Abstract
Aims:
Cranial vault reconstruction can be performed with a variety of autologous or alloplastic materials. We describe our experience using high-density porous polyethylene (HDPE) cranial hemisphere for cosmetic and functional restoration of skull defects. The porous nature of the implant allows soft tissue ingrowth, which decreases the incidence of infection. Hence, it can be used in proximity to paranasal sinuses and where previous alloplastic cranioplasties have failed due to implant infection.
Materials and Methods:
We used the HDPE implant in seven patients over a three-year period for reconstruction of moderate to large cranial defects. Two patients had composite defects, which required additional soft tissue in the form of free flap and tissue expansion.
Results:
In our series, decompressive craniectomy following trauma was the commonest aetiology and all defects were located in the fronto-parieto-temporal region. The defect size was 10 cm on average in the largest diameter. All patients had good post-operative cranial contour and we encountered no infections, implant exposure or implant migration.
Conclusions:
Our results indicate that the biocompatibility and flexibility of the HDPE cranial hemisphere implant make it an excellent alternative to existing methods of calvarial reconstruction.
KEY WORDS: Calvarial defect, Cranioplasty, high-density porous polyethylene cranial hemispheres
INTRODUCTION
Bony defects in the cranial skeleton can occur as a result of congenital defects, acquired injuries or ablative tumour resection. In the present day scenario, road traffic accidents and combat-associated craniomaxillofacial injuries are on the rise. Increased survival because of body armour and advanced emergency and trauma medicine has resulted in patients once not considered amenable for survival being aggressively treated. These patients are surviving devastating head injuries and ultimately require reconstructive surgery. Reconstruction of the calvarium is required for both cosmetic and functional reasons, i.e., mechanical protection of the brain and prevention of low pressure syndrome.[1] Since successful spontaneous calvarial reconstruction only occurs in infants younger than two years of age,[2] a variety of materials have been proposed to restore such defects including autogenous bone grafts, allogenic banked bone, alloplastic materials (such as calcium ceramics, polymers, etc.) and tissue-engineered bone scaffolds seeded with osteoprogenitor cells and growth factors.[3] The multitude of methods reflects that each technique has its own shortcomings as well as the need for new and improved treatment options.[4]
We chose the high-density porous polyethylene (HDPE) implant since[5–11] the porous nature of the implant allows soft tissue ingrowth, there is less incidence of infection, it can be used in proximity to paranasal sinuses and it is also feasible where previous alloplastic cranioplasties have failed due to infection.
Furthermore, using the HDPE cranial hemisphere implant with its pre-formed convexity makes the reconstruction simpler and faster to perform than using sheets and is particularly useful for moderate to large skull defects.[12]
MATERIALS AND METHODS
We used the HDPE implant in seven patients over a three-year period, from October 2007 to December 2010, for reconstruction of moderate to large cranial defects (>8 cm). Cranial defects were classified as small, medium and large in respect of their largest diameter,[5] which is the most important parameter particularly for stability [Table 1].
Table 1.
Size of calvarial defect
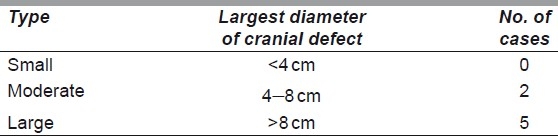
All of our patients were males aged 19 to 55 years. Decompressive craniectomy following trauma was the commonest aetiology. All defects were located in the fronto-temporo-parietal region of the skull. All cases underwent cranioplasty as a secondary reconstruction. The average interval following the initial surgery was 5 months (range, 3–9 months). The recommended interval following the last neurosurgery is 3 to 6 months in order to allow intracranial pressure to return to normal, the tissue bed to mature and decrease chances of infection.[13] Computed tomography (CT) scan with 3D reconstruction was the imaging modality of choice for accurate evaluation of the bony defect.
A detailed written informed consent was obtained from all patients after explaining the various options for cranioplasty and the nature, merits and possible adverse effects of the implant. All the procedures were performed under general anaesthesia and preceded by the infiltration of a solution containing adrenaline (1 in 5 lacs dilution) and hyaluronidase (1500 IU) in 500 cc Ringer's lactate. The infiltration serves the dual purpose of achieving haemostasis and creating a plane of dissection between the dura and overlying tightly adherent skin, scar tissue and in one case–skin graft.
Operative details
In order to avoid placing the implant directly under the surgical incision, the most common approach used was the bicoronal approach in four patients (57%) and a new incision in one patient (14%), although we did place it through the pre-existing incision in two patients (29%). In all cases, we used the HDPE cranial hemispheres that were further shaped as per the defect requirements with Mayo scissors or scalpel to a size slightly larger than the defect, so that it does not dip into the defect and produce a visible step in the contour. Implant edges were feathered with a scalpel blade to obtain a smooth contour to the surrounding bone or a high-speed drill may be used to create a shelf at the edge of the craniotomy defect. Additional moulding of the convexity can be achieved by placing the implant in a hot sterile bath for several minutes. Fixation was performed with titanium miniplates and screws or polydiaxone sutures [Figure 1a]. In very large defects, dural hitch sutures are taken between the dura and pericranium/galea which will hold the dura tightly against the bone/implant to prevent accumulation of fluid/haematoma in the extradural space. For the surgical closure, well-vascularised tissue was used for obliteration of dead space and for implant coverage. A galeopericranial flap [Figure 1b] was used in five patients (71%), one patient had previous free latissimus dorsi (LD) flap which was re-draped over the implant and one patient had tissue-expanded scalp flaps rotated and transposed over the implant.
Figure 1a.
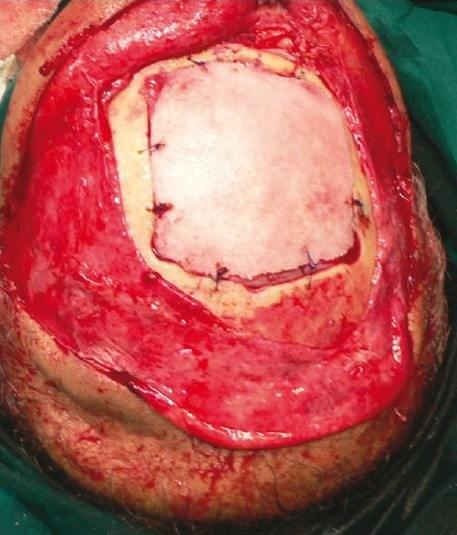
Fixation of Medpor implant to edges of the skull defect with PDS sutures
Figure 1b.
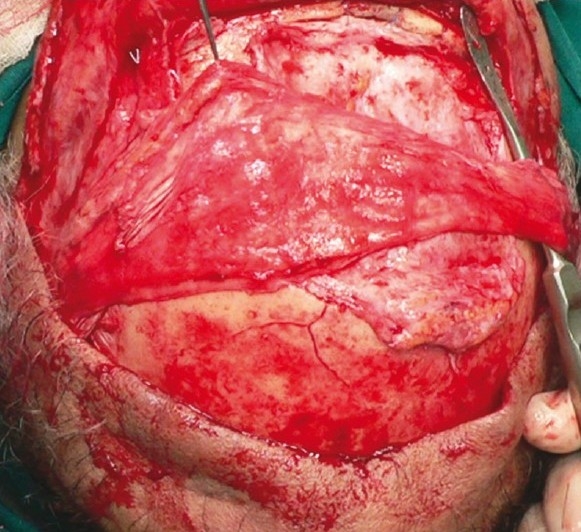
Bipedicled galeopericranial flap raised and transposed over the implant
All patients received pre-operative antibiotic prophylaxis. The antibiotic was continued for seven to ten days post-operatively. The average hospital stay was seven days. The patients were followed up to observe for any complications or complaints related to the implant. The average follow-up period was 18 months (longest–3 years and shortest–6 months).
RESULTS
The HDPE cranial hemisphere was used in seven patients, following neurosurgeries that required cranioplasty. In our study, the implant was most commonly used after a partial/hemi-craniectomy for post-traumatic decompression as a delayed secondary reconstruction. Cranial bony defect localisation can be seen in Table 2.
Table 2.
Location of cranial bone defects
The defect sizes were 10 cm on average in the greatest dimension, range 7 to 19 cm. Requirements of each defect were evaluated in terms of bone and soft tissue cover [Table 3].
Table 3.
Bone/soft tissue requirement of the defect

We had good aesthetic results [Figures 2a and b] with no patient dissatisfaction or complications such as infection, implant exposure, etc., in the post-operative period.
Figure 2a.
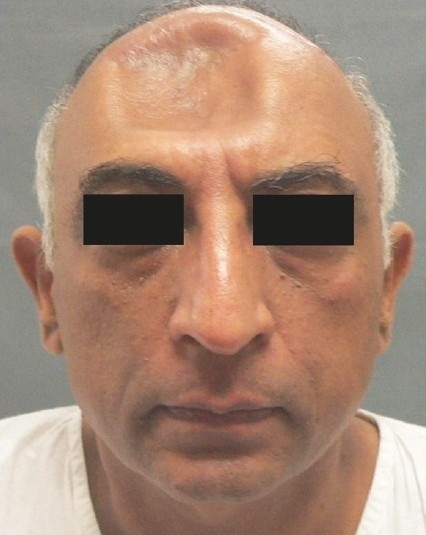
Pre-operative view of patient with frontal craniectomy defect following transpalatal bullet injury
Figure 2b.
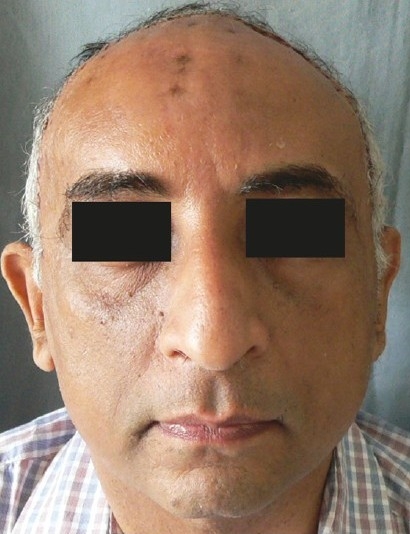
Good long-term post-operative result of the same patient at 1½ year
Case report 1
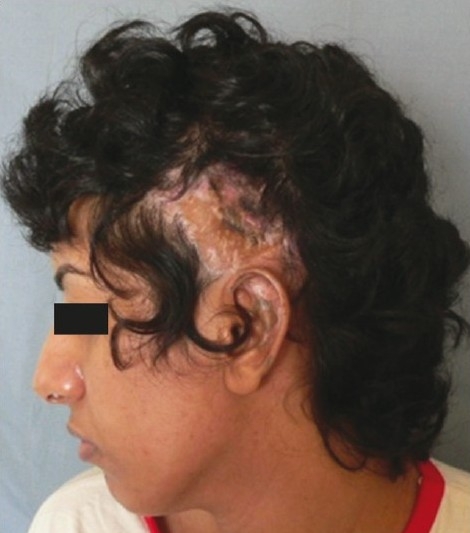
This patient had a post-decompressive craniectomy defect in the frontal region [Figure 3a], had undergone three previous cranioplasties, once with his own banked bone flap and twice with alloplastic materials (polymethylmethacrylate [PMMA] and silicone), all of which had got infected and needed removal. He presented to us with a persistent discharging sinus and tethered forehead skin [Figure 3b]. On exploration, a piece of polypropylene (Prolene) mesh, which is often the backing for silicone implants, was found in the sinus tract [Figure 3c] and removed and the defect filled with a local galeopericranial flap. In view of the post-surgical scarring, we felt that the soft tissue quality was too poor for stable implant coverage. Hence, we opted for a free LD musculocutaneous flap to restore the skin deficit and provide bulk to fill in the dead space and simulate bony contour [Figures 4a and b]. The problem that followed was repeated sagging of the flap due to its inherent bulk and lack of periosteal attachments despite two revision procedures. Therefore, a final decision was taken to place an HDPE implant under cover of the free flap [Figure 5a and b]. Late post-operative results after final stage of reconstruction are seen in Figure 6.
Figure 3.
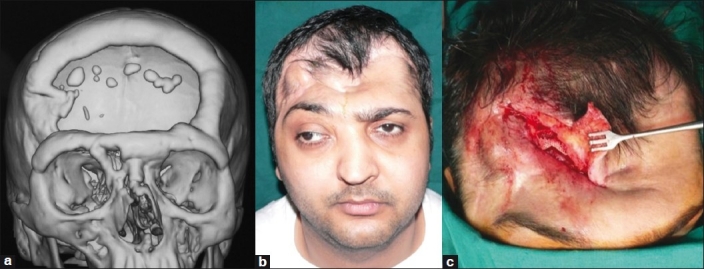
(a) Three-dimensional CT scan showing large frontal bone defect in a case of complicated cranioplasty, (b) Pre-operative view of the patient showing puckered forehead skin with sinus, (c) Intra-operative photo showing remnant of prolene mesh
Figure 4a.
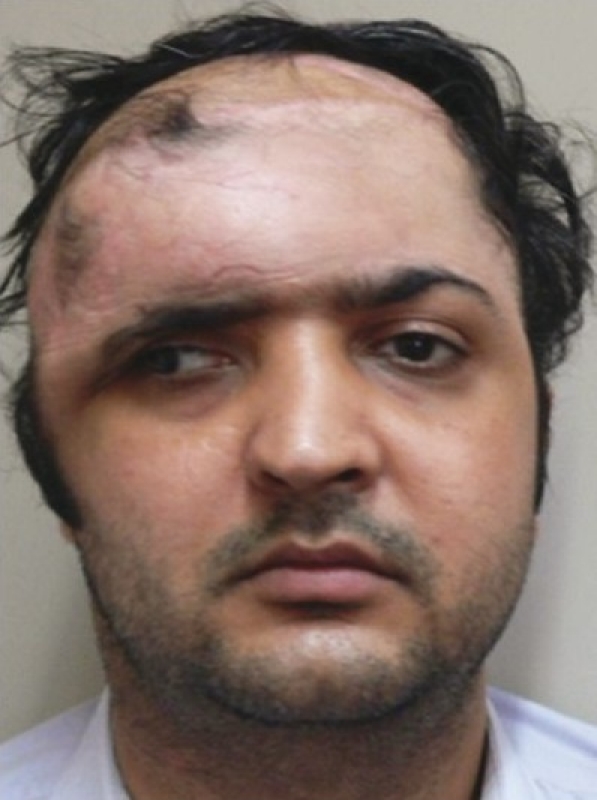
Post-operative result following free LD flap with sagging of forehead over the right eyebrow
Figure 4b.
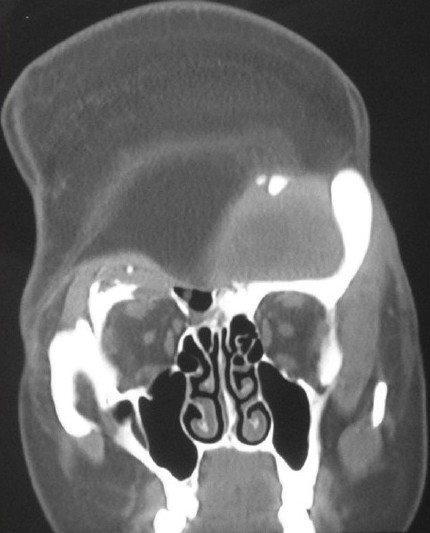
Post-operative CT scan showing excess bulk of the LD flap
Figure 5a.
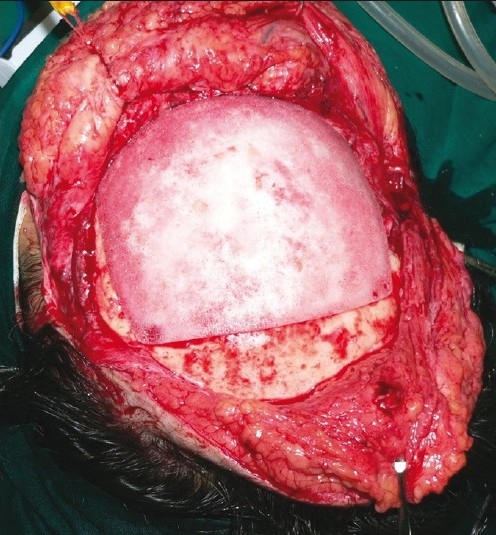
Intra-operative view of Medpor cranial hemisphere in situ
Figure 5b.
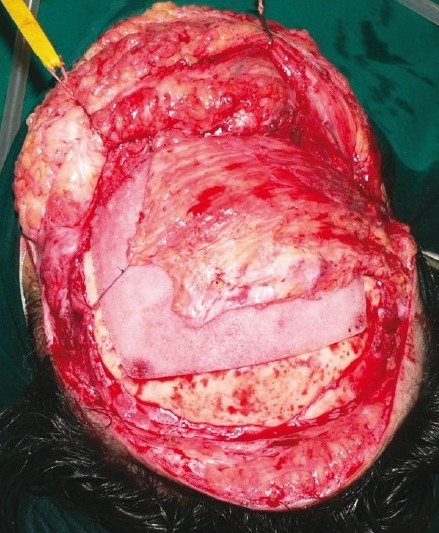
LD muscle re-draped over the implant for soft-tissue coverage
Figure 6.
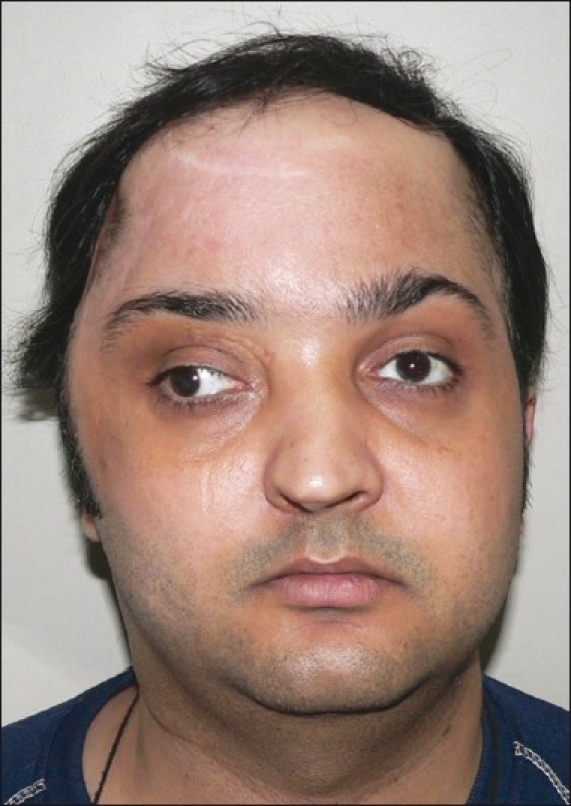
Early post-operative result at one year showing stable contour (frontal view)
Case report 2
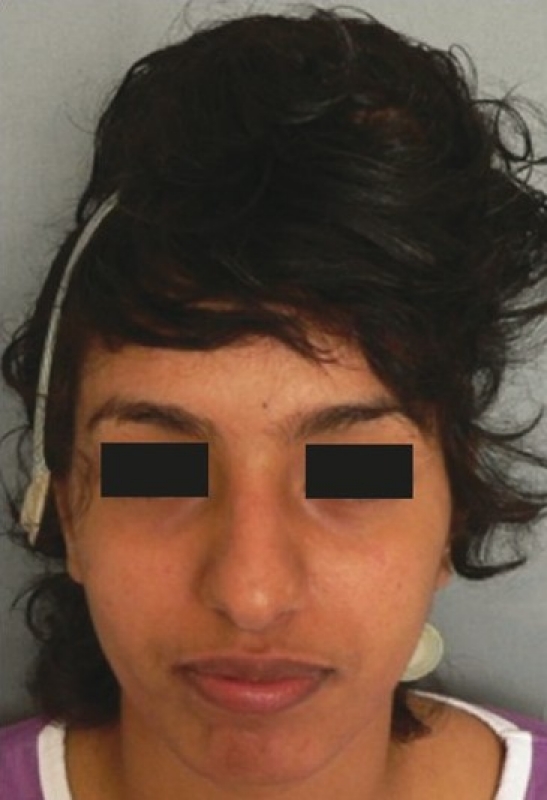
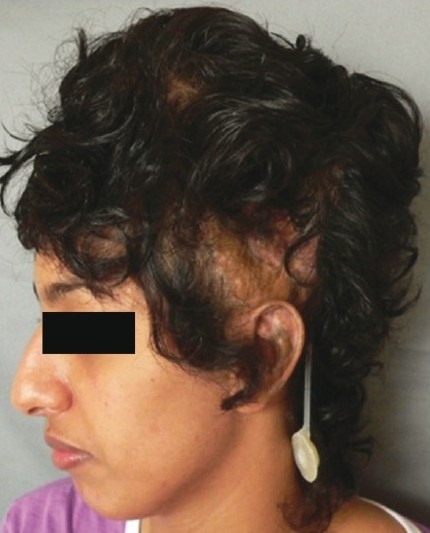
The patient had a post-traumatic composite skull defect with skin graft placed directly over the dura. She initially presented with a cerebrospinal fluid leak and unstable scar which were managed conservatively [Figures 7a and b]. Six months after the wounds had settled, we planned a secondary reconstruction of her composite defect with tissue expansion to provide skin cover and HDPE to replace bone. Two tissue expanders were placed as shown in Figures 8a and b. Serial tissue expansion was carried out over a period of eight weeks [Figures 9a and b]. At the time of final surgery, hydrodissection was essential prior to reflecting the skin graft without breaching the underlying adherent dura. A posteriorly based scalp flap was transposed and a laterally based flap was advanced to cover the HDPE implant placed in the calvarial defect [Figures 10a and b]. Post-operative results show excellent cosmetic restoration of the hair-bearing scalp [Figures 11a and b].
Figure 7a.
CT scan of patient with left temporal region composite defect
Figure 7b.
Pre-operative view of patient showing skin graft with unstable scar on left temple
Figure 8a.
Crescentric tissue expander placed in left parietal region through radial incision near midline
Figure 8b.
Rectangular tissue expander placed in left mastoid region
Figure 9a.
Serial tissue expansion over 8 weeks (frontal view)
Figure 9b.
Lateral view of tissue expanders in situ showing external ports
Figure 10a.
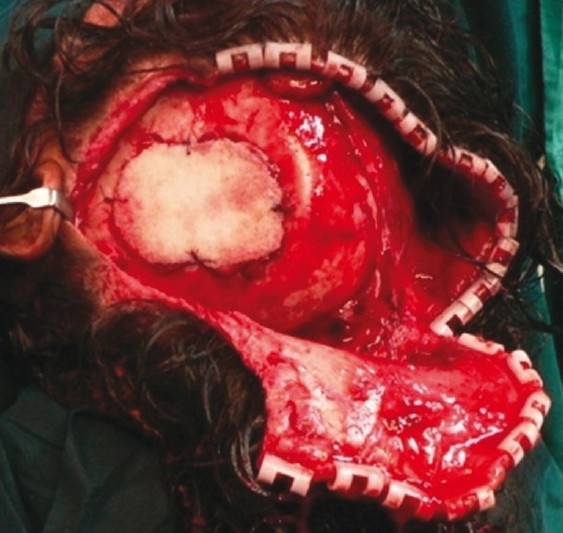
Medpor implant contoured to defect size
Figure 10b.
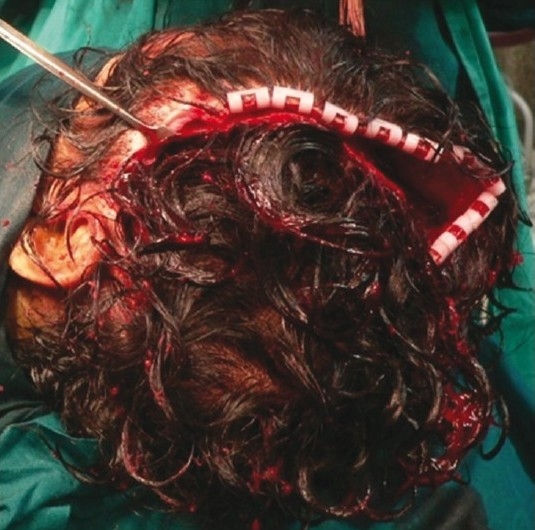
Scalp flaps transposed/advanced over the implant
Figure 11a.
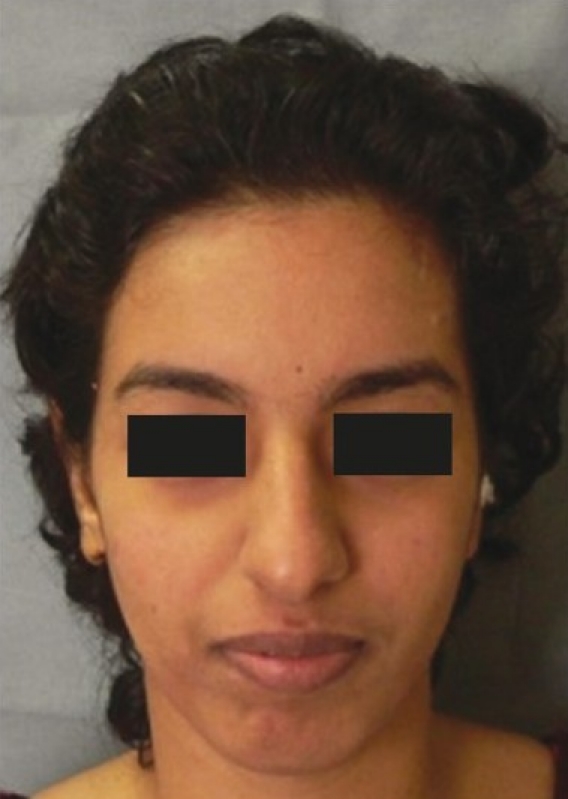
Post-operative frontal view at 6 months
Figure 11b.
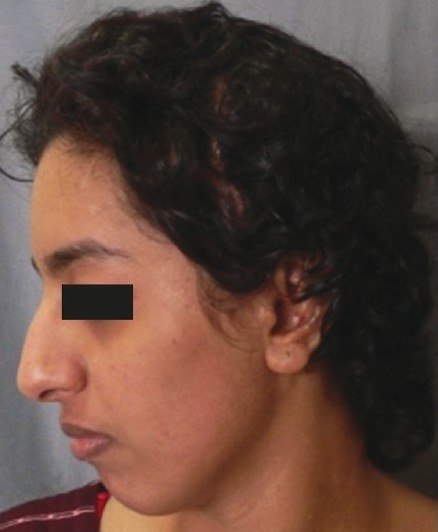
Post-operative frontal view at 6 months
DISCUSSION
Trauma is the predominant aetiology for skull defects as seen in our study and corroborated by various authors.[9,11,14] Whatever the cause, these defects lead to functional (neurologic) debility, social embarrassment due to disfigurement and an economic burden. The need for their reconstruction has lead to the development of many reconstructive techniques using autografts, allografts and various alloplastic materials.
The donor sites for autogenous materials include split rib grafts, iliac crest bone graft from the inner table and split calvarial bone graft from the inner or outer table.
Though autogenous bone grafts are the ‘gold standard’ for craniomaxillofacial reconstruction,[15] they have several disadvantages including donor site morbidity, graft resorption, additional operative time and insufficient donor resources, especially in very large defects.[16,17]
Allogenic bone carries the risk of disease transmission, immunological reactions[18] and possibly graft-versus-host disease.
The options for alloplastic materials include the following:
Polymers-PMMA, silicone, HDPE (Medpor), polyether-ether ketone
Calcium ceramics-calcium hydroxyapatite (HA), calcium phosphates
Titanium mesh
The properties of an ideal bone substitute include[11] biocompatibility, ease of moulding to the defect with fast setting time, durability, radiolucency to allow radiographic assessment, non-conductivity, easily available, sterilisable, inexpensive, osteoconductive and osteoinductive.
However, alloplastic materials such as those mentioned above are not the most appropriate options for the paediatric or adolescent patient.[19] As the cranial vault of these patients undergoes further ossification and remodelling, autogenous or allogenic bone grafts are a better option because of their capacity for osseointegration and grow with the child's skull.[20,21]
One of the most widely used alloplastic materials is PMMA, also known as bone cement. However, PMMA has many disadvantages including[3,16,22] the exothermic reaction produced during the curing process and significant rate of infection when used adjacent to the paranasal sinuses.
Other types of bone cement are calcium phosphate and HA which have advantages such as biocompatibility and osteoconductivity.[23] However, because of the unacceptably high complication rate with the use of calcium-based bone cements in large skull defects, many authors believe that their use is contraindicated.[16,24]
Titanium mesh is highly inert, easily shaped and allows tissue integration.[3,4] However, it is radio-opaque and produces image artefacts on post-operative CT and MRI scans, making it less preferred to radiolucent alloplasts.[9]
HDPE is composed of HDPE microspheres that are sintered to create a framework of interconnecting pores approximately 100 to 250 μm in diameter. It is available in a variety of shapes and sizes. For calvarial reconstruction, cranial hemispheres whose shape approximates the contour of a half cranium are available. They have a 4-6 mm thickness and are available in left and right versions.[12] They are particularly useful in the frontal and parietotemporal regions where excessive bending of the HDPE sheets is needed to match the skull curvature and give a good cosmetic result. In large skull defects (>8 cm), too much bending of the implant also decreases the strength of the construct. Customised implants are created from high-resolution, three-dimensionally reconstructed CT scans. They are currently very expensive and hence not commonly used. Cranial hemispheres provide a cheaper, off-the-shelf alternative to these customised implants.[25]
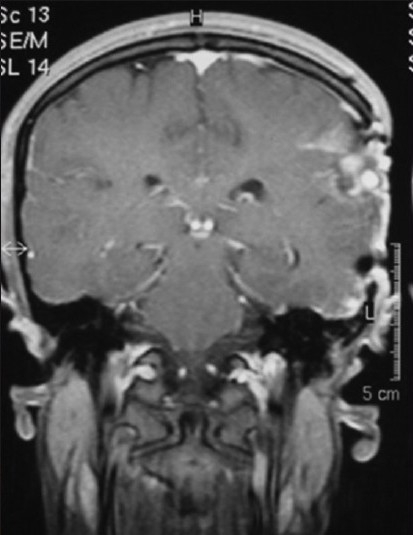
The HDPE implant material is easy to use, can be carved with sharp instruments, sheets can be easily cut with scissors, sutures can be passed through it and it is easily stabilised with miniplates and screws.[26,27] It remains stable after many years of use in human beings with studies having a follow-up period of more than 30 years.[28,29] These characteristics make the surgical procedure simpler than with other cranioplasty materials. The simplicity of implantation shortens the operative time, as verified by Hong et al. in a study comparing porous polyethylene implant with PMMA. The absolute operation times were shorter using the HDPE implant and the differences were statistically significant (P=0.030).[12] The radiolucent property of HDPE is an advantage while doing post-operative neuroimaging with CT or MRI scans[22] [Figure 12].
Figure 12.
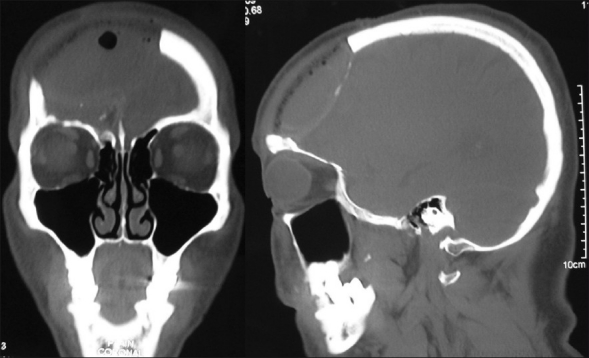
Post-operative CT scans illustrating the radiolucent property of Medpor implant
The large and stable pores of HDPE promote a rapid bony and fibrous ingrowth into the implant which in turn minimises capsule formation, anchors the implant and maintains the local host immune response.[30,31] Couldwell et al. used scanning electron microscopy to conclusively demonstrate the ingrowth of fibrovascular host tissue into the Medpor implant after 3 months.[5] Several studies have found that there was bleeding from the cut surface of the implant during revision surgery.[8,32,33] Hong et al. did serial follow-up CT images of patients which showed an increase in the Hounsfield (HF) units of the implant, particularly at the marginal areas which was considered to be indirect evidence for the ingrowth of vascularity, soft tissue and bone from the bony edge of the defect.[12] Thus, the vascular ingrowth protects the implant from infection and the several millimetres of bone ingrowth into the implant serves as a stable interface with a high tensile strength that anchors the implant.[30,34] This property also makes HDPE safe in reconstruction adjacent to the paranasal sinuses which is advantageous while reconstructing the frontal bone.[9,28] Mucosal overgrowth occurs even after post-operative radiation therapy.[9]
Well-vascularised tissue should be used for obliteration of dead space and implant coverage to minimise chances of exposure post-operatively in case of wound dehiscence.[35,36] A galeopericranial flap serves this purpose and was used in 5 out of 7 patients in our series (71%). The implant may be placed deep to temporalis muscle, tissue-expanded local flaps or free flaps, as the situation demands. Reduced blood supply of the scalp skin following repeated surgeries necessitated a prior free flap in one patient which was re-draped over the implant at the time of HDPE insertion. One patient had a composite defect involving skin and bone for which tissue expansion was carried out. This has the added advantage of increasing scalp vascularity, providing a thick capsule for implant cover and restoration of hair-bearing skin. It is particularly useful after wound infection, because of scalp atrophy, poor nutritional condition and tense sutures.[37] As far as possible, the surgical incision should be remote from the underlying implant. Thus, in the event of wound gape, the chances of implant exposure are reduced. To this end, we have used the bicoronal approach in the majority of patients (four out of seven). HDPE is safe to use even when previous alloplast cranioplasties have failed, as seen in our first case report. Hong et al. had a similar experience with a patient with two cranioplasties and two revision operations owing to infection. But his third trial was successful with HDPE, without infection.[12]
Overall, our patients were satisfied with the aesthetic results of the alloplast augmentation, as evaluated during post-operative follow-ups, over an 18-month period on average (range, 3 years to 6 months). We have achieved excellent cosmetic results and no implant-related complications such as infection, extrusion, migration or exposure of the implant. Our experience is confirmed by various authors including Romano et al.[6] (140 cases of open facial fractures), Yaremchuk[8] (370 facial implants over 11 years, Liu et al.[9] (611 cases of small to medium cranioplasties with HDPE), Couldwell et al.[5] (25 cranioplasties) and Hong et al.[12] (10 patients of cranioplasty) who have reported good results with no implant-related complications.
The only potential drawback with HDPE is that the ingrowth of soft tissue into the inner surface of the implant may render removal difficult in cases that demand re-operation, though there are no reports of any experience with this issue.[5] A possible solution is to coat the inner surface of the implant to selectively prevent dural adherence.
Lastly, the future of cranioplasty lies in tissue engineering using bone morphogenic proteins,[38] osteoprogenitor cells and tissue scaffolds to stimulate new bone formation.[3,39] This holds the promise of overcoming the disadvantages of both autogenous and alloplastic materials for cranial bone reconstruction.
CONCLUSIONS
The properties of the HDPE implant make it an easy, safe and effective alternative to the existing methods of cranial contour correction. This method provides similar cosmetic results to standard alloplast cranioplasty with no donor site morbidity, excellent and stable contour enhancement, decreased risk of infection due to soft tissue ingrowth and minimal long-term complication rate. The HDPE cranial hemispheres further save time and cost, give better strength and a superior aesthetic result while performing large and/or complex cranial vault reconstructions.
ACKNOWLEDGEMENT
Dr. Charudutta Choudhary for doing the free latissimus dorsi flap.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.Schiffer J, Gur R, Nisim U, Pollak L. Symptomatic patients after craniectomy. Surg Neurol. 1997;47:231–7. doi: 10.1016/s0090-3019(96)00376-x. [DOI] [PubMed] [Google Scholar]
- 2.Fong KD, Warren SM, Loboa EG, Henderson JH, Fang TD, Cowan CM, et al. Mechanical strain affects dura mater biological processes: Implications for immature calvarial healing. Plast Reconstr Surg. 2003;112:1312–27. doi: 10.1097/01.PRS.0000079860.14734.D6. [DOI] [PubMed] [Google Scholar]
- 3.Neovius E, Engstrand T. Craniofacial reconstruction with bone and biomaterials: Review over the last 11 years. J Plast Reconstr Aesthet Surg. 2010;63:1615–23. doi: 10.1016/j.bjps.2009.06.003. [DOI] [PubMed] [Google Scholar]
- 4.Rubin JP, Yaremchuk MJ. Complications and toxicities of implantable biomaterials used in facial reconstructive and aesthetic surgery: A comprehensive review of the literature. Plast Reconstr Surg. 1997;100:1336–53. doi: 10.1097/00006534-199710000-00043. [DOI] [PubMed] [Google Scholar]
- 5.Couldwell WT, Chen TC, Weiss MH, Fukushima T, Dougherty W. Cranioplasty with the Medpor porous polyethylene Flexblock implant. Technical note. J Neurosurg. 1994;81:483–6. doi: 10.3171/jns.1994.81.3.0483. [DOI] [PubMed] [Google Scholar]
- 6.Romano JJ, Iliff NT, Manson PN. Use of Medpor porous polyethylene implants in 140 patients with facial fractures. J Craniofac Surg. 1993;4:142–7. doi: 10.1097/00001665-199307000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Frodel JL, Lee S. The use of high-density polyethylene implants in facial deformities. Arch Otolaryngol Head Neck Surg. 1998;124:1219–23. doi: 10.1001/archotol.124.11.1219. [DOI] [PubMed] [Google Scholar]
- 8.Yaremchuk MJ. Facial skeletal reconstruction using porous polyethylene implants. Plast Reconstr Surg. 2003;111:1818–27. doi: 10.1097/01.PRS.0000056866.80665.7A. [DOI] [PubMed] [Google Scholar]
- 9.Liu JK, Gottfried ON, Cole CD, Dougherty W, Couldwell WT. Porous polyethylene implant for cranioplasty and skullbase reconstruction. Neurosurg Focus. 2004;16:ECP1. doi: 10.3171/foc.2004.16.3.14. [DOI] [PubMed] [Google Scholar]
- 10.Menderes A, Baytekin C, Topcu A, Yilmaz M, Barutcu A. Craniofacial reconstruction with high-density porous polyethylene implants. J Craniofac Surg. 2004;15:719–24. doi: 10.1097/00001665-200409000-00004. [DOI] [PubMed] [Google Scholar]
- 11.Cenzi R, Farina A, Zuccarino L, Carinci F. Clinical outcome of 285 Medpor grafts used for craniofacial reconstruction. J Craniofac Surg. 2005;16:526–30. doi: 10.1097/01.scs.0000168761.46700.dc. [DOI] [PubMed] [Google Scholar]
- 12.Hong KS, Kang SH, Lee JB, Chung YG, Lee HK, Chung HS. Cranioplasty with the Porous Polyethylene Implant (Medpor) for Large Cranial Defect. J Korean Neurosurg Soc. 2005;38:96–101. [Google Scholar]
- 13.Manson PN, Crawley WA, Hoopes JE. Frontal cranioplasty: Risk factors and choice of cranial vault reconstructive material. Plast Reconstr Surg. 1986;77:888–904. [PubMed] [Google Scholar]
- 14.Foustanos AP, Anagnostopoulos D, Kotsianos G, Rapidis AD. Cranioplasty: A review of 10 cases. J Maxillofac Surg. 1983;11:83–6. doi: 10.1016/s0301-0503(83)80020-4. [DOI] [PubMed] [Google Scholar]
- 15.Salyer KE, Taylor DP. Bone grafts in craniofacial surgery. Clin Plast Surg. 1987;14:27–35. [PubMed] [Google Scholar]
- 16.Maas CS, Merwin GE, Wilson J, Frey MD, Maves MD. Comparison of biomaterials for facial bone augmentation. Arch Otolaryngol Head Neck Surg. 1990;116:551–6. doi: 10.1001/archotol.1990.01870050051005. [DOI] [PubMed] [Google Scholar]
- 17.Moreira-Gonzalez A, Jackson IT, Miyawaki T, Barakat K, DiNick V. Clinical outcome in cranioplasty: Critical review in long-term follow-up. J Craniofac Surg. 2003;14:144–53. doi: 10.1097/00001665-200303000-00003. [DOI] [PubMed] [Google Scholar]
- 18.Breidahl AF, Geofrey L, Holmes AD. Allogeneic Membranous Bone Grafting for a Large Calvarial Defect. Plast Reconstr Surg. 2005;115:43–9e. doi: 10.1097/01.prs.0000153041.69937.10. [DOI] [PubMed] [Google Scholar]
- 19.Josan VA, Sgouros S, Walsh AR, Dover MS, Nishikawa H, Hockley AD. Cranioplasty in children. Childs Nerv Syst. 2005;21:200–4. doi: 10.1007/s00381-004-1068-2. [DOI] [PubMed] [Google Scholar]
- 20.Brevi BC, Magri AS, Toma L, Sesenna E. Cranioplasty for repair of a large bone defect with autologous and homologous bone in children. J Pediatr Surg. 2010;45:E17–20. doi: 10.1016/j.jpedsurg.2010.01.011. [DOI] [PubMed] [Google Scholar]
- 21.Gosain AK, Chim H, Arneja JS. Application-Specific Selection of Biomaterials for Pediatric Craniofacial Reconstruction: Developing a Rational Approach to Guide Clinical Use. Plast Reconstr Surg. 2009;123:319–30. doi: 10.1097/PRS.0b013e318193478c. [DOI] [PubMed] [Google Scholar]
- 22.Couldwell WT, Stillerman CB, Dougherty W. Reconstruction of the skull base and cranium adjacent to sinuses with porous polyethylene implant: Preliminary report. Skull Base Surg. 1997;7:57–63. doi: 10.1055/s-2008-1058609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gosain AK, Song L, Riordan P, Amarante MT, Nagy PG, Wilson CR, et al. A 1-year study of osteoinduction in hydroxyapatite-derived biomaterials in an adult sheep model: Part I. Plast Reconstr Surg. 2002;109:619–30. doi: 10.1097/00006534-200202000-00032. [DOI] [PubMed] [Google Scholar]
- 24.Zins JE, Moreira-Gonzalez A, Papay FA. Use of Calcium-Based Bone Cements in the Repair of Large, Full-Thickness Cranial Defects: A Caution. Plast Reconstr Surg. 2007;120:1332–42. doi: 10.1097/01.prs.0000279557.29134.cd. [DOI] [PubMed] [Google Scholar]
- 25.Implant far cranial defects - Product Marketplace - Cranial Hemispheres from MEDPOR Implants - Advertising - Brief Article. Ear, Nose and Throat Journal. 2002 Apr [Google Scholar]
- 26.Wellisz T, Dougherty W, Gross J. Craniofacial applications for the Medpor porous polyethylene Flexblock implant. J Craniofac Surg. 1992;3:101–7. doi: 10.1097/00001665-199209000-00009. [DOI] [PubMed] [Google Scholar]
- 27.Haug RH, Likavec MJ. A lag screw technique for fixation of cranioplasty implants. J Oral Maxillofac Surg. 1992;50:1343–4. doi: 10.1016/0278-2391(92)90243-s. [DOI] [PubMed] [Google Scholar]
- 28.Dougherty W, Wellisz T. The natural history of alloplastic implants in orbital floor reconstruction: An animal model. J Craniofac Surg. 1994;5:26–33. doi: 10.1097/00001665-199402000-00007. [DOI] [PubMed] [Google Scholar]
- 29.Rubin LR. Polyethylene as a bone and cartilage substitute: A 32 year retrospective. In: Rubin LR, editor. Biomaterials in Reconstructive Surgery. St Louis: C V Mosby; 1983. pp. 474–93. [Google Scholar]
- 30.Spector M, Flemming WR, Kreutner A. Bone growth into porous high density polyethylene. J Biomed Mater Res. 1976;10:595–603. doi: 10.1002/jbm.820100416. [DOI] [PubMed] [Google Scholar]
- 31.Foustanos AP, Anagnostopoulos D, Kotsianos G, Rapidis AD. Cranioplasty: A review of 10 cases. J Maxillofac Surg. 1983;11:83–6. doi: 10.1016/s0301-0503(83)80020-4. [DOI] [PubMed] [Google Scholar]
- 32.Romo T, 3rd, Sclafani AP, Sabini P. Use of porous high-density polyethylene in revision rhinoplasty and in the platyrrhine nose. Aesthetic Plast Surg. 1998;22:211–21. doi: 10.1007/s002669900193. [DOI] [PubMed] [Google Scholar]
- 33.Chen CT, Hu TL, Lai JB, Chen YC, Chen YR. Reconstruction of traumatic nasal deformity in Orientals. J Plast Reconstr Aesthet Surg. 2010;63:257–64. doi: 10.1016/j.bjps.2008.10.006. [DOI] [PubMed] [Google Scholar]
- 34.Eppley BL, Sadove AM. Effects of material porosity on implant bonding strength in a craniofacial model. J Craniofac Surg. 1979;1:191–202. doi: 10.1097/00001665-199001040-00008. [DOI] [PubMed] [Google Scholar]
- 35.Rahn AO, Boucher LJ. In: Maxillofacial prosthetics: Principles and concepts. Philadelphia: WB Saunders Co; 1970. p. 95. [Google Scholar]
- 36.Zins JE, Claude-Jean L, Serdar N. Controversies in Skull Reconstruction. J Craniofac Surg. 2010;21:1755–60. doi: 10.1097/SCS.0b013e3181c34675. [DOI] [PubMed] [Google Scholar]
- 37.Miyazawa T, Azuma R, Nakamura S, Kiyosawa T, Shima K. Usefulness of scalp expansion for cranioplasty in a case with postinfection large calvarial defect: A case report. Surg Neurol. 2007;67:291–5. doi: 10.1016/j.surneu.2006.04.019. [DOI] [PubMed] [Google Scholar]
- 38.Elsalanty ME, Por YC, Genecov DG, Salyer KE, Wang Q, Barcelo CR, et al. Recombinant human BMP-2 enhances the effects of materials used for reconstruction of large cranial defects. J Oral Maxillofac Surg. 2008;66:277–85. doi: 10.1016/j.joms.2007.06.626. [DOI] [PubMed] [Google Scholar]
- 39.Arnaud E. Advances in cranioplasty with osteoinductive biomaterials: Summary of experimental studies and clinical prospects. Childs Nerv Syst. 2000;16:659–68. doi: 10.1007/s003810000321. [DOI] [PubMed] [Google Scholar]


