Dear Editor,
Diplopia after Botulinum Toxin A (BTX) injection for facial rejuvenation is rare, transient and recurrent with re-injections.[1] It is mostly associated with inferior oblique paresis[1–3] or rarely with lateral rectus paresis.[4] Here, we report a superior oblique paresis following BTX injection for facial rejuvenation. A literature search on Google and PubMed did not show a report.
A 48-year-old man presented with blurred vision since three weeks that disappeared on tilting the head to the left. Three weeks prior, he had received BTX for facial rejuvenation. Blurred vision was noticed on the next day. It was his sixth treatment with BTX in the last three years. His reports indicated that 48U BTX was administered [Fig. 1]. The injections were given in the sitting position and the brow injection on the right was allegedly administered ‘too’ close to the eye. There was no history of recent head injury, diabetes, hypertension or hyperlipidemia.
Figure 1.
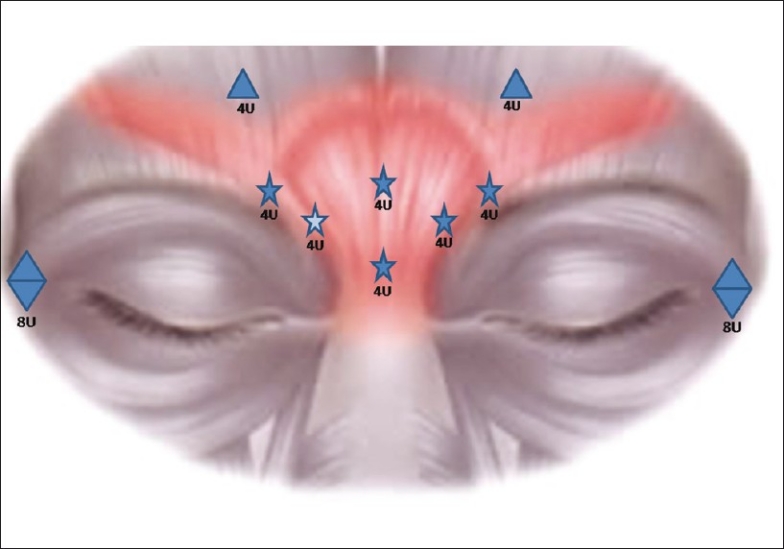
Sketch showing the dose distribution and injection sites of BTX. The potential likely site resulting in superior oblique paresis is marked by a highlighted star
His best corrected distance and near vision was 20/20, N6 in both eyes. Examination of the pupils, anterior segment and posterior segment was normal. The orthoptic examination revealed an abnormal head posture (10 degrees tilt to the left). In forced primary position, there was a right hypertropia of 2Δ that increased to 6Δ in levoversion and 6Δ on right head tilt. Hypertropia disappeared in dextroversion and with left head tilt. Extraocular movements were normal. Hess chart demonstrated mild weakness of the right superior oblique [Fig. 2]. Double Maddox rod test was normal. Neutralization of diplopia in free space confirmed the findings of the cover test. Fundus examination revealed trace extorsion in the right eye and no torsion in the left.
Figure 2.
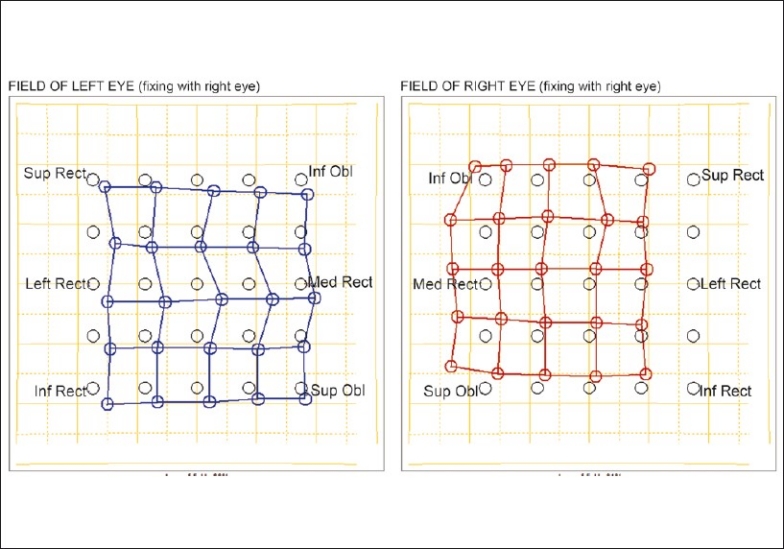
Hess chart showing small hypertropia in the right eye associated with ipsilateral superior oblique muscle underaction
Other cranial nerves functioned normally. Neuroimaging of the brain was advised. However, the patient was lost to follow-up and reported back after one month with normal ocular motility and no diplopia.
BTX-induced chemo-denervation is a minimally invasive, focused, safe and effective therapy for facial rejuvenation.[1] Minute quantities (units) and volume of the drug injected, better anatomical knowledge of the affected muscles and improved techniques have significantly reduced the incidence of complications. Nevertheless, diplopia after BTX for facial rejuvenation occurs in 2.1% patients.[1] In most cases, the pattern of the palsy remains unidentifiable or affects the inferior oblique muscle. BTX-induced superior oblique palsy is not reported.
Typical complaints, pattern of ocular motility disturbances, history of BTX injection prior to the onset of symptoms and the self-limiting nature of the disease in this patient indicate transient superior oblique paresis due to BTX.
Potential risk factors in this patient were inherent susceptibility (causing higher intraorbital diffusion), proximity of the needle tip to the trochlea (faulty technique), deep penetration of the needle into the orbital septum (faulty technique) and increased diffusion of the drug following repeated injections.[3] The clinicians engaged in BTX for facial rejuvenation should inform the patients about the possibility of this potential complication. Adequate technical modifications and/or adjustment in the dose and/or volume may be necessary to avoid this complication, especially with repeated treatments.
References
- 1.Dutton JJ, Fowler AM. Botulinum toxin in ophthalmology. Surv Ophthalmol. 2007;52:13–31. doi: 10.1016/j.survophthal.2006.10.003. [DOI] [PubMed] [Google Scholar]
- 2.Aristodemou P, Watt L, Baldwin C, Hugkulstone C. Diplopia associated with the cosmetic use of botulinum toxin a for facial rejuvenation. Ophthal Plast Reconstr Surg. 2006;22:134–6. doi: 10.1097/01.iop.0000203304.22031.b5. [DOI] [PubMed] [Google Scholar]
- 3.Wutthiphan S, Kowal L, O’Day J, Jones S, Price J. Diplopia following subcutaneous injections of botulinum A toxin for facial spasms. J Pediatr Ophthalmol Strabismus. 1997;34:229–34. doi: 10.3928/0191-3913-19970701-10. [DOI] [PubMed] [Google Scholar]
- 4.Chen CS, Miller NR. Botulinum toxin injection causing lateral rectus palsy. Br J Ophthalmol. 2007;91:843. doi: 10.1136/bjo.2006.109926. [DOI] [PMC free article] [PubMed] [Google Scholar]


