Abstract
Obstetric brachial plexus injury (OBPI), also known as birth brachial plexus injury (BBPI), is unfortunately a rather common injury in newborn children. Incidence varies between 0.15 and 3 per 1000 live births in various series and countries. Although spontaneous recovery is known, there is a large subset which does not recover and needs primary or secondary surgical intervention. An extensive review of peer-reviewed publications has been done in this study, including clinical papers, review articles and systematic review of the subject. In addition, the authors’ experience of several hundred cases over the last 15 years has been added and has influenced the ultimate text. Causes of OBPI, indications of primary nerve surgery and secondary reconstruction of shoulder, etc. are discussed in detail. Although all affected children do not require surgery in infancy, a substantial proportion of them, however, require it and are better off for it. Secondary surgery is needed for shoulder elbow and hand problems. Results of nerve surgery are very encouraging. Children with OBPI should be seen early by a hand surgeon dealing with brachial plexus injuries. Good results are possible with early and appropriate intervention even in severe cases.
KEY WORDS: Birth brachial plexus injury, obstetric palsy, primary surgery
INTRODUCTION
Obstetric brachial plexus injury (OBPI), also known as birth brachial plexus injury (BBPI), is unfortunately a rather common injury in newborn children. Incidence varies between 0.15 and 3 per 1000 live births in various series and countries.[1] Although spontaneous recovery is known, there is a large subset which does not recover and needs primary or secondary surgical intervention. The first known documentation was by Smellie in 1764,[2] and Duchenne in 1872[3] surmised that traction was the cause of the palsy. Erb described a similar palsy in adults in 1874[4] and suggested that traction or compression of the C5 and C6 roots could produce the injury.
This article reviews peer-reviewed publications including clinical papers, review articles and systematic review of the subject. In addition, the authors’ experience of several hundred cases over the last 15 years has been added and has influenced the ultimate text. Causes of OBPI, indications of primary nerve surgery and secondary reconstruction of shoulder, etc. are discussed in detail.
INCIDENCE
There is a wide variation in reported figures of incidence ranging from 0.15 to 3 per 1000 live births. These figures reflect health care availability, reporting methods, referral bias and population differences. In general, a figure of 1:1000 live births is generally agreed upon as an average of various series. Spontaneous recovery is reported in all series, but varies from as high as 90% to as low as 30%.[1,5–13] Once again, referral bias and surveys based on tertiary centres are the cause of this disparity. It is, however, now agreed that recovery is less than what was originally thought and that more children need monitoring and early intervention to improve their outcome.
CAUSES
Foetal
Macrosomia
Breech
Maternal
Diabetes in pregnancy
Shoulder dystocia
Small stature/cephalopelvic disproportion
Primi or multiparity
Prolonged second stage of labour
MECHANISM AND PATHOPHYSIOLOGY
The generally accepted mechanism in cases of shoulder dystocia is traction to the neck caused by pull of the obstetrician's hand or instruments like forceps or vacuum. In this scenario, the neck on the side of the anterior shoulder is stretched and this stretch causes a “strain” on the brachial plexus on that side, causing a varying degree of injury. This mechanism would typically cause anterior shoulder involvement, which would imply a higher incidence of right-sided injuries because left occipito anterior (LAO) is the most common presentation. However, posterior shoulder involvement is seen frequently[14] and the right side is not the more common side in literature. Our own data, which have been documented, show that in a cohort of 305 patients, 60% were right sided, 37 % were left sided and 3% were bilateral. If we had gone by LAO statistics we should have had 90% right side involvement.
Therefore, other mechanisms have been looked at. For example, some papers have discussed whether it is really an intrauterine injury. Jennet et al.[14] have shown that almost half the cases they reviewed did not have shoulder dystocia and concluded that it could be caused by intrauterine maladaptation and not birth trauma. Others like Gherman[15] have described that OBPI has occurred following caesarean sections and also that shoulder dystocia does not always lead to OBPI. There is some electrophysiological evidence[16] to show that OBPI could have occurred in the intrauterine period since denervation potentials are seen on day 1 after delivery which ought not be possible in case it occurred at the moment of delivery. It has been shown that the Posterior shoulder can get stuck on the Sacral promontory and cause injury through a stretch on that side[14] while the baby is in early stage of labour before any question about shoulder dystocia and traction. Bicornuate uterus has also given rise to OBPI with phrenic palsy.[17]
In contrast, Gilbert et al.[18] found that the majority (92%) of the high-risk patients (diabetic women delivered by operative vaginal delivery with infants of 4.5 kg birth weight) did not have OBPP and caesarean delivery would have been unnecessary. Although macrosomia is commonly associated with OBPP, Rouse et al.[19] found no benefit with elective caesarean delivery in women with estimated foetal weights of 4.5 kg, unless they were also diabetic. Lindsay et al.[20] found that a policy of elective caesarean section for macrosomia would necessitate 148–258 caesarean sections to prevent a single persistent injury and avoidance of operative vaginal delivery would require 50–99 caesarean sections per injury prevented.
Thus, there is no agreement among scholars about the mechanism or active prevention and this has medico-legal implications. Lawyers for aggrieved parents routinely blame the obstetrician for the injury, but this may not be supported by evidence in the literature.
Pathophysiology
The injury caused to the roots of the plexus can be of a varied nature and can affect some or all roots. The classical injury is a C5, C6 palsy, but all roots can be involved. The level and nature of root involvement varies from a neuropraxia to varying levels of axonotomesis to neurotomesis. In the worst injuries, even a root avulsion is possible and one can find ganglia in the neck as shown in Figure 1. Clinical examination and electrophysiology with or without magnetic resonance imaging (MRI) can help in largely determing the type and extent of injury. Sunderland's[21] well-known classification is useful to understand the nature of the injury. Broadly speaking, for the surgeon, there are three different kinds of lesions:
Figure 1.
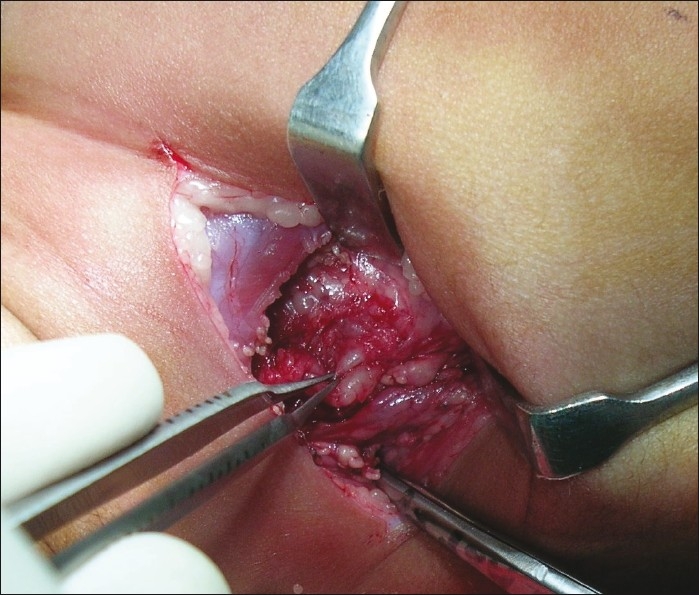
Avulsed ganglia noted on supraclavicular exploration in a child
Neuroma in continuity: This represents a postganglionic rupture, i.e. a Sunderland type 2, 3 or 4 injury which is healed with fibrosis and some axons may be attempting to go across the scar tissue. Occasionally, it is a conducting neuroma with useful function. However, if seen early enough, it is far better to resect and reconstruct [Figure 2].
Rupture: This is a postganglionic neurotomesis, i.e. Sunderland type 5 causing a separation of proximal and distal ends often bridged by scar tissue [Figure 3].
Avulsion: This is a preganglionic lesion showing avulsed ganglia in the neck or a pseudomeningocoele [Figures 1 and 4].
Figure 2.
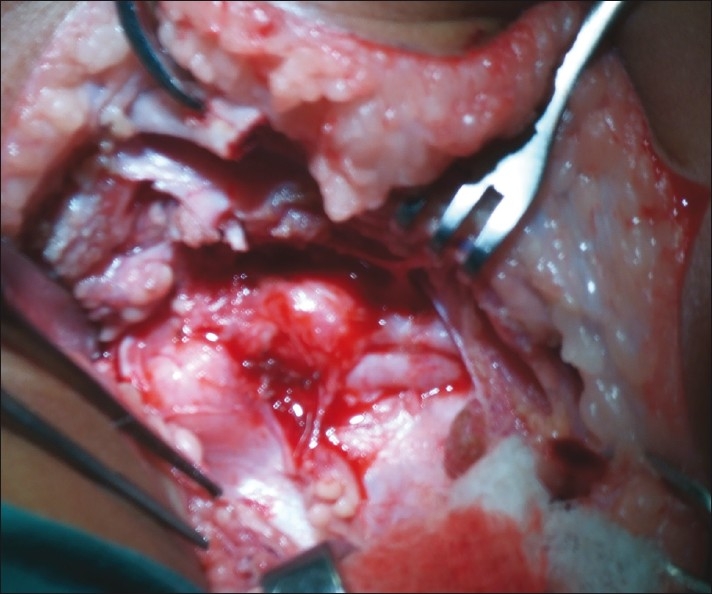
Example of neuroma in continuity
Figure 3.
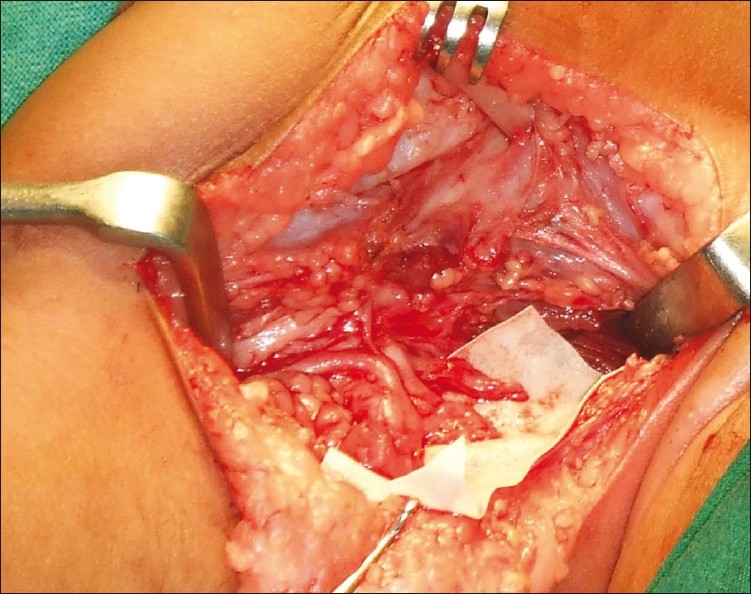
Example of ruptured plexus
Figure 4.
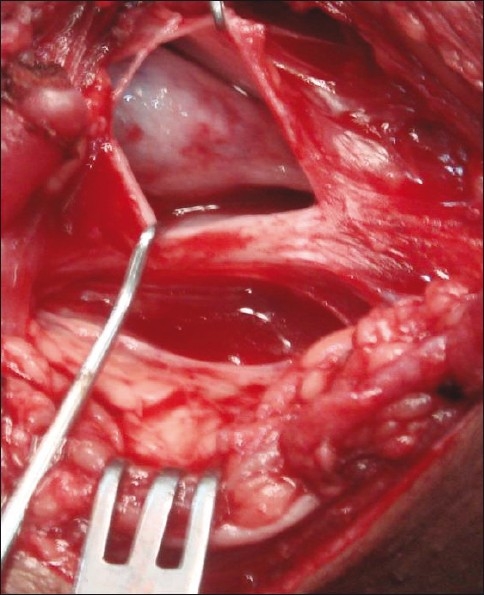
Pseudomeningocoele in neck
SPONTANEOUS RECOVERY
In literature, spontaneous recovery in over 90% of cases is mentioned in several papers.[5–13,22,23] However, a systematic review by Pondaag et al.[1] showed that no study presented a prospective, population-based cohort that was scored with a proper scoring system with adequate follow-up. They concluded that no scientifically sound evidence exists to support the common perception of complete spontaneous recovery. In their opinion, the incidence of children requiring surgery was 20–30% rather than the 10% often mentioned in literature.
INITIAL TREATMENT
Initial treatment consists of coming to a proper diagnosis, which includes a careful history taking, detailed clinical examination, including a check for associated injuries like fractures of the clavicle and humerus. Electrophysiology is recommended at 4 weeks initially to confirm the diagnosis and get a baseline reading of the involvement of various nerves and muscles as well as sensory parameters. Counselling of parents is critical and clear open communication is strongly recommended. Gentle mobilisation of all joints of the affected limb is suggested to avoid stiffness. “Gift wrapping” the baby in the typical traditional Indian style after morning bath is discouraged as it deprives the child of a chance to use that limb spontaneously. Repeat examinations are carried out every 4–6 weeks until 3 months. At this time, a decision is made about the need for surgery, which in turn depends on clinical and electrophysiological findings.
IMAGING
The following modalities are available:
Plain X-ray of arm and chest for fractures and phrenic palsy
Computed tomography (CT) myelography
MR scans
There are several papers discussing these modalities.[24–32] At one time, CT myelography was the gold standard to decide on root avulsion in adult palsy. Currently, MRI is considered very useful, at least in the adult palsy. Although there are reports of the use of MR scans, the authors do not routinely perform MR scans in infants. Clinical exam and electrophysiology can give adequate evidence of the status of the plexus and the indication for surgery.
INDICATIONS FOR SURGERY
In case of global involvement, involvement of the hand or a flail upper limb, there is no real dispute about indication or timing of surgery. The dispute arises in the upper plexus lesions of Narakas Type I and II [Table 1]. The author follows Prof. Gilbert's criteria of using the biceps brachii as an indicator of recovery in upper plexus lesions. Following the landmark theses by Tassin,[33] it was generally agreed that a lack of antigravity biceps function at 3 months is an indication for surgery in C5C6+/–C7 lesions. The logic behind this caveat is that the biceps is the only C5–C6 innervated muscle whose function cannot be duplicated by other muscles, and therefore is a good uncluttered indicator of C5C6 recovery. The shoulder, for example, is moved by multiple muscles, which have various innervations and represent many neurotomes, and is therefore considered unsuitable for making this judgement call. Biceps recovery indicates the good health of C5–C6 and the upper trunk all the way to the upper arm. Clarke and his group[34,35] developed an alternative Active Movement Scale (AMS) in Toronto [Table 2]. They use this scoring and are prepared to wait for a much longer observation period to come to a decision. They also use the “Cookie” test around 8–9 months in children who are on the borderline of surgery and conservative treatment to make a final call regarding the indication of surgery. While there is merit in this system, the resource requirement in terms of physical and occupational therapists dedicated to this clinic, etc. exceeds that which is typically available. In addition, the system is cumbersome to administer at least in our circumstances at this point in time and may not offer a great advantage over Gilbert's rather simpler indication.
Table 1.
Narakas classification

Table 2.
Toronto active movement scale of clarke and curti
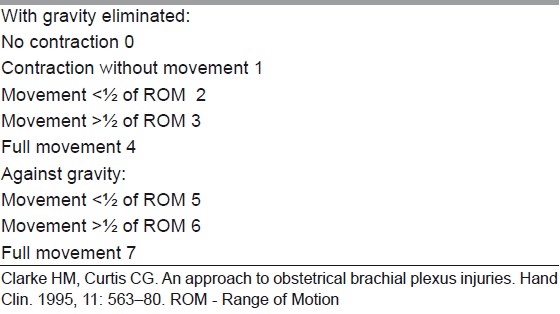
Waters, in a review,[36] has summarised the issue with the following observation: “Infants who recover partial antigravity upper trunk muscle strength in the first 2 months of life should have a full and complete recovery over the first 1–2 years of life. Infants who do not recover antigravity biceps strength by 5–6 months of life should have microsurgical reconstruction of the brachial plexus, as successful surgery will result in a better outcome than natural history alone. Infants with partial recovery of C5–C6–C7 antigravity strength during months 3 through 6 of life will have permanent, progressive limitations of motion and strength; they also are at risk for the development of joint contractures in the affected limb.”
The author MRT personally uses Gilbert's criteria for the decision, i.e. if a child with upper plexus lesion does not have a biceps function exceeding MRC grade 3 (antigravity movement) at 3 months of age, then surgery is recommended for nerve repair. In cases where there is visible biceps contraction but triceps co-contraction preventing elbow flexion, or biceps being 2+ but not really 3, etc., we can wait and watch with/without botox to triceps up to 6 months by which time the picture is clear. If the bicpes continues to be under MRC grade 3, then surgery is better than conservative treatment.
SURGICAL TREATMENT
Surgery was pioneered by Kennedy (1903),[37] Sever (1916)[38] and Wyeth and Sharpe (1917).[39] However, Sever's results and paper were a dampener for future work for almost 50 years. It was only later that Gilbert,[40] Narakas,[41] Kawabata,[42] Millesi[43] and others started the surgical treatment again in the 80s, which gave remarkably impressive results.
Surgery in these indications consists of a complete exploration of the supra and infraclavicular plexus and nerve repair using microsurgical techniques based on merits, i.e. after assessing the injury and its pathophysiology, the surgeon can decide on the strategy to be used. Thatte et al.[44] have recently published a detailed paper describing the technique for exposure of the brachial plexus, which can be used for further reading. Typically, the preference in a total palsy is to hand function, while in an upper plexus it is of course to reconstruct the nerves for elbow and shoulder function. The suprascapular nerve (SSN) supplying the supra and infraspinatus is a crucial nerve for shoulder abduction and external rotation and needs to be targeted with priority, either from one of the roots or separately with the spinal accessory (XI) nerve as the donor. The lower trunk/medial cord for hand and musculocutaneous nerve (MCN) for biceps are the other priorities.
Intraplexus neurotisation thus uses existing healthy root stumps, which are ruptured for reconstruction by joining them with the distal target trunks, cords or nerves using nerve grafts. The source of nerve grafts is both sural nerves and the ipsilateral medial cutaneous nerve of forearm (MCNF) and superficial radial nerve (SRN), especially in global severe palsies if grafts are falling short. Not all cases may be of rupture; there can be avulsions as well, and therefore no roots may be available. Depending on the number of roots available, a policy is evolved for neurotisation.
Global palsy strategy
Four or five roots available: Very rare; just direct them to respective trunks/cords.
Three roots available: One each to medial, lateral and posterior cords. XI to SSN depending on the pathoanatomy of the upper trunk lesion.
Two roots available: Root 1 to lower trunk/medial cord, Root 2 to lateral cord (or shared between lateral and posterior cords) and XI to SSN. Some authorities would prefer Root 2 to posterior division of upper trunk/posterior cord and 2 or 3 intercostal nerves (ICNs) to biceps. Their point is getting a strong triceps and a stable shoulder with deltoid and triceps as well as the rotator cuff is important to balance a well-recovered biceps. This is logical; however, the addition of ICNs adds to surgical time, blood loss and increases surgical risks and morbidity and needs to considered carefully before attempting. In well-equipped centres with blood bank support, ICU for children and experienced anaesthesiologists, it can be done if the child is fit.
One root available: Root to lower trunk/medial cord + XI to SSN, 2 or 3 ICNs to biceps. Shoulder will need secondary transfers if possible.
Zero roots – rare total avulsion: Opp. C7 root (posterior division) to lower trunk/medial cord and XI to MCN or XI to SSN and ICNs to MCN plus one ICN to long head of triceps. If the child is healthy and fit for prolonged anaesthesia-or stagger in a second session in a couple of months if there is any concern on this count.
Upper plexus: C5–C6
Both roots available: Typically C5 to anterior division of upper trunk and C6 to posterior division of upper trunk.
One root available: Root to anterior division of upper trunk (or both divisions if they are of really good quality with plenty of healthy axons) and XI nerve to SSN. ICNs can be used for additional neurotisation depending on situation and fitness.
DISTAL NERVE TRANSFERS
The entire protocol given above is without considering the additional use of distal nerve transfers in upper plexus lesion. Following the description by Oberlin[45] and Somsak[46] of nerve transfers for elbow flexion and deltoid reinnervation, the whole landscape in adult plexus injury changed dramatically. This philosophy is now coming in play in the OBPI reconstruction as well in selected cases. Oberlin[45] described the use of motor fascicles of the ulnar nerve for reinnervation of the biceps via the MCN.
Subsequently, this has been modified to ulnar to biceps and median to brachialis. Somsak[46] described the use of the branch to long head of triceps for the anterior division of the axillary nerve to reinnervate the deltoid. The interesting point about distal transfers is the speed of neurotisation, since the nerve is coapted very close to the hilum of the muscle and the growing axons reach the end plates much faster.
In upper plexus lesions (C5, C6), these transfers allow for leaving the neuroma undisturbed and doing a triple transfer consisting of XI to SSN, Oberlin and Somsak transfers to reconstruct shoulder abduction and external rotation, as well as elbow flexion. If the C7 is involved, then the triceps is often not functioning and the Somsak transfer is not possible; it also often means wrist extension is affected. In such cases, ICNs can be used for the deltoid as described by Somsak[47] and intraplexal neurotisation can be used for the C7 posterior division to help reinnervate both triceps and wrist extension.
There is considerable disagreement about this. The two European doyens, Gilbert and Raimondi,[48] argue that if roots are available, it is preferable to do a classical reinnervation and reserve distal transfers for later use. This allows reinnervation of all muscles supplied by those trunks/cords in a proper manner and yet leaves the distal transfers in reserve in case of unfavourable outcome. As mentioned earlier, distal transfers can be done at a late stage as they reach the muscle very rapidly. There is also no conclusive evidence in the form of a properly conducted prospective study to show that distal transfers give better results compared to classical neurotisation in the long run.
In the Indian scenario, distal transfers have a great benefit of targeting select muscles with no visible function without cutting the neuroma. Thus, for late referrals where some movements are seen, these are not lost even temporarily due to resection of the neuroma. This has social implications in this country because many parents and grandparents are not willing to risk losing existing movements (however inadequate they may be) to gain an eventual better result. Gilbert has reported similar social taboos in the Middle East.
AUTHORS’ CHOICE
The authors prefer to explore every plexus irrespective of whether a distal transfer is subsequently planned [Figure 5]. If healthy roots are available, then we prefer to use them and keep distal transfers in reserve. Figure 6a–c shows function at 4 years after intraplexal neurotisation in infancy. Videos (www.ijps.org) show function in another child with Narakas Grade IV palsy following intraplexal neurotisation. However, if there is paucity of good roots or avulsion, then a judicious combination of intraplexus neurotisation and distal transfers is preferred. Videos (www.ijps.org) show the results of contralateral C7 transfer to medial cord in a root avulsion case. Ultimately, each surgeon has to be aware of all options and use his/her judgement to make informed choices. All the options given above are open to interpretation and individual modifications based on personal experience and the situation faced during surgery.
Figure 5.
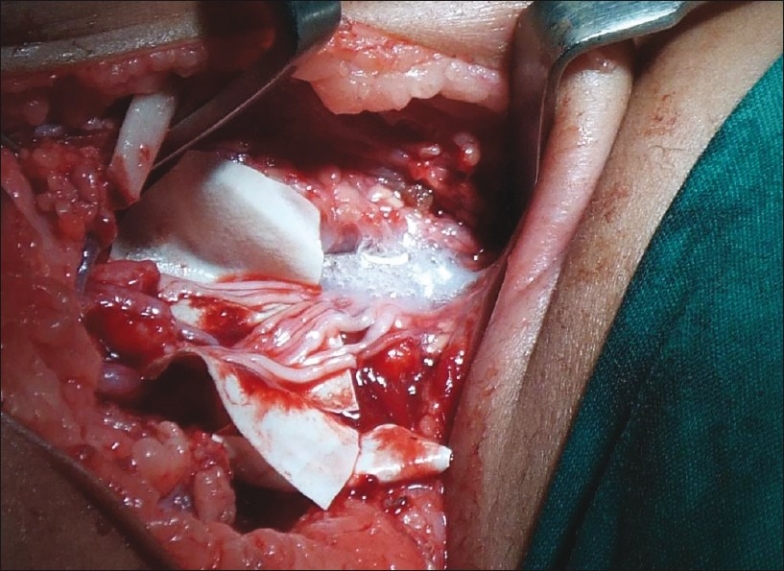
Exposed plexus with intraplexus repair using nerve grafts
Figure 6.
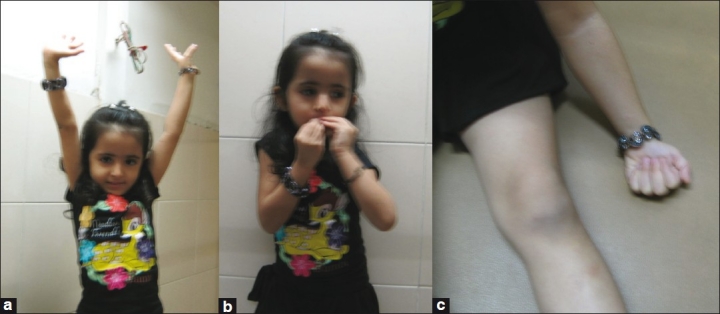
(a) Shoulder function; (b) elbow function; (c) hand function
SECONDARY SURGERY
Secondary surgery is performed to treat either untreated older children or as a follow-up to primary nerve reconstruction. Secondary surgery involves surgery on shoulder, elbow as well as the hand.
Shoulder
Typically, shoulder surgery is performed to treat an internal rotation (IR) contracture combined with poor abduction. This is typically caused by co-contraction of abductors with adductors and internal with external rotators. The deltoid and supraspinatus along with the trapezius and serratus anterior are involved in various stages of shoulder abduction. The infraspinatus and teres minor are external rotators, while four big muscles, viz. pectoralis major (PM), teres major (TM), latissimus dorsi (LD) and subscapularis (SS), are involved in adduction and IR. In the recovering plexus (both natural and in those with nerve reconstruction), there is a tendency of mix-up of the growing axons to target muscles. This results in co-contraction of these groups. In such a battle, the four big muscles mentioned above are the invariable winners leading to an IR contracture and an inability to abduct fully. If the anterior and posterior axillary folds of these children are felt during an attempted abduction, this co-contraction of one or both folds (containing the PM, TM and LD, respectively) can be easily felt and helps determine the choice of muscle to release and transfer. IR contracture, if allowed to persist, leads to glenoid deformation and posterior subluxation of the humeral head. It is advisable to prevent this by early surgery in these instances to restore function and avoid deformity. Mallet's score[49] is often used to assess the shoulder function before and after surgery [Table 3]. Gilbert[50] also used a scoring system for shoulder [Table 4]. The author in the same journal in the June 2011 issue[51] has published a detailed discussion on the shoulder surgery.
Table 3.
The Mallet score
Table 4.
Gilbert score for shoulder results

Elbow
Surgery is directed towards restoration of either flexion or extension. For elbow flexion, typical transfers are triceps to biceps and Steindler's flexoroplasty. Rarely, an LD transfer can be theoretically used, but the author has no experience of it in OBPI. For extension, the transfer of choice is deltoid to triceps. The posterior half of the deltoid is detached and extended with fascia lata to attach it to the triceps insertion.
Hand
Raimondi[52] [Table 5] describes an excellent system of assessing the hand. A plethora of tendon transfers can be used to improve hand function. They are based on standard tendon transfers done for peripheral nerve lesions and very valuable insights gained through the voluminous Indian work on leprosy tendon transfers. But there are a few caveats for transfers in the hand following OBPI, given as follows:
Table 5.
Raimondi score for hand function

OBPI cases have relatively weaker donors to achieve the desired function as the donor muscle too is often reinnervated. Full function, therefore, may not be achieved.
Children keep recovering hand function for 3–4 years both naturally as well as after nerve surgery (unless of course you know that a particular nerve is not targeted at all).
It is better to splint with appropriate splints in the interim.
Start transfers only after about 3.5–4 years to get optimum results.
DISCUSSION
OBPI has seen a renaissance since the 1980s. It is now clear it is not only Erb's palsy or C5, C6 injury. In a study by Gilbert et al.,[53] C5, C6 was damaged in 46.5% and C5, C6, C7 in 28.9%. All roots were damaged in 24.5% of which almost all were avulsions. In the authors’ own series, for those who underwent primary nerve surgery. C5, C6 Narakas Gr. I was 29%, C5,C6,C7 Narakas Group II was 24%, C5-T1 Narakas Group III was 40% and C5-T1 with Horner's Narakas Froup IV was 7%. Thus our data too showed the same fact.
It now appears quite clear that unless strong social and cultural factors (this can be very common in India where the family is not willing to temporarily lose any existing movement) intervene, it is better to resect a neuroma than to do neurolysis. This has been well documented by Clarke's group.[54] Several review articles have contributed to this discussion.[1,34,36,53–57] On the whole, everyone agrees about expert consultation in neonates, early diagnosis and follow-up and quick decision about nerve suirgery if the various assessment modalities, i.e. either Gilbert's or the AMS score from Toronto, indicate the need.
Assessment of results and each other's results has always been a problem. This makes the work of people doing systematic reviews very difficult.[1] Capek et al.[54] have shown that Mallet's score and the Toronto AMS are both reliable for documenting function, etc. In the authors’ view, the Raimondi Hand score[52] too is a very useful tool for assessment and documentation.
Finally we would conclude that surgery for OBPI is rewarding, early decisions are very helpful in long term results and that these children need to be seen early by experts in the field.
CONCLUSIONS
OBPI is a relatively neglected field in this country.
Currently it is showing one of the fastest growths amongst surgeons interested in nerve surgery.
Considering the incidence, the need and potential for doing some good is immense.
Early primary nerve surgery, when indicated, is strongly recommended.
In late referrals, distal nerve transfers and secondary reconstruction can yield useful results.
No child should be abandoned; there is always some useful function that can be improved.
The brachial plexus surgeon can never comment about medico-legal liability, as he/she is unaware of the emergency that could have developed intrapartum, which necessitated the traction manoeuvres.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Pondaag W, Malessy M, van Dijk JG, Thomeer R. Natural history of obstetric brachial plexus palsy: A systematic review. Dev Med Child Neurol. 2004;46:138–44. doi: 10.1017/s0012162204000258. [DOI] [PubMed] [Google Scholar]
- 2.Smellie W. Collection of preternatural cases and observations in Midwifery. Vol. 3. London: Wilson and Durham; 1764. [Google Scholar]
- 3.Duchenne GB. De l’Electrisation Localisee et de son Application a la Pathologie et a la Therapeutique. 3rd ed. Paris: Bailliere; 1872. [Google Scholar]
- 4.Erb WH. Ueber eine eigenthumliche localisation von lahmungen in plexus brachialis. Verh Dtsch Natur Med. 1874;2:130. [Google Scholar]
- 5.Greenwald AG, Schute PC, Shiveley JL. Brachial plexus birth palsy: A 10- year report on the incidence and prognosis. J Pediatr Orthop. 1984;4:689–92. doi: 10.1097/01241398-198411000-00006. [DOI] [PubMed] [Google Scholar]
- 6.van Ouwerkerk WJ, van der Sluijs JA, Nollet F, Barkhof F, Slooff AC. Management of obstetric brachial plexus lesions: State of the art and future developments. Childs Nerv Syst. 2000;16:638–44. doi: 10.1007/s003810000319. [DOI] [PubMed] [Google Scholar]
- 7.Waters PM. Comparison of the natural history, the outcome of microsurgical repair, and the outcome of operative reconstruction in brachial plexus birth palsy. J Bone Joint Surg [Am] 1999;81:649–59. doi: 10.2106/00004623-199905000-00006. [DOI] [PubMed] [Google Scholar]
- 8.Bradley WG, Daroff RB, Fenichel GM, Marsden CD. Neurology in Clinical Practice. 2nd ed. Boston: Butterworth- Heinemann; 1996. [Google Scholar]
- 9.Greenberg MS. Handbook of Neurosurgery. 5th ed. New York: Thieme; 2001. [Google Scholar]
- 10.Laurent JP, Lee RT. Birth-related upper brachial plexus injuries in infants: Operative and non-operative approaches. J Child Neurol. 1994;9:111–7. doi: 10.1177/088307389400900202. [DOI] [PubMed] [Google Scholar]
- 11.Painter MJ, Bergman I. Obstetrical trauma to the neonatal central and peripheral nervous system. Semin Perinatol. 1982;6:89–104. [PubMed] [Google Scholar]
- 12.Shenaq SM, Berzin E, Lee R, Laurent JP, Nath R, Nelson MR. Brachial plexus birth injuries and current management. Clin Plast Surg. 1998;25:527–36. [PubMed] [Google Scholar]
- 13.Terzis JK, Papakonstantinou KC. Management of obstetric brachial plexus palsy. Hand Clin. 1999;15:717–36. [PubMed] [Google Scholar]
- 14.Jennett RJ, Tarby TJ, Kreinick CJ. Brachial plexus palsy: An old problem revisited. Am J Obstet Gynecol. 1992;166:1673–6. doi: 10.1016/0002-9378(92)91555-o. [DOI] [PubMed] [Google Scholar]
- 15.Gherman RB, Murphy Goodwin T, Ouzounian JG, Miller DA, Paul RH. Brachial plexus palsy associated with cesarean section: An in utero injury? Am J Obstet Gynecol. 1997;177:1162–4. doi: 10.1016/s0002-9378(97)70034-6. [DOI] [PubMed] [Google Scholar]
- 16.Koenigsberger MR. Brachial plexus palsy at birth: Intrauterine or due to delivery trauma? Ann Neurol. 1980;8:228. [Google Scholar]
- 17.Dunn DW, Engle WA. Brachial plexus palsy: Intrauterine onset. Pediatr Neurol. 1985;1:367–9. doi: 10.1016/0887-8994(85)90074-8. [DOI] [PubMed] [Google Scholar]
- 18.Gilbert M, Nesbitt T, Danielsen B. Associated Factors in 1611 Cases of Brachial Plexus Injury. Obstet Gynecol. 1999;93:536–40. doi: 10.1016/s0029-7844(98)00484-0. [DOI] [PubMed] [Google Scholar]
- 19.Rouse DJ, Owen J, Goldenberg RL, Cliver SP. The effectiveness and costs of elective cesarean delivery for fetal macrosomia diagnosed by ultrasound. JAMA. 1996;276:1480–6. [PubMed] [Google Scholar]
- 20.Lindsay B, Kolderup LB, Laros RK, Musci TJ. Incidence of persistent birth injury in macrosomic infants: Association with mode of delivery. Am J Obstet Gynecol. 1997;177:37–42. doi: 10.1016/s0002-9378(97)70435-6. [DOI] [PubMed] [Google Scholar]
- 21.Sunderland S. Nerves and Nerve Injuries. London: Churchill Livingstone; 1978. [Google Scholar]
- 22.Bradley WG, Daroff RB, Fenichel GM, et al. Neurology in clinical practice. 2nd ed. Boston: Butterworth-Heinemann; 1996. [Google Scholar]
- 23.Michelow BJ, Clarke HM, Curtis CG, Zuker RM, Seifu Y, Andrews DF. The natural history of obstetrical brachial plexus palsy. Plast Reconstr Surg. 1994;93:675–80. [PubMed] [Google Scholar]
- 24.Nagano A, Ochiai N, Sugioka H, Hara T, Tsuyama N. Usefulness of myelography in brachial plexus injuries. J Hand Surg Br. 1989;14:59–64. doi: 10.1016/0266-7681(89)90017-x. [DOI] [PubMed] [Google Scholar]
- 25.Petras AF, Sobel DF, Mani JR, Lucas PR. CT myelography in cervical nerve root avulsion. J Comput Assist Tomogr. 1985;9:275–9. doi: 10.1097/00004728-198503000-00009. [DOI] [PubMed] [Google Scholar]
- 26.Piatt JH, Jr, Hudson AR, Hoffman HJ. Preliminary experiences with brachial plexus exploration in children: Birth injury and vehicular trauma. Neurosurgery. 1988;22:715–23. doi: 10.1227/00006123-198804000-00016. [DOI] [PubMed] [Google Scholar]
- 27.Popovich MJ, Taylor FC, Helmer E. MR imaging of birth-related brachial plexus avulsion. AJNR Am J Neuroradiol. 1989;10(Suppl 5):S98. [PMC free article] [PubMed] [Google Scholar]
- 28.Sherrier RH, Sostman HD. Magnetic resonance imaging of the brachial plexus. J Thorac Imag. 1993;8:27–33. [PubMed] [Google Scholar]
- 29.Urabe F, Matsuishi T, Kojima K, Abe T, Utsunomiya H, Okudera T. MR imaging of birth brachial palsy in a two-month-old infant. Brain Dev. 1991;13:130–1. doi: 10.1016/s0387-7604(12)80121-5. [DOI] [PubMed] [Google Scholar]
- 30.Vielvoye GJ, Hoffmann CF. Neuroradiological investigations in cervical root avulsion. Clin Neurol Neurosurg. 1993;95(Suppl):S36–8. doi: 10.1016/0303-8467(93)90033-d. [DOI] [PubMed] [Google Scholar]
- 31.Wehrli FW. Fast-scan magnetic resonance: Principles and applications. Magn Reson Q. 1990;6:165–236. [PubMed] [Google Scholar]
- 32.Gupta RK, Mehta VS, Banerji AK, Jain RK. MR evaluation of brachial plexus injuries. Neuroradiology. 1989;31:377–81. doi: 10.1007/BF00343859. [DOI] [PubMed] [Google Scholar]
- 33.Tassin JL. Thesis. Paris: Universite Paris VII; 1983. Paralysies obstetricales du plexus brachial: Evolution spontanee, resultats des interventions reparatrices precoces. [Google Scholar]
- 34.Michelow BJ, Clarke HM, Curtis CG, Zuker RM, Seifu Y, Andrews DF. The natural history of obstetrical brachial plexus palsy. Plast Reconstr Surg. 1994;93:675–81. [PubMed] [Google Scholar]
- 35.Clarke HM, Curtis CG. An approach to obstetrical brachial plexus injuries. Hand Clin. 1995;11:563–81. [PubMed] [Google Scholar]
- 36.Waters PM. Update on management of pediatric brachial plexus palsy. J Pediatr Orthop. 2005;25:116–26. doi: 10.1097/00004694-200501000-00025. [DOI] [PubMed] [Google Scholar]
- 37.Kennedy R. Suture of the brachial plexus in birth paralysis of the upper extremity. Br Med J. 1903;1:98–301. doi: 10.1136/bmj.1.2197.298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Sever JW. Obstetric paralysis: Its etiology, clinical aspects and treatment, with a report of four hundred and seventy cases. Arch Pediatr Adolesc Med. 1916;12:541–7. [Google Scholar]
- 39.Wyeth JA, Sharpe W. The field of neurological surgery in a general hospital. Surg Gynecol Obstet. 1917;24:29–36. [Google Scholar]
- 40.Gilbert A, Tassin JL. Reparation chirurgicale du plexus brachial dans la paralysie bstetricale. Chirurgie. 1984;110:70–5. [PubMed] [Google Scholar]
- 41.Narakas AO. Obstetrical brachial plexus injuries. In: Lamb DW, editor. The Paralysed Hand. Edinburgh: Churchill Livingstone; 1987. pp. 116–35. [Google Scholar]
- 42.Kawabata H, Masada K, Tsuyuguchi Y. Early microsurgical reconstruction in birth palsy. Clin Orthop. 1987;215:233–42. [PubMed] [Google Scholar]
- 43.Millessi H. Brachial plexus injuries: Nerve grafting. Clin Orthop. 1988;237:43–56. [PubMed] [Google Scholar]
- 44.Thatte MR, Agashe M, Rathod C, Lad P, Mehta R. An approach to the supraclavicular and infraclavicular aspects of the brachial plexus. Tech Hand Up Extrem Surg. 2011;15:188–97. doi: 10.1097/BTH.0b013e3182164b15. [DOI] [PubMed] [Google Scholar]
- 45.Oberlin C, Beal D, Leechavengvongs S, Salon A, Dauge MC, Sarcy JJ. Nerve transfer to biceps muscle using a part of ulnar nerve for C5/C6 avulsion of the brachial plexus. Anatomical study and report of 4 cases. J Hand Surg. 1994;19:232–7. doi: 10.1016/0363-5023(94)90011-6. [DOI] [PubMed] [Google Scholar]
- 46.Leechavengvongs S, Witoonchart K, Uerpairojkit C, Thuvasethakul P. Nerve transfer to deltoid muscle using the nerve to the long head of the triceps, part II: A report of 7 cases. J Hand Surg Am. 2003;28:633–8. doi: 10.1016/s0363-5023(03)00199-0. [DOI] [PubMed] [Google Scholar]
- 47.Malungpaishrope K, Leechavengvongs S, Uerpairojkit C, Witoonchart K, Jitprapaikulsarn S, Chongthammakun S. Nerve transfer to deltoid muscle using the intercostal nerves through the posterior approach: An anatomic study and two case reports. J Hand Surg Am. 2007;32:218–24. doi: 10.1016/j.jhsa.2006.12.005. [DOI] [PubMed] [Google Scholar]
- 48.Gilbert A, Raimondi P. Presentations in Club Narakas meeting Lisbon Mallet, J. Paralysie obstandricale. Revue de Chimrgie Orthopedique et Reparatrice de L’Appareil Moteur. 2011;58(Suppl):116. [Google Scholar]
- 49.Pagnotta A, Haerle M, Gilbert A. Long-term results on abduction and external rotation of the shoulder after latissimus dorsi transferfor sequelae of obstetric palsy. Clin Orthop Relat R. 2004;426:199–205. doi: 10.1097/01.blo.0000138957.11939.70. [DOI] [PubMed] [Google Scholar]
- 50.Thatte MR, Agashe MV, Rao A, Rathod CM, Mehta R. Clinical outcome of shoulder muscle transfer for shoulder deformities in obstetric brachial plexus palsy: A study of 150 cases. Indian J Plast Surg. 2011;44:21–8. doi: 10.4103/0970-0358.81441. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Raimondi P. Presented at the International Meeting on Obstetric Brachial Plexus Palsy. The Netherlands: Heerlen; 1993. Evaluation of results in obstetric brachial plexus palsy: The hand. [Google Scholar]
- 52.Gilbert A. Whitaker I. Obstetrical brachial plexus lesions, J Hand Surg. 1991;16B:489–91. doi: 10.1016/0266-7681(91)90100-3. [DOI] [PubMed] [Google Scholar]
- 53.Capek L, Clarke HM, Curtis CG. Neuroma-in-continuity resection: Early outcome in obstetrical brachial plexus palsy. Plast Reconstr Surg. 1998;102:1555–62. doi: 10.1097/00006534-199810000-00032. [DOI] [PubMed] [Google Scholar]
- 54.Hale HB, Bae DS, Waters PM. Current concepts in the management of brachial plexus birth palsy. J Hand Surg. 2010;35A:322–31. doi: 10.1016/j.jhsa.2009.11.026. [DOI] [PubMed] [Google Scholar]
- 55.Borschel GH, Clarke HM. Obstetrical Brachial Plexus Palsy Plast. Reconstr Surg. 2009;124(Suppl):144e. doi: 10.1097/PRS.0b013e3181a80798. [DOI] [PubMed] [Google Scholar]
- 56.Bahm J, Ocampo-Pavez C, Disselhorst-Klug C, Sellhaus B, Weis J. Obstetric Brachial Plexus Palsy, Treatment Strategy, Long-Term Results, and Prognosis. Dtsch Arztebl Int. 2009;106:83–90. doi: 10.3238/arztebl.2009.0083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Bae DS, Waters PM, Zurakowski D. Reliability of three classification systems measuring Active motion in brachial plexus birth palsy. J Bone Joint Surg Am. 2003;85-A:1733–8. doi: 10.2106/00004623-200309000-00012. [DOI] [PubMed] [Google Scholar]


