Abstract
Aim:
To study the indications, risk factors, postoperative course, and long-term survival of corneal transplants done for optical purposes.
Design:
Retrospective case series.
Materials and Methods:
Data were obtained by reviewing the records of 181 patients operated at our institute (H.V. Desai Eye Hospital) between October 2005 and October 2007 for optical penetrating keratoplasty. Patients with less than one year of follow up, pediatric cases, therapeutic, tectonic, and lamellar keratoplasties were excluded. Kaplan Meier survival analysis was used to calculate median survival time of grafts and to see correlation between nine variables viz. age, gender, corneal vascularization, previous failed grafts, previous Herpes Simplex keratitis, post-perforation corneal scars, donor tissue quality, graft size, type of surgery and follow-up. These variables were also used for univariate and multivariate analysis using Cox Proportional Hazard Regression Modeling.
Results:
Median survival of the cohort was 27 months (95% confidence interval: 20.47-33.52). One- and two-year survival rates were 65% and 52.5%, respectively. Median survival was significantly lower in poor prognosis cases (14 months) than good prognosis cases (27 months, P = 0.0405). Graft survival was lower in vascularized corneas (18.55 months, P = 0.030) and in post-perforation corneal scars (17.96 months, P = 0.09, borderline significance). Multivariate analysis showed that the same factors were predictive of graft failure.
Conclusion:
Long-term survival of grafts at our center is different from centers in western world. More high-risk cases, paucity of excellent quality donor corneas, and differences in patient profile could be the contributory factors.
Keywords: Kaplan Meier survival analysis, multivariate Cox Proportional Hazard Regression analysis, optical penetrating keratoplasties
Corneal blindness is one of the most challenging public health problems all over the world, especially in developing countries like India, where it is one of the leading causes of visual disability.[1]
Penetrating keratoplasty (P.K.) is the mainstay of surgical treatment of corneal blindness is a well well-established fact. However, the indications and outcome of P.K.s in developed western world and developing countries is completely different.[2,3] We, a tertiary care eye institute in Western India, detail our experience with P.K.s, report our success rate, and attempt to identify factors that influence a successful visual outcome after the surgery.
Materials and Methods
This is a retrospective case series where we obtained the data by reviewing the records of patients operated for optical P.K. 181 patients operated for optical P.K. at our institute between October 2005 and October 2007 and those who completed at least one-year follow-up were included for analysis. In patients who underwent multiple P.K.s, or bilateral P.K.s, (at our institute) only the first graft done at our institute was included for analysis. Patients presenting with failed grafts, who had undergone one or more grafts somewhere else, were included in analysis as previous failed grafts. Exclusion criteria were pediatric cases, therapeutic, tectonic, lamellar grafts, and patients who followed up for less than a year.
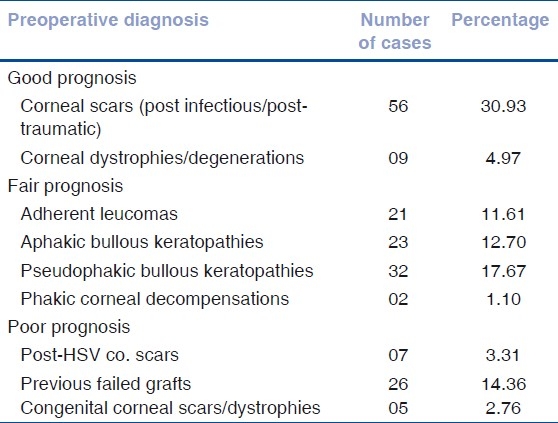
Based on the preoperative diagnosis [Table 1], patients were classified into three categories of graft survival viz. Good prognosis, which included quiet eyes with inactive corneal scars, corneal degenerations, and dystrophies with no corneal vascularization; Fair prognosis, which included patients of aphakic or pseudophakic bullous keratopathy, adherent leucomas, and corneal scars following perforations, descemetoceles. Eyes with up to one or two quadrants of deep stromal vascularization were included. Poor prognosis category consisted of congenital corneal scars, preexisting glaucoma, unstable ocular surface, postchemical injury corneal scars, post- Herpes Simplex Keratitis (HSV) keratitis corneal scars, and all eyes with >2 quadrants deep vessels.[4] The patients operated for Congenital Hereditary Endothelial Dystrophy(CHED) or congenital corneal scars were bilaterally blind, so after explaining about guarded visual prognosis (due to amblyopia), these patients were operated with expectation of gaining ambulatory vision postoperatively.
Table 1.
Indications for penetrating keratoplasty
In situ corneoscleral rim excision was done for all eye donations and donor tissue was collected in M.K. medium with all aseptic precautions. Tissue was labeled as Excellent quality if epithelium was intact, stroma compact without any opacities or haze, no Descemet's Membrane (DM) folds, and endothelial cell density (ECD) >2 500; Good quality if minimal exposure keratitis was present with light stromal haze, few DM folds, and ECD between 2 000 and 2 500; and Fair quality if significant exposure was present, moderate degree of stromal haze with central DM folds, and ECD between 1 800 and 2 000.[5] Tissue evaluation was done by slit lamp observation and with Bioptics LSM 12000 specular microscope, by ophthalmologists who had done their fellowship training in cornea. Excellent quality tissue was used for young adults and bilaterally blind patients.
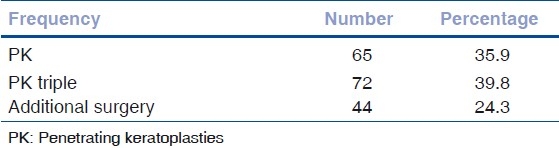
All surgeries were done under peribulbar anesthesia with supplementary IV sedation whenever required. Surgeries were done by two equally experienced surgeons who had done their fellowship training. In all cases, the donor grafts were 0.5 mm larger than the recipient. Commonly used graft sizes were 8.0 × 7.5mm, 7.5 × 7.0, and 8.5 × 8.0 mm. We used the manual disposable trephines for all cases. In all cases, interrupted suturing with 10/0 nylon was done. Surgeries were divided into P.K., P.K. triple (PK with cataract extraction with IOL), or PK with additional surgery like (anterior vitrectomy, pupilloplasty, intraocular lens explant, etc.) for further analysis [Table 2]. Postoperatively, patients were given prednisolone acetate (1%) eye drops, topical antibiotics, and artificial tears, antiglaucoma drugs if needed. Topical steroids were tapered over one year. For post-herpetic cases (n = 13), prophylactic oral acyclovir was given for one year. We did not use systemic immunosuppressive. Routine follow-up schedule was every weekly for first month, biweekly for second month, monthly for 3 to 6 months, and every three months for one year. Patients compliant with this schedule were called “Regular Follow Ups.”
Table 2.
Penetrating keratoplasties and associated procedures
Selective suture removal was started from third month onward, after assessing tightness of sutures on slit lamp examination, and calculating astigmatism by retinoscopy, keratometry, or topography. Best corrected visual acuity was determined at postoperative one year or later.
Graft rejection was defined as presence of one or more of the following signs: Mild if there were 1 to 5 keratic precipitates (KP), sub epithelial infiltrates, increased corneal thickness without increase in aqueous cells; Severe Rejection if >5 KPs, inflammatory cells in the stroma (not due to infection), endothelial rejection line, or increased thickness with aqueous cells.[6]
Graft clarity was graded as Grade 4 if grafts were optically clear with excellent view of iris details, Grade 2-3 (borderline) if there was moderate to significant corneal haze with or without good view of iris details, and Grade 1-0 (failed) for opaque grafts with poor view of iris and anterior segment details.[6] Good visual improvement was defined as postoperative vision improvement ≥two lines on Snellen's compared with preoperative vision, Moderate as one line improvement, and No improvement if vision remained same or worsened. Four patients with postoperative glaucoma required cyclocryotherapy. Graft resuturing for traumatic graft dehiscence was done for two patients. One patient with graft infection needed therapeutic keratoplasty. YAG laser capsulotomy was done in one patient with posterior capsule opacification.
Graft failure was defined as irreversible loss of optical clarity with the date of onset taken when the patient presented to cornea clinic with signs of irreversible rejection (for 3 months or more) or with failed graft.
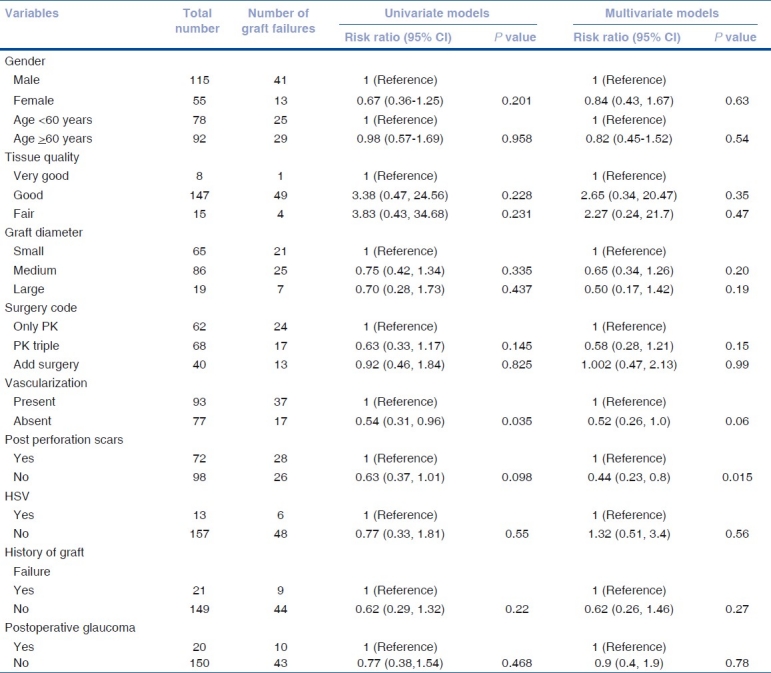
Data was presented using number (%) or mean (SD). Time to graft failure among operated patients was defined as outcome of interest. Patients with no graft failure during follow-up period were considered as “Censored cases.” Kaplan-Meier survival analysis was performed to calculate median survival (failure free) time of the grafts [Table 3]. Kaplan–Meier survival analysis was also performed by ten correlates for graft failure viz. age, gender, corneal vascularization, previous failed grafts, HSV keratitis, post-perforation corneal scars, donor tissue quality, graft size and type of surgery, and postoperative glaucoma. Log rank statistics was used to test the equality of estimates of survival functions among different strata of above correlates. Univariate and multivariate Cox proportional hazards models were used to assess the relationship between risk factors and graft failure event [Table 4]. The relative risk (RR) estimates the relative hazard estimates from the model. Results were considered as significant with a two-sided P value of <0.05. Data analysis was performed using SPSS (version 15.0, SPSS, USA).
Table 3.
Kaplan Meier survival analysis
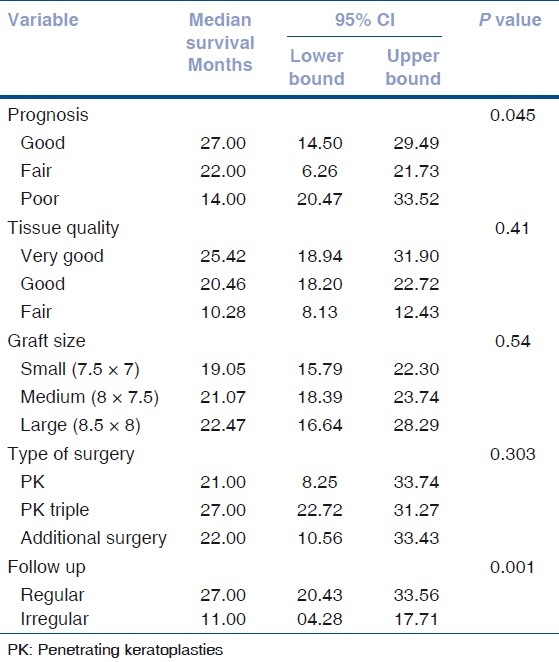
Table 4.
Univariate and multivariate Cox Regression models for risk factors predicting graft failure
Results
Of 181 patients, 125 (69.1%) were males and 56 (30.93%) females. Mean age of the patients was 55.24 ± 17.90 years. Patients were followed for median follow-up of 9 months (Inter Quartile Range (IQR): 3-15 months duration).
114 (63%) patients had preoperative vision of light perception and projection of rays (PLPR) and 67 (37.1%) had better than PLPR vision.
Of 181 patients, 120 completed at least one year of follow-up. At one year, 77 (64.16%) grafts remained optically clear (Grade 4 clarity), 11 (9.16%) had borderline clarity (Grade 2-3), and 32 (26.66%) grafts failed.
Good visual improvement was seen in 59 (49.17%), Moderate visual improvement in 25 (20.83%), and vision did not improve in 36 (30%).
Kaplan meier univariate analysis
Of 181 patients observed, adequate data for survival analysis could be obtained in 170 patients. Eleven patients did not even come for the first follow-up; therefore, these cases were excluded. Since the event of interest in survival analysis is graft failure, for the remaining 170 cases, estimation of survival function was possible even though some of them did not complete one year of follow-up. Of 170 patients, graft failure was observed among 54 (31.76%) patients during two-year follow-up period. Median survival of grafts in the cohort was 27 months (95% confidence interval [CI]: 20.47 – 33.52). 65% of the grafts survived at 12 months, whereas 52.5% of the grafts survived at 24 months [Fig. 1].
Figure 1.
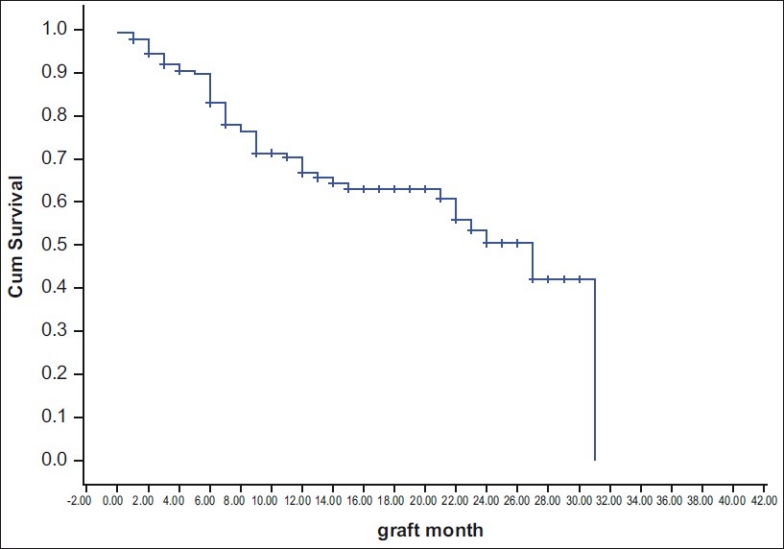
Kaplan Meier curve for graft failure
Graft survival did not differ between 2 gender groups (male – 23 months, females - 30 months, P = 0.119) and also between 2 recipient age groups (<60 years - 29 months, >60 years - 27 months, P = 0.950). Graft survival in good prognosis cases was 27 months (95% CI: 20.42 - 33.52) and in poor prognosis cases was 14 months (95% CI: 6.26 - 21.73, P = 0.045) [Fig. 2].
Figure 2.
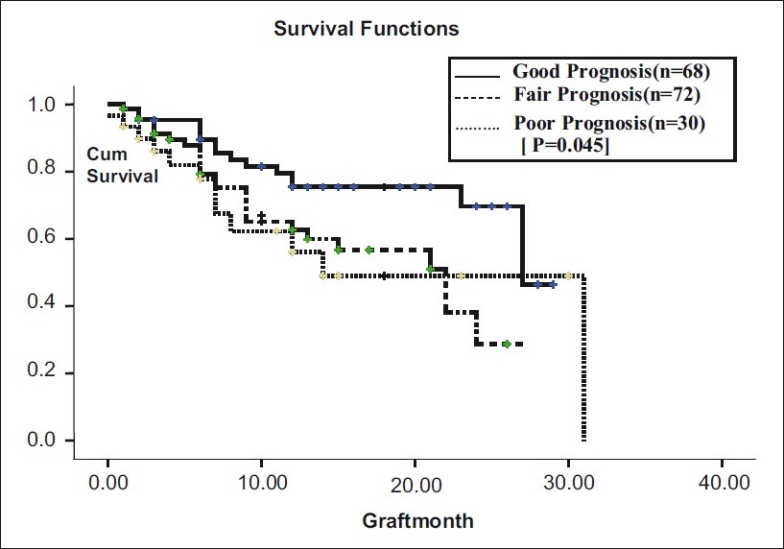
Prognosis vs graft survival
The prognosis of the case was determined on the basis of prior corneal vascularization, preexisting corneal perforation followed by scaring, history of herpes simplex keratitis (HSK), and history of previous ipsilateral corneal graft. So, these four variables were used for further analysis.
The extent of vascularization of recipient bed correlates strongly with the graft survival, as demonstrated in the Australian Corneal Graft Registry (ACGR) report[7] So, we used quadrants of vascularization as one of the factors for grouping our cases into fair prognosis (intermediate risk) category and poor prognosis (high risk) category.[4] In order to study the relation between individual risk factors like vascularization and graft survival, rather than low-risk vs high-risk group, our further analysis is designed accordingly.
In eyes with more than two quadrants of vascularization (n = 45), and those with less than two quadrants of vascularization (n = 48), median graft survival was 18.55 months (95% CI: 15.6 – 21.4). In nonvascularized corneas (n = 77), median graft survival was 22.69 months (95% CI: 19.83 - 25.55). Graft survival was significantly different with P = 0.06 [Fig. 3]. In corneal scars following perforation (n = 72), graft survival was 17.96 months ± 1.43 (95% CI: 15.14 – 20.78), whereas cases in which there was no perforation (n = 98), median graft survival was 22.56 months ± 1.403 (95% CI: 19.81 - 5.31, P = 0.09, borderline significant) [Fig. 4]. In post-HSV keratitis cases (n = 13), graft survival was 16.9 months (95% CI: 11.56 - 22.27), whereas in non-HSV keratitis (n = 157), graft survival was 20.95 months (95% CI: 18.72, 23.73, P = 0.544) [Fig. 5]. In previous failed grafts (one or more grafts done elsewhere) (n = 21), median graft survival was 14 months (95% CI: 5.55 - 22.44 months). This was much lower than eyes with first-time grafts (n = 149) in which median graft survival was 27 months (95% CI: 21.11 – 32.88); however, this difference was not statistically significant (P = 0.207) [Fig. 6]. In eyes with postoperative glaucoma, the median graft survival was 18.35 months (95% CI: 13.94 - 22.76) as compared with eyes without postoperative glaucoma (median, 21.59 months; 95% CI: 19.30-23.88). This too was not statistically significant (P = 0.460) [Fig. 7].
Figure 3.
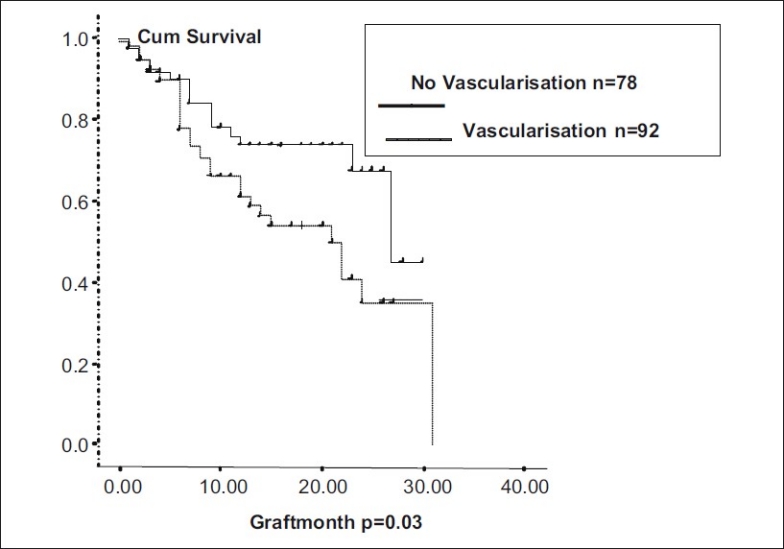
Graft survival in vascularized corneas
Figure 4.
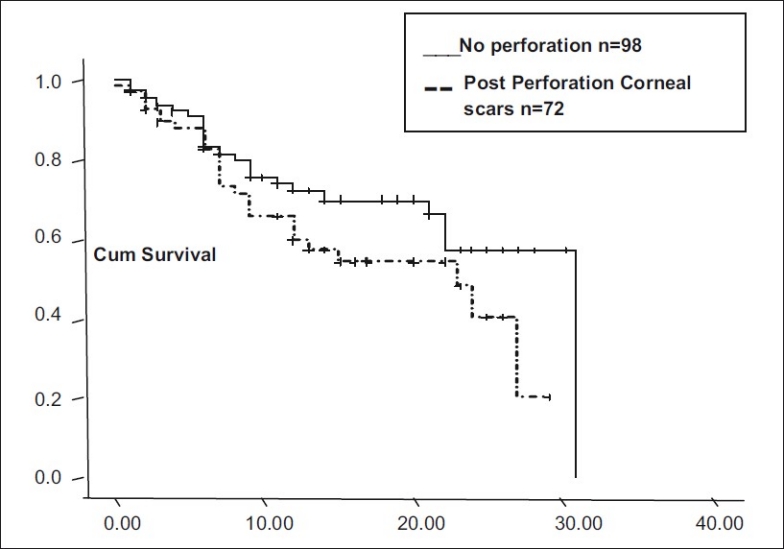
Graft survival in corneal perforations
Figure 5.
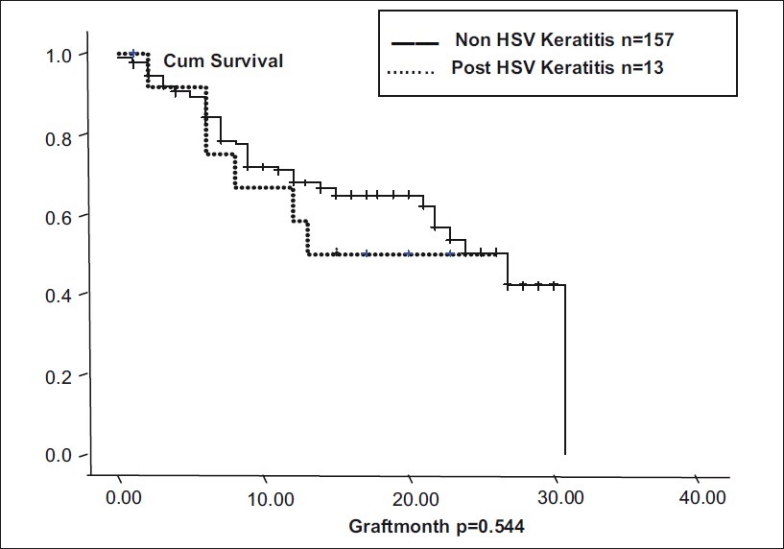
Graft survival in HSV corneal scars
Figure 6.
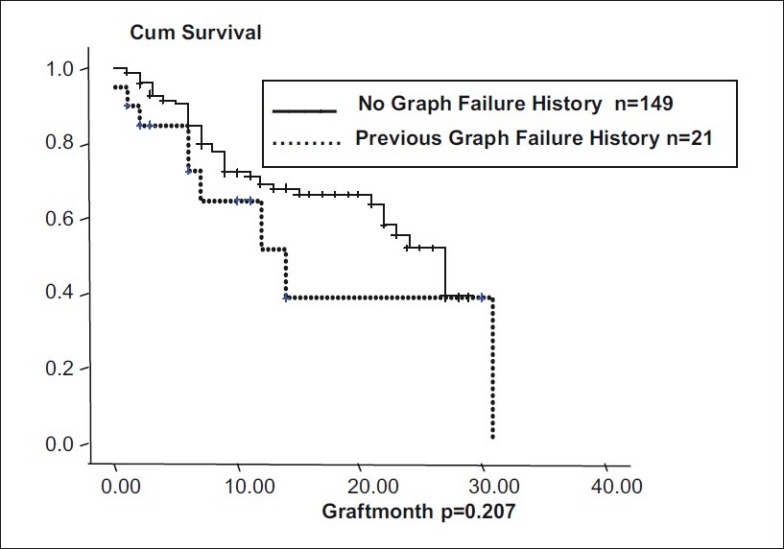
Graft survival in previous failed grafts
Figure 7.
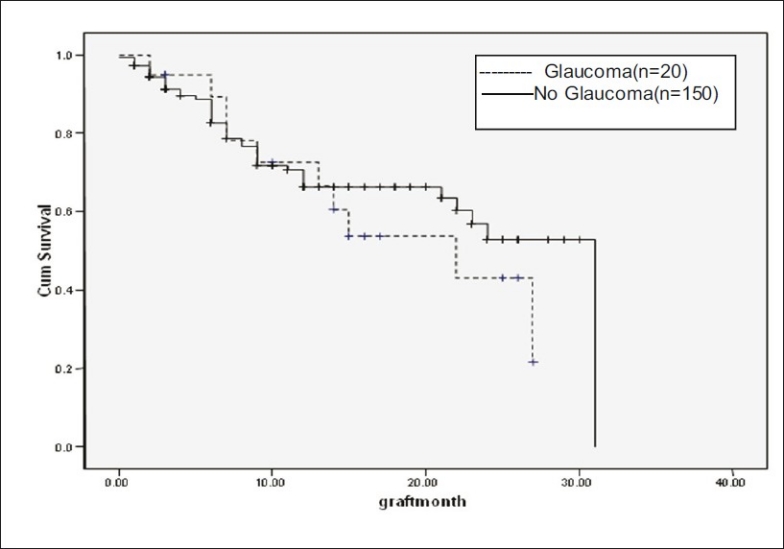
Postoperative glaucoma
In our study, cases for which very good quality donor corneas (n = 8) were used, had longer graft survivals (25.42 months) as compared with other cases (n = 147, good quality graft; n = 15, fair quality grafts), but this difference was not statistically significant (P = 0.41) [Fig. 8].
Figure 8.
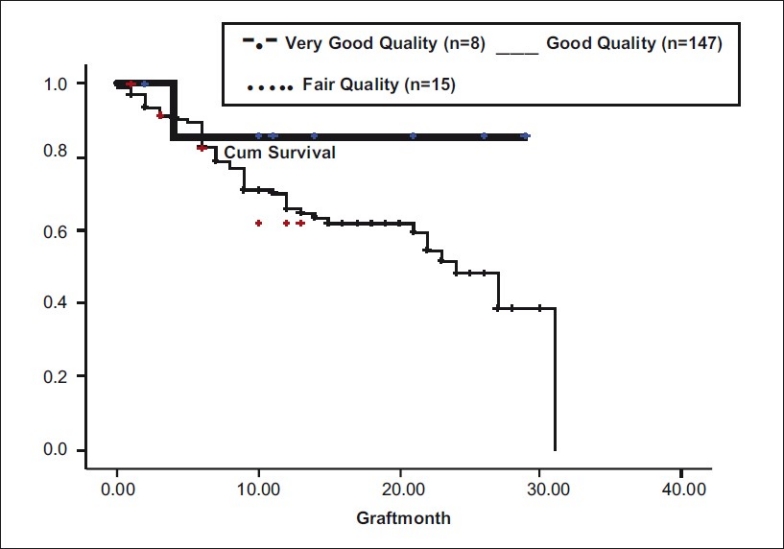
Donor tissue quality vs graft survival
In our series, we found that graft survival did not differ significantly in small size grafts (7.5 × 7 mm) (n = 66), medium size (8 × 7.5 mm) (n = 85), or large size grafts (8.5 × 8 mm) (n = 19) (P = 0.549) [Fig. 9].
Figure 9.
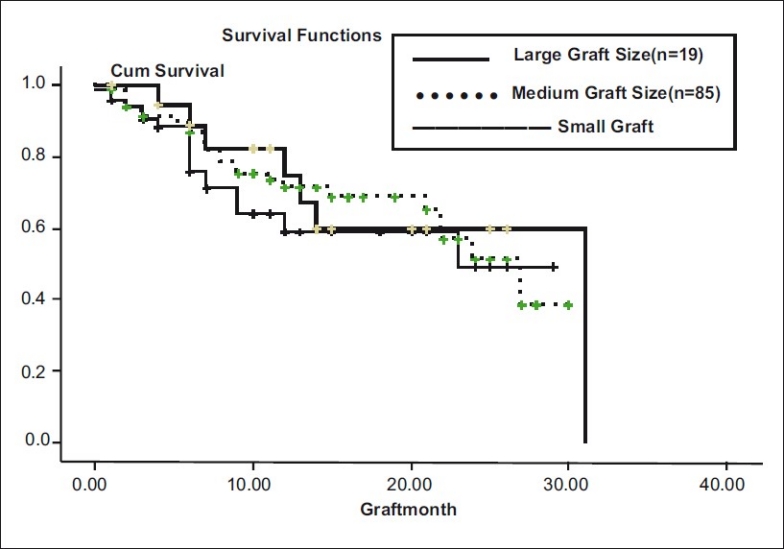
Graft size vs graft survival
Graft survival did not differ statistically (P = 0.303) in different types of surgeries namely PK, (n = 62), PK triple (n = 68), and PK with additional surgery (n = 40) [Fig. 10]. Additional surgeries included anterior vitrectomy (15), pupilloplasty (4), conjunctival limbal autografts (6), and IOL explants/exchanges (15).
Figure 10.
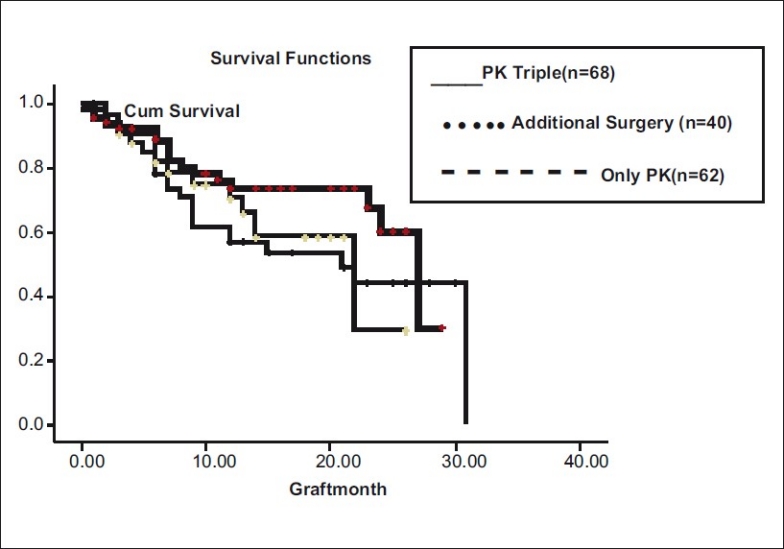
Type of surgery vs graft survival
In patients who followed up regularly (n = 64), medial graft survival time was 27 months (95% CI: 20.43 - 33.56 months) as compared with irregular follow ups (n = 106) in whom median graft survival time was 11 months (95% CI: 4.28 - 17.71 months, P = 0.001) [Fig. 11].
Figure 11.
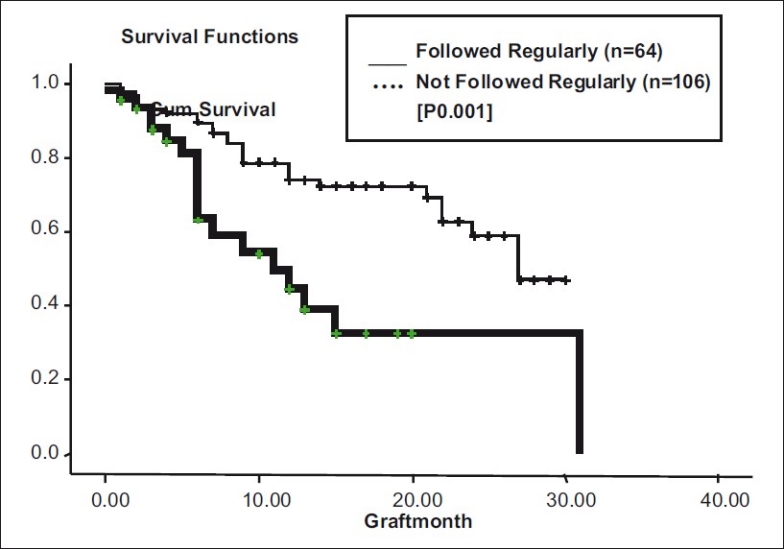
Follow-up vs graft survival
Table 3 summarizes the median survival time in months of corneal grafts and association between covariates and graft failure.
Kaplan Meier Multivariate Cox Proportional hazard regression analysis was performed to evaluate the combined effect of ten risk factors [Table 4]. Of these, vascularization (P = 0.048) and scars following perforation (P = 0.012) were identified as significant risk factors for graft failure. Immunological rejection (n = 15), surface problems like persistent epithelial defects (n = 8), graft infections (n = 8), postoperative glaucoma (10), traumatic graft dehiscence (n = 1), and primary endothelial failure (n = 1) were the causes of graft failure in our series.
Discussion
Corneal transplant surgery is the most commonly performed allograft and is said to be the most successful solid organ transplants, with short-term survival rates (1 year) as high as 90%.[8] However, the long-term success rate diminishes to 73% at 5 years, 60% at 10 years, and 46% at 15 years as reported in ACGR.[9] Reports from various graft registries of the developed countries show the indications for surgery being mainly keratoconus, other corneal dystrophies, followed by aphakic and pseudophakic bullous keratopathies.[2,3,7] However, the scenario in developing world is quite different. Firstly, the patient profile and indications for surgery differ. According to a study done in Nepal, corneal scars following infectious keratitis, adherent leucomas, and corneal perforations were the major indications for surgery.[10] A study done in India to analyze survival rate of corneal transplants in a large series shows survival rates at 1, 2, and 5 years for first-time grafts to be 79.6%, 68.7%, and 46.5%, respectively.[11] They are different from the western studies essentially due to differences in patient profile, different indications for surgery, differences in methods of storage of corneas, and socioeconomic factors affecting healthcare provision.
Our study shows much lower survival rates (at one year - 65%, at 2 years - 52.5%). There is a variety of reasons for this. In our series, only one-third of our cases (n = 65, 35.91%) were good prognosis cases like corneal dystrophies (gelatinous droplet keratopathy, 1; granular dystrophy, 1; macular dystrophy, 1), corneal degenerations (6), and central/paracentral scars (posttraumatic18, postinfectious 38). Most of our patients (64%) are high-risk cases, which are at increased immunological risk of graft rejection due to factors like vascularized corneas, previous ipsilateral grafts, peripheral anterior synechiae, irregular anterior chamber, bullous keratopathies, herpetic eye disease with deep vascularization, etc. Secondly, most of our corneal collection is through voluntary eye donations by home calls, our donors being in the age group 60 years and above, with average quality of donor tissue and comparatively lower endothelial cell counts.[12,13] Hospital Cornea Retrieval Programs more often give a higher yield and younger donor tissue.
Also, majority of our patients are illiterate with poor socioeconomic status. So, postoperative care and follow up is a major challenge.
In our study, we found significant correlation between prognosis of the case and graft survival. Median survival in good prognosis cases (corneal dystrophies and degenerations, corneal scars, etc) was 27 months (95% CI: 20.42 - 33.52), whereas in poor prognosis cases (post-HSV corneal scars, previous failed grafts) was 14 months (95% CI: 6.26 - 21.73), the difference being statistically significant. Survival analysis of corneal transplants done at L.V. Prasad Eye Institute, India,[11] also shows higher survival for corneal dystrophies (56%, 5-year survival rate) and lowest survival for previous failed grafts (21.2%, 5-year survival rate), although they have described results in terms of survival rates and not median survival time.
In our analysis, we found that extent of deep vascularization did not correlate statistically with graft survival, but the presence or absence of it did. In our series, patients with vascularized corneas had 51% more risk of failure. Graft survival in vascularized corneas was 18.55 months and 22.69 months in nonvascularized corneas. The Singapore Corneal Transplant Study (SCTS) also found lower graft survival in vascularized corneas compared with nonvascularized (P = 0.017).[12] Another study by Price et al. too showed eyes with deep stromal vascularization three times more likely to experience rejection and failure (RR: 2.7, CI: 1.6 - 4.8, P<0.01).[14] In our study, patients with previous corneal perforations had 45% more risk of failure (multivariate analysis). The sequel following perforation like vascularization, anterior synechiae with irregular anterior chamber, iridocorneal scar, and secondary glaucoma could have been responsible for lower survival rates. SCTS has also shown corneal perforation as a very significant risk factor for graft failure (HR - 3.16, CI: 1.92 - 5.19, P = 0.001).[12] Both these are well-established risk factors for graft failure, reported by various studies.[14,15] We found lower average survival time in post-HSV keratitis (16.9 months) compared with non-HSV keratitis (20.95 months). This agrees with other reports like the study from Switzerland, in which at 5 years, cumulative probability of graft survival in HSK patients was 40.85% compared with 50.15% in non-HSK.[16] Causes of graft failure in HSK patients were rejection (83.33%) and recurrence of HSK (16.66%). In our study, graft survival was lower (14 months) in previous failed grafts than that in first-time grafts (27 months). Prior graft failure as a risk factor in subsequent grafts is a well-known fact established by Khodadoust, where rejection rate of 40% after first graft, 68% after second, and 80% after third graft was found.[17] We did not find statistically significant correlation between surgical factors like graft size, PK, and PK with associated procedure with graft survival. This is perhaps due to the fact that like most other reputed centers, we follow well-established practice patterns of surgical and postoperative treatment. We also found that patients who followed up regularly had better graft survival (27 months) than those who did not follow up (11 months). This once again emphasizes the need for good patient compliance and regular follow-up. In our series, major cause of graft failure was allograft rejection (34.88), followed by surface problems (18.6%), graft infections (18.6%), and glaucoma (23.25%). A study done by Dandona et al. shows similar causes, e.g., rejection (29.2%), increased intraocular pressure (16.9%), infections (15.4%), and surface problems (12.7%).[18] 70% of our patients showed some improvement in vision over preoperative vision. Causes of non-improvement in vision were failed grafts (32), preexisting amblyopia (2), and preexisting optic disc pallor (2).
There are several limitations to our study. We did not assess in details the effect of several donor tissue-related variables like death to in situ excision time, preservation time, age of the donor, HLA matching or ABO grouping of donor- recipient. Also, we have not taken into account effect of factors like preexisting glaucoma, inflammation, type of suturing, postoperative anterior or posterior uveitis, systemic immunosuppressant, and rejection episodes. Reversible graft rejection is an important risk factor, responsible for about one-third of corneal graft failures in ACGR. Also, our study shows survival rates for a relatively short period, i.e., 1 to 3 years. We are in a process of collecting and analyzing data over longer periods like 5 to 7 years. Nearly one-third to half of our patients have been lost to follow-up (n = 69) causing several limitations to analysis. Still to conclude, short-term success and survival of corneal grafts in this part of the developing world is reasonably good. Our study has validated the normally accepted facts regarding outcome and survival of corneal grafts. Further improvements in eye banking facilities, adopting Hospital Cornea Retrieval Programme to procure young donor corneas, and better patient counseling to ensure good follow-up are needed to improve long-term survival of corneal grafts.
Acknowledgments
We acknowledge Mrs. Swapna Deshpande for her help in statistical analysis, Eyebank technician Mr. Satish Kurpad, the staff of eye bank and cornea department, Ms. Swapna Dike and Mr. Ajit Joshi for technical support, and Dr. Salil Gadkari for his valuable guidance and inputs.
Footnotes
Source of Support: Nil.
Conflict of Interest: None declared.
References
- 1.John N, Jose R, Vashist P, Murthy GV. National Program for control of blindness, Rapid Assessment of Avoidable Blindness in India Report 2006-2007 [Google Scholar]
- 2.Cosar CB, Sridhar MS, Cohen EJ, Held EL, Alvim Pde T, Rapuano CJ, et al. Indications for penetrating keratoplasty and associated procedures, 1996-2000. Cornea. 2002;21:148–51. doi: 10.1097/00003226-200203000-00003. [DOI] [PubMed] [Google Scholar]
- 3.Siganos CS, Tsiklis NS, Miltsakakis DG, Georgiadis NS, Georgiadou IN, Kymionis GD, et al. Changing indications for penetrating Keratoplasty in Greece, 1982-2006: A multicenter study. Cornea. 2010;29:372–4. doi: 10.1097/ICO.0b013e3181bd44a1. [DOI] [PubMed] [Google Scholar]
- 4.2nd ed. Vol. 2. Krachmer, Mannis: Holland Part IX, Section 1; Cornea, Surgery of the cornea and conjunctiva; p. 1426. [Google Scholar]
- 5.Saini JS, Reddy M, Sharma S, Wagh S. Donor corneal tissue evaluation. Indian J Ophthalmol. 1996;44:3–13. [PubMed] [Google Scholar]
- 6.McDonnell PJ, Enger C, Stark WJ, Stulting RD. Corneal thickness changes after high-risk penetrating keratoplasty.Collaborative Corneal Transplantation Study Group. Arch Ophthalmol. 1993;111:1374–81. doi: 10.1001/archopht.1993.01090100082032. [DOI] [PubMed] [Google Scholar]
- 7.Coster DJ, Williams KA. The impact of corneal allograft rejection on the long-term outcome of corneal transplantation. Am J Ophthalmol. 2005;140:1112–22. doi: 10.1016/j.ajo.2005.07.024. [DOI] [PubMed] [Google Scholar]
- 8.Williams KA, Muehlberg SM, Lewis RF, Coster DJ. How successful is corneal transplantation? A report from the Australian Corneal Graft Register. Eye (Lond) 1995;9:219–27. doi: 10.1038/eye.1995.43. [DOI] [PubMed] [Google Scholar]
- 9.Williams KA, Lowe M, Bartlett C, Kelly TL, Coster DJ. Risk factors for human corneal graft failure within the Australian corneal graft registry. Transplantation. 2008;86:1720–4. doi: 10.1097/TP.0b013e3181903b0a. [DOI] [PubMed] [Google Scholar]
- 10.Tabin GC, Gurung R, Paduyal G, Reddy HS, Hobbs CL, Wiedman MS, et al. Penetrating Keratoplasty in Nepal. Cornea. 2004;23:589–96. doi: 10.1097/01.ico.0000121712.36593.0d. [DOI] [PubMed] [Google Scholar]
- 11.Dandona L, Naduvilah TJ, Janarthanan M, Raghu K, Rao, Gullapalli N, et al. Survival analysis and visual outcome in a large series of corneal transplants in India. Br J Ophthalmol. 1997;81:726–31. doi: 10.1136/bjo.81.9.726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Tan DT, Janardhanan P, Zhou H, Chan YK, Htoon HM, Ang LP, et al. Penetrating Keratoplasty in Asian Eyes, The Singapore Corneal Transplant Study. Ophthalmology. 2008;115:975–82 e1. doi: 10.1016/j.ophtha.2007.08.049. [DOI] [PubMed] [Google Scholar]
- 13.Bourne WM, Nelson LR, Hodge DO. Central corneal endothelial changes over a ten year period. Invest Ophthalmol Vis Sci. 1997;38:779–82. [PubMed] [Google Scholar]
- 14.Price MO, Thompson RW, Jr, Price FW., Jr Risk factors for various causes of failure in initial corneal grafts. Arch Ophthalmol. 2003;121:1087–92. doi: 10.1001/archopht.121.8.1087. [DOI] [PubMed] [Google Scholar]
- 15.Sit M, Weisbrod DJ, Naor J, Slomvic AR. Corneal graft outcome study. Cornea. 2001;20:129–33. doi: 10.1097/00003226-200103000-00002. [DOI] [PubMed] [Google Scholar]
- 16.Halbersted M, Machens M, Galenbek KA, Böhnke M, Garweg JG. Outcome of Corneal Grafting in patients with stromal keratitis of Herpetic and non-herpetic origin. Br J Ophthalmol. 2002;86:646–52. doi: 10.1136/bjo.86.6.646. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Khadadoust AA. The allograft rejection reaction: The leading cause of late failure of clinical corneal grafts. In: Jones BR, editor. Corneal Graft Failure. New York: Elsevier; 1973. [Google Scholar]
- 18.Dandona L, Naduvilah TJ, Janarthan M, Rao GN. Causes of corneal graft failure in India. Indian J Ophthalmol. 1998;46:149–52. [PubMed] [Google Scholar]


