Abstract
Background
Several metrics of glucose variability have been proposed to date, but an integrated approach that provides a complete and consistent assessment of glycemic variation is missing. As a consequence, and because of the tedious coding necessary during quantification, most investigators and clinicians have not yet adopted the use of multiple glucose variability metrics to evaluate glycemic variation.
Methods
We compiled the most extensively used statistical techniques and glucose variability metrics, with adjustable hyper- and hypoglycemic limits and metric parameters, to create a user-friendly Continuous Glucose Monitoring Graphical User Interface for Diabetes Evaluation (CGM-GUIDE©). In addition, we introduce and demonstrate a novel transition density profile that emphasizes the dynamics of transitions between defined glucose states.
Results
Our combined dashboard of numerical statistics and graphical plots support the task of providing an integrated approach to describing glycemic variability. We integrated existing metrics, such as SD, area under the curve, and mean amplitude of glycemic excursion, with novel metrics such as the slopes across critical transitions and the transition density profile to assess the severity and frequency of glucose transitions per day as they move between critical glycemic zones.
Conclusions
By presenting the above-mentioned metrics and graphics in a concise aggregate format, CGM-GUIDE provides an easy to use tool to compare quantitative measures of glucose variability. This tool can be used by researchers and clinicians to develop new algorithms of insulin delivery for patients with diabetes and to better explore the link between glucose variability and chronic diabetes complications.
Introduction
As the use of continuous glucose monitors becomes more prevalent in the management of type 1 diabetes mellitus (T1DM), metrics for interpreting and connecting continuous glucose monitoring (CGM) data to therapeutic algorithms of insulin delivery are essential for the most effective use of continuous glucose monitors in clinical care, with the ultimate goal of preventing chronic diabetes complications including nephropathy and kidney failure, retinopathy and blindness, peripheral neuropathy, and cardiovascular disease. Glucose variability is under consideration as a possible link to diabetes complications, with studies reporting its role in promoting increased oxidative stress1 and vascular pathology.2,3 Currently, the statistics and metrics used to reflect glucose dynamics include, but are not limited to, the overall SD from a mean glucose value, percentage of values within, above, or below specified thresholds, area under the curve, mean amplitude of glycemic excursion (MAGE),4 mean of daily differences (MODD),5 and continuous overall net glycemic action (CONGA) (for the indicated n hours).6 Additional metrics include the M-value,7 average daily risk range,8 Glycemic Risk Assessment Diabetes Equation scores,9 and J-index.10 Inconsistencies, however, can arise from the miscalculation, misinterpretation, and misuse of these metrics in disparate data types and applications.
Of primary concern is the practical implementation of rigorous glucose variability metrics and glycemic statistics in the clinic, where there is currently a lack of software that provides easy quantification and comparison of glucose variability data. Here we provide a standardized approach for visualizing the dynamics associated with crossing into different regions of glycemia through the development of a novel histogram plot—from here onward referred to as the “transition density profile”—that assesses the severity and frequency of glucose transitions per day as they move between critical glycemic zones. Also, to address the translational gap between existing metrics and their actual use in clinical practice, we have created the Continuous Glucose Monitoring Graphical User Interface for Diabetes Evaluation (CGM-GUIDE©) to integrate the evaluation of CGM data.
Materials and Methods
CGM data were provided by the laboratory of R.P.-B. and were collected on an iPro™ CGM system (Medtronic, Northridge, CA) in adult patients with T1DM under normal daily conditions for up to 5 days. The study was approved by the Institutional Review Board at the University of Michigan (Ann Arbor), and all subjects signed a written informed consent. Here we present detailed analysis from four subjects with T1DM to appreciate the full functionality of CGM-GUIDE. Reference metric values for those without diabetes were reported in independent studies by McDonnell et al.,6 based on CGM traces for 10 healthy controls without diabetes, by Cameron et al.,11 based on CGM traces for 12 healthy controls without diabetes, and Fox et al.12 based on CGM traces for 74 healthy controls without diabetes (Table 1). Each patient's interstitial glucose levels (from here forward referred to as “glucose”) were sampled at 5-min intervals for up to 140 h. CGM-GUIDE was designed using Matlab version 2008b (The MathWorks, Natick, MA) with descriptions of user inputs and novel CGM-GUIDE outputs, including transition speeds and the transition density profile, as well as standard statistics and metrics. Gaps or null data points are automatically deleted and not included in the calculated times or areas.
Table 1.
Integrated Glycemic Variability Assessment for Patients with Diabetes and Controls Without Diabetes
| Metric | Subject 1 | Subject 2 | Subject 3 | Subject 4 | T1DM group mean | Mean of controls without diabetes* | Fold difference |
|---|---|---|---|---|---|---|---|
| Mean glucose (mg/dL) | 173.6 | 229.8 | 121.9 | 144.1 | 167±47 | 96.84 | 1.7 |
| SD | 80.8 | 90.1 | 55.2 | 69.5 | 74±15 | 14.13 | 5.3 |
| MODD | 107.8 | 100.0 | 57.5 | 54.3 | 80±28 | 14.41 | 5.6 |
| CONGA(1) | 60.4 | 54.5 | 35.7 | 41.9 | 48±11 | 12.91 | 3.7 |
| CONGA(2) | 87.1 | 80.9 | 54.8 | 64.1 | 72±15 | 15.89 | 4.5 |
| CONGA(4) | 111.1 | 114.6 | 71.5 | 87.5 | 96±20 | 18.26 | 5.3 |
| MAGE | 171.8 | 155.3 | 90.1 | 117.6 | 134±37 | 32.14 | 4.2 |
| Time (%) | |||||||
| Hyperglycemic | 39.7 | 68.9 | 15.6 | 23.2 | 37±24 | 0 | — |
| Hypoglycemic | 4.8 | 3.8 | 22.0 | 10.0 | 10±8 | 1.70 | 1.6 |
| AUC (mg×min/dL)/day | |||||||
| Hyperglycemic | 43,813 | 96,761 | 7,050 | 23,425 | (4.3±3.9)×104 | ||
| Hypoglycemic | 726 | 911 | 5,356 | 2,071 | 2,265±2,144 | ||
| Percentage of time spent in each bin (%) | |||||||
| <50 mg/dL | 1.0 | 1.5 | 9.7 | 3.3 | 4±4 | ||
| 50–70 mg/dL | 4.3 | 3.6 | 12.4 | 7 | 7±4 | ||
| 70–180 mg/dL | 60.8 | 36 | 62.3 | 68.6 | 57±14 | ||
| 180–220 mg/dL | 14.7 | 21 | 10.6 | 6.9 | 13±6 | ||
| 220–300 mg/dL | 19.1 | 37.7 | 5.1 | 15 | 19±13 | ||
Metric calculations are shown for patients with type 1 diabetes mellitus (T1DM) versus average reference values from subjects without diabetes. Reference values from subjects without diabetes for mean glucose, percentage of time hyperglycemic, and percentage of time hypoglycemic were obtained from the study of McDonnell et al.6 on 10 healthy controls.
A twofold lower hypoglycemic percentage (1.7%) has been more recently reported in a study by the Juvenile Diabetes Research Foundation Continuous Glucose Monitoring Study Group using 74 healthy controls without diabetes.12 Reference values from subjects without diabetes for the remaining metrics were obtained from the study of Cameron et al.11 on 12 healthy controls without diabetes.
AUC, area under the curve; CONGA, continuous overall net glycemic action (number in parentheses represents h); MAGE, mean amplitude of glycemic excursions; MODD, mean of daily differences.
Definitions
Time interval
Time interval (in min) is the interval at which the CGM glucose data were collected.
Bin thresholds
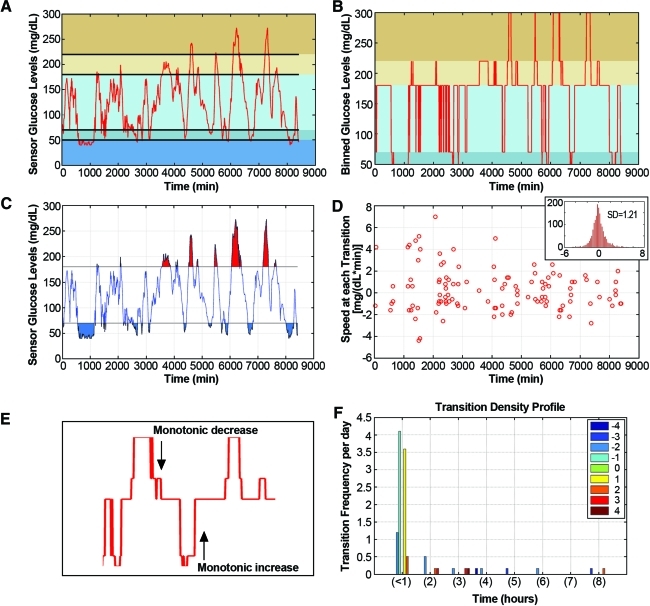
The bounding threshold values (in mg/dL) are those into which raw CGM data will be binned, such that Bin(1) contains all raw values that satisfy the condition [threshold(1)<glucose≤threshold(2)] (Fig. 1).
FIG. 1.
Transition density profile: a cumulative measure of severity and frequency of glycemic change. (A) Raw continuous glucose monitoring data from a diabetes patient with mean glucose equal to 121.9 mg/dL. Colored horizontal bands indicate different user-defined glucose ranges. (B) Raw continuous glucose monitoring data are categorized into bins according to user-defined threshold ranges, defined here as (0, 50, 70, 180, 220, 300 mg/dL). The percentage of time spent in each bin is displayed in red. (C) Area under the curve above and below the hyper-/hypoglycemic limit of 180 (red) and 70 (blue) mg/dL, respectively. The area under the curve is a measure of hyper- and hypoglycemic severity in conjunction with the (D) transition speed, or the rate of change in glucose at the transition between each threshold. (Thresholds are defined as 0, 50, 70, 120, 180, 220, and 300 mg/dL.) (Inset) Histogram of the slopes over each 5-min time interval. (E) Example of binned continuous glucose monitoring data where a monotonic increase and decrease was observed. The duration of these monotonic increases/decreases was then calculated for the (F) transition density profile. The number of transitions per day where transitions are described as the magnitude of every continuous monotonic change in blood glucose levels, after smoothing, sorted into the number of thresholds crossed (e.g., 2, 4, −3, −5) and separated into the time interval necessary to complete each change (e.g., <1 h, between 1 and 2 h, 2 and 3 h, etc.). Negative numbers indicate monotonic decreases. Color graphics available online at www.liebertonline.com/dia
Hyperglycemic/hypoglycemic limit
All glucose values greater than the hyperglycemic limit (in mg/dL) and less than or equal to the hypoglycemic limit (in mg/dL) will be considered hyper- or hypoglycemic, respectively.
Time hyperglycemic/hypoglycemic
Total time (in h) spent in the hyper- or hypoglycemic condition was estimated. A linear model was used to approximate the time spent in the hyper-/hypoglycemic ranges during the threshold crossing. Glycemic time is expressed both in hours and as a percentage of the total CGM period.
Time spent in each bin
The total time (in h) spent in each bin (as defined above in “Bin thresholds”) was estimated.
Mean glucose
Mean glucose (in mg/dL) is the arithmetic mean of all raw CGM glucose values.
SD
The sample SD of all raw CGM glucose values was taken.
MODD
MODD is the mean of all valid absolute value differences between glucose concentrations measured at the same time of day on two consecutive days (Table 2).
Table 2.
Standard Measures of Glucose Variability
| Name | Formula | Description | Reference |
|---|---|---|---|
| Mean | 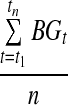 |
n=number of observations (number of glucose readings for a given individual) | |
| SD | 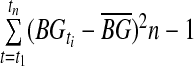 |
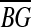 =mean glucose reading =mean glucose reading |
|
| n=number of observations | |||
| MODD | 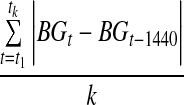 |
k=number of observations where there is an observation at the same time 24 h (1,440 min) ago | Molnar et al.5 |
| CONGA(n) | 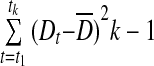 |
k=number of observations where there is an observation n×60 min ago | McDonnell et al.6 |
| where Dt=BGt−BGt−m | m=n×60 | ||
and 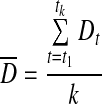
|
|||
| MAGE | 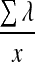 |
λ=the absolute value difference between sequential glucose peaks and nadirs | Fritzsche et al.13 |
| if λ>v | x=number of valid observations | Baghurst14 | |
| v=1 SD of mean glucose | Service et al.4 |
BG, glucose reading at time t min after start of observations; CONGA(n), continuous overall net glycemic action (h); MAGE, mean amplitude of glycemic excursions; MODD, mean of daily differences; ti, time (in min) after start of observations when the ith observation is taken.
CONGA(n)
CONGA(n) represents the SD of all valid differences between a current observation and an observation (n) hours earlier (Table 2).
MAGE
MAGE is calculated as the arithmetic average of absolute value differences between adjacent glucose peaks and nadirs, where the differences exceed 1 SD from the mean. The algorithm was described by Fritzsche et al.13
Area under the curve
The area under the curve is the graphical representation of two sets of cumulative areas under the curve—a hyperglycemic set and a hypoglycemic set. The areas were computed using a trapezoidal numerical integration function.
Input data plot
The input data plot is the line plot of the raw CGM data. The horizontal lines indicate the user-defined glycemic thresholds (Fig. 1A and B).
Transition speed
CGM-GUIDE provides two assessments of the slopes of a patient's CGM data: (1) a newly developed scatter plot of slopes at transition points (Fig. 1C) and (2) a histogram of the distribution of slopes between every recorded time interval (Fig. 1D). The histogram presents the distribution of slopes between every two consecutive glucose measurements in the patient CGM data; the SD of the slope is also calculated as a potential indicator of the rate of glucose fluctuations. The scatter plot displays the slopes of points flanking transitions from one user-defined threshold into another (as described in “Transition density profile”).
Transition density profile
First, raw CGM data are partitioned into bins based on user-defined threshold values, and exact transition points are established (Fig. 1B). Second, the magnitude of every continuous monotonic change in glucose levels, after smoothing, is sorted into the number of thresholds crossed (e.g., 2, 4, −3, −5); negative numbers indicate monotonic decreases, and positive numbers indicate monotonic increases in glucose levels (Fig. 1E). Third, transitions are separated into the time interval necessary to complete each change (i.e., <1 h, between 1 and 2 h, 2 and 3 h, etc.). Finally, the frequency of each monotonic threshold crossing per day is plotted against the time interval needed to cross the indicated number of thresholds (Fig. 1F). Gaps or null data points are recorded, and the appropriate times are added to the corresponding threshold transitions.
Safeguards and compatibility
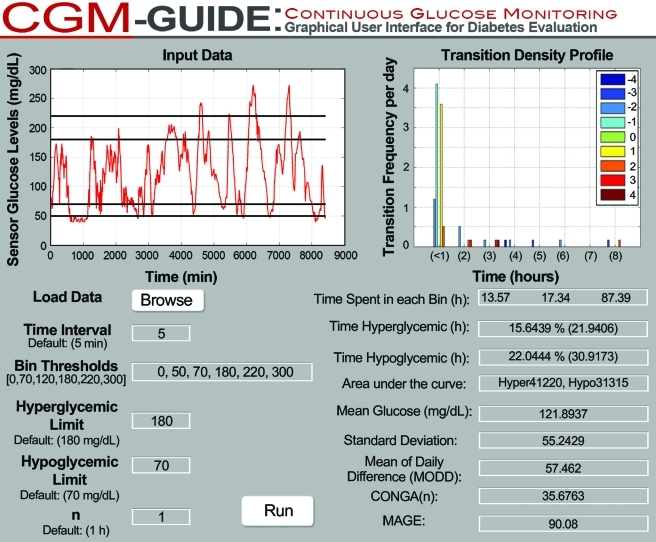
The data collection time interval entered by the user is checked against published statistical limits within which variability metrics can be accurately assessed. When an interval exceeds limits, an error message states, “Data collected at intervals greater than 1 hour affect the accuracy of MAGE, and intervals greater than 2–4 h affect the accuracy of SD and CONGA(n) where n=1, 2, or 4.”15 Additionally, CGM-GUIDE (Fig. 2) utilizes basic Excel data files, of glucose readings only, as inputs. Output files from all available CGM monitors are convertible to this format.
FIG. 2.
The CGM-GUIDE Interface. CGM-GUIDE allows for user-defined input of the threshold ranges, the hyper- and hypoglycemic limits, and the continuous overall net glycemic action (CONGA) “n” value. Glucose variability metrics [SD, MODD, CONGA(n), and mean amplitude of glycemic excursions (MAGE)] are calculated as described in Materials and Methods in conjunction with glycemic statistics (time spent within thresholds, time spent in hyperglycemic/hypoglycemic conditions, area under the curve, and mean glucose). Displays for area under the curve, transition speed, and slope histogram plots (Fig. 1C and D) are available through a plot menu option. Color graphics available online at www.liebertonline.com/dia
Results
Mean, SD, and time in glycemic states
To highlight the methodology behind the integrated glycemic variability assessment, we provide information on a group of four T1DM patients, in different strata of mean glucose (below 125 mg/dL, between 125 and 150 mg/dL, between 150 and 200 mg/dL, and above 200 mg/dL) that capture the capability of the CGM-GUIDE. Data captured with the CGM system show that the T1DM group had an average mean glucose concentration of 167±47 mg/dL, which is a 72% increase above the average reference value for control patients without diabetes (96.8 mg/dL), and an average SD of 74±15 mg/dL, 5.3-fold higher than the average SD of controls without diabetes (14.1 mg/dL) (Table 1).
Because critical limits used to assess glucose control may be heterogeneously standardized for different applications, to accommodate for non-uniformity, we allow variable thresholding of the CGM data and calculate the time spent within each user-defined range. We chose threshold ranges of (0–50; 50–70; 70–180; 180–220; and 220–300) and observed all four patients within these ranges. For example, Subject 3 was observed in each range 9.7%, 12.4%, 62.3%, 10.6%, and 5.1% of the time, respectively (Fig. 1B). To determine if the lower mean glucose observed for Subject 3 is reflective of good glycemic control, as measured by percentage of time out of normal glycemia, Subject 3 was observed to experience hyperglycemia (≥180 mg/dL) 15.6% and hypoglycemia (<70 mg/dL) 22.0% of the monitored time compared with Subjects 1, 2, and 4, who experienced hypoglycemia 4.8%, 3.8%, and 10%, respectively, and hyperglycemia 39.7%, 68.9%, and 23.3%, respectively. This suggests Subject 3's lower mean glucose reflects a higher incidence of hypoglycemia and not necessarily better glucose control compared with Subjects 1, 2, and 4. The controls without diabetes never reached the hyperglycemic threshold and spent an average of 1.7% of the time below the hypoglycemic limit (Table 1).
MODD, CONGA(n), and MAGE
As a measure of inter-day glucose variation, we calculated the mean absolute value of daily differences (MODD) between glucose concentrations (Table 2). In the T1DM group, the average daily variation was 80±28 mg/dL, compared with an average MODD value of 14.4 mg/dL in those without diabetes. Because of inter-day comparisons, MODD is dependent on patients' adherence to a regular meal and insulin schedule. Clinical experience has indicated that CGM traces with high MODD values can be indicative of irregular habits and thus require detailed contemporaneous lifestyle information prior to interpretation (Table 1).6
As a supplement to MODD, we analyzed the differences in glucose over a fixed interval using CONGA(n), which calculates the SD of glucose differences n h apart. Because of the flexibility of CONGA(n), we can assess time increments shorter than 24 h to provide a measure of intra-day glycemic variation and reduce dependence on the rigorous tracking of patient habits. CONGA(1), CONGA(2), and CONGA(4) for the T1DM group were found to be 48±11, 72±15, and 96±20 mg/dL, respectively. These values are four- to fivefold greater than the corresponding mean control values in those without diabetes of 12.9, 15.9, and 18.3 mg/dL, respectively (Table 1). For healthy controls, the time period, n, used to calculate CONGA has minimal effect on the metric value. For patients with diabetes, however, CONGA(n) values have been shown to increase with n (1 to 8 hours), gradually leveling off as n approaches 4 h.11
MAGE considers adjacent glucose peaks and nadirs whose absolute differences exceed 1 SD from the mean and calculates the arithmetic average of these differences. T1DM patients with brittle glucose concentrations are therefore expected to have higher MAGE values than normal, healthy individuals. Correspondingly, the T1DM group was found to have a MAGE value of 134±37 mg/dL, fourfold greater than the average MAGE of 32.1 mg/dL found in healthy controls (Table 1).
Area under the curve, slope histogram, and transition speed scatterplot
For a more visual interpretation of glucose control, we calculated the area under the curve plots to depict the degree of glucose deviation above or below glycemic limits.16 In the T1DM group, the area above the hyperglycemic limit (180 mg/dL) was (4.3±3.9)×104 mg·min/dL, whereas the area below the hypoglycemic limit (70 mg/dL) was (2.3±2.1)×103 mg·min/dL (Fig. 1C).
We next assessed the glucose rates of change as a direct way to quantify and analyze the severity of fluctuations in glucose levels. CGM-GUIDE calculates the rate of change between every two consecutive CGM measurements and then consolidates all the slopes and presents them in a histogram (Fig. 1D, inset). The SD of the slope distribution is representative of the dispersion in speed of glucose changes and was measured as 1.2 mg/(dL·min) for the subject with the lowest mean glucose level, Subject 3 (Fig. 1D, inset). It is notable that this SD of slopes is distinct from the SD measured previously, which is a measurement of the variation in glucose concentrations.
To differentiate from slope histogram, which displays glucose rates of change calculated at every sampling point, we also assessed the transition speed scatterplot that focuses on glucose dynamics during threshold crossings. CGM-GUIDE calculates the glucose rate of change across each threshold intersection and then plots the slope against the intersection time (Fig. 1D). Expressing these “transition speeds” in a graphical manner allows for ready visualization of dangerous glucose excursions and for early detection of potentially severe hyper- or hypoglycemia that could be addressed by adjusting insulin therapy. Subject 3 experienced fast transitions across thresholds, with an absolute value above 4, in 7.8% of transitions crossed (Fig. 1D).
Transition density profiles
We developed a novel transition density profile that reports the frequency, relative magnitude, and time taken for glucose levels to cross user-defined thresholds. Transition density profiles permit easy assessment of glucose dynamics across critical thresholds of glycemia (for calculation, see Materials and Methods). Subject 3's transition density profile depicted multiple large monotonic changes in glucose values (Fig. 1F). Large changes occurring over shorter timeframes are assumed to cause greater stress to the body than similar transitions occurring over longer timeframes. For example, the (−2 level) transitions that required between 1 and 2 h to complete would not cause the same level of stress as the more rapid (−2 level) transitions observed in less than 1 h (Fig. 1F).
Discussion
We present here a clinician-friendly CGM-GUIDE that simultaneously calculates statistics, including mean glucose and (percentage) time spent in hyperglycemic and hypoglycemic conditions, along with data thresholding based on user-specified glucose ranges. Thresholding allows for any range of glucose concentrations to be independently assessed for time spent in these ranges and for transition speeds between ranges to be estimated. Uniquely, CGM-GUIDE calculates the most widely used glucose variability metrics—SD, MODD, CONGA(n), and MAGE—and presents them visually all in one setting, like a dashboard. Data from the most popular CGM collection monitors and software systems including the Freestyle Navigator® CoPilot Health Management System (Abbott, Abbott Park, IL), CareLink™ Personal Therapy Management (Medtronic), MiniMed solutions pumps (Medtronic), and DexCom Data Manager 3 SEVEN® PLUS (DexCom, San Diego, CA) can be adapted for use with CGM-GUIDE. The aggregated format provides a more complete picture of glycemic control while facilitating comparisons between glucose variability and various insulin algorithms for the potential design of more accurate insulin adjustments. Additionally, CGM-GUIDE provides interactive graphical representations of CGM data, including (1) raw data with a display of user-defined threshold ranges, (2) area under the curve above or below user-defined hyper- and hypoglycemic limits, (3) a new graph of transition speeds across user-defined thresholds, (4) a histogram of slopes, and (5) a novel histogram plot—the transition density profile—indicating the magnitude and frequency of monotonic increases or decreases in the glucose data, over the time duration necessary for transitions to occur.
Continuous glucose monitors have afforded patients and physicians the flexibility to track glucose trends throughout the day, to assess individualized response to exercise and various stressors, and to evaluate nocturnal glucose trends, including frequency and trends of hypoglycemia. As the use of continuous glucose monitors increases, conclusions previously drawn from finger prick profiles can be tested against this more robust dataset and used to guide optimization in individualized insulin regimens and effective prevention of severe hypo- and hyperglycemia. Ultimately, data obtained with this tool may be used to assess the relationship with chronic diabetes complications and disease progression in either future long-term prospective clinical studies and/or with data previously collected during former follow-up studies. Cameron et al.11 compared the performance of many of the metrics listed above and identified inherent properties and applications associated with each metric. Some of the metrics, such as average daily risk range, are more appropriate for routine self-monitored blood glucose data,9 whereas CONGA(n) was designed specifically for CGM data.17 Apart from the necessity of differentiating between methods for analyzing self-monitored blood glucose versus CGM readings, many investigators acknowledge the subjectivity of certain extant glucose variability metrics. For example, researchers have criticized the M-value and MAGE—two of the earliest glucose variability measurements formulated—for their reliance on glucose reference points and subjective definitions for glycemic peaks and nadirs.17 Nonetheless, MAGE is still one of the most commonly used metrics for describing glucose variability in diabetes studies. Only as of 2011 did a standardized computer algorithm become available for the calculation of MAGE.13,14
Rapid variability in glycemia has been thought to incorporate additional “stress” to a patient's system and has been reported to induce increased oxidative stress,1 and these in concert were proposed to contribute to the development of microvascular complications.2,3 There exist, however, conflicting conclusions on the relationship between glucose variability and these aforementioned complications.3,18 For example, some statistical models developed using the glycemic profile data collected during the landmark Diabetes Control and Complications Trial (DCCT) suggested a relationship between fluctuations in glucose and the development of retinopathy, nephropathy, and neuropathy.19 However, recent independent analyses of DCCT glycemia data performed by the DCCT coordinating center have contradicted these findings and reported that the SD of glucose does not relate to the progression of microvascular complications in general.20,21 Similarly, Siegelaar et al.21 used SD and MAGE to evaluate the relationship between DCCT glucose data and complications, concluding that glucose variability as assessed by these measures does not contribute to the development of peripheral and autonomic neuropathy.
The inconsistent results on the relationship between some measures of glucose dynamics and the development of specific diabetes complications reported by these investigators may be, however, due to the insensitivity of glucose data as obtained from only five- to seven-point single monitor glucose profiles in accurately assessing glucose variability. These discrete measures of glucose provide only limited information on a patient's glucose dynamics over a 24-h period compared with the study of CGM data. In addition, as discussed earlier, statistical measurements of SD do not provide a comprehensive assessment of glucose variability, especially when the dataset contains a relatively sparse set of glucose measurements. In particular, recent work suggests the calculations of SD and CONGA(4) become unreliable when data measurements are taken more than 2–4 h apart, whereas MAGE becomes unreliable at observation intervals >1 h.15
Furthermore, different measures of glucose variability may be appropriate for assessing different physiological conditions. Many studies that use an individual metric in isolation to correlate glucose variability with clinical complications may observe correlations but are not complete in their assessment of variability. Cameron et al.11 demonstrated that glucose variability metrics, although correlated with each other in patients without diabetes, are not correlated in populations with diabetes. Thus, studies in populations with diabetes that look at only one or two measures of glucose variability are somewhat misleading because they do not take into account the range of available glycemic metrics.22–24
In contrast to (analyses of) diabetes patients, analyses of critically ill adult and pediatric intensive care unit patients without diabetes have indicated correlations between glucose variability and mortality regardless of illness or severity.18 Glucose variability, as measured by SD, has been shown to be a significant predictor of mortality in the adult intensive care unit by three independent groups25–27 and in two different pediatric intensive care units,28,29 suggesting a need for glucose control in patients without diabetes. These findings, along with the observation that mortality was observed to significantly increase with glucose variability in different strata of mean glucose levels,27 suggest that glucose variability is a predictor of mortality independent from mean glucose level. Consequently, the calculation and monitoring of glucose variability would be of added importance for the general care of adult and pediatric patients in intensive care units.
In summary, the development of CGM-GUIDE provides researchers and clinicians with an easy-to-use tool for a superior assessment of a patient's glucose landscape. The interface calculates and displays multiple metrics from CGM data, offering not only a multifaceted approach to studying glucose variability, but also a means to investigate glucose variability using more information-rich datasets. CGM-GUIDE will be made publicly available as a web-based application, similar to other recent glycemic calculators,30 and will provide enhanced graphic and user-defined options. Clinicians initiating CGM use in their practice will also have the option to request advanced education and training classes in using CGM-GUIDE to open discussion about glucose variability as continued snapshots of patient progress over time. By combining metrics into an aggregate tool, we anticipate the use of integrated glycemic variability assessments to guide clinicians in designing better insulin delivery algorithms for patients with T1DM and potentially supplement the current use of hemoglobin A1c in the global assessments of the relationship between glucose variability and the development of diabetes complications.
Acknowledgments
We thank Dr. Gil Ommen for his helpful discussions and editing. These studies were supported by grants NSF-DMS 0634590 to P.W.N., 1R01HL102334-01 to R.P.-B., SSP#12961 Medtronic Agreement to R.P.-B, and NSF-AGEP to R.A.R. and UM-SUBMERGE undergraduate research scholarships to L.-H.Y. and H.S. ©2011 The Regents of the University of Michigan.
Author Disclosure Statement
No competing financial interests exist.
References
- 1.Monnier L. Mas E. Ginet C. Michel F. Villon L. Cristol J-P. Colette C. Activation of oxidative stress by acute glucose fluctuations compared with sustained chronic hyperglycemia in patients with type 2 diabetes. JAMA. 2006;295:1681–1687. doi: 10.1001/jama.295.14.1681. [DOI] [PubMed] [Google Scholar]
- 2.Kilpatrick ES. Rigby AS. Atkin SL. Effect of glucose variability on the long-term risk of microvascular complications in type 1 diabetes. Diabetes Care. 2009;32:1901–1903. doi: 10.2337/dc09-0109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zaccardi F. Pitocco D. Ghirlanda G. Glycemic risk factors of diabetic vascular complications: the role of glycemic variability. Diabetes Metab Res Rev. 2009;25:199–207. doi: 10.1002/dmrr.938. [DOI] [PubMed] [Google Scholar]
- 4.Service FJ. Molnar GD. Rosevear JW. Ackerman E. Gatewood LC. Taylor WF. Mean amplitude of glycemic excursions, a measure of diabetic instability. Diabetes. 1970;19:644–655. doi: 10.2337/diab.19.9.644. [DOI] [PubMed] [Google Scholar]
- 5.Molnar GD. Taylor WF. Ho MM. Day to day variation of continuously monitored glycemia: a further measure of diabetic instability. Diabetologia. 1972;8:342–348. doi: 10.1007/BF01218495. [DOI] [PubMed] [Google Scholar]
- 6.McDonnell CM. Donath SM. Vidmar SI. Werther GA. Cameron FJ. A novel approach to continuous glucose analysis utilizing glycemic variation. Diabetes Technol Ther. 2005;7:253–263. doi: 10.1089/dia.2005.7.253. [DOI] [PubMed] [Google Scholar]
- 7.Schlichtkrull J. Munck O. Jersild M. The M-value, an index of blood-sugar control in diabetics. Acta Med Scand. 1965;177:95–102. doi: 10.1111/j.0954-6820.1965.tb01810.x. [DOI] [PubMed] [Google Scholar]
- 8.Kovatchev BP. Otto E. Cox D. Gonder-Frederick L. Clarke W. Evaluation of a new measure of blood glucose variability in diabetes. Diabetes Care. 2006;29:2433–2438. doi: 10.2337/dc06-1085. [DOI] [PubMed] [Google Scholar]
- 9.Hill NR. Hindmarsh PC. Stevens RJ. Stratton IM. Levy JC. Matthews DR. A method for assessing quality of control from glucose profiles. Diabet Med. 2007;24:753–758. doi: 10.1111/j.1464-5491.2007.02119.x. [DOI] [PubMed] [Google Scholar]
- 10.Wojcicki JM. “J”-index. A new proposition of the assessment of current glucose control in diabetic patients. Horm Metab Res. 1995;27:41–42. doi: 10.1055/s-2007-979906. [DOI] [PubMed] [Google Scholar]
- 11.Cameron FJ. Donath SM. Baghurst PA. Measuring glycaemic variation. Curr Diabetes Rev. 2010;6:17–26. doi: 10.2174/157339910790442592. [DOI] [PubMed] [Google Scholar]
- 12.Fox LA. Beck RW. Xing D. Variation of interstitial glucose measurements assessed by continuous glucose monitors in healthy, nondiabetic individuals. Diabetes Care. 2010;33:1297–1299. doi: 10.2337/dc09-1971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fritzsche G. Kohnert KD. Heinke P. Vogt L. Salzsieder E. The use of a computer program to calculate the mean amplitude of glycemic excursions. Diabetes Technol Ther. 2011;13:319–325. doi: 10.1089/dia.2010.0108. [DOI] [PubMed] [Google Scholar]
- 14.Baghurst PA. Calculating the mean amplitude of glycemic excursions from continuous glucose monitoring data: an automated algorithm. Diabetes Technol Ther. 2011;13:296–302. doi: 10.1089/dia.2010.0090. [DOI] [PubMed] [Google Scholar]
- 15.Baghurst PA. Rodbard D. Cameron FJ. The minimum frequency of glucose measurements from which glycemic variation can be consistently assessed. J Diabetes Sci Technol. 2010;4:1382–1385. doi: 10.1177/193229681000400612. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Salardi S. Zucchini S. Santoni R. Ragni L. Gualandi S. Cicognani A. Cacciari E. The glucose area under the profiles obtained with continuous glucose monitoring system relationships with HbA1c in pediatric type 1 diabetic patients. Diabetes Care. 2002;25:1840–1844. doi: 10.2337/diacare.25.10.1840. [DOI] [PubMed] [Google Scholar]
- 17.Rodbard D. Bailey T. Jovanovic L. Zisser H. Kaplan R. Garg SK. Improved quality of glycemic control and reduced glycemic variability with use of continuous glucose monitoring. Diabetes Technol Ther. 2009;11:717–723. doi: 10.1089/dia.2009.0077. [DOI] [PubMed] [Google Scholar]
- 18.Siegelaar SE. Holleman F. Hoekstra JB. DeVries JH. Glucose variability; does it matter? Endocr Rev. 2010;31:171–182. doi: 10.1210/er.2009-0021. [DOI] [PubMed] [Google Scholar]
- 19.The effect of intensive treatment of diabetes on the development and progression of long-term complications in insulin-dependent diabetes mellitus. The Diabetes Control and Complications Trial Research Group. N Engl J Med. 1993;329:977–986. doi: 10.1056/NEJM199309303291401. [DOI] [PubMed] [Google Scholar]
- 20.Kilpatrick ES. Rigby AS. Atkin SL. The effect of glucose variability on the risk of microvascular complications in type 1 diabetes. Diabetes Care. 2006;29:1486–1490. doi: 10.2337/dc06-0293. [DOI] [PubMed] [Google Scholar]
- 21.Siegelaar SE. Kilpatrick ES. Rigby AS. Atkin SL. Hoekstra JB. Devries JH. Glucose variability does not contribute to the development of peripheral and autonomic neuropathy in type 1 diabetes: data from the DCCT. Diabetologia. 2009;52:2229–2232. doi: 10.1007/s00125-009-1473-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruttomesso D. Crazzolara D. Maran A. Costa S. Dal Pos M. Girelli A. Lepore G. Aragona M. Iori E. Valentini U. Del Prato S. Tiengo A. Buhr A. Trevisan R. Baritussio A. In Type 1 diabetic patients with good glycaemic control, blood glucose variability is lower during continuous subcutaneous insulin infusion than during multiple daily injections with insulin glargine. Diabet Med. 2008;25:326–332. doi: 10.1111/j.1464-5491.2007.02365.x. [DOI] [PubMed] [Google Scholar]
- 23.Kilpatrick ES. Rigby AS. Goode K. Atkin SL. Relating mean blood glucose and glucose variability to the risk of multiple episodes of hypoglycaemia in type 1 diabetes. Diabetologia. 2007;50:2553–2561. doi: 10.1007/s00125-007-0820-z. [DOI] [PubMed] [Google Scholar]
- 24.White NH. Chase HP. Arslanian S. Tamborlane WV. Comparison of glycemic variability associated with insulin glargine and intermediate-acting insulin when used as the basal component of multiple daily injections for adolescents with type 1 diabetes. Diabetes Care. 2009;32:387–393. doi: 10.2337/dc08-0800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dossett LA. Cao H. Mowery NT. Dortch MJ. Morris JM., Jr May AK. Blood glucose variability is associated with mortality in the surgical intensive care unit. Am Surg. 2008;74:679–685. doi: 10.1177/000313480807400802. discussion 685. [DOI] [PubMed] [Google Scholar]
- 26.Egi M. Bellomo R. Stachowski E. French CJ. Hart G. Variability of blood glucose concentration and short-term mortality in critically ill patients. Anesthesiology. 2006;105:244–252. doi: 10.1097/00000542-200608000-00006. [DOI] [PubMed] [Google Scholar]
- 27.Krinsley JS. Glycemic variability: a strong independent predictor of mortality in critically ill patients. Crit Care Med. 2008;36:3008–3013. doi: 10.1097/CCM.0b013e31818b38d2. [DOI] [PubMed] [Google Scholar]
- 28.Hirshberg E. Larsen G. Van Duker H. Alterations in glucose homeostasis in the pediatric intensive care unit: hyperglycemia and glucose variability are associated with increased mortality and morbidity. Pediatr Crit Care Med. 2008;9:361–366. doi: 10.1097/PCC.0b013e318172d401. [DOI] [PubMed] [Google Scholar]
- 29.Wintergerst KA. Buckingham B. Gandrud L. Wong BJ. Kache S. Wilson DM. Association of hypoglycemia, hyperglycemia, and glucose variability with morbidity and death in the pediatric intensive care unit. Pediatrics. 2006;118:173–179. doi: 10.1542/peds.2005-1819. [DOI] [PubMed] [Google Scholar]
- 30.Czerwoniuk D. Fendler W. Walenciak L. Wojciech M. GlyCulator: a glycemic variability calculation tool for continuous glucose monitoring data. J Diabetes Sci Technol. 2011;5:447–451. doi: 10.1177/193229681100500236. [DOI] [PMC free article] [PubMed] [Google Scholar]