Abstract
Introduction:
Idiosyncratic drug reactions (IDRs) are unexpected responses to a drug. The spectrums of severe cutaneous reactions include Stevens–Johnson Syndrome (SJS), SJS/Lyell Syndrome and Toxic Epidermal Necrolysis (TEN). The conditions are associated with high mortality. This study was designed to determine the causal agents, patterns of presentations, review the management and make recommendations to reduce the incidence and mortality of this class of drug reactions.
Materials and Methods:
A retrospective study was made of patients seen with IDR in the Lagos State University Teaching Hospital, LASUTH, between January, 2004 and December, 2008. They were cases admitted with bullous skin eruptions with associated systemic symptoms.
Results:
Sixty-seven patients were seen, with 45 (67.2%) satisfying the inclusion criteria. Fifteen males and 30 females were involved, giving a male to female (M:F) ratio of 1:2. Their ages ranged from 7 to 79 years (mean, 40.02 ± 17.89 years). Peak incidences occurred among the 20–24 and 30–34 year age groups. The causal agents were antibiotics (48.89%), sulphonamides (24.44%), herbal preparations (17.78%) and artemisinin drugs (8.89%).
Conclusions:
The age groups with the peak incidence are the most likely to indulge more in drug abuse in environments with poor drug control. Diagnosis of SJS, SJS/TEN and TEN were missed in many patients at first contact due to the progressive nature of the conditions. Patients needed reviews at regular intervals when IDR was suspected. Health education to prevent drug abuse is important and herbal preparations should be scientifically studied to determine the efficacy and side-effects.
KEY WORDS: Idiosyncratic drug reactions, Stevens Johnson Syndrome, Toxic Epidermal Necrolysis, toxic epidermal necrolysis
INTRODUCTION
Idiosyncratic drug reactions (IDRs) are unexpected responses to a drug, different from its pharmacologic action.[1] The reactions can result from the use of wide range of agents, and range from transient fever, minimal maculopapular rash to fatal toxic epidermal necrolysis (TEN).[2] A variety of agents may induce IDR, but drugs are more commonly involved.
Adverse cutaneous reactions to drugs affect 2–3% of all hospitalized patients.[3] Only 2% of these patients are severely affected.[4] The spectrum of severe cutaneous reactions includes Stevens-Johnson syndrome (SJS), SJS/ Lyell Syndrome (LS) and TEN.
Ferdinand von Hebra[5] in 1866 was the first to document the unexpected cutaneous reactions to administered drugs. In 1922, Stevens and Johnson described two patients with skin eruptions and fever. These were accompanied by stomatitis and ophthalmitis.[3] Alan Lyell[6] in 1956 described four cases of severe skin eruptions, resembling scalding of the skin. In these cases, epidermal necrolysis was accompanied by widespread mucocutaneous manifestations. These associated symptoms and signs came to be known as LS or TEN. These manifestations include perioral mucocutaneous sloughing, conjunctivitis, pharyingitis and pruritis, among others. Fever often precedes the mucocutaneous manifestations and mucous membranes are commonly affected 1–3 days before the skin manifestations.[3] The cutaneous lesions are often confluent and may lead to wide areas of epidermal detachments.[7] The preceding fever is caused by drugs or release of toxins from epidermal necrosis or both.[3] When the extent of the epidermal loss is not more than 10% of the total body surface area (TBSA), the condition is known as SJS. When the TBSA is greater than 30%, it is known as LS or TEN. SJS/TEN is used in describing cases between 10% and 30%.[8,9] The diffuse epidermal exfoliation is similar to the skin loss in superficial partial thickness or second degree burns.[10] Cutaneous tissue separates at the dermo–epidermal junction. The separation is responsible for the Nikolsky sign, which is seen clinically as skin with wet dressing appearance.[11]
Patients with the above conditions express lytically active Fas ligands (Fasl) on their keratinocytes. A normal keratinocyte expresses the death receptor–Fas (CD95)– on the membrane and Fasl in its intracellular space and rarely demonstrates apoptosis. Fas belongs to the TNG-NGF family of receptors.[12] On stimulation by causal drugs, peripheral blood mononuclear cells secrete significantly high amount of soluble Fasl. The soluble Fasl may then initiate Fas–Fasl interaction, which results in nuclear apoptosis and epidermal necrolysis.[12]
Drugs that have been implicated as causative agents include sulphonamides, penicillin, antibiotics, non-steroidal anti-inflammatory drugs (NSAID) and anti-epileptics.[13,14] Recently, Ugburo and others reported TEN in three patients from Lagos, Nigeria, who took Artemisinin derivatives for the treatment of malaria.[15]
Mortality from SJS, SJS/TEN and TEN ranges from between 25% to 70%.[16] The morbidity is also considerable.[17] Prognostic factors include age, TBSA involved, severe anaemia, lymphopaenia, neutropaenia, serum urea nitrogen level and visceral organ involvement.[3] These are scored using (a) Simplified Acute Physiology Score (SAPS I and SAPS II) and (b) SCOREN.[3,18]
Early diagnosis of the condition and commencement of appropriate management has been known to reduce the morbidity and high mortality that is associated with the condition.[19] To facilitate early diagnosis in our environment, a retrospective study of SJS, SJS/TEN and TEN cases that were seen in LASUTH between January 2004 and December 2008 was carried out to determine the causal agents, study the pattern of presentations, note the modes of management and patient outcome as well as complications. We also examined factors involved in the incidence, morbidity and mortality with a view to suggesting ways to reduce them.
MATERIALS AND METHODS
A retrospective study of all patients with severe bullous skin eruptions admitted into LASUTH between January 2004 and December 2008 was carried out. The case notes were retrieved from the medical records department and analysed for the following information: Age, sex, medications, suspected initiating agent, initial and final diagnosis, anatomic areas affected, TBSA involved, histopathology findings, modes of management, complications developed and treatment outcome.
The criteria for inclusion in the study were: History of fever preceding the bullous eruptions, confluent blisters, widespread loss of epidermal sheets of varying TBSA, involvement of mucosal surfaces, severe systemic symptoms and variable involvements of the eyes, gastrointestinal, haematologic and respiratory systems.
Patient care
The patients were jointly managed by the Plastic Surgeons and the Dermatologist. All antibiotics and any other suspected causal agents were stopped on admission. The patients were managed either in Isolation or in Burns Wards. Wound management consisted of thorough but gentle cleaning with saline solution, wound biopsy for microbiological and histopathological studies and puncturing of the blisters. Extreme care was taken to preserve the overlying epidermal sheet in situ. These served as the biologic dressing for the wounds. Petroleum jelly or moist exposed burn ointment (MEBO) was applied to all the involved surfaces and they were covered with Sofratulle gauze. Gamgee dressing was finally applied. Dressings were changed on alternate days initially and twice-weekly later when the exudates reduced. Blood transfusion was given whenever the haemoglobin level dropped below 8 gm/dl. Fluid resuscitation was commenced with a wide bore cannula using Parkland formula. Seventy-five percent of the calculated volume was infused over 24 h. Ringers lactate fluid was used for the initial resuscitation and alternated with 5% dextrose in normal saline after the initial 48 h. The amount of fluid required was adjusted daily based on the changing TBSA and the hourly urinary output of patients. Hourly urinary output of between 50 and 60 ml was maintained in the adults and 1.2 ml/kg/h in children. Urethral catheterization was carried out but catheters were removed as soon as haemodynamic stability was achieved and patients were ambulant.
Adequate nutrition was maintained in the patients. Oral feeding was commenced 48 h after admission with diluted milk drinks and graduated to the local diet as tolerated. Additional protein in the form of boiled eggs was added to the meals.
The oxygen saturation level was monitored in all the patients. Oxygen-enriched air using intranasal catheters was administered in all patients with arterial blood oxygen saturation levels below 95%. Chest physiotherapy was commenced and continued throughout the duration of admission.
Appropriate antibiotics were used only in patients with confirmed wound infections, and the drugs were based on the sensitivity pattern.
The eyes of the patients were routinely examined by the Ophthalmologists and managed with topical chloramphenicol eye drop and ointment when involved.
Oral hygiene was maintained in all patients with saline mouth wash in the morning, at nights and after all oral feedings.
Patients were discharged from the hospital to follow- up clinics when there was clinical evidence of complete mucocutaneous wound healing.
RESULTS
A total of 67 patients with cutaneous eruptions were seen at LASUTH during the study period, and 45 (67.2%) satisfied the inclusion criteria for the study. There were 15 males and 30 females, giving a male to female (M:F) ratio of 1:2. The patients’ age ranged between 7 and 79 years, with a mean of 40.02 ± 17.89 years. The distribution of epidermal necrolysis among the age groups is as shown in Figure 1. The peak incidences occurred among the 20–24 and 30–34 year age groups.
Figure 1.
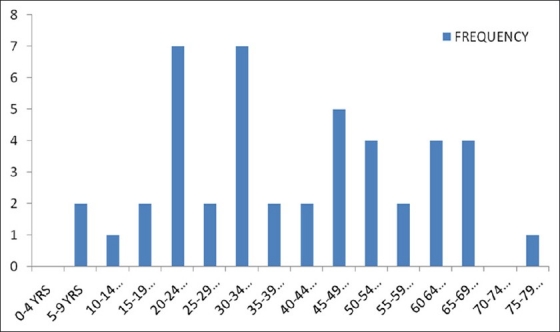
Distribution of epidermal necrolysis among the different age groups
Polypharmacy was widely practiced among the patients. This made the identification of causal agents difficult in some cases and raised the possibility of more than one agent in others. The following drugs were implicated: antibiotics in 22 (48.89%) cases, sulphonamides in 11 (24.44%), herbal preparations in eight (17.78%) and dihydroartemisinin in four (8.89%) cases. The antibiotics involved were Ampicillin, Ceftriaxone, Penincillin and Tetracycline. The sulphonamides were Trimethoprim, Sulfamethoxazole and Co-Trimoxazole. Dihydroartemisinin drug was taken as part of Artemisinin Combination Therapy (ACT), the World Health Organization (WHO)-recommended standard treatment regime for malaria. Two patients combined Artemisinin with Amodiaquine, one combined it with Chloroquine and the last patient combined it with Lumefantrine.
Drug eruptions were the initial diagnosis in 32 (71.11%) cases, Erythema multiforme (EM) in five (11.11%) and SJS in eight (17.78%). No patient was diagnosed as TEN at the initial contact with the Physician. The final diagnoses were SJS in 21 (46.67%), SJS/LS in 16 (35.55%) and TEN in eight (17.78%) patients.
The TBSA involved in patients with epidermal necrolysis ranged between 4 and 60%, with a mean of 19.42 ± 15.72%.
The distribution of the epidermal loss is as shown in Table 1. The trunk was affected in 75% of the cases, the oral mucosa was affected in 60% and the respiratory tract was the least affected [Figure 2].
Table 1.
Anatomical distribution of epithelial necrolysis and frequency
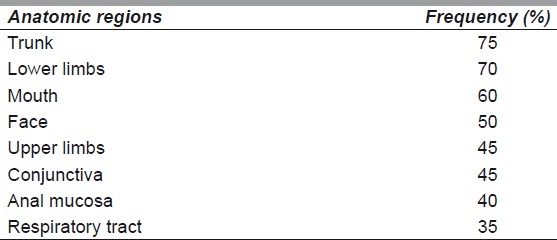
Figure 2.
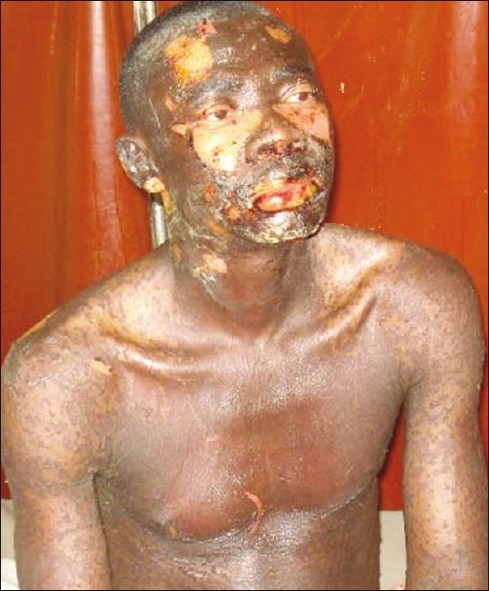
Toxic epidermal necrolysis. Note the involvement of the skin of the trunk, upper extremities and the oral mucosa
Histopathology findings were obtained in three (6.67%) patients, and confirmed the diagnosis of SJS and TEN.
The patients were on admission for between 7 and 28 days, with a mean of 13.87 ± 7.13 days. Patients with SJS/LS and TEN were on admission for longer periods compared with cases of SJS.
Five (62.50%) of the cases with TEN and seven (43.75%) with SJS/LS died. Three patients out of these had septicemia, five had multiple organ failures while autopsy could not be performed on the rest due to sociocultural and religious reasons. Two (9.52%) patients with SJS were lost to follow-up and were presumed dead. These two were HIV infection positive. The diagnosis was made during the course of management for the mucocutaneous lesions.
Dyschromic skin changes were the only recorded complication, and these occurred in all the survivors.
DISCUSSION
IDRs result from genetic differences between individuals.[1,2] They represent a qualitative abnormality and may lead to extensive dermoepidermal disruption and tissue loss. Unlike hypersensitivity reactions, there is no previous sensitization to a particular chemical agent or any that is structurally similar in idiosyncratic reactions, although both are adverse drug reactions (ADRs). There was no history of previous sensitization or mild reactions in all the patients in this study.
SJS and TEN are rare conditions, occurring in 1.8 per million persons per year.[16] Previous studies showed that the conditions are more common among the female gender and among the elderly.[20,21] A female preponderance was also found in the present study. The incidence was however found to be highest among the 20–24 and 30–34 year age groups. This may be due to the dynamics of the society that was studied. These age groups are very active and most likely to get exposed to the disease conditions that may necessitate the intake of the causal drugs. They are also the most likely to indulge more in drug abuse by taking drugs that are not prescribed by medical doctors.
SJS, SJS/TEN and TEN were found to have been caused by a variety of drugs.[3] Studies by Roujeau et al.[22] showed sulphonamides as the most common agents followed by antibiotics. The sulphonamides include, Co-Trimoxazole and Trimethoprim and Sulphamethoxazole. These were mostly used to treat infective conditions. Associated antibiotics include Cephalosporins, Quinolones, Aminopenincillin, Tetracycline and Macrolides. Other agents were Anti-epileptics, Allopurinol and NSAID.[22] The most common causal agents in the present study were antibiotics. This may be because control of drug sale is poor in the study environment and most drugs can be purchased without medical prescription.
Sulphonamides were used in the study to treat infective conditions and as part of the drug regime in the treatment of malaria. Lagos is located in the malaria endemic zone. Various drugs including Pyrimethamine–Sulfadoxine combinations are used in the treatment of malaria parasitaemia. Co-Trimoxazole and Trimethoprim are commonly used to manage infective conditions. These factors may be responsible for the prominence of sulphonamides in the study.
The use of herbal remedies is increasing in popularity among urban dwellers in several countries. They are seen as being cheaper, beneficial, free of side-effects, closer to nature and complementary to Western medicine.[23,24] The herbal remedy practitioners (Herbologists) are also seen as being closer to the people and understanding their feelings better than the Western-oriented medical practitioners.[25] Concomitant use of herbal drugs with orthodox medications among low-income earning Nigerians may be approaching 100%.[26] The compositions of these remedies are, in many cases, not standardized and not subjected to scientific scrutiny. The possibility of drug interaction between the herbal remedies and the orthodox medications being responsible for some of the reactions that were observed in the study cannot be ruled out. The herbal remedies alone may also be the causal agents.
The WHO-recommended ACT for the standard management of malaria[27] was to reduce the incidence of the malaria parasites’ resistance to the antimalaria medications. TEN, occurring with the use of ACT in a pregnant woman, had been previously reported from this centre.[15] Three cases occurring in other patients were subsequently seen and managed during the study period. A yet-to-be-known genetic variation may be responsible for the reaction.
The spectrum of severe ADR with cutaneous eruptions include EM, SJS, TEN and drug eruption with eosinophilia and systemic symptoms (DRESS), among others. EM is an acute, self-limiting skin disease symmetrically distributed to the extremities. It is divisible into EM minor and EM major. EM minor is mild, often recurrent and post-infectious to diseases like herpes, mycoplasma and tuberculosis. There is no mucosal involvement. EM major is severe, with widespread skin involvement, and is often confluent. It affects the trunk, and Nikolsky sign is positive [Figure 3]. The extent of the disease is best defined at the worst stage and not when the patient is first seen.[3] It is believed by some workers that SJS and TEN are the extreme forms of progressively evolving EM while DRESS is the form with one or multiple internal organ involvement, occurring in a delayed fashion.[3] In the study, the initial diagnosis in 11.11% of the patients was EM. No diagnosis of TEN was made at first contact. This is not surprising in view of the progressive nature of the disease. Thus, it is important to review these patients at regular intervals for proper diagnosis and appropriate management.
Figure 3.
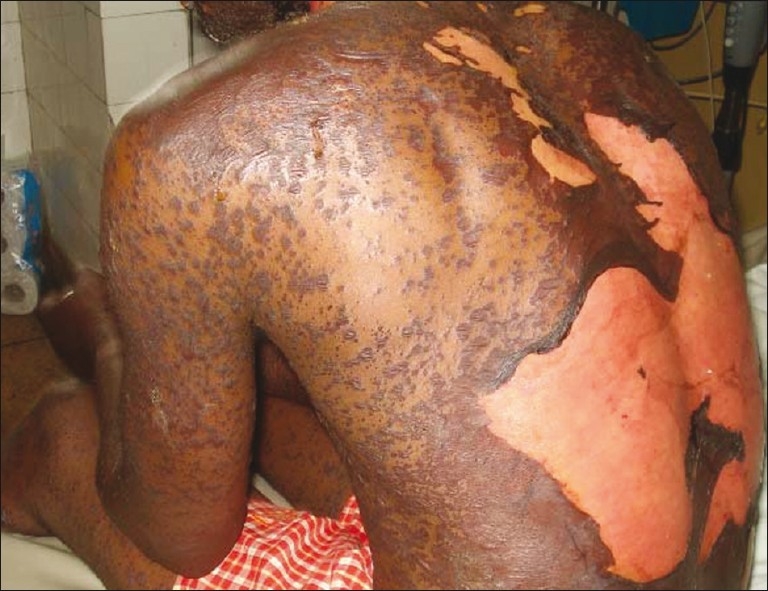
Toxic epidermal necrolysis affecting the trunk and the extremities. The cutaneous lesions on the trunk were confluent with wide epidermal detachments
Histopathology findings in SJS and TEN are typical. The pathology is localized to the dermoepidermal junction. The junction is a complex area and is associated with mechanical, biochemical and immunological functions.[28] Intimate relationship is maintained between the epithelial basement membrane of the epidermis and the papillary layer of the dermis by laminin, which anchors the two layers together. The interaction of the Fas–Fasl ligand triggers the cascade of reactions that ultimately causes apoptosis and loss of keratinocytes.[12] Separation of the epithelium from the dermis follows and is pathognomonic of the condition.
The skin of the trunk, lower extremities and the oral mucosa were affected in most of the cases that were seen in the study. The anal mucosa was the least affected. This may be due to the fact that the anal region is often not examined, especially in the absence of symptomatologies that are related to the area. Diagnoses of respiratory tract mucosal involvements were only made on clinical grounds, when signs of respiratory problems are present. The arterial oxygen saturation levels were closely monitored in all patients with respiratory tract involvement. Supplementary oxygen was supplied with intranasal catheters whenever the saturation levels dropped to 95%. Chest physiotherapy was also commenced and continued until the patients were discharged. The patients were also adequately motivated. There was no need to use mechanical ventilators in any of the cases. In our practice, the parameters that are used to determine whether a patient requires mechanical ventilatory support include decreasing level of mental alertness in the presence of obvious respiratory tract mucosal involvement, restlessness, evidences of respiratory distress including airway obstruction, decreasing arterial oxygen saturation with increasing carbon dioxide levels, decreasing serum pH values and 90% oxygen saturation level despite the administration of supplementary oxygen therapy.
Re-epithelialisation of the wound occurs from the epidermal appendages that are left in the exposed dermis. This takes an average of 3 weeks, similar to what happens in the healing of second degree burns.[13] The periods of hospital stay of the patients in the study were also within this range.
Previous studies utilized various advantages provided by the environment of the burn wards in the management of the wounds of patients with epidermal necrolysis.[29] Similarly, the patients in this study were managed in the same environment. The use of biological dressings had been advocated for the coverage of the wounds in those studies.[3,30] Biological dressings were obtained from various sources in the studies.[21,30] In the present study, the patients’ own detached epidermis overlying the wounds was used in most of the cases. Various types of biological dressings, natural and synthetic, that have been advocated for use were not available, unaffordable or contraindicated for religious and/or sociocultural reasons in the immediate environment of this study. The epidermis used in the study was an autograft and was thus not immunoreactive in the patients. It equally combined all the advantages of biological dressings. Previous studies in our centre and elsewhere had shown the beneficial effects of MEBO when used in the dressing of second degree burns.[31,32] MEBO was used in the dressing of the exposed dermis. Various management modalities have been used in treating the systemic effects of epidermal necrolysis.[12,15,30] In recent times, the use of intravenous immunoglobulin (IVIG) has been strongly advocated.[3,12,22] IVIG is used to block the Fas receptors and prevent the formation of Fas–Fasl ligand, thereby stopping nuclear apoptosis in the keratinocytes.[12,22] IVIG also has powerful anti-inflammatory effects, with prophylaxis against septicemia.[21,22] While some workers were able to show the beneficial effects of IVIG,[22] others could not.[3] In this study, IVIG was not used in any of the patients. This was due to the fact that it was not readily available. The use of corticosteroids in the management of patients with TEN has remained debatable.[16] While some workers advocate for it to be used at the early stages of the illness for it to suppress the inflammatory reactions, others are of the opinion that the use may increase the chances of sepsis, prolong the hospital stay and increase the mortality.[33] Corticosteroids were not used in the present study. This was due to the fact that most of our cases were not seen at the early stages when these drugs could be beneficial to the patients. We also felt that due to the possibilities of wound contaminations that the cases could have had as a result of the delayed presentations at our hospital, the use of corticosteroids will increase the possibilities of wound infection and systemic invasion.
Epidermal necrolysis could be dangerous with an unacceptably high mortality rate as shown in this study. Efforts should be made to reduce the incidence as much as possible. This could be done by avoiding the intake of the implicated causal agents when this is possible. Access to drugs should be controlled and indiscriminate intake discouraged by appropriate agencies using health enlightenment programmes. The use of herbal preparations has come to stay. The preparations however should be made available for proper scientific studies. This will enable the users to recognize any possible side-effects. The out-patient physicians are the ones that will likely make the initial contacts with the cases. They should always be aware of the possibility of these cases and commence appropriate management as soon as possible. Adequate facilities should also be provided to manage the cases by the specialists.
Footnotes
Source of Support: Nil
Conflict of Interest: None declared.
REFERENCES
- 1.British National Formulary 56. London: BMJ Group; 2008. p. 11. [Google Scholar]
- 2.Leape LL, Breanan TA, Laird N. The nature of adverse events in hospitalized patients. Results of the Harvard Medical Study II. N Engl J Med. 1991;324:377–84. doi: 10.1056/NEJM199102073240605. [DOI] [PubMed] [Google Scholar]
- 3.Wolf R, Orion E, Marcos B, Matz H. Life-threatening acute adverse cutaneous drug reactions. Clin Dermatol. 2005;23:171–81. doi: 10.1016/j.clindermatol.2004.06.012. [DOI] [PubMed] [Google Scholar]
- 4.Alanko K, Stubb S, Kauppinan K. Cutaneous drug reactions: Clinical types and causative agents. A five-year survey of in patients (1981-1985) Acta Dem Venereol. 1989;69:223–6. [PubMed] [Google Scholar]
- 5.Fabbri P, Panconesi F. Erythema multiforme (minus and mains) and drug intake. Clin Dermatol. 1993;11:479–89. doi: 10.1016/0738-081x(93)90154-5. [DOI] [PubMed] [Google Scholar]
- 6.Lyell A. Drug-induced toxic epidermal necrolysis: I. An overview. Clin Dermatol. 1993;11:491–2. doi: 10.1016/0738-081x(93)90155-6. [DOI] [PubMed] [Google Scholar]
- 7.Castana O, Makrodimou M, Michelakis D, Tsandoulas Z, Alexakis D. Diseases mimicking a burn- Outcome and treatment. Ann Burn Fire Disasters. 2005;18:130–2. [PMC free article] [PubMed] [Google Scholar]
- 8.Marinkovich MP. Blistering Diseases. Department of Dermatology, Stanford University School of Medicine. [last accessed on 2011 Apr 05]. Available from: http://telemedicine.org/blister.htm .
- 9.Brambilla G, Brucato F, Angrisano A, Palmieri G. Treatment of toxic epidermal necrolysis (TEN) Ann Burn Fire Disasters. 2002;15:17–21. [Google Scholar]
- 10.Atiyeh BS, Kayle DI, Nasser AA. Burn like syndromes. Ann Burns Fire Disasters. 1999;12:39–43. [Google Scholar]
- 11.Petkov T, Pehlivanov G, Grozdev I, Kavaklieva S, Tsankov N. Toxic epidermal necrolysis as a dermatological manifestation of drug hypersensitivity syndrome. Eur J Dermatol. 2007;17:422–7. doi: 10.1684/ejd.2007.0241. [DOI] [PubMed] [Google Scholar]
- 12.French LE, Trent JT, Kerdel FA. Use of intravenous immunoglobulin in toxic epidermal necrolysis and Stevens-Johnson syndrome: Our current understanding. Int Immunopharmacol. 2006;6:543–9. doi: 10.1016/j.intimp.2005.11.012. [DOI] [PubMed] [Google Scholar]
- 13.Cabral L, Riobom F, Diogo C. Toxic epidermal necrolysis. Ann Burn Fire Disasters. 2004;17:90–102. [Google Scholar]
- 14.Roujeau JC, Guillaume JC, Fabre JP, Penso D. Toxic epidermal necrolysis: Incidence and drug aetiology in France. Arch Dermatol. 1996;126:37–43. doi: 10.1001/archderm.126.1.37. [DOI] [PubMed] [Google Scholar]
- 15.Ugburo AO, Ilombu CA, Temiye EO, Fadeyibi IO, Akinola OI. Severe idiosyncratic reaction (Lyell Syndrome) after ingesting Dihydroartemisinin. Niger J Clin Pract. 2009;12:224–7. [PubMed] [Google Scholar]
- 16.Lissia M, Mulas P, Bulla A, Rubino C. Toxic epidermal necrolysis (Lyell,s disease) Burns. 2010;36:152–63. doi: 10.1016/j.burns.2009.06.213. [DOI] [PubMed] [Google Scholar]
- 17.Spies M, Sanford AP, Wolf SE, Ail-Low JF, Herndon DN. Treatment of extensive toxic epidermal necrolysis in children. Paediatrics. 2001;108:1162–8. doi: 10.1542/peds.108.5.1162. [DOI] [PubMed] [Google Scholar]
- 18.Bastuji-Garin S, Fouchard N, Bertocchi M, Roujeau JC, Revuz P, Wolkenstein P. SCORTEN: A severity-of-illness score for toxic epidermal necrolysis. J Invest Dermatol. 2000;115:149–53. doi: 10.1046/j.1523-1747.2000.00061.x. [DOI] [PubMed] [Google Scholar]
- 19.Stern RS. Improving outcome of patient with toxic epidermal necrolysis and Steven-Johnson syndrome. Arch Dermaltol. 2000;136:410–1. doi: 10.1001/archderm.136.3.410. [DOI] [PubMed] [Google Scholar]
- 20.Becker DS. Toxic epidermal necrolysis. Lancet. 1998;331:1417–20. doi: 10.1016/S0140-6736(97)11369-1. [DOI] [PubMed] [Google Scholar]
- 21.Ugburo AO, Ilombu CA, Temiye EO. A 12-year retrospective study of non-burn skin loss (burn-like syndromes) at a tertiary burns unit in a developing country. Burn. 2008;34:637–43. doi: 10.1016/j.burns.2007.08.022. [DOI] [PubMed] [Google Scholar]
- 22.Stella M, Clemente A, Bollero D, Risso D, Dalmasso P. Toxic epidermal necrolysis and Stevens-Johnson Syndrome (SJS): Experience with high-dose intravenous immunoglobullins and topical conservative approach. A retrospective analysis. Burns. 2007;33:452–9. doi: 10.1016/j.burns.2006.08.014. [DOI] [PubMed] [Google Scholar]
- 23.De Smet PA. Herbal remedies. N Engl J Med. 2002;347:2046–56. doi: 10.1056/NEJMra020398. [DOI] [PubMed] [Google Scholar]
- 24.Kaptchuk TJ, Eisenberg DM. The persuasive appeal of alternative medicine. Ann Int Med. 1998;129:1061–5. doi: 10.7326/0003-4819-129-12-199812150-00011. [DOI] [PubMed] [Google Scholar]
- 25.Hewson MG. Traditional healers in Southern Africa. Ann Int Med. 1998;128:1029–38. doi: 10.7326/0003-4819-128-12_part_1-199806150-00014. [DOI] [PubMed] [Google Scholar]
- 26.Awobusuyi JO. Alternative Medical Remedies in Clinic practice: Why do Patients use Native Medicines? Pharm J Lagos State Univ Teach Hosp. 2006;1:3–5. [Google Scholar]
- 27.Media Center Press Release-Prospectives in Health Magazine-PAHO Today. WHO Dihydroartemisinin Htm [Google Scholar]
- 28.Burgeson RE, Lunstrum GP, Rokosova B, Rimberg CS, Rosenbaum LM, Keene DR. The structure and function of type VII collagen. Ann NY Acad Sci. 1990;580:32–43. doi: 10.1111/j.1749-6632.1990.tb17915.x. [DOI] [PubMed] [Google Scholar]
- 29.McGee T, Munstard A. Toxic epidermal necrolysis syndrome: Mortality rate reduced by early referral to regional burn center. Plast Reconstr Surg. 1998;102:1018–22. doi: 10.1097/00006534-199809040-00014. [DOI] [PubMed] [Google Scholar]
- 30.Acikel C, Eren F, Ergun O, Celikoz B. Topical treatment of toxic epidermal necrolysis using Omiderm and glycerol preserved human cadaver skin. Ann Burns Fire Disaster. 2002;15:2. [Google Scholar]
- 31.Jewo PI, Fadeyibi IO, Babalola OS, Saalu LC. A comparative study of the wound healing properties of Moist Exposed Burn Ointment (MEBO) and silver sulphadiazine. Ann Burns Fire Disaster. 2009;22:79–82. [PMC free article] [PubMed] [Google Scholar]
- 32.Jurjus A, Atiyeh BS, Abdallah IM, Jurjus RA, Hayek SN, Jaoude MA, et al. Pharmacological modulation of wound healing in experimental burns. Burns. 2007;33:892–907. doi: 10.1016/j.burns.2006.10.406. [DOI] [PubMed] [Google Scholar]
- 33.Chave TA, Mortimer NJ, Sladden MJ, Hall AP, Hutchinson PE. Toxic epidermal necrolysis: current evidence, practical management and future directions. Br J Dermatol. 2005;153:241–53. doi: 10.1111/j.1365-2133.2005.06721.x. [DOI] [PubMed] [Google Scholar]


