxidative metabolism. This leads to leakage of electrons from the mitochondria which may generate more (O2-•).[13]
Ischemia could also cause degradation of purine nucleotides with increased activity of xanthine oxidase and generating more (O2-•).[12]
Figure 1 explains the possible biochemical changes observed following ischemia-reperfusion (I/R). Overexpression of G6PDH in endothelial cells demonstrated a significant reduction in ROS accumulation when exposed to hydrogen peroxide, xanthine-xanthine oxidase, or tumor necrosis factor-alpha (TN-α).[15] The regulation of G6PDH gene expression by chronic hypoxia indicated that the G6PDH mRNA level and activity were increased in PC12 cells by hypoxia. These results suggest the dependence of G6PDH gene expression on hypoxia-induced transcription factor 1 (HIF) and intracellular redox status, and the differential hypoxic regulation of glucose-metabolizing enzymes.[16]
It was interesting to observe that the reduced glutathione level, in this study, significantly increased in torsed testis compared to sham-operated controls [Table 1] in an attempt to boost the body defense mechanism against the deleterious ROS. GSH, as a co-substrate of glutathione peroxidase, plays an essential protective role against oxygen free radicals and prevents peroxidation of membrane lipids.[17,18] Overexpressing G6PDH maintained intracellular glutathione stores when exposed to oxidants because of increased activity of glutathione reductase.[15]
It follows that the changes of glutathione status provided important information in the cellular oxidative events and tissue accumulation and/or release of GSSG was a sensitive and accurate index of oxidative stress.[19] Free radicals accumulation in ischemia can cause lipid peroxidation and thus brings about damage in lipid bilayer cellular membranes.
When the testicular tissue was detorsioned after 30 min and reperfused for designated time periods of the experimental conditions, the testis regained the oxygen and nutrients supply. Reperfusion for different time periods of 1 h, 24 h, 48 h and 1 week maintained an increase in MDA levels as compared to sham-operated controls. MDA is one of the most sensitive indicators of lipid peroxidation.[18] MDA levels significantly reduced in 1 h reperfused group compared to the torsed testis group. It supports the contention that with the initial reperfusion of 1 h, the tissue was attempting to revert back to its normalcy. A decline in MDA indicates that some defense mechanism(s) have been induced to combat the mechanisms involved in ROS production.
The levels of MDA were significantly higher in the 24 h reperfusion group with the maximum levels showed in 48 h and 1 week reperfusion groups compared to the torsed testis group. Detorsion increased the MDA level if the initial torsion had been less than 3 h.[20] The levels of NO2 – NO3, malondialdehyde, 8-hydroxy deoxyguanosine, and myeloperoxidase were significantly greater in the ischemia-reperfusion (I-R) group subjected to induction of 30-min ischemia and 60-min reperfusion than in the control.[21] Numerous studies have reported an increase in oxidative stress in the testis after repair of testicular torsion, and all have reported its adverse effects on testicular function, including germ cell loss and disruption of the seminiferous epithelium.[22,23] Thus, testicular torsion, when repaired before infarction and necrosis, causes an IR injury that is a classic inducer of oxidative stress.
The reduced glutathione levels were observed to be decreased in 1 h, 24 h, and in 1 week reperfusion group as compared to torsed and control groups in this study. Normal vascular endothelial cells respond to a modest H2O2 challenge by increasing G6PDH activity to respond to a decrease in cellular GSH levels and enhance glutathione recycling. G6PDH-deficient bovine aorta endothelial cells (BAEC) experience a more pronounced oxidant stress, as shown by the marked increase in cellular ROS accumulation and the corresponding decrease in GSH levels.[24]
Left testicular artery and vein occluded for 1 h, followed by reperfusion of 3 h, 24 h, or 30 days increased the level of reduced glutathione by 24 h of reperfusion, but malondialdehyde remained unchanged.[25] This decrease may possibly be attributed to the leakage due to necrotic cellular damage during oxygen reperfusion injury. Reperfusion after a short period of ischemia normalizes the testicular glutathione status, whereas reperfusion after a prolonged period of ischemia results in a release of GSH from the tissue with a further depauperation of the tissue content.[17]
The reduced availability of cellular GSH becomes a rate limiting factor for detoxification of oxygen metabolites most likely hydrogen peroxide and lipid hydroperoxide. At the same time, an important accumulation and release of oxidized glutathione occurs, causing a further reduction of the GSH/GSSG ratio and a shift of cellular thiol redox state toward oxidation. Even short periods of ischemia, for 3 h or less, can lead to a high levels of oxidative stress in the testes, depletion of testicular glutathione levels and the consequent disruption of spermatogenesis.[26]
Significantly, the level of peroxidative damage observed in testicular tissue increases following detorsion, indicating the induction of reperfusion injury.[27]
Although oxidative stress is clearly a dominant feature in the etiology of male infertility, the underlying causative mechanisms remain unresolved. At the level of the testes, oxidative stress is capable of disrupting the steroidogenic capacity of Leydig cells[28] as well as the capacity of the germinal epithelium to differentiate normal spermatozoa.[29]
CONCLUSION
The increase in MDA levels seen throughout the experimental period indicates the oxidant stress caused by I/R in the testicular tissue. The oxidant-antioxidant system of the testicular tissue is altered during torsion as well as detorsion which results in the altered activities involved in the key enzyme of the hexose monophosphate shunt pathway, glucose-6-phosphate dehyrogenase activity along with a reduction of glutathione (G.SH) content.
The increase in G6PDH activity during torsion and followed by an increase in detorsion indicate the tissue's response to counter the oxidant stress caused by reduced blood supply. The G.SH content which is used to reduce the levels of super oxide radicals appears to be less to counter the increased demands of the tissue under oxidant stress. The reduced form of G.SH may be less than the oxidized form G.S.S.G due probably to reduced levels of NADPH generated by the hexose mono phosphate shunt pathway (HMP) [Figure 1]. The conversion of G.S.S.G to G.SH by aldose reductase enzyme requires NADPH as coenzyme.[13–15] This may be due to faster consumption of G.SH though there is an increased G6PDH activity. Continued exposure to such oxidant stress may lead to decreased capacity of the tissue to perform its physiological function such as testicular steroidogenesis and spermiogenesis shown in this study.
ACKNOWLEDGEMENT
The authors are grateful to the support given by Libyan NASR for the study
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Peltola V, Mäntylä E, Huhtaniemi I, Ahotupa M. Lipid peroxidation and antioxidant enzyme activities in the rat testis after cigarette smoke inhalation or administration of polychlorinated biphenyls or polychlorinated naphthalenes. J Androl. 1994;15:353–61. [PubMed] [Google Scholar]
- 2.Quinn PG, Payne AH. Oxygen-mediated damage of microsomal cytochrome P-450 enzymes in cultured Leydig cells. Role in steroidogenic desensitization. J Biol Chem. 1984;259:4130–5. [PubMed] [Google Scholar]
- 3.Chen H, Liu J, Luo L, Baig MU, Kim JM, Zirkin BR. Vitamin E, aging and Leydig cell steroidogenesis. Exp Gerontol. 2005;40:728–36. doi: 10.1016/j.exger.2005.06.004. [DOI] [PubMed] [Google Scholar]
- 4.Free MJ, Schluntz GA, Jaffe RA. Respiratory gas tensions in tissues and fluids of the male rat reproductive tract. Biol Reprod. 1976;14:481–8. doi: 10.1095/biolreprod14.4.481. [DOI] [PubMed] [Google Scholar]
- 5.Becker EJ, Prillaman HM, Turner TT. Microvascular blood flow is altered after repair of testicular torsion in the rat. J Urol. 1997;157:1493–8. [PubMed] [Google Scholar]
- 6.Beutler E, Duron O, Kelly BM. Improved method for the determination of blood glutathione. J Lab Clin Med. 1963;61:882–8. [PubMed] [Google Scholar]
- 7.Lohr GW, Waller HD. 3rd ed. Wehnheim: Varlag Chemie; 1974. Glucose-6-Phosphate Dehydrogenase. Methods of Enzymatic Analysis; p. 636. [Google Scholar]
- 8.Makarem A. Clinical chemistry principles and techniques. In: Henery RF, Cannon DC, Winkelman JW, editors. 2nd ed. Hagerstown: Harper and Row; 1974. pp. 1128–35. [Google Scholar]
- 9.Ohakawa H, Ohishi N, Yagi K. Assay for lipid peroxidase in animal tissues by thiobarbituricacid reaction. Anal Biochem. 1979;95:351–8. doi: 10.1016/0003-2697(79)90738-3. [DOI] [PubMed] [Google Scholar]
- 10.Lowry OH, Rosebroug NV, Farr AL, Rendall RJ. Protein measurement with the folin phenol reagent. J Biol Chem. 1951;193:256–75. [PubMed] [Google Scholar]
- 11.Guimarães SB, Vasconcelos PR. In vivo acute changes in ATP and glucose concentrations in ipsilateral testicles of prepubertal rats following unilateral torsion. Acta Cir Bras [serial online] 2002;17:105–10. [Google Scholar]
- 12.Unsal A, Eroglu M, Avci A, Cimentepe E, Guven C, Derya Balbay M, et al. Protective role of natural antioxidant supplementation on testicular tissue after testicular torsion and detorsion. Scand J Urol Nephrol. 2006;40:17–22. doi: 10.1080/00365590500407514. [DOI] [PubMed] [Google Scholar]
- 13.Turner TT, Bang HJ, Lysiak JL. The molecular pathology of experimental testicular torsion suggests adjunct therapy to surgical repair. J Urol. 2004;172:2574–8. doi: 10.1097/01.ju.0000144203.30718.19. [DOI] [PubMed] [Google Scholar]
- 14.Tian WN, Braunstein LD, Apse K, Pang J, Rose M, Tian X, et al. Importance of glucose-6-phosphate dehydrogenase activity in cell death. Am J Physiol. 1999;276:C1121–31. doi: 10.1152/ajpcell.1999.276.5.C1121. [DOI] [PubMed] [Google Scholar]
- 15.Leopold JA, Zhang Y-Y, Scribner AW, Stanton RC, Loscalzo J. Glucose-6-phosphate dehydrogenase overexpression decreases endothelial cell oxidant stress and increases bioavailable nitric oxide. Arterioscler Thromb Vas Biol. 2003;23:411–7. doi: 10.1161/01.ATV.0000056744.26901.BA. [DOI] [PubMed] [Google Scholar]
- 16.Gao L, Mejías R, Echevarría M, López-Barneo J. Induction of the glucose-6-phosphate dehydrogenase gene expression by chronic hypoxia in PC12 cells. FEBS Letters. 2004;569:256–60. doi: 10.1016/j.febslet.2004.06.004. [DOI] [PubMed] [Google Scholar]
- 17.Curello S, Ceconi C, Bigoli C, Ferrari R, Albertini A, Guarnieri C. Change in the cardiac glutathione status after ischemia and reperfusion. Experientia. 1985;41:42–3. doi: 10.1007/BF02005863. [DOI] [PubMed] [Google Scholar]
- 18.Gutteridge JM. Lipid peroxidation and antioxidants as biomarkers of tissue damage. Clin Chem. 1995;41:1819–28. [PubMed] [Google Scholar]
- 19.Curello S, Ceconi C, Cargnoni A, Ferrari R, Albertini A. Improved procedure for determining glutathione plasma as an index of myocardial oxidative stress. Clin Chem. 1987;33:1448–9. [PubMed] [Google Scholar]
- 20.Duru FI, Noronha CC, Akinwande AI, Okanlawon AO. Effects of torsion, detorsion and melatonin on testicular malondialdehyde level. West Afr J Med. 2007;26:312–5. doi: 10.4314/wajm.v26i4.28333. [DOI] [PubMed] [Google Scholar]
- 21.Tamamura M, Saito M, Kinoshita Y, Shimizu S, Satoh I, Shomori K, et al. Protective effect of edaravone, a free-radical scavenger, on ischaemia reperfusion injury in the rat testis. BJU Int. 2010;105:870–6. doi: 10.1111/j.1464-410X.2009.08798.x. [DOI] [PubMed] [Google Scholar]
- 22.Rodriguez MG, Rival C, Theas MS, Lustig L. Immunohistopathology of the contralateral testis of rats undergoing experimental torsion of the spermatic cord. Asian J Androl. 2006;8:576–83. doi: 10.1111/j.1745-7262.2006.00146.x. [DOI] [PubMed] [Google Scholar]
- 23.Lysiak JJ, Zheng S, Woodson R, Turner TT. Caspase-9-dependent pathway to murine germ cell apoptosis: Mediation by oxidative stress, BAX, and caspase-2. Cell Tissue Res. 2007;328:411–9. doi: 10.1007/s00441-006-0341-y. [DOI] [PubMed] [Google Scholar]
- 24.Leopold JA, Cap A, Scribner AW, Stanton RC, Loscalzo J. Glucose-6-phosphate dehydrogenase deficiency promotes endothelial oxidant stress and decreases endothelial nitric oxide bioavailability. FASEB J. 2001;15:1771–3. doi: 10.1096/fj.00-0893fje. [DOI] [PubMed] [Google Scholar]
- 25.Heskimoglu A, Kurcer Z, Aral F, Baba F, Sahna E, Atessahin A. Lycopene, an antioxidant carotenoid, attenuates testicular injury caused by ischemia/reperfusion in rats. Tohoku J Exp Med. 2009;218:141–7. doi: 10.1620/tjem.218.141. [DOI] [PubMed] [Google Scholar]
- 26.Aitken RJ, Roman SD. Antioxidant systems and oxidative stress in the testes. Oxid Med Cell Longev. 2008;1:15–24. doi: 10.4161/oxim.1.1.6843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Guimarães SB, Aragão AA, Santos JM, Kimura Ode S, Barbosa PH, Vasconcelos PR. Oxidative stress induced by torsion of the spermatic cord in young rats. Acta Cir Bras. 2007;22:30–3. doi: 10.1590/s0102-86502007000100005. [DOI] [PubMed] [Google Scholar]
- 28.Hales DB, Allen JA, Shankara T, Janus P, Buck S, Diemer T, et al. Mitochondrial function in Leydig cell steroidogenesis. Ann N Y Acad Sci. 2005;1061:120–34. doi: 10.1196/annals.1336.014. [DOI] [PubMed] [Google Scholar]
- 29.Naughton CK, Nangia AK, Agarwal A. Pathophysiology of varicoceles in male infertility. Hum Reprod Update. 2001;7:473–81. doi: 10.1093/humupd/7.5.473. [DOI] [PubMed] [Google Scholar]


