Abstract
CD4+ T helper type 2 (Th2) cells characterized by their expression of IL-4, IL-5, IL-9 and IL-13 are required for immunity to helminth parasites1 and promote the pathological inflammation associated with asthma and allergic diseases2. Polymorphisms in the gene encoding the cytokine thymic stromal lymphopoietin (TSLP) are associated with the development of multiple allergic disorders in humans, suggesting that TSLP is a critical regulator of allergic diseases3-6. Supporting genetic analyses, exaggerated TSLP production is associated with asthma, atopic dermatitis and food allergies in patients, and studies in murine systems demonstrated that TSLP promotes Th2 cytokine-mediated immunity and inflammation5, 7-12. However, the mechanisms through which TSLP promotes Th2 cytokine responses remain poorly defined. Here we demonstrate that TSLP promotes systemic basophilia, that disruption of TSLP-TSLPR interactions results in defective basophil responses and that TSLPR-sufficient basophils can restore Th2 cell-dependent immunity in vivo. TSLP acted directly on bone marrow- resident progenitors to selectively promote basophil responses. Critically, TSLP could elicit basophil responses in both IL-3-sufficient and IL-3-deficient environments and genome-wide transcriptional profiling and functional analyses identified heterogeneity between TSLP-elicited versus IL-3-elicited basophils. Further, activated human basophils expressed the TSLPR and basophils isolated from eosinophilic esophagitis (EoE) patients were heterogeneous. Collectively, these studies identify previously unrecognized heterogeneity within the basophil cell lineage and indicate that expression of TSLP may influence susceptibility to multiple allergic diseases by regulating basophil hematopoiesis and eliciting a population of functionally distinct basophils that promote Th2 cytokine-mediated inflammation.
Keywords: TSLP, Th2 cytokine responses, innate immunity, basophils, hematopoiesis
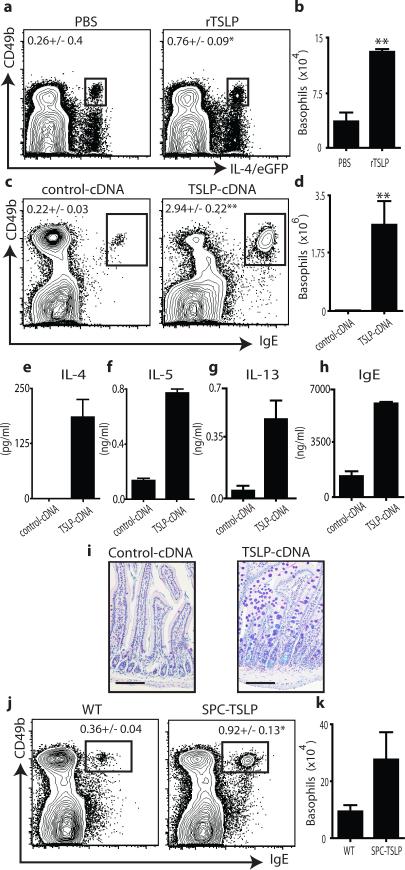
Previous in vitro studies suggested that TSLP could promote Th2 cytokine- mediated inflammation by influencing dendritic cell (DC), mast cell and lymphocyte populations13. However, the IL-4-expressing cell populations that TSLP targets in vivo remain poorly defined. Following administration of recombinant TSLP (rTSLP) to IL- 4/eGFP (enhanced green fluorescent protein) reporter mice, an increase in the frequency and total number of basophils was observed in the spleen (Fig. 1a,b). Mice receiving hydrodynamic tail vein injections of cDNA plasmid encoding TSLP (TSLP- cDNA)14 also exhibited significant increases in the frequency and total number of basophils in the spleen (Fig. 1c,d), blood, lung and bone marrow (Supplementary Fig. 1a-f). Basophils, along with DCs, are capable of contributing to the induction of optimal Th2 cytokine responses15-20. Consistent with this, exaggerated basophil responses in TSLP-cDNA-treated mice were associated with increased systemic production of Th2 cytokines (Fig. 1e-g), elevated IgE levels (Fig. 1h) and the development of Th2 cytokine- associated intestinal inflammation (Fig. 1i). In addition to reported expression by mast cells and basophils, epithelial cells at barrier surfaces are potent sources of TSLP13. Therefore, we tested whether epithelial cell-derived TSLP could influence peripheral basophil populations. Mice that constitutively express a TSLP transgene under the surfactant protein C promoter (SPC-TSLP) in lung epithelial cells21 exhibited increased frequencies and total numbers of basophils in the spleen (Fig. 1j,k).
Figure 1. TSLP promotes peripheral basophilia.
IL-4/eGFP reporter mice were treated with PBS or rTSLP and splenic basophils were (a) identified and (b) quantified. WT mice were treated with control-cDNA or TSLP-encoding (TSLP-cDNA) plasmid and splenic basophils were (c) identified and (d) quantified. Serum (e) IL-4, (f) IL-5, (g) IL-13 and (h) total IgE were quantified. (i), Histologic analysis of small intestine. Scale bars, 100 μm. (j), Splenic basophils from control mice (WT), or (SPC-TSLP) mice were identified (j) and (k) quantified. Results are representative of at least three (a-d and j-k) or two (e-i) separate experiments containing 3-5 (a-d), 5 (e-i) or 6-12 (j-k) mice per group. Statistical analyses performed using a two-tailed students t test (*, p<0.05), (**, p<0.01).
To test whether TSLP-TSLPR signaling influences infection-induced basophilia in vivo, we employed a helminth model in which protective immunity is dependent on TSLP-TSLPR signaling10. While Trichuris muris-infected wild-type (WT) mice exhibited increased basophils in the periphery, Tslpr-/- mice failed to exhibit infection-induced basophilia (Supplementary Fig. 2a). Critically, TSLP-elicited basophils isolated from Tslpr+/+ mice and adoptively transferred into normally susceptible T. muris-infected Tslpr-/- mice were sufficient to restore Th2 cytokine responses (Supplementary Fig. 2b,c), IgE production (Supplementary Fig. 2d), goblet cell hyperplasia (Supplementary Fig. 2e,f), secretion of goblet cell-derived RELMβ(Supplementary Fig. 2g) and significantly reduce worm burdens (Supplementary Fig. 2h). These data demonstrate that basophil-restricted TSLPR expression is sufficient to promote protective Th2 cytokine responses in vivo.
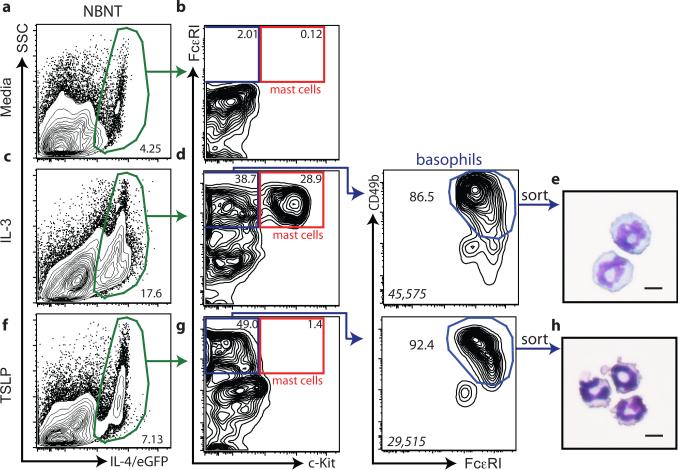
TSLP was originally identified as a lymphocyte growth factor capable of regulating the differentiation of lymphocytes from hematopoietic progenitor cells13. These findings provoked the hypothesis that in addition to influencing peripheral basophil responses, TSLP may regulate basophil hematopoiesis in the bone marrow. To directly test whether TSLP could elicit the population expansion of basophils from bone marrow cells, we adopted a basophil differentiation assay using IL-4/eGFP reporter mice22. When bone marrow was cultured in media alone, 4.25% of the non-B non-T (NBNT) cells expressed IL-4/eGFP (Fig. 2a), but no mast cells or basophils were present (Fig. 2b). As previously reported22, bone marrow cells cultured in the presence of IL-3 exhibited increased frequencies of IL-4/eGFP+ NBNT cells (17.6%) (Fig. 2c) and increased mast cell and basophil populations (Fig. 2d, e). In contrast, TSLP-stimulated bone marrow cultures yielded increased frequencies of IL-4/eGFP+ cells (7.13%) (Fig. 2f) and basophils but did not yield mast cell populations (Fig. 2g, h). Consistent with these data, bone marrow from WT mice cultured in the presence of IL-3 exhibited increased frequencies and total numbers of basophils, while bone marrow cultured in the presence of TSLP alone exhibited increased frequencies and total numbers of basophils but not mast cells (Supplementary Fig. 3a-h). Collectively, these data illustrate that TSLP can elicit the selective population expansion of mature basophils from bone marrow- resident cells.
Figure 2. TSLP preferentially expands basophil populations from bone marrow- resident cells.
Bone marrow-resident cells were taken from IL-4/eGFP reporter mice and cultured in the presence of (a) media, (c) IL-3 or (f) TSLP, and the frequency of IL- 4/eGFP+ NBNT cells was determined. (b, d, g), The frequencies of IL-4/eGFP+, NBNT, Siglec-F- basophils and mast cells were determined. Cytology of IL-4/eGFP+, CD49b+, FcεRI+, c-Kit- (e) IL-3-elicited or (h) TSLP-elicited basophils. Results are representative of at least three separate experiments. Italicized numbers in basophil gates represent the MFI levels of CD49b staining. Scale bar, 10 μm.
Basophil populations develop from precursors (BaP) that are characterized as CD34+, c-Kit-, FcεRI+, NBNT cells23. BaPs isolated from bone marrow expressed both chains of the TSLPR complex (IL-7Rα and TSLPR), suggesting they may be TSLP- responsive (Supplementary Fig. 4a). To test whether IL-3 or TSLP could induce basophil maturation, sorted bone marrow-resident BaPs (Supplementary Fig. 4b) were cultured in the presence of media, IL-3 or TSLP and mature basophil populations were evaluated on day 5 post-culture. Greater than 30% of BaPs cultured in the presence of media alone retained their progenitor-like phenotype as measured by expression of CD34 (Supplementary Fig. 4c). In contrast, IL-3-stimulated BaPs were predominantly CD34- and exhibited elevated CD49b expression, a phenotype consistent with mature basophils (Supplementary Fig. 4c,d). Critically, TSLP-stimulated BaPs were also predominantly CD34- and exhibited elevated levels of CD49b expression (Supplementary Fig. 4c,d). Consistent with whole bone marrow cultures (Fig. 2e,h), IL-3- or TSLP-treated cultures yielded more basophils than media alone controls and IL-3- elicited basophils were larger in size than TSLP-elicited basophils (Supplementary Fig. 4e,f). To compare the ability of IL-3 or TSLP to enhance basophil survival, basophils were sort-purified from the blood and spleen of WT mice and cultured in the presence of media, IL-3 or TSLP for 24 hours. While most mature basophils died when cultured in media alone, 24.6% of basophils survived in the presence of IL-3 and 13.1% of the basophils survived in the presence of TSLP (Supplementary Fig. 5a). These data demonstrate that both IL-3 and TSLP can promote basophil maturation from bone marrow-resident precursor cells and promote survival of basophils in the periphery.
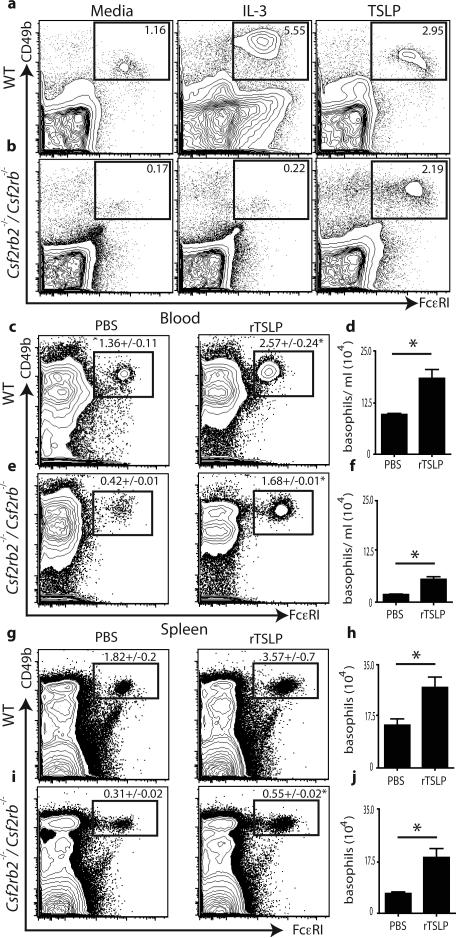
IL-3 is reported to be essential for optimal basophil activation, population expansion and survival24. To test whether TSLP-elicited basophil responses were dependent on IL-3-IL-3R signaling, bone marrow cells from WT or mice deficient in both IL-3Rβc and IL-3RβIL-3 (Csf2rb2-/-/Csf2rb-/-)25 were cultured in the presence of media, IL-3 or TSLP. Addition of IL-3 to WT bone marrow cultures increased the frequency of basophils compared to media alone controls (Fig. 3a), while the addition of IL-3 to Csf2rb2-/-/Csf2rb-/- bone marrow cultures had no effect on basophil populations (Fig. 3b). Similar to IL-3 stimulation, addition of TSLP to WT bone marrow cultures increased the frequency of basophils (Fig. 3a). Critically, addition of TSLP to Csf2rb2-/-/Csf2rb-/- bone marrow cultures also increased the frequency of basophils (Fig. 3b), demonstrating that TSLP can promote the population expansion of basophils from bone marrow cells independently of IL-3-IL-3R signaling. However, basophils were readily identified in the spleen and bone marrow of Csf2rb2-/-/Csf2rb-/- × Tslpr-/- mice, or Csf2rb2-/-/Csf2rb-/- mice treated with neutralizing anti-TSLP mAb (Supplementary Fig. 5b,c), demonstrating that signaling via IL-3-IL-3R and TSLP-TSLPR is not essential for basophil development. To test whether TSLP could elicit mature basophil responses independently of IL-3-IL-3R signaling in vivo, WT or Csf2rb2-/-/Csf2rb-/- mice were injected with PBS or rTSLP and peripheral basophil responses were examined. TSLP-treated WT mice exhibited increased frequencies and total numbers of basophils in the blood (Fig. 3c,d) and spleen (Fig. 3g,h) compared to PBS treated controls. Critically, rTSLP treatment significantly increased both the frequency and total number of basophils in the blood (Fig. 3e,f) and spleen (Fig. 3i,j) of Csf2rb2-/-/Csf2rb-/- mice. Collectively, these data illustrate that TSLP promotes peripheral basophilia in both IL-3-sufficient and IL-3-deficient environments.
Figure 3. TSLP-elicited basophilia is independent of IL-3-IL-3R signaling.
Bone marrow cultures from (a) WT or (b) Csf2rb2-/-/ Csf2rb-/- mice. WT or Csf2rb2-/-/ Csf2rb-/- mice were treated with PBS or rTSLP and (c,e) blood and splenic (g,i) basophils were identified. (d,f,h,j), Total numbers of basophils were quantified. Flow cytometry plots are gated on live NBNT cells. (a,b), Results are representative of at least 3 separate experiments. (c-j), Results are representative of 3 separate experiments. (WT + PBS n=7, Csf2rb2-/-/ Csf2rb-/- + PBS n=7, WT + TSLP n=7, Csf2rb2-/-/Csf2rb-/- + TSLP n=7). Statistical analysis was performed using a two-tailed students t test (*, p<0.05).
To test if IL-3-elicited versus TSLP-elicited basophil populations were phenotypically distinct, flow cytometric analysis of basophil-associated surface markers was performed. While IL-3-elicited and TSLP-elicited basophils exhibited similar expression of CD200R and CD69, IL-3-elicited basophils exhibited elevated expression of CD11b and CD62L (Fig. 4a). In contrast, TSLP-elicited basophils exhibited elevated expression of CD123, IL-33R (T1/ST2) and IL-18Rα (Fig. 4b). Collectively, these data demonstrate that IL-3- versus TSLP-elicited basophils exhibit distinct surface marker expression. Genome-wide transcriptional profiling of IL-3-elicited versus TSLP-elicited basophils also revealed remarkable differences in patterns of gene expression between the two basophil populations. Gene set enrichment analysis (GSEA)26 of complete transcriptional profiles revealed that IL-3-elicited basophils reflected gene expression associated with monocyte and dendritic cell maturation, matrix metalloproteinase production and TNFα signaling (Fig. 4c, Supplementary Fig. 6). In contrast, TSLP- elicited basophils exhibited a gene expression profile enriched for linoleic acid metabolism, arachidonic acid metabolism, cell communication and the expression of cell adhesion molecules (Fig. 4c, Supplementary Fig. 6). Real-time PCR analysis also revealed selective differential expression of mRNA encoding basophil-associated proteases mast cell protease (mcpt)2 and mcpt7 between the two populations (Supplementary Fig. 7a). Collectively, these results demonstrate phenotypic heterogeneity between IL-3-elicited versus TSLP-elicited basophils and suggest that either the two basophil populations represent distinct developmental stages of basophils or that they are functionally heterogeneous mature basophil populations.
Figure 4. Murine and human basophil populations exhibit heterogeneity.
IL-3- elicited or TSLP-elicited murine basophils were stained (shaded) for (a), CD200R, CD69, CD11b, CD62L, (b) CD123, T1/ST2 and IL-18Rα expression and compared to fluorescence minus one (FMO) controls. Numbers represent MFI levels. Results are representative of at least 3 separate experiments. (c), GSEA of microarray data comparing IL-3-elicited and TSLP-elicited basophils. (d), Activated human basophils stained for TSLPR. (e), Representative histograms demonstrating ST2 expression (shaded) compared to isotype controls. (f), Percentage of basophils from control or EoE patients expressing ST2 compared to isotype controls. Statistical analysis was performed using a two-tailed students t test.
To test whether phenotypic differences in the two basophil populations were associated with functional heterogeneity, a panel of basophil activation assays was employed. IL-3-elicited basophils exhibited similar degranulation and β-hexosaminidase release27 (a hallmark of basophil effector function28) as mast cells in response to IgE- mediated FcεRI crosslinking (Supplementary Fig. 7b). Consistent with distinct effector functions, TSLP-elicited basophils exhibited minimal degranulation (Supplementary Fig. 7b). Surface marker expression on IL-3-elicited versus TSLP-elicited basophils suggested that these two populations may differ in their responsiveness to cytokines (Fig 4b). To directly test this, IL-3- or TSLP-elicited basophils were sort-purified and stimulated with media alone, IL-3, IL-18 or IL-33, and production of cytokines and chemokines were assessed. TSLP-elicited basophils produced significantly more IL-4, IL-6 (Supplementary Fig. 8a) and more CCL3, CCL4 and CCL12 (Supplementary Fig. 8b) in response to IL-3 stimulation compared to IL-3-elicited basophils. TSLP-elicited basophils also produced significantly more IL-4, IL-6, TNFα (Supplementary Fig. 8a), CCL3, CCL9, CCL12 (Supplementary Fig. 8b), and Cxcl2 (Supplementary Fig. 8c) in response to IL-18 stimulation when compared to IL-3-elicited basophils. Finally, TSLP- elicited basophils produced more IL-4, IL-6 (Supplementary Fig. 8a), CCL3, CCL12 (Supplementary Fig. 8b) and Cxcl12 (Supplementary Fig. 8c) in response to IL-33 stimulation than IL-3-elicited basophils. Collectively, these data indicate that IL-3-elicited and TSLP-elicited basophils exhibit distinct responsiveness and functional potential following stimulations with IL-3, IL-18, or IL-33.
To test whether IL-3-independent, TSLP-dependent basophil responses exist in vivo, a TSLP-dependent murine model of atopic dermatitis29 was employed. Topical treatment with the vitamin D analogue MC903 resulted in increased levels of TSLP (Supplementary Fig. 9a). Further, MC903-treatment resulted in the accumulation of basophils in the skin of WT mice (Supplementary Fig. 9b). MC903-induced basophil responses were TSLP-dependent, as treatment of WT mice with anti-TSLP diminished the accumulation of basophil populations (Supplementary Fig. 9b). MC903 treatment of Csf2rb2-/-/Csf2rb-/- mice also resulted in a robust accumulation of basophils in the skin (Supplementary Fig. 9c) and MC903-induced, IL-3-independent basophil responses were significantly reduced following anti-TSLP treatment (Supplementary Fig. 9c). Collectively, these data are the first to demonstrate IL-3-independent, TSLP-dependent basophil responses in vivo. To test whether TSLP-dependent basophils contribute to the induction of Th2 cell responses, MC903 treatment of WT mice was extended to 7 days. MC903 treatment resulted in the robust accumulation of basophils in the skin (Supplementary Fig. 9d) and a marked increase in IL-4 and IL-5 production in the draining lymph nodes (LNs) (Supplementary Fig. 9f). Critically, diphteria toxin-mediated depletion of basophils30 reduced the accumulation of basophil in the skin (Supplementary Fig. 9e) and decreased IL-4 and IL-5 production (Supplementary Fig. 9f), demonstrating that TSLP-dependent basophils contribute to Th2 cytokine responses in vivo.
To test whether human basophils can respond to TSLP, peripheral blood from healthy human donors was taken and TSLPR expression on basophils was evaluated. Greater than 70% of activated peripheral human basophils expressed TSLPR (Fig. 4d), suggesting that human basophils can be TSLP-responsive. The food allergy-associated disease EoE is associated with gain-of-function polymorphisms in the gene encoding TSLP and elevated expression of TSLP3-6, 13. To examine whether basophil heterogeneity exists when human basophils develop in a TSLP-rich environment associated with food allergy, basophil responses were examined in healthy control or EoE patients. No differences in expression of HLA-DR, CD28, CD40, CD86, CD69 or CD203c were observed between control and EoE patients (data not shown). However, consistent with TSLP-induced basophils in mice, basophils from EoE patients expressed significantly higher levels of the IL-33R (ST2) compared to basophils from healthy controls (Fig. 4e,f). These data suggest that heterogeneity in basophil populations may exist in humans and correlate with susceptibility to allergic inflammation.
Collectively, these data identify that TSLP can selectively promote basophil hematopoiesis from bone marrow-resident precursors and elicit mature basophil responses in the periphery in an IL-3-sufficient or IL-3-deficient environment. The demonstration that TSLP-elicited basophils exhibit distinct transcriptional and functional profiles indicates that similar to other immune cell lineages, basophils are a heterogeneous cell population that exhibit distinct phenotypic and functional characteristics depending on the cytokine milieu in which they develop or are activated. The identification of a role for TSLP in promoting basophil hematopoiesis, peripheral basophilia and altering the functional capacity of basophils (Supplementary Fig. 10) may provide a biological mechanism through which local production of TSLP at one barrier surface can confer susceptibility to pan-allergic diseases at multiple mucosal sites.
METHODS SUMMARY
B6 or BALB/c, IL-4/eGFP reporter mice, or Csf2rb2-/-/Csf2rb-/- mice were treated by intraperitoneal (i.p.) injection with PBS or 10 μg of rTSLP daily for 4-7 days and basophil populations were evaluated. Mice were injected intravenously (i.v.) with 10 μg of control or TSLP encoding cDNA plasmid, basophils and small intestine pathology were evaluated on days 14-28 post-injection. WT and Tslpr-/- mice were infected with T. muris and on day 10 post-infection, one group of Tslpr-/- mice received 100×103 TSLPR- sufficient basophils elicited from TSLP-cDNA injected H2K-Bcl2-transgenic mice. All groups received 10 μg injections of rTSLP every other day starting at day 11 post- infection. On day 21 post-infection, Th2 cytokine responses, serum IgE, pathology, RELM β and worm burdens were evaluated. Bone marrow (BM) from WT, IL-4/eGFP reporter mice, or Csf2rb2-/-/Csf2rb-/- mice was cultured in the presence of 10 ng/ml of IL- 3, or 1 μg/ml of TSLP for 5 days. BM-derived basophils were sorted on day 5 post- culture, RNA was isolated and gene expression profiles were determined using an Affymetrix HT Mouse 430 PM platform and analysis was performed using gene set enrichment analysis (GSEA) software through http://www.broadinstitute.org/gsea/index.jsp. BM-resident BaPs were sorted from WT whole BM and cultured in the presence of 10 ng/ml of IL-3, or 1 μg/ml of TSLP for five days and basophils were evaluated by flow cytometry and cytology. WT, Csf2rb2-/- /Csf2rb-/-, or BaS-TRECK mice were treated with 50μl of 100% ethanol or 50μl of 2nm MC903 dissolved in 100% ethanol topically for 3-7 days. Groups were treated (i.p.) with 1mg of Rat IgG isotype control or anti-TSLP antibody on d.0 of ethanol or anti-MC903 treatment. Skin-draining LN cells were isolated from ethanol-treated or MC903-treated mice on day 7 and stimulated with anti-CD3 and anti-CD28 for 48 hours.
Supplementary Material
Acknowledgements
We thank members of the Artis lab for critical reading of the manuscript, Alison Budelsky, Todd Martin, Bo-Rin Park Yoon, Jeannette Bigler and Marty Timour (Amgen) for assistance with experiments and microarrays and the University of Pennsylvania flow cytometry core for assistance with sorting. Work in the Artis lab is supported by the National Institutes of Health (AI61570, AI74878, AI87990 and AI083480 (to D.A.), F32 fellowship AI085828 (to M.C.S.), F31 training grant GM082187 (to S.A.S), T32 training grant AI060516 (to D.A.H.)) and the Burroughs Wellcome Fund (to D.A.).
Footnotes
Authors Contributions:
M.C.S., S.A.S., D.A.H., B.S.K., M.B.H., H.K.J., L.A.S., M.R.C. and D.A. designed and performed the research. S.F.Z. and T.K. provided reagents. E.C.D., A.C. and J.M.S. collected and provided human samples. M.C.S., S.A.S., D.A.H., B.S.K., T.A.D. and D.A. analyzed the data. M.C.S. and D.A. wrote the paper.
References
- 1.Anthony RM, Rutitzky LI, Urban JF, Jr., Stadecker MJ, Gause WC. Protective immune mechanisms in helminth infection. Nat Rev Immunol. 2007;7:975–987. doi: 10.1038/nri2199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Umetsu DT, McIntire JJ, Akbari O, Macaubas C, DeKruyff RH. Asthma: an epidemic of dysregulated immunity. Nat Immunol. 2002;3:715–720. doi: 10.1038/ni0802-715. [DOI] [PubMed] [Google Scholar]
- 3.Harada M, et al. TSLP Promoter Polymorphisms are Associated with Susceptibility to Bronchial Asthma. Am J Respir Cell Mol Biol. doi: 10.1165/rcmb.2009-0418OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sherrill JD, et al. Variants of thymic stromal lymphopoietin and its receptor associate with eosinophilic esophagitis. J Allergy Clin Immunol. 126:160–165. e163. doi: 10.1016/j.jaci.2010.04.037. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Rothenberg ME, et al. Common variants at 5q22 associate with pediatric eosinophilic esophagitis. Nat Genet. 42:289–291. doi: 10.1038/ng.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Hunninghake GM, et al. TSLP polymorphisms are associated with asthma in a sex-specific fashion. Allergy. doi: 10.1111/j.1398-9995.2010.02415.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ying S, et al. Thymic stromal lymphopoietin expression is increased in asthmatic airways and correlates with expression of Th2-attracting chemokines and disease severity. J Immunol. 2005;174:8183–8190. doi: 10.4049/jimmunol.174.12.8183. [DOI] [PubMed] [Google Scholar]
- 8.Al-Shami A, et al. A role for thymic stromal lymphopoietin in CD4(+) T cell development. J Exp Med. 2004;200:159–168. doi: 10.1084/jem.20031975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ito T, et al. TSLP-activated dendritic cells induce an inflammatory T helper type 2 cell response through OX40 ligand. J Exp Med. 2005;202:1213–1223. doi: 10.1084/jem.20051135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Taylor BC, et al. TSLP regulates intestinal immunity and inflammation in mouse models of helminth infection and colitis. J Exp Med. 2009;206:655–667. doi: 10.1084/jem.20081499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Mou Z, et al. Overexpression of thymic stromal lymphopoietin in allergic rhinitis. Acta Otolaryngol. 2009;129:297–301. doi: 10.1080/00016480802225884. [DOI] [PubMed] [Google Scholar]
- 12.Soumelis V, et al. Human epithelial cells trigger dendritic cell mediated allergic inflammation by producing TSLP. Nat Immunol. 2002;3:673–680. doi: 10.1038/ni805. [DOI] [PubMed] [Google Scholar]
- 13.Ziegler SF, Artis D. Sensing the outside world: TSLP regulates barrier immunity. Nat Immunol. 11:289–293. doi: 10.1038/ni.1852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Sebestyen MG, et al. Mechanism of plasmid delivery by hydrodynamic tail vein injection. I. Hepatocyte uptake of various molecules. J Gene Med. 2006;8:852–873. doi: 10.1002/jgm.921. [DOI] [PubMed] [Google Scholar]
- 15.Perrigoue JG, et al. MHC class II-dependent basophil-CD4+ T cell interactions promote T(H)2 cytokine-dependent immunity. Nat Immunol. 2009;10:697–705. doi: 10.1038/ni.1740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sokol CL, et al. Basophils function as antigen-presenting cells for an allergen-induced T helper type 2 response. Nat Immunol. 2009;10:713–720. doi: 10.1038/ni.1738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Yoshimoto T, et al. Basophils contribute to T(H)2-IgE responses in vivo via IL-4 production and presentation of peptide-MHC class II complexes to CD4+ T cells. Nat Immunol. 2009;10:706–712. doi: 10.1038/ni.1737. [DOI] [PubMed] [Google Scholar]
- 18.Hammad H, et al. Inflammatory dendritic cells--not basophils--are necessary and sufficient for induction of Th2 immunity to inhaled house dust mite allergen. J Exp Med. 207:2097–2111. doi: 10.1084/jem.20101563. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Ohnmacht C, et al. Basophils orchestrate chronic allergic dermatitis and protective immunity against helminths. Immunity. 33:364–374. doi: 10.1016/j.immuni.2010.08.011. [DOI] [PubMed] [Google Scholar]
- 20.Tang H, et al. The T helper type 2 response to cysteine proteases requires dendritic cell-basophil cooperation via ROS-mediated signaling. Nat Immunol. doi: 10.1038/ni.1883. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zhou B, et al. Thymic stromal lymphopoietin as a key initiator of allergic airway inflammation in mice. Nat Immunol. 2005;6:1047–1053. doi: 10.1038/ni1247. [DOI] [PubMed] [Google Scholar]
- 22.Gessner A, Mohrs K, Mohrs M. Mast cells, basophils, and eosinophils acquire constitutive IL-4 and IL-13 transcripts during lineage differentiation that are sufficient for rapid cytokine production. J Immunol. 2005;174:1063–1072. doi: 10.4049/jimmunol.174.2.1063. [DOI] [PubMed] [Google Scholar]
- 23.Iwasaki H, Akashi K. Myeloid lineage commitment from the hematopoietic stem cell. Immunity. 2007;26:726–740. doi: 10.1016/j.immuni.2007.06.004. [DOI] [PubMed] [Google Scholar]
- 24.Lantz CS, et al. Role for interleukin-3 in mast-cell and basophil development and in immunity to parasites. Nature. 1998;392:90–93. doi: 10.1038/32190. [DOI] [PubMed] [Google Scholar]
- 25.Scott CL, et al. Reassessment of interactions between hematopoietic receptors using common beta-chain and interleukin-3-specific receptor beta-chain-null cells: no evidence of functional interactions with receptors for erythropoietin, granulocyte colony-stimulating factor, or stem cell factor. Blood. 2000;96:1588–1590. [PubMed] [Google Scholar]
- 26.Haining WN, Wherry EJ. Integrating genomic signatures for immunologic discovery. Immunity. 32:152–161. doi: 10.1016/j.immuni.2010.02.001. [DOI] [PubMed] [Google Scholar]
- 27.Lenox LE, et al. Mutation of tyrosine 145 of lymphocyte cytosolic protein 2 protects mice from anaphylaxis and arthritis. J Allergy Clin Immunol. 2009;124:1088–1098. doi: 10.1016/j.jaci.2009.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Siracusa MC, Perrigoue JG, Comeau MR, Artis D. New paradigms in basophil development, regulation and function. Immunol Cell Biol. 88:275–284. doi: 10.1038/icb.2010.1. [DOI] [PubMed] [Google Scholar]
- 29.Li M, et al. Induction of thymic stromal lymphopoietin expression in keratinocytes is necessary for generating an atopic dermatitis upon application of the active vitamin D3 analogue MC903 on mouse skin. J Invest Dermatol. 2009;129:498–502. doi: 10.1038/jid.2008.232. [DOI] [PubMed] [Google Scholar]
- 30.Sawaguchi M, S.T., Mukai K, Ishiwata K, Oboki K, Nakae S, Kambayashi T, Watanabe N, Karasuyama H, Kubo M. Basophils and mast cells differently regulate allergic response; in vivo demonstration by diphtheria toxin based deletion system. International Congress of Immunology. 2011 WS/PP-043-04. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.