Abstract
Objective
The objective of this study was to determine the role of NO in TNF-α–induced matrix damage, compared to IL-1β in bovine cartilage explant cultures.
Methods
Cartilage explants were subjected to treatment with TNF-α (100 ng/ml), IL-1β (10 ng/ml) and to the nitric oxide synthase inhibitor, N-methylarginine (L-NMA; 1.25 mM) for 26, 50 or 120 hours (5 days). The collected medium was analyzed for sGAG, nitrate and nitrite, MMP activity by zymography, and aggrecan degradation by immunoblotting of aggrecan G1 and aggrecan-G1-NITEGE fragments. RNA was extracted from the 26 and 50 hour treated explants for real-time PCR analyses.
Results
TNF-α and IL-1β treatment caused a 3–5 fold increase in sGAG release with an increase in aggrecanase specific aggrecan breakdown and an increase in nitrate and nitrite production. L-NMA treatment inhibited almost 50% of the sGAG release caused by TNF-α treatment, with concomitant decrease in the aggrecanase-specific-NITEGE neo-epitope of aggrecan released into the medium. No L-NMA effect was identified with IL-1β. TNF-α and IL-1β both increased ADAMTS4 and ADAMTS5 transcription with no effect by L-NMA, suggesting that nitric oxide regulates aggrecanase activity at a post-transcriptional level in response to TNF-α. TNF-α and IL-1β both caused an increase in protease transcription (MMP3, MMP13, ADAMTS4 and ADAMTS5) and in pro-inflammatory enzymes iNOS and COX2, as well as a decrease in matrix protein transcription, including collagen II, aggrecan, fibromodulin and link, protein (IL-1β only), and an increase MMP-3 and MMP-9 secretion. L-NMA had no effect on gene transcription or MMP secretion.
Conclusion
Nitric oxide regulates aggrecanase activity at a post-transcriptional level in response to TNF-α treatment while having no effect on IL-1β treated cartilage explants.
Keywords: tumor necrosis factor alpha, nitric oxide, interleukin 1, matrix degradation, aggrecan, aggrecanase
Introduction
Nitric oxide (NO) is a neutral free radical product of nitric oxide synthase (NOS) with potent biological effects through its actions on cGMP production as well as through various oxidative and nitrosative chemistries that have been shown to modulate gene expression and to alter protein structure and function (1). NO production by chondrocytes was first discovered by Stadler et al. who showed that, in vitro, chondrocytes produce substantial amounts of NO in response to interleukin 1 beta (IL-1β) and to lipopolysaccharide (LPS) (2). Since these first reports, numerous studies have shown that chondrocytes or cartilage explant cultures from most species produce NO via inducible nitric oxide synthase (iNOS) in response to IL-1β, IL-1α, LPS, and tumor necrosis factor alpha (TNF-α) (3–4), as well as IL-17, IL-18, interleukin 1 converting enzyme (ICE), and fibronectin fragments (5–7).
The inflammatory cytokines TNF-α and IL-1 contribute to disease processes in rheumatoid arthritis (RA) and likely in osteoarthritis (OA). TNF-α was identified in synovial exudates of RA and OA joints (8, 9). In RA patients, TNF-α may contribute to IL-1 production as determined by anti-TNF-α therapy (10), and TNF-α alone or in combination with IL-1 may cause cartilage breakdown and a decrease in new matrix synthesis by chondrocytes in vitro (11). TNF-α production by OA synovial cells and in synovial fluid and serum may be elevated in OA (10, 12, 13), and OA cartilage explants may be more sensitive to IL-1 and TNF-α treatment (14–16). TNF-α receptor, TNF-R p55, is elevated in chondrocytes near OA lesions, and this expression correlates with sGAG depletion (17). These data together suggest that TNF-α as well as IL-1 may play a role in cartilage breakdown in OA.
To determine whether NO production plays a role in mediating the pro-catabolic and anti-anabolic effects of inflammatory mediators, in vitro studies have used the NOS inhibitors, L-NMA (L-N-methyl-arginine), Nω-nitro-L-arginine methyl ester (L-NAME), aminoguanidine, and L-NIO (N-iminoethyl-L-ornithine), to evaluate the role of NO in IL-1-induced changes in chondrocyte metabolism and matrix degradation in explant, hydrogel, and monolayer culture. With the exception of bovine explant studies (18), inhibiting NOS partially reversed IL-1-induced inhibition of proteoglycan synthesis in cartilage explants or chondrocyte cultures (4, 19, 20). TNF-α can decrease proteoglycan synthesis in a NO dependent manner (21), and the exogenous NO donor, SNAP, may also decrease proteoglycan synthesis. Cao et al. found that NO decreased collagen synthesis(22). Studies on matrix degradation have shown that inhibiting NO production may enhance (18, 23, 24) or have no effect (25) on IL-1β-induced aggrecan degradation as measured by sGAG release. IL-1-induced NO was also found to enhance gelatinase (2, 26, 27) and alter stromelysin (MMP-3) (18, 26) expression or activity. While most studies on inhibition of NOS are associated with IL-1 treatment, other inflammatory cytokines, such as TNF-α, are capable of mediating cartilage damage and enhancing NO production. Thus, understanding the contributions of NO with other cytokines may be important in determining their role in cartilage degradation.
The purpose of this study was to characterize the role of NO in matrix degradation in response to TNF-α and compare it to the effects of NO following IL-1β treatment using a non-specific NOS inhibitor, N-methylarginine (L-NMA). We found that inhibition of NOS by L-NMA decreased sGAG release in response to TNF-α by almost 50%, with a concomitant decrease in release of aggrecan-G1-NITEGE fragments specific for aggrecanase-mediated aggrecan degradation. No L-NMA effect was seen with IL-1β treatment. L-NMA did not alter ADAMTS4 or ADAMTS5 transcription in response to cytokine treatment. Gene transcription profiling of a panel of inflammatory molecules, proteases, and matrix proteins showed that TNF-α and IL-1β both inhibited transcription of matrix proteins including collagen II, aggrecan, link protein, and fibromodulin, while enhancing matrix proteases and inflammatory factors such as MMP-3, MMP-13, iNOS, and COX2, all with no effect of L-NMA. Overall these data suggest that NO plays a role in TNF-α–induced aggrecan release at a post-transcriptional level by altering ADAMTS4 or ADAMTS5 protein expression or post-translational modification, and that TNF-α and IL-1β appear to promote aggrecan degradation through different mechanisms of aggrecanase regulation.
Methods
Reagents
ITS medium supplement and NOS inhibitor, N-methyl-arginine, were from Sigma (St Louis, MI). Recombinant human IL-1β and TNFα were from R&D systems (Minneapolis, MN), PAGE gels were from BioRad (Hercules, CA). Protease-free chondroitinase and keratanase II were from Seikagaku (Japan). Common chemicals were purchased from ICN, Mallenkrodt, or Sigma.
Cartilage explant harvest and culture
Articular cartilage disks were obtained from the patello-femoral groove of 1–2 week old bovine calves as described previously (28). Cartilage-bone cylinders (9-mm-diameter) were cored from the patello-femoral groove, perpendicular to the joint surface. Two 1-mm-thick slices were then microtomed from the middle zone and a 6-mm diameter dermal punch was then used to core a 6-mm diameter by 1-mm thick disk from the center of each of the 9-mm slice. The explants were cultured in high glucose DMEM with 1% ITS as described previously with medium change every other day prior to treatment (29). Explants were weighed two days prior to the start of cytokine treatment.
TNF–α, IL-1β, and L-NMA treatments
Cartilage explants were allowed to rest 7 days in culture, and were then treated with 10 ng/ml IL-1β or 100 ng/ml TNF–α or left untreated in 2 ml of medium without 1% ITS. After 2 hours, half the explants were further treated with 1.25 mM L-NMA. Experiments utilized 5–8 different joints (5–8 different animals) with at least two explants per joint per condition. Every 24 hours over 5 days, cultures underwent a 10% (200 µl) medium removal and supplementation. After day 5 of treatment, medium and explants were collected, pooled to each treatment group, and stored at −80 °C. For real time PCR analyses explants were cultured for 24 or 48 hours after the addition of L-NMA and snap frozen in liquid nitrogen.
Sulfated Glycosaminoglycan (sGAG) Assay
sGAG released to the medium was measured as an indicator of aggrecan degradation and assessed via the dimethylmethylene blue (DMMB) assay using shark chondroitin C as a standard, as described previously (30).
Nitrate/Nitrite Analysis
Medium samples were diluted 1:2 in water prior to nitrate and nitrite analysis by Griess assay (31). Total nitrogen oxides were determined by cadmium column reduction of nitrate to nitrite followed by direct nitrite detection by Griess reaction. Nitrite was assayed directly, and nitrate was calculated as the difference between the total nitrogen oxides and nitrite.
Gelatin and Casein Zymography for MMPs
Zymograms were performed as described (32). Briefly, conditioned medium from day 5 was mixed with 4X non-reducing SDS-sample buffer and electrophoresed in 10%/12% gelatin or casein zymogram gels. Proteases were renatured in 2.5% triton X-100 solution, and placed in solution of 50 mM Tris and 5 mM CaCl2 for 18 hours at 37°C. Gels were then fixed and stained with Coomassie brilliant blue and destained until bands became visible and distinct. EDTA was added to the incubation buffer to verify MMP activity. MMP-3 and MMP-9 were verified by immunoblot.
Desalting and deglycosylation
Samples were desalted by ethanol precipitation or by dialysis with deglycosylation. The medium equivalent of 10–20 µg sGAG was precipitated by the addition of 3 volumes of ice-cold ethanol. The samples were resuspended (50 mM Tris-Acetate, 10mM EDTA) and sequentially deglycosylated beginning with 5 mU of protease-free chondroitinase ABC for three hours followed by 0.1 mU of keratanase II and 0.1 mU of endo-β-galactosidase for an additional four hours. Equal amounts of sGAG were loaded for aggrecan western blots.
Western blot analysis
Concentrated samples (10–15 µl) were subjected to denaturing SDS-PAGE on a 4–15% gradient gel run at 15 mA for 2 hours. Proteins were then transferred to Immobilon (PVDF) membrane, and proteins were detected using a monoclonal antibody to the aggrecan-NITEGE neoepitope in the interglobular domain of aggrecan associated with aggrecanase degradation (from C. Flannery, Wyeth Pharmaceuticals).
RNA extraction and real time PCR
For each condition (untreated, L-NMA, TNF-α ±, L-NMA, IL-1β± L-NMA), 2–3 cartilage explants per condition per joint, from a total of 8 joints (8 animals) were taken at 26 and 50 hours of culture, pulverized under liquid nitrogen and homogenized in Trizol (Invitrogen, San Diego,CA). As described previously (33), homogenates were transferred to Phase Gel tubes according to the manufacturer’s instructions (Eppendorf, Hamburg, Germany) and spun at 10,000 rpm for 10 minutes at 4°C. The clear RNA containing supernatant was removed from Phase Gel tubes and subjected to RNAeasy mini-kit clean-up (Qiagen, Chatsworth, CA) according to manufacturer’s instructions. RNA quantification was determined by nano-drop method measuring absorbance at 260 nm and 280 nm, which gave the concentration of RNA extracted from the tissue and the purity of the extract; the average 260/280 absorbance ratio for all samples was 2.12 ± 0.1. Equal amounts of RNA were subjected to reverse transcription using AmpliTaq-Gold reverse transcription kit (ABI, Foster City, CA) to generate cDNA for real time PCR analysis. Real time PCR analysis was performed with Applied Biosystems SYBR-Green master mix in combination with cDNA and primers, using the ABI prism 7900HT real time 384-well plate PCR instrument. Both forward and reverse primers were designed using Primer3 software based on the bovine genomic sequence (33). Primers from 32 genes relevant to cartilage homeostasis were used: 18S-RNA, aggrecan, collagen II, fibromodulin, fibronectin, proteoglycan link protein, MMP1, MMP3, MMP9, MMP13, ADAMTS4, ADAMTS5, TIMP1, TIMP2, TIMP3, COX2, iNOS, G3PDH, β-actin, IGF-1, IGF2, TGF-β, TNF-α, IL-1β, IL-4, IL-6, TXNIP, HSP90, CD44, HAS2, bFGF, and OP-1. Standard curve analysis was performed on each primer to determine the efficiency of amplification and proper primer concentration. The measured cycle threshold (CT) was determined and converted to relative copy number using information from standard curves for comparisons.
Data analysis and statistics
Real time PCR data for each gene were first normalized to 18S-RNA; data from each sample were then normalized to the untreated control sample within each sample set (animal), and these normalized data were pooled across all animals and are reported as the mean +/− SEM (n=8) for each condition. Principal component analysis and k-means clustering were performed on the PCR data using components of the Matlab Statistics toolbox (33). Cluster analysis was performed(33) on a (31 × 5 × 2) matrix of data derived from the set of 31 genes (i.e., all genes but 18S), 5 treatment conditions (each normalized to untreated control), and 2 time points (26 or 50 hrs). Each time point was also clustered individually to test the robustness of the co-expressed gene groupings. The Kruskal-Wallis test and Wilcoxon sign-rank test with Bonferroni correction for multiple comparisons were used to determine statistical significance of the L-NMA effect and of pair-wise comparisons between treatments, respectively. For the sGAG and nitrate/nitrite assays, multi-way ANOVA followed by a post-hoc Student’s t-test with Bonferroni correction for multiple comparisons were used to determine statistical significance defined as p < 0.05. All statistical analyses were performed using Matlab (Natick, MA) or Systat 11 (Richmond, CA).
Results
Nitrate/Nitrite
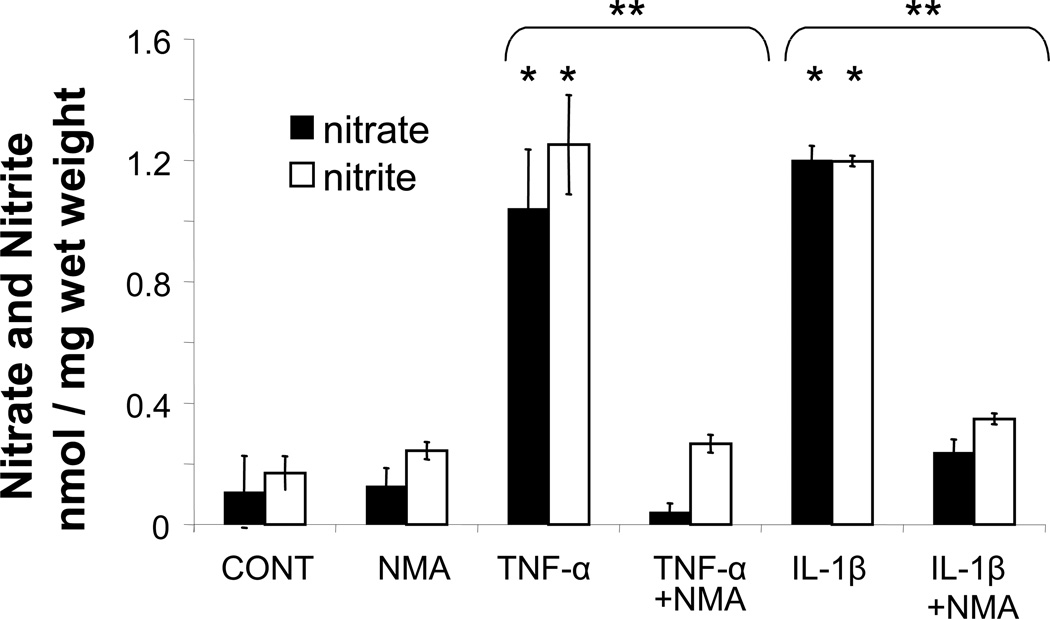
Nitrite and total nitrate plus nitrite were determined by the Griess assay as described by Green et al. (31). IL-1β and TNF–α both increased accumulated nitrate and nitrite compared to the untreated control (p < 0.001) (Figure 1). The relative amounts of nitrite and nitrate were similar between the two cytokine treatments by day 5 (data not shown). Treatment of samples with L-NMA inhibited NO production to levels at or near untreated controls.
Figure 1.
Five-day accumulated total nitrate and nitrite release to the medium (nmol/mg wet weight) as a measure of NO production plotted as mean +/− SEM from greater than 3 explants per joint pooled from from 5 different joints (animals) (* p<0.001 cytokine compared to control and ** p<0.01 cytokine compared to cytokine + NMA)
Sulfated glycosaminoglycan release
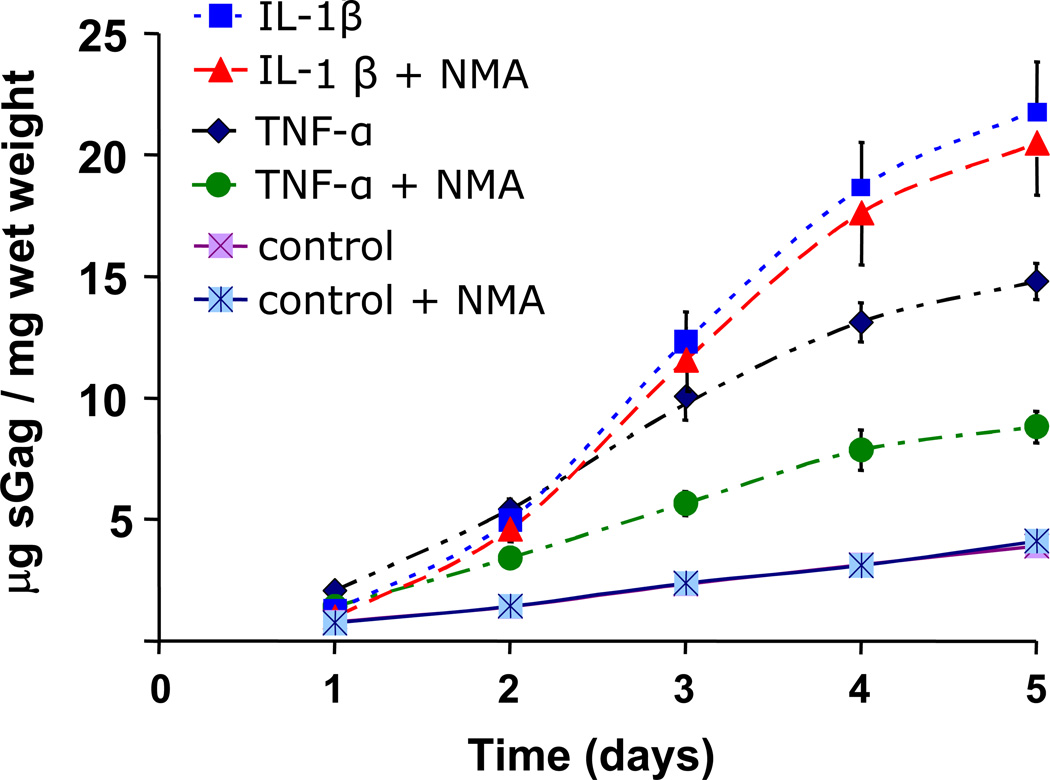
sGAG loss, which is known to be predominantly the result of aggrecan degradation in cartilage explants, was measured by DMMB assay. TNF–α and IL-1β caused 3–4 fold increase in sGAG release to the medium compared to control samples (Figure 2; p<0.001 for TNF-α and IL-1β compared to controls at all time points). Treatment with L-NMA partially inhibited TNF–α induced sGAG release (p<0.003, all time points). However with the exception of the 24-hour time point, L-NMA had no effect on IL-1β-induced sGAG release (p = 0.015 first 24 hours; p > 0.745 for all other points). No L-NMA effect was seen in the absence of cytokine treatment. These results suggest that NO was partially responsible for sGAG release in response to TNF–α treatment, but had little or no effect on IL-1β treatment.
Figure 2.
Accumulated sGAG release to medium over five day cytokine treatment in the presence or absence of L-NMA plotted as mean +/− SEM. IL-1β and TNF-α alone cause a 3–5 fold increase in sGAG release compared to untreated controls (p<0.001 for all points). L-NMA inhibited roughly half the sGAG release caused by treatment with TNF-α (p<0.01 for all time points).
Western analysis for aggrecanase generated aggrecan-G1 neo-epitope
To investigate whether L-NMA was able to decrease sGAG release by decreasing aggrecanase mediated proteolysis of aggrecan, immunoblots were performed to probe for the aggrecan-NITEGE-COOH neoepitope generated by aggrecanase activity (Figure 3). A 60–80 kDa band was visible in the TNF-α and IL-1β treated lanes but not in the untreated (control) lanes of the immunoblot. While the addition of L-NMA to IL-1β treatment had no effect on the anti-NITEGE-stained band, samples treated with TNF–α and L-NMA showed a marked decrease compared to the sample treated with TNF-α alone. This result suggests that the decrease in sGAG release seen with the addition of L-NMA to TNF–α treatment was related to decreased aggrecanase-specific proteolysis of aggrecan.
Figure 3.
Anti-Aggrecan-G1-NITEGE-COOH Western blot to probe mechanism of sGAG loss in response to IL-1β and TNF-α treatment with and without NOS inhibitor, L-NMA. A band is visualized in all cytokine-treated conditions; however, the TNF-α + L-NMA band is significantly lighter than that with TNF-α alone or with IL-1β ± L-NMA. This immunoblot is representative of five different experiments and corresponding blots.
Zymography for Metalloproteinase Measurements
To examine MMP expression and activity, zymography was performed on medium samples at the end of the experiment. Samples subjected to IL-1β treatment and, to a lesser extent, TNF-α treatment showed a diffuse clearing containing a doublet between 50 kDa and 75 kDa (with the lower band most prominent) by casein zymography (Figure 4A), representing secreted pro-MMP-3. No activated MMP-3 band (at 45 kDa) was visible in any of the samples. Gelatin zymography (Figure 4B) demonstrated two bands running in between 75 kDa and 50 kDa in all samples, which represent the pro- and the active forms of MMP-2. In addition, a ~90 kDa band indicative of pro-MMP-9 was present only in the cytokine treated samples. No differences in MMP-2, MMP-3, or MMP-9 were seen with L-NMA treatment, as assessed by zymography.
Figure 4.
Casein (4A) and Gelatin (4B) zymograms of 5 day culture medium. (A) The casein zymogram for both IL-1β and IL-1β - L-NMA contains a 54 kDa band, corresponding to pro-MMP-3 that appears as a doublet, with a lighter upper band and a brighter lower band. A faint doublet is seen for TNF-α treatment and, in addition, a 90 kDa band is seen with all cytokine treatments. (B) The gelatin zymogram contained a 72 kDa band (arrow) and a lower 65 kDa band in all samples, corresponding to the pro- and active forms of MMP-2, respectively. Only cytokine treated samples had a 92 kDa band corresponding to MMP-9. L-NMA had no effect on MMP-3, MMP-9 or MMP-2 expression or activation as seen in medium samples.
Real time PCR analyses
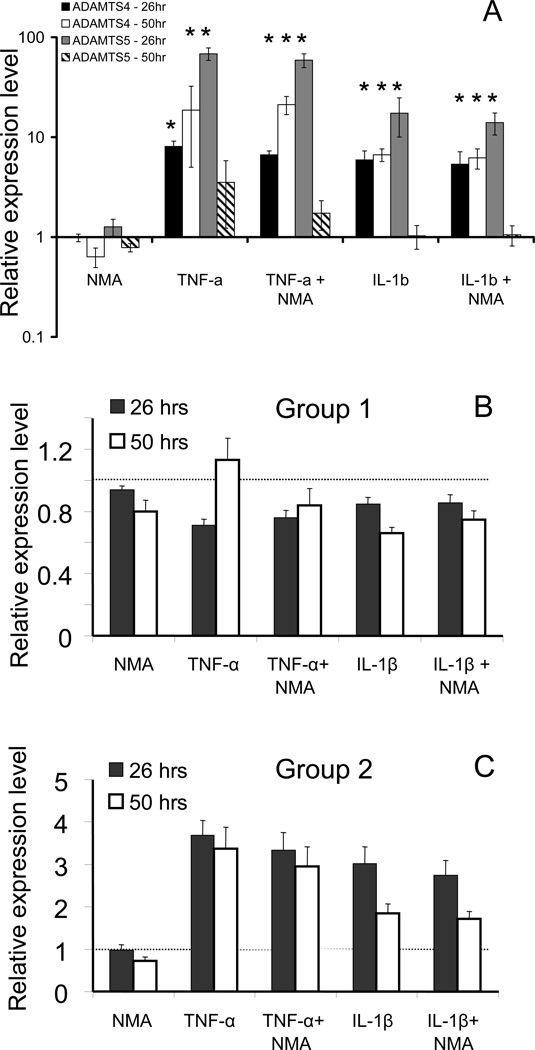
To determined whether cytokine ± L-NMA treatments altered transcription of aggrecanases, other proteases, matrix molecules, cytokines, and growth factors, real time PCR analysis of a 32-gene set was performed on explants from 8 different animals subjected to 26 and 50 hours of IL-1β or TNF-α treatment ± L-NMA, or L-NMA alone. Relative copy numbers were normalized to 18S-RNA and expressed in terms of the fold increase or decrease compared to untreated control samples within each set. Expression of both ADAMTS4 and ADAMTS5 was elevated in response to TNF-α and IL-1β treatment (though little increase at 50 hours for ADAMTS5), with no statistically significant effect of added L-NMA at either the 26 hour or the 50 hour time point (Figure 5A). To elucidate the main expression patterns and identify groups of genes co-expressed under different treatment conditions, gene clustering was performed. The results are shown in principal component space (Figure 6); the first three principal components accounted for ~95% of the variance in the data. K-means clustering (31 genes, 5 normalized conditions, 2 time points) showed that the genes segregated well into two spatially distinct groups with group centroids as indicated (Figure 6). The centroid profiles (showing each condition for each group) are represented in Figure 5B,C as the mean ± SEM of each centroid. Group 1 genes (Figure 5B) had little response or were slightly down regulated by IL-1β and TNF-α treatment, while Group 2 genes were up regulated by IL-1β and TNF-α (Figure 5C). For each individual gene in Groups 1 and 2, the average-fold-change ± SEM is listed in Table 1 (with TNF-α and IL-1β each compared to control, TNF-α compared to TNF-α + L-NMA and IL-1β compared to IL-1β + L-NMA); asterisks represent statistical significance for the comparison (p<0.05). While addition of L-NMA often slightly decreased gene transcription compared to IL-1β or TNF-α alone, this decrease was not statistically significant for any individual gene.
Figure 5.
Relative gene expression by real-time PCR and clustering analyses. (5A) Expression of ADAMTS4 and ADAMTS5 at 26 and 50 hour time points, relative to controls, in response to treatment with L-NMA alone, IL-1β ± L-NMA and TNF-α ± L-NMA. (5B) Centroid profile for gene cluster 1 (group-1 genes) which showed decreased expression or were unchanged in response to cytokine treatment. Centroids represent 26 and 50 hour time points clustered together. (5C) Centroid profile for gene cluster 2, showing genes that were upregulated in response to cytokine treatment. (Groups produced from clustering 26 hours alone (see Table 1) vs. 26 and 50 hours together were identical, except IGF-2 and TIMP-3 in Table 1 swapped groups. The * indicates statistical significance compared to the untreated control sample by pair-wise comparison (Wilcoxan Sign Rank test with Bonferroni correction for multiple comparison; p<0.05)
Figure 6.
Projection plot of gene behavior represented by the first three principal components. The genes readily separated into 2 clusters which correspond to genes that respond positively to IL-1β and TNF-α treatment (triangles = Group 2 of Table 1) and those genes that either did not respond or responded negatively to the cytokine treatment (squares; Group 1). The black circles indicate the group centroids which are significantly different from each other (p<0.001; Student’s t-Test).
Table 1.
Genes probed by real time PCR, represented as the mean fold change over control +/−SEM at 26 hours. Groups were determined using clustering analysis of data from the 26 hour time point. The expression data for the individual genes show similar trends at the 50 hour time point.
| GENE Group 1 |
NMA | TNF-α | TNF-α / TNF+NMA |
IL-1β | IL-1β / IL-1b+NMA |
|---|---|---|---|---|---|
| Aggrecan | 0.98+/−0.06 | 0.74+/−0.07* | 1.17+/−0.14 | 0.91+/−0.05 | 1.12+/−0.24 |
| Collagen II | 0.98+/−0.13 | 0.73+/−0.11 | 1.14+/−0.27 | 0.81+/−0.10 | 1.31+/−0.19 |
| Fibromodulin | 1.18+/−0.12 | 0.59+/−0.06* | 0.98+/−0.08 | 0.86+/−0.06 | 0.91+/−0.05 |
| Link protein | 1.0+/−0.10 | 0.46+/−0.06* | 0.95+/−0.07 | 0.81+/−0.08 | 1.09+/−0.15 |
| TIMP-2 | 0.99+/−0.12 | 0.53+/−0.03* | 1.12+/−0.16 | 0.50+/−0.08* | 0.76+/−0.12 |
| TIMP-3 | 0.61+/−0.11 | 1.1+/−0.37 | 4.07+/−3.18 | 0.97+/−0.27 | 1.52+/−0.53 |
| MMP-1 | 0.88+/−0.09 | 0.92+/−0.08 | 1.02+/−0.10 | 0.98+/−0.09 | 1.15+/−0.08 |
| G3DPH | 0.90+/−0.12 | 0.72+/−0.15 | 0.93+/−0.21 | 0.89+/−0.19 | 0.82+/−0.16 |
| b-actin | 0.95+/−0.08 | 0.81+/−0.06 | 0.92+/−0.05 | 0.86+/−0.07 | 1.03+/−0.07 |
| IGF-1 | 1.0+/−0.13 | 0.50+/−0.12* | 1.05+/−0.21 | 0.64+/−0.10 | 1.33+/−0.27 |
| IL-4 | 0.82+/−0.11 | 0.78+/−0.11 | 1.10+/−0.26 | 0.91+/−0.13 | 1.27+/−0.23 |
| IL-6 | 0.77+/−0.13 | 0.82+/−0.15 | 1.56+/−0.38 | 1.1+/−0.16 | 1.33+/−0.30 |
| TXNIP | 0.92+/−0.09 | 0.55+/−0.05* | 0.87+/−0.09 | 0.66+/−0.05* | 0.91+/−0.06 |
| HAS2 | 1.0+/−0.12 | 0.49+/−0.03* | 0.95+/−0.11 | 0.47+/−0.04* | 1.01+/−0.12 |
| Group 2 | |||||
| Fibronectin | 0.96+/−0.08 | 1.60+/−0.15* | 1.16+/−0.10 | 1.41+/−0.15* | 1.20+/−0.16 |
| MMP-3 | 1.0+/−0.18 | 11+/−2.7* | 1.75+/−0.43 | 34+/−9.1* | 1.80+/−0.53 |
| MMP-9 | 1.1+/−0.24 | 115+/−60 | 1.11+/−0.24 | 74+/−54 | 0.79+/−0.17 |
| MMP-13 | 0.83+/−0.17 | 12+/−4.4* | 1.87+/−0.66 | 76+/−23* | 1.31+/−0.17 |
| ADAMTS4 | 0.98+/−0.09 | 8.0+/−1.1* | 1.24+/−0.16 | 5.9+/−1.4* | 1.26+/−0.14 |
| ADAMTS5 | 1.26+/−0.23 | 68+/−9.1* | 1.28+/−0.24 | 17.4+/−6.9* | 1.15+/−0.17 |
| TIMP-1 | 10.1+/−0.16 | 4.6+/−1.3* | 1.40+/−0.38 | 3.4+/−0.64* | 1.24+/−0.19 |
| COX2 | 1.2+/−0.11 | 11.4+/−2.7* | 1.13+/−0.20 | 17.6+/−5.0* | 1.28+/−0.27 |
| NOS2 | 1.5+/−0.57 | 4513+/−1495* | 1.04+/−0.31 | 894/−183* | 1.94+/−0.70 |
| IGF-2 | 0.99+/−0.10 | 0.92+/−0.08 | 0.94+/−0.17 | 1.1+/−0.11 | 0.96+/−0.11 |
| TGF-beta | 0.92+/−0.07 | 2.3+/−0.20 | 0.91+/−0.06 | 1.7+/−0.18* | 1.10+/−0.08 |
| TNF-alpha | 2.0+/−0.98 | 2.5+/−1.6 | 0.91+/−0.13 | 3.7+/−2.6 | 1.27+/−0.22 |
| IL-1beta | 0.95+/−0.12 | 4.4+/−2.8 | 1.35+/−0.25 | 4.3+/−2.4 | 1.33+/−0.34 |
| HSP90 | 0.85+/−0.07 | 2.2+/−0.27* | 1.21+/−0.11 | 1.3+/−0.14 | 1.06+/−0.08 |
| CD44 | 1.0+/−0.17 | 20+/−3.0* | 1.06+/−0.09 | 11+/−2.1* | 1.27+/−0.16 |
| bFGF | 9.0+/−7.0 | 1722+/−1109 | 10.56+/−5.86 | 61+/−48 | 8.66+/−4.91 |
| OP-1 | 1.6+/−0.41 | 80+/−16* | 1.32+/−0.28 | 26+/−10* | 1.42+/−0.31 |
The * indicates statistical significance compared to the untreated control sample by pair-wise comparison (Wilcoxan Sign Rank test with Bonferroni correction for multiple comparison; p<0.05).
Data represent experiments from eight animals with 2 or 3 explants pooled per animal for RNA extraction.
Discussion
IL-1β and, to a lesser extent, TNF–α, can induce chondrocyte-mediated matrix degradation, with loss of aggrecan typically preceding collagen degradation (34). Previous studies explored the role of NO in IL-1β–induced aggrecan degradation; however, to our knowledge, this is the first study to test the effect of NO on TNF-α–induced sGAG release and to explore its underlying mechanism. Cartilage explants treated with the NOS inhibitor, L-NMA, showed marked inhibition of TNF–α–induced sGAG release and concomitant production of aggrecan G1-NITEGE fragments, indicating a decrease in aggrecan proteolysis by aggrecanase. In contrast, L-NMA did not alter IL-1β–induced sGAG release or the generation of aggrecan-G1-NITEGE fragments. Real time PCR analyses showed no significant effect of L-NMA alone on expression of ADAMTS4 or ADAMTS5 or a panel of 30 other matrix genes, proteases and cytokines. Together, these results suggest that NO plays a role in the post-transcriptional regulation of aggrecanases in response to TNF-α while NO has no effect on IL-1β induced aggrecan degradation. Thus, TNF-α and IL-1β utilize different pathways of expression or activation of aggrecanases to mediate aggrecanolysis, with that of TNF-α being regulated in part by an NO mediated post-transcriptional processing of ADAMTS4 and ADAMTS5.
NO can mediate its effects through enhancing cGMP, increasing protein nitrosation or protein nitration which may serve as an important regulatory mechanism at the level of the cell or individual proteins. NO concentration and chemistry, which depends in part of oxygen free radicals, may determine its role as a second signal mediator. Treatment of cartilage with cytokines caused an increase in NO production (Figure 1, p<0.001 for IL-1β and TNF-α vs. control for both nitrate and nitrite). In addition, TNF–α or IL-1β resulted in similar amounts of nitrate and nitrite, each corresponding to approximately half of the NO production, suggesting that NO chemistry was similar for both cytokine treatments. L-NMA decreased NO to control levels for both cytokines (cytokine + L-NMA vs. control not significant). Equal nitrate and nitrite production with both TNF-α and IL-1β treatment suggests that the effects of NO likely depend on cellular processes induced with each cytokine treatment and not NO chemistry.
The role of NO in matrix degradation associated with TNF-α may be quite different than that with IL-1β. In this study, L-NMA treatment inhibited TNF-α–induced sGAG loss by ~50% (Figure 2). While we know of no previous reports of the effects of NO treatment with TNF-α alone, van Bezooijen et al. (35) showed no measurable effect of L-NMA on cartilage damage induced by the combination of TNF-α and IL-17, compared to the cytokine combination alone as assessed by Alcian blue staining for sGAG. We found that L-NMA had no effect on sGAG loss following IL-1β treatment (Figure 2), similar to the findings of Bird et al.(25) using equine cartilage explants. In contrast, Stefanovic-Racic found that L-NMA in the presence of IL-1β significantly increased sGAG release compared to IL-1β alone, using bovine, human, and rabbit explants(18, 23). Regarding other matrix degrading signals, Pichika et al. (36) showed that inhibiting NO resulted in a matrix protective effect when cartilage was treated with fibronectin fragments, similar to the effect seen with TNF-α (Figure 2). The effects of NO appear to depend on the cytokines or other signals with which it is produced and with which it signals. NO appears to play a distinctly degradative role in TNF-α (but not in IL-1β) treated cartilage.
Because aggrecanases are known to mediate aggrecan degradation in response IL-1β and TNF-α (37, 38), we hypothesized that the mechanism of L-NMA inhibition of TNF–α-induced sGAG release was through a decrease in aggrecanase cleavage of aggrecan at the Glu373-Ala374 site in the interglobular domain (37, 39). Immunoblot analysis of the medium using an antibody to the aggrecan-NITEGE373 fragment showed that L-NMA + TNF-α resulted in less fragment release than TNF-α alone (Figure 3), consistent with decreased sGAG release (Figure 2). And while L-NMA did not alter sGAG release induced by IL-1β, there was no decrease in aggrecan-NITEGE373 fragment release either. These results suggest that NO is partially responsible for TNF-α–induced aggrecanase activity in cartilage explants.
While aggrecanases are the main mediator of aggrecanolysis early in response to IL-1β and TNF-α treatment, NO has also been implicated in activation of MMPs through the disruption of the cysteine switch present in the zymogens(40). Therefore, zymography was used to evaluate the effects of L-NMA on expression and activation of selected MMPs in response to cytokine treatment. IL-1β (and to a lesser extent, TNF-α) increased pro-MMP-3 secretion to the medium with no effect of L-NMA on activation or secretion (Figure 4A). Pro-MMP-9 was expressed in response to cytokine treatment, while MMP-2 was present under all conditions. L-NMA had no effect on MMP-2 or MMP-9 secretion or activation. MMP-3 and MMP-9 protein in the medium correlated well with the gene expression data (Figure 5,6; Table 1). These zymography results were also similar to those of Dozin et al.(41) who saw no effect of NOS inhibition on MMPs with chondrocyte monolayers treated with IL-1α and TNF-α in combination. Zymography failed to show evidence of an active MMP-3 band and no effect of L-NMA was observed, suggesting NO mediates aggrecan degradation in response to TNF-α by enhancing aggrecanase and not MMP-3 activity.
While ADAMTS1, 8, 9, 15, 16, and 18 have aggrecanase activity(42, 43), only ADAMTS4 and 5 are known to potently cleave the Glu373-Ala374 site forming the aggrecan-NITEGE fragment(44). We thus focused on ADAMTS4 and ADAMTS5 to explore the possible mechanism by which NO may be mediating an increase in aggrecanase activity. Because ADAMTS4 and ADAMTS5 proteins could not be reliably identified in tissue or medium samples (data not shown), we examined the effects of NO inhibition on gene expression of ADAMTS4 and 5 as well as a panel of genes associated with cartilage homeostasis (Table 1). We hypothesized that changes in expression of aggrecanases in response to cytokine and L-NMA treatments would likely occur approximately 24 hours before sGAG release to the medium, allowing time for protein expression and diffusion of aggrecan fragments out of the tissue. Since the rate of sGAG release was maximal between days 2 and 4 of treatment (Figure 2), real time PCR analysis was performed on explants at 26 and 50 hours after treatment with IL-1β or TNF-α ± L-NMA. While IL-1β and TNF-α treatment caused significant changes in gene expression of many of the genes tested including ADAMTS4 and ADAMTS5 (Figure 5, Table 1), L-NMA treatment did not alter transcription of any genes evaluated in this study at either 26 or 50 hours.
Previous studies using young bovine explants showed significant increases in transcription of ADAMTS4 after 24 hours of treatment with IL-1α (45) and IL-1β (46), while ADAMTS5 was up-regulated by TNF-α (46). Here, we observed a strong increase in ADAMTS5 expression with TNF-α and IL-1β at 26 hours but little increase at 50 hours (Figure 5A). In contrast, ADAMTS4 showed a sustained increase in expression at 26 and 50 hours with both IL-1β and TNF-α (Figure 5A). Interestingly, while TNF-α caused a greater average increase in ADAMTS4 and 5 transcription than IL-1β (Table 1), IL-1β treatment resulted in greater sGAG release. In addition, L-NMA did not significantly alter transcription of either ADAMTS4 or 5 in response to either cytokine treatment. Thus, the NO mediated regulation of ADAMTS4 and/or 5 activity in response to TNF-α is likely post-transcriptional and may be at the level of either translation or post-translational modification such as proteolysis(45). ADAMTS4 and 5 do undergo proteolysis, which is known to change its binding affinity and proteolytic specificity of aggrecan cleavage sites(44, 45, 47). Although less is known about ADAMTS5, some work on ADAMTS4 suggests that its activity may be regulated in part through a combination of proteolysis and/or protein-protein interactions rather than by protein expression(45, 48, 49). Further work to explore the role of NO in modulating possible ADAMTS4 activators or inhibitors may be warranted. While it is possible that NO could play a more direct role in ADAMTS4 and/or 5 activity through direct chemical modification, this would still require a difference in ADAMTS4 and 5 protein expression between TNF-α and IL-1β in order to explain a TNF-α specific NO effect. But based on our findings, changes in aggrecanase transcription cannot account for the effects of L-NMA on TNF-α induced aggrecan degradation or the differential effects of L-NMA between IL-1β and TNF-α.
While previous studies have not reported changes in expression via qPCR for the broad panel of genes examined here (Table 1), we note several useful comparisons. Sasaki et al. reported a NO dependent increase in bFGF and MMP-9 gene expression by rabbit chondrocytes in monolayer in response to IL-1β(50). While we did find an increase in the mean transcription level of bFGF and MMP-9 at 26 and 50 hours with TNF-α and IL-1β (not statistically significant), no L-NMA effect was observed. In the present study, inducible nitric oxide synthase (iNOS), responsible for chondrocyte production of NO, was elevated in response to both IL-1β and TNF-α (p<0.05), as was COX-2, MMP-3, and MMP-13 but not IL-6 or MMP-1. With the exception of IL-6 and MMP-1, these findings were consistent with the reported effects of IL-1β on human chondrocytes(51) and effects on MMPs in human OA cartilage using TNF-α and IL-1β receptor inhibitors(52) as well as equine cartilage in response to TNF-α(53). Decreases in matrix geme expression (e.g., collagen II, aggrecan, fibromodulin, and link protein) were more pronounced at 50 hours than 26 hours suggesting that effects of IL-1β and TNF-α on matrix gene expression may occur later compared to the up regulation of matrix degrading enzymes(54) (p<0.05 TNF-α and IL-1β compared to control for collagen alpha 1(II), aggrecan, fibromodulin, and link protein (IL-1 only) at 50 hrs). The inhibitory effect of IL-1β and TNF-α on aggrecan and collagen synthesis is well characterized.
In summary, NO plays an enhancing role in TNF-α (but not IL-1β induced aggrecanase-mediated aggrecan degradation. Inhibiting NO production with L-NMA caused no changes in ADAMTS4 or ADAMTS5 gene transcription; however, L-NMA partially inhibited aggrecanase-mediated aggrecan degradation in response to TNF-α suggesting that NO must play a role in regulating aggrecanases at a post-transcriptional level. At the same time, inhibiting NOS had no detectable effects on IL-1β or TNF-α induced MMP expression, activation, or the gene expression of various other matrix proteins, cytokines or growth factors after 26 or 50 hours of cytokine treatment. IL-1β and TNF-α–enhanced transcription of inflammatory genes such as iNOS and COX2 as well as matrix degrading enzymes MMP-3 and MMP-13 at 26 hours, and both cytokines decreased collagen II, aggrecan, link protein and fibromodulin at 50 hours, consistent with known effects of these cytokines and with no effect of L-NMA. These findings support the hypothesis of a post-transcriptional role for NO in regulating aggrecanase activity in response to TNF-α treatment, and the hypothesis that TNF-α and IL-1β regulate aggrecanase activity though different mechanisms.
Acknowledgements
We thank J. Glogowski for technical assistance with the nitrate/nitrite analysis, and Dr. Carl Flannery for the aggrecan-G1-NITEGE neo-epitope antibody and ADAMTS4 and ADAMTS5 antibody used in this study. This research was funded in part by NIH grants AR45779 and CA26731, and a fellowship from NDSEG fellowship (ALS).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Miersch S, Mutus B. Protein S-nitrosation: biochemistry and characterization of protein thiol-NO interactions as cellular signals. Clin Biochem. 2005;38(9):777–791. doi: 10.1016/j.clinbiochem.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 2.Stadler J, Stefanovic-Racic M, Billiar TR, Curran RD, McIntyre LA, Georgescu HI, et al. Articular chondrocytes synthesize nitric oxide in response to cytokines and lipopolysaccharide. J. Immunol. 1991;147(11):3915–3920. [PubMed] [Google Scholar]
- 3.Palmer RM, Hickery MS, Charles IG, Moncada S, Bayliss MT. Induction of nitric oxide synthase in human chondrocytes. Biochem Biophys Res Commun. 1993;193(1):398–405. doi: 10.1006/bbrc.1993.1637. [DOI] [PubMed] [Google Scholar]
- 4.Hauselmann HJ, Oppliger L, Michel BA, Stefanovic-Racic M, Evans CH. Nitric oxide and proteoglycan biosynthesis by human articular chondrocytes in alginate culture. FEBS Lett. 1994;352(3):361–364. doi: 10.1016/0014-5793(94)00994-5. [DOI] [PubMed] [Google Scholar]
- 5.Olee T, Hashimoto S, Quach J, Lotz M. IL-18 is produced by articular chondrocytes and induces proinflammatory and catabolic responses. J Immunol. 1999;162(2):1096–1100. [PubMed] [Google Scholar]
- 6.Cai L, Suboc P, Hogue DA, Fei DT, Filvaroff EH. Interleukin 17 induced nitric oxide suppresses matrix synthesis and protects cartilage from matrix breakdown. J Rheumatol. 2002;29(8):1725–1736. [PubMed] [Google Scholar]
- 7.Boileau C, Martel-Pelletier J, Moldovan F, Jouzeau JY, Netter P, Manning PT, et al. The in situ up-regulation of chondrocyte interleukin-1-converting enzyme and interleukin-18 levels in experimental osteoarthritis is mediated by nitric oxide. Arthritis Rheum. 2002;46(10):2637–2647. doi: 10.1002/art.10518. [DOI] [PubMed] [Google Scholar]
- 8.Yocum DE, Esparza L, Dubry S, Benjamin JB, Volz R, Scuderi P. Characteristics of tumor necrosis factor production in rheumatoid arthritis. Cell Immunol. 1989;122(1):131–145. doi: 10.1016/0008-8749(89)90154-8. [DOI] [PubMed] [Google Scholar]
- 9.Di Giovine FS, Nuki G, Duff GW. Tumor necrosis factor in synovial exudates. Annals of the Rheum. 1988;47:768–772. doi: 10.1136/ard.47.9.768. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Brennan FM, Chantry D, Jackson A, Maini R, Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989;2(8657):244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- 11.Saklatvala J. Tumour necrosis factor alpha stimulates resorption and inhibits synthesis of proteoglycan in cartilage. Nature. 1986;322(6079):547–549. doi: 10.1038/322547a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hrycaj P, Stratz T, Kovac C, Mennet P, Muller W. Microheterogeneity of acute phase proteins in patients with clinically active and clinically nonactive osteoarthritis. Clin Rheumatol. 1995;14(4):434–440. doi: 10.1007/BF02207678. [DOI] [PubMed] [Google Scholar]
- 13.Schlaak JF, Pfers I, Meyer Zum Buschenfelde KH, Marker-Hermann E. Different cytokine profiles in the synovial fluid of patients with osteoarthritis, rheumatoid arthritis and seronegative spondylarthropathies. Clin Exp Rheumatol. 1996;14(2):155–162. [PubMed] [Google Scholar]
- 14.Pelletier JP, DiBattista JA, Roughley P, McCollum R, Martel-Pelletier J. Cytokines and inflammation in cartilage degradation. Rheum Dis Clin North Am. 1993;19(3):545–568. [PubMed] [Google Scholar]
- 15.Ismaiel S, Atkins RM, Pearse MF, Dieppe PA, Elson CJ. Susceptibility of normal and arthritic human articular cartilage to degradative stimuli. Br J Rheumatol. 1992;31(6):369–373. doi: 10.1093/rheumatology/31.6.369. [DOI] [PubMed] [Google Scholar]
- 16.Westacott CI, Ismaiel S, Langkamer VG, Atkins RM, Elson CJ. Transactions of the Orthopaedic REsearch Society. San Francisco, CA: 1993. Human Articular Cartilage Degradation and Chondrocyte Expression of TNF-alpha Receptors; p. 739. 1993. [Google Scholar]
- 17.Westacott CI, Barakat AF, Wood L, Perry MJ, Neison P, Bisbinas I, et al. Tumor necrosis factor alpha can contribute to focal loss of cartilage in osteoarthritis. Osteoarthritis Cartilage. 2000;8(3):213–221. doi: 10.1053/joca.1999.0292. [DOI] [PubMed] [Google Scholar]
- 18.Stefanovic-Racic M, Morales TI, Taskiran D, McIntyre LA, Evans CH. The role of nitric oxide in proteoglycan turnover by bovine articular cartilage organ cultures. J Immunol. 1996;156(3):1213–1220. [PubMed] [Google Scholar]
- 19.Taskiran D, Stefanovic-Racic M, Georgescu H, Evans C. Nitric oxide mediates suppression of cartilage proteoglycan synthesis by interleukin-1. Biochem Biophys Res Commun. 1994;200(1):142–148. doi: 10.1006/bbrc.1994.1426. [DOI] [PubMed] [Google Scholar]
- 20.Hickery MS, Palmer RMJ, Charles IG, Moncada S, Bayliss MT. Transactions of the Orthopaedic Research Society. 1994. Feb 21–24, The role of Nitric Oxide in the IL-1- and TNF-alpha induced inhibition of proteoglycan synthesisi in human articular cartilage; p. 77. 1994; 1994. [Google Scholar]
- 21.Goodstone NJ, Hardingham TE. Tumour necrosis factor alpha stimulates nitric oxide production more potently than interleukin-1beta in porcine articular chondrocytes. Rheumatology (Oxford) 2002;41(8):883–891. doi: 10.1093/rheumatology/41.8.883. [DOI] [PubMed] [Google Scholar]
- 22.Cao M, Westerhausen-Larson A, Niyibizi C, Kavalkovich K, Georgescu HI, Rizzo CF, et al. Nitric oxide inhibits the synthesis of type-II collagen without altering Col2A1 mRNA abundance: prolyl hydroxylase as a possible target. Biochem J. 1997;324(Pt 1):305–310. doi: 10.1042/bj3240305. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stefanovic-Racic M, Mollers MO, Miller LA, Evans CH. Nitric oxide and proteoglycan turnover in rabbit articular cartilage. J Orthop Res. 1997;15(3):442–449. doi: 10.1002/jor.1100150318. [DOI] [PubMed] [Google Scholar]
- 24.Bird JL, May S, Bayliss MT. Nitric oxide inhibits aggrecan degradation in explant cultures of equine articular cartilage. Equine Vet J. 2000;32(2):133–139. doi: 10.2746/042516400777591651. [DOI] [PubMed] [Google Scholar]
- 25.Bird JL, Wells T, Platt D, Bayliss MT. IL-1 beta induces the degradation of equine articular cartilage by a mechanism that is not mediated by nitric oxide. Biochem Biophys Res Commun. 1997;238(1):81–85. doi: 10.1006/bbrc.1997.7246. [DOI] [PubMed] [Google Scholar]
- 26.Murrell GA, Jang D, Williams RJ. Nitric oxide activates metalloprotease enzymes in articular cartilage. Biochem Biophys Res Commun. 1995;206(1):15–21. doi: 10.1006/bbrc.1995.1003. [DOI] [PubMed] [Google Scholar]
- 27.Tamura T, Nakanishi T, Kimura Y, Hattori T, Sasaki K, Norimatsu H, et al. Nitric oxide mediates interleukin-1-induced matrix degradation and basic fibroblast growth factor release in cultured rabbit articular chondrocytes: a possible mechanism of pathological neovascularization in arthritis. Endocrinology. 1996;137(9):3729–3737. doi: 10.1210/endo.137.9.8756539. [DOI] [PubMed] [Google Scholar]
- 28.Sah RL, Kim YJ, Doong JY, Grodzinsky AJ, Plaas AH, Sandy JD. Biosynthetic response of cartilage explants to dynamic compression. J Orthop Res. 1989;7(5):619–636. doi: 10.1002/jor.1100070502. [DOI] [PubMed] [Google Scholar]
- 29.Kisiday JD, Kurz B, DiMicco MA, Grodzinsky AJ. Evaluation of medium supplemented with insulin-transferrin-selenium for culture of primary bovine calf chondrocytes in three-dimensional hydrogel scaffolds. Tissue Eng. 2005;11(1–2):141–151. doi: 10.1089/ten.2005.11.141. [DOI] [PubMed] [Google Scholar]
- 30.Farndale RW, Buttle DJ, Barrett AJ. Improved quantitation and discrimination of sulphated glycosaminoglycans by use of dimethylmethylene blue. Biochim Biophys Acta. 1986;883(2):173–177. doi: 10.1016/0304-4165(86)90306-5. [DOI] [PubMed] [Google Scholar]
- 31.Green LC, Wagner DA, Glogowski J, Skipper PL, Wishnok JS, Tannenbaum SR. Analysis of nitrate, nitrite, and [15N]nitrate in biological fluids. Anal Biochem. 1982;126(1):131–138. doi: 10.1016/0003-2697(82)90118-x. [DOI] [PubMed] [Google Scholar]
- 32.Clark IM. Matrix Metalloproteinase Protocols. Clifton, NJ: Humana Press; 2001. [Google Scholar]
- 33.Fitzgerald JB, Jin M, Dean D, Wood DJ, Zheng MH, Grodzinsky AJ. Mechanical compression of cartilage explants induces multiple time-dependent gene expression patterns and involves intracellular calcium and cyclic AMP. J Biol Chem. 2004;279(19):19502–19511. doi: 10.1074/jbc.M400437200. [DOI] [PubMed] [Google Scholar]
- 34.Pratta MA, Yao W, Decicco C, Tortorella MD, Liu RQ, Copeland RA, et al. Aggrecan protects cartilage collagen from proteolytic cleavage. J Biol Chem. 2003;278(46):45539–45545. doi: 10.1074/jbc.M303737200. [DOI] [PubMed] [Google Scholar]
- 35.Van Bezooijen RL, Van Der Wee-Pals L, Papapoulos SE, Lowik CW. Interleukin 17 synergises with tumour necrosis factor alpha to induce cartilage destruction in vitro. Ann Rheum Dis. 2002;61(10):870–876. doi: 10.1136/ard.61.10.870. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Pichika R, Homandberg GA. Fibronectin fragments elevate nitric oxide (NO) and inducible NO synthetase (iNOS) levels in bovine cartilage and iNOS inhibitors block fibronectin fragment mediated damage and promote repair. Inflamm Res. 2004;53(8):405–412. doi: 10.1007/s00011-004-1279-8. [DOI] [PubMed] [Google Scholar]
- 37.Sandy JD, Flannery CR, Neame PJ, Lohmander LS. The structure of aggrecan fragments in human synovial fluid. Evidence for the involvement in osteoarthritis of a novel proteinase which cleaves the Glu 373-Ala 374 bond of the interglobular domain. J Clin Invest. 1992;89(5):1512–1516. doi: 10.1172/JCI115742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Arner EC, Hughes CE, Decicco CP, Caterson B, Tortorella MD. Cytokine-induced cartilage proteoglycan degradation is mediated by aggrecanase. Osteoarthritis Cartilage. 1998;6(3):214–228. doi: 10.1053/joca.1998.0114. [DOI] [PubMed] [Google Scholar]
- 39.Flannery CR, Lark MW, Sandy JD. Identification of a stromelysin cleavage site within the interglobular domain of human aggrecan. Evidence for proteolysis at this site in vivo in human articular cartilage. J Biol Chem. 1992;267(2):1008–1014. [PubMed] [Google Scholar]
- 40.Gu Z, Kaul M, Yan B, Kridel SJ, Cui J, Strongin A, et al. S-nitrosylation of matrix metalloproteinases: signaling pathway to neuronal cell death. Science. 2002;297(5584):1186–1190. doi: 10.1126/science.1073634. [DOI] [PubMed] [Google Scholar]
- 41.Dozin B, Malpeli M, Camardella L, Cancedda R, Pietrangelo A. Response of young, aged and osteoarthritic human articular chondrocytes to inflammatory cytokines: molecular and cellular aspects. Matrix Biol. 2002;21(5):449–459. doi: 10.1016/s0945-053x(02)00028-8. [DOI] [PubMed] [Google Scholar]
- 42.Zeng W, Corcoran C, Collins-Racie LA, Lavallie ER, Morris EA, Flannery CR. Glycosaminoglycan-binding properties and aggrecanase activities of truncated ADAMTSs: comparative analyses with ADAMTS-5, -9, -16 and -18. Biochim Biophys Acta. 2006;1760(3):517–524. doi: 10.1016/j.bbagen.2006.01.013. [DOI] [PubMed] [Google Scholar]
- 43.Porter S, Clark IM, Kevorkian L, Edwards DR. The ADAMTS metalloproteinases. Biochem J. 2005;386(Pt 1):15–27. doi: 10.1042/BJ20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Tortorella MD, Liu RQ, Burn T, Newton RC, Arner E. Characterization of human aggrecanase 2 (ADAM-TS5): substrate specificity studies and comparison with aggrecanase 1 (ADAM-TS4) Matrix Biol. 2002;21(6):499–511. doi: 10.1016/s0945-053x(02)00069-0. [DOI] [PubMed] [Google Scholar]
- 45.Patwari P, Gao G, Lee JH, Grodzinsky AJ, Sandy JD. Analysis of ADAMTS4 and MT4-MMP indicates that both are involved in aggrecanolysis in interleukin-1-treated bovine cartilage. Osteoarthritis Cartilage. 2005;13(4):269–277. doi: 10.1016/j.joca.2004.10.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Caterson B, Flannery CR, Hughes CE, Little CB. Mechanisms involved in cartilage proteoglycan catabolism. Matrix Biol. 2000;19(4):333–344. doi: 10.1016/s0945-053x(00)00078-0. [DOI] [PubMed] [Google Scholar]
- 47.Flannery CR, Zeng W, Corcoran C, Collins-Racie LA, Chockalingam PS, Hebert T, et al. Autocatalytic cleavage of ADAMTS-4 (Aggrecanase-1) reveals multiple glycosaminoglycan-binding sites. J Biol Chem. 2002;277(45):42775–42780. doi: 10.1074/jbc.M205309200. [DOI] [PubMed] [Google Scholar]
- 48.Pratta MA, Scherle PA, Yang G, Liu RQ, Newton RC. Induction of aggrecanase 1 (ADAM-TS4) by interleukin-1 occurs through activation of constitutively produced protein. Arthritis Rheum. 2003;48(1):119–133. doi: 10.1002/art.10726. [DOI] [PubMed] [Google Scholar]
- 49.Gao G, Plaas A, Thompson VP, Jin S, Zuo F, Sandy JD. ADAMTS4 (aggrecanase-1) activation on the cell surface involves C-terminal cleavage by glycosylphosphatidyl inositol-anchored membrane type 4-matrix metalloproteinase and binding of the activated proteinase to chondroitin sulfate and heparan sulfate on syndecan-1. J Biol Chem. 2004;279(11):10042–10051. doi: 10.1074/jbc.M312100200. [DOI] [PubMed] [Google Scholar]
- 50.Sasaki K, Hattori T, Fujisawa T, Takahashi K, Inoue H, Takigawa M. Nitric oxide mediates interleukin-1-induced gene expression of matrix metalloproteinases and basic fibroblast growth factor in cultured rabbit articular chondrocytes. J Biochem (Tokyo) 1998;123(3):431–439. doi: 10.1093/oxfordjournals.jbchem.a021955. [DOI] [PubMed] [Google Scholar]
- 51.Saas J, Haag J, Rueger D, Chubinskaya S, Sohler F, Zimmer R, et al. IL-1beta, but not BMP-7 leads to a dramatic change in the gene expression pattern of human adult articular chondrocytes--portraying the gene expression pattern in two donors. Cytokine. 2006;36(1–2):90–99. doi: 10.1016/j.cyto.2006.10.005. [DOI] [PubMed] [Google Scholar]
- 52.Kobayashi M, Squires GR, Mousa A, Tanzer M, Zukor DJ, Antoniou J, et al. Role of interleukin-1 and tumor necrosis factor alpha in matrix degradation of human osteoarthritic cartilage. Arthritis Rheum. 2005;52(1):128–135. doi: 10.1002/art.20776. [DOI] [PubMed] [Google Scholar]
- 53.Richardson DW, Dodge GR. Dose-dependent effects of corticosteroids on the expression of matrix-related genes in normal and cytokine-treated articular chondrocytes. Inflamm Res. 2003;52(1):39–49. doi: 10.1007/s000110300012. [DOI] [PubMed] [Google Scholar]
- 54.Aigner T, McKenna L, Zien A, Fan Z, Gebhard PM, Zimmer R. Gene expression profiling of serum- and interleukin-1 beta-stimulated primary human adult articular chondrocytes--a molecular analysis based on chondrocytes isolated from one donor. Cytokine. 2005;31(3):227–240. doi: 10.1016/j.cyto.2005.04.009. [DOI] [PubMed] [Google Scholar]