Abstract
CD56bright lymphocytes appear in the uterus 3–5 days post-ovulation co-incident with the onset of stromal cell decidualization. While the source of these uterine immune cells is not defined, a subset of blood CD56bright cells exhibits enhanced capacity to adhere to decidual vascular endothelium during the peri-ovulatory period of menstrual cycles. Here, the effects of early pregnancy on the adhesive capacity of CD56bright cells to bind uterine substrates were examined in a time-course study of 18 infertile women undergoing natural cycles prior to transfer of frozen/thawed embryos and 18 infertile women undergoing controlled ovarian stimulation. There were 3 pregnancies in the natural cycle group and 7 in the hormone stimulated cohort. Hormone levels, and number and quality of transferred embryos were similar between pregnant and non-pregnant cycles. However, adhesive function of CD56bright cells increased prior to ovulation in hormone-treated women who became pregnant and prior to embryo transfer in naturally cycling women who became pregnant. This pattern of incremental adhesion, which was less frequently observed in unsuccessful cycles, suggests a role for NK cells in implantation. These results support the notion that temporal control of NK cell homing to the uterine microenvironment is prerequisite to pregnancy.
Keywords: Cell Trafficking, NK Cells, Luteinizing Hormone, Decidua
Within hours of ovulation, under the influence of steroid hormones secreted by corpus luteum, the human endometria begins a transformation from fibroblast-like cells to plump secretory decidual cells. Decidualization of human endometria is associated with a rapid increase in the number of three unique maternal immune cell types; decidual (d)NK cells (also known as LGL), macrophages and immature dendritic cells (1–4). Natural killer cells likely migrate in response to increased local chemokines CXCL9 and 10 (Mig and IP-10) secreted by endometria under the influence of progesterone (P4) (5). These cells differentiate to become a unique lymphocyte subset characterized by retained expression of alpha 4 integrin, LFA-1, CCR1, 2 and 5, CXCR3 and 4, CX3CR1, increased expression of CD56 and de novo expression of estrogen receptor (ER)β and many express the proliferation marker Ki-67(6;7). NK cells form close associations with EVT and with spiral arteries, where they are thought to regulate depth of invasion through secretion of cytokines such as IL-4, IL-10 and IFNγ (7;8). In turn, cytokines secreted by macrophages modulate both NK cell activation and cytokine secretion.
In mice, through secretion of interferon (IFN)γ (9;10), uterine (u)NK cells function to support terminal decidual cell differentiation and initiate changes to spiral artery structure that enable pregnancy-associated dilation and elongation (11;12). Human dNK cells, like their murine uNK cell counterparts, express major angiogenic molecules such as vascular endothelial and placental growth factors (13). If the roles of uNK cells in mice are analogous to those of dNK cells in humans, dNK cell support of decidualization can be linked to implantation success and thus fertility.
Murine uNK cell progenitors reside outside the uterus and are found in all lymphoid tissues, with enrichment in spleen during pregnancy (14). Mobilization of blood-borne lymphocytes to tissue depends on their sequential adhesive interactions with endothelial cells under wall shear stress induced by hemodynamic flow (15;16). L-selectin adhesion molecule initiates rolling and tethering of lymphocytes to specialized endothelial cells, while chemokines mediate integrin-dependent firm adhesion, prior to extravasation. Using an in-vitro assay of this functional interaction (adhesion of lymphocytes to endothelium in frozen tissue-sections under shear), we found that lymphocytes from human peripheral blood adhering to microvessels of gestation day (gd) 6–8 mouse decidua were enriched for the CD56bright NK cells (14;17). While CD56bright NK cells comprise about 1% of blood lymphocytes, they were 75% of the adherent cells. Blood CD56bright NK cells intensely express L-selectin homing receptor (18;19) and display a specific array of chemokine receptors, CCR5 and 7, CXCR3 and 4 and CX3CR1 (20). Expression of these molecules predispose CD56bright cells to tissue-selective homing. Adhesion of CD56bright cells to uterine substrates from pregnant animals was shown to be L-selectin-dependent (14;17;21). Furthermore, murine lymphocytes from pregnant or hormone treated (E2 or P4) animals showed increased L-selectin binding activity. We postulate that hormonal fluctuations during the menstrual cycle induce novel regulatory events in lymphocyte-endothelial cell adhesion pathways that contribute to traffic of uNK progenitor cells from lymphoid tissue into blood, then subsequently into decidualizing endometrium (14).
The number of dNK cells in the human uterus increases dramatically 3–5 days post-ovulation (1;22). We recently demonstrated that the potential of a subset of peripheral blood NK cells for trafficking from the circulation was enhanced during a brief, peri-ovulatory window which extends from 2–3 days prior to the LH surge to 1 day after the LH surge in unmedicated menstrual cycles of fertile, regularly cycling women (21). The CD56bright cell adhesion was restricted to vascular endothelium only in the decidua basalis of pregnant mouse uteri (21). A similar increase in adhesion of CD56bright cells could be induced by culture with 17-β estradiol (E2), luteinizing hormone (LH) or very low doses of progesterone (P4) (21). Higher doses of P4 returned CD56bright cell adhesion to baseline levels. This suggests that a subset of blood CD56bright cells may initiate mobilization to the uterus in response to factors induced by rising plasma E2 and to the amount of LH found at LH surge, resulting in increased trafficking ability, which is terminated by high post-ovulation levels of P4. Together with the finding that LH primes uterine receptivity in menopausal women attempting pregnancy using Assisted Reproductive Technology (23), the data suggests that events induced at LH surge may synchronize dNK progenitor cell migration to the uterus with uterine decidualization or that LH-induced activation of dNK progenitor cells enables migration to the uterus where dNK cells themselves contribute to the optimal decidualization necessary for implantation.
Here, we address two questions; firstly, whether adhesion of CD56bright cells changes in the interval from ovulation to the establishment of pregnancy and secondly, whether exogenous hormone treatment alters the trafficking potential of blood NK cells. Two groups of patients undergoing Assisted Reproductive Technology were assessed for functional uterine homing potential of their circulating CD56bright cells before and after embryo transfer. The patients were either having an unmanipulated cycle before transfer of banked frozen embryos or receiving exogenous hormones for controlled ovarian hyperstimulation, oocyte collection, in vitro fertilization and transfer of freshly prepared embryos. A relationship was found between lymphocyte adhesive function and the establishment of pregnancy.
Materials and Methods
Human subjects and blood sampling
Women of reproductive age with primary female infertility of various etiologies, some of whom also had male partner infertility, were recruited to participate in this study by physicians in the Reproductive Endocrinology and Infertility Program at the London Health Sciences Centre. The study was approved by the Health Sciences Research Ethics Board at the University of Western Ontario, and all recruited patients were fully informed of potential risks and signed consent forms prior to participation. The women were divided into two groups; those undergoing a natural monitored cycle for transfer of frozen embryos (FET) and those entered in an exogenous, controlled ovarian hyperstimulation (COH) protocol for in vitro fertilization. Exclusion criteria included lack of response to treatment or lack of embryos suitable for transfer.
Sequential analysis of serum hormone levels was performed for clinical monitoring of each treatment cycle. For study purposes, every other day, an additional 8.5 mL of blood were drawn according to the schedule shown in Table 1. Encoded samples were processed within 3 h of collection. Lymphocytes were separated on standard Ficol-Hypaque gradients, adjusted to 5×107 cells/mL in 37°C RPMI 1640 medium (Sigma) with no additives and used immediately in adhesion and flow cytometric assays, as previously described (17;21).
Table I.
Schedule of blood sampling
| Group | Sample | Assays performed |
|---|---|---|
|
|
||
| FET | cycle d8 to LH surge | lymphocyte adhesion, flow cytometry, serum LH, E2 |
| COH | stimulation d9 to βhCG | lymphocyte adhesion, flow cytometry, serum E2, |
| COH | oocyte pickup (OPU) | lymphocyte adhesion, flow cytometry, serum E2, P4 |
| FET/COH | embryo transfer (ET) | lymphocyte adhesion, flow cytometry, serum E2, P4 |
| FET/COH | luteal day (LD)18, 40 | lymphocyte adhesion, flow cytometry, serum P4, β-hCG |
Each woman in the study contributed six sets of repeated measurements over time (hereinafter referred to as profiles), namely adhesion, percent CD56bright cells, percent CD56dim cells, E2, LH, and P4. For the FET group, blood was drawn starting at cycle day 7. For all women, day of embryo transfer was treated as designated time 0, day of LH surge was designated as analysis time −1. For the COH cohort, day of OPU and hCG were assigned as analysis time −1 and −2, respectively. Blood samples taken before these days were assigned analysis times of −3, −4, −5, −6 and −7. Since the menstrual cycle length of individual women differed, not all women had measurements at all timepoints. Samples taken after ET were labeled as luteal day (LD)18 and LD40, the respective days on which biochemical pregnancy (βhCG detection >5 IU/L) and clinical pregnancy (ultrasonographic fetal heartbeat detection) were determined.
Mice and tissue dissections
C57Bl/6J (Jackson Laboratories, Bar Harbor, ME) aged 8–16 wk were used for timed matings with the morning of the copulation plug designated gd0. All procedures were performed under approved animal utilization protocols (Animal Care Committee, University of Guelph). Mice were euthanized on gd7, uteri were removed and implantation sites snap frozen as described previously (17). Mid-saggital sections were cryosectioned at 12 μm immediately prior to assay within 14 days of harvest as previously described (21).
Antibodies
To detect CD56+ cells, aliquots of 5×106 lymphocytes were pre-labeled with mouse anti-CD56-PE (Immunotech, Beckman Coulter, Mississauga, ON) at 1:100 for 20 min RT. When cell numbers permitted, cell aliquots were incubated with 10 μg/ml function blocking mAb specific for human L-selectin (CD62L, BD Pharmingen, Mississauga, ON). The cell suspension was characterized by flow cytometric analysis using the following panel of mouse anti-human antibodies (Caltag Laboratories); CD4 R-PE-8 FITC (1:100), CD19 FITC (1:10), CD33-4D3 R-PE (1:10), CD34 FITC (1:10) and the above described anti-CD56-PE. Isotype controls were mouse IgG2a FITC/R-PE (1:100), mouse IgG1 FITC (1:10), mouse IgG1 R-PE (1:10).
Flow cytometry
Cells suspended in PBS were labeled for 30 min RT, then washed twice with PBS containing 2% BSA and 0.01% Na azide. Immediate analyses of cell data were performed on a FACScan using CellQuest™ software (Becton-Dickenson, San Jose, CA). Lymphocytes were defined by forward and side scatter properties.
Assay of cell adhesion under shear to frozen tissue sections
A modified Stamper Woodruff adhesion assay (14;17) was performed, layering 5 ×106 CD56-PE labeled lymphocytes, in the presence or absence of function blocking antibodies to L-selectin, in 100 μL RPMI 1640 medium with no additives onto cryosections of mouse uterine tissues. After 30 min of rotation at 112 rpm in a cold chamber, non-adherent cells were rinsed off and the tissue fixed. Double-blind analysis was performed by 2 independent researchers counting adherent CD56bright cells in 25 high power fields (HPF, 400×).
Measurements of hormone concentrations
Hormonal assays were conducted by immunochemistry (Architect, Abbott Diagnostics, Mississauga, ON) and were available for each of the 241 blood samples evaluated in this study.
Statistical analyses
The mixed linear regression model, an extension of repeated measures analysis of variance and linear regression, was used to estimate mean profiles and to test whether there were differences in the profiles between the two groups. Profiles were modeled using straight lines, piece-wise linear splines, quadratics or cubic functions, and likelihood ratio tests were used to determine which of these shapes provided an adequate fit to the data while remaining parsimonious. The mixed model methodology correctly adjusts for correlation that exists between measurements made on the same woman. It also corrects for potential biases that can be caused by differing profile lengths and missing data points. We used likelihood ratio tests to test for differences in the shapes of the mean profiles between women who subsequently became pregnant and those who did not.
For some analyses, the residuals were highly skewed, and in these cases, the dependent variable was transformed using the log (base e) transformation. Analyses were performed using SigmaStat and SAS. All p-values reported are from mixed model likelihood ratio tests, unless otherwise stated.
Results
In this center, FET has an historical pregnancy success rate of 20%. Twenty-five women were recruited as participants and monitored through the early phase of their natural cycle as they prepared for transfer of banked frozen embryos from previous hyperstimulation cycles. Within this group, 18 received frozen embryos and 7 were canceled due to inadequate follicular development, poor endometrial development or poor embryo quality. The pregnancy success rate for our study was 16.8%; 3 women became clinically pregnant, 1 had a biochemical pregnancy (β-hCG of 23 IU/L at LD18, which disappeared before LD40) and the remaining 14 were unsuccessful. In this center, COH has an historical clinical pregnancy rate of 30%. Thirty women were recruited to the study, with 18 women completing the treatment protocol to embryo transfer. The clinical pregnancy rate was 39%; 7 had clinical pregnancies, 3 had biochemical pregnancies (6, 8 and 54 IU/L) and the remaining 8 did not become pregnant.
The number of blood samples collected before embryo transfer (ET) varied considerably between individuals, with 1 to 6 pre-ET samples. However, the average number of pre-ET samples did not differ between the groups (p=0.78, t-test). There was no difference in the number or the quality of embryos transferred between pregnant and non-pregnant groups (Table II).
Table II.
Summary of transferred embryos
| FET Group | COH Group | |||
|---|---|---|---|---|
|
| ||||
| Pregnant | Non-pregnant | Pregnant | Non-pregnant | |
| n=3 | n=15 | n=7 | n=11 | |
| # embryos/transfer | 3 | 2.5 | 2.42 | 2.45 |
| # embryos by grade | ||||
| A (%) | 0 (0%) | 4 (10.5%) | 1 (5.9%) | 1 (3.7%) |
| B (%) | 6 (66.7%) | 16 (42.1%) | 6 (35.3%) | 11 (40.7%) |
| C (%) | 3 (33.3%) | 18 (47.4%) | 10 (58.8%) | 15 (55.6%) |
Definition of Embryo Grades: A = regularly shaped, equally sized blastomeres with no fragmentation; B = irregularly shaped and/or unequally sized blastomeres with no fragmentation; C = embryos with minor (<25%) fragmentation irrespective of blastomere shape or size.
Functional adhesive properties of CD56bright cells
FET cohort
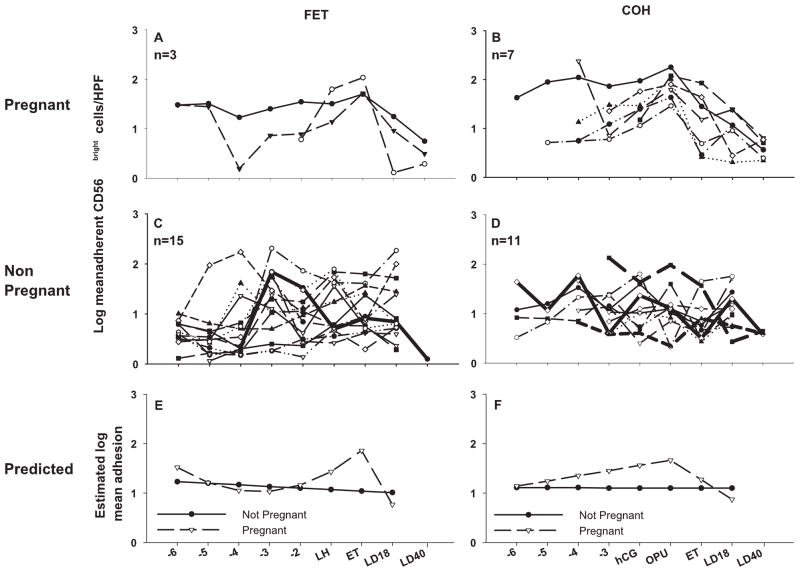
The data were grouped into those who became pregnant (n=3) (Figure 1) and those who did not become pregnant (n=15). The non-pregnant group also includes data from one woman who had a biochemical pregnancy (shown as a heavier line). The pattern of mean adhesion over time differed significantly (p=0.008) between women who subsequently had successful pregnancy compared to those who did not. This increase was blocked in the presence of function-blocking antibody to L-selectin (data not shown). Specifically, in the pregnant group, the mean log number of adherent cells showed a steady increase from day ET-3, peaking on day of ET, followed by a decrease back to baseline levels at 18 days post-ovulation (LD18). In the group of women who were not successful in becoming pregnant or maintaining implantation, the mean log number of adherent cells remained constant over the study interval. From the mean log adhesion data, a model of the predicted pattern of adhesion between those who became pregnant and those who did not was constructed (Figure 1E). There was no significant difference in the average number of blood samples analyzed in the pregnant versus non-pregnant groups (p=0.271, t-test).
Figure 1.
Log mean number of CD56bright cells per high power field (HPF, 400×) which adhere to mouse gestation day (gd) 7 decidual endothelium, plotted against day of blood collection. In A and B, data are presented from individuals who became pregnant in their FET and COH cycles respectively. Adhesion data from individuals who did not establish pregnancy in FET and COH cycles are shown in C and D respectively. The heavily weighted lines represent data from 1 subject in the FET group and 3 subjects in the COH group who had pregnancies not sustained to LD40 (biochemical). Predicted adhesion curves are shown in E and F for the FET group and the COH group.
As shown in Table III, three of the 14 women who were diagnosed with female factor as the underlying cause of infertility became pregnant. No woman classified with male factor or idiopathic cause became pregnant in the FET group. The relationship between pregnancy outcome and underlying cause of infertility was not statistically significant (p=1.0, Fisher’s Exact test).
Table III.
Outcome by diagnosis of infertility - FET
| FET | Female Factor | Idiopathic | Male Factor | Total |
|---|---|---|---|---|
| Pregnant | 3 | 0 | 0 | 3 |
| Not | 11 | 3 | 1 | 15 |
| Total | 14 | 3 | 1 | 18 |
COH cohort
The data for women receiving exogenous hormone treatment were grouped into those who became pregnant (n=7) and those who did not become pregnant (n=11) (Figure 1). The non-pregnant group includes 3 women who exhibited biochemical pregnancy (shown as heavier lines). We found that women who became clinically pregnant showed an increase in mean log adhesion which peaked on day of OPU, followed by a decline which continued to LD18. Again, this increase was not evident when lymphocytes were pre-treated with a function-blocking antibody to L-selectin (data not shown). In women who were unsuccessful in becoming pregnant or sustaining an implanting embryo, log adhesion remained constant. This difference in adhesion profiles was statistically significant (p=0.0002) (Figure 1F). While there is some reason to believe that ova mature faster in more fertile women, there were no differences in the number of blood samples analyzed per woman in the pregnant versus non-pregnant groups (p=0.0967, t-test).
In the women who underwent COH, two of the eight women classified with female factor infertility became pregnant, as did both women with idiopathic presentation and three of five women with male factor as the underlying cause (Table IV). However, the relationship between diagnosis and pregnancy outcome was not significant (p=0.3102, Fisher’s Exact Test).
Table IV.
Outcome by diagnosis of infertility - COH
| COH | Female Factor | Idiopathic | Male Factor | Total |
|---|---|---|---|---|
| Pregnant | 2 | 2 | 3 | 7 |
| Not | 6 | 0 | 5 | 11 |
| Total | 8 | 2 | 8 | 18 |
Cytometric analysis of PBL
FET cohort
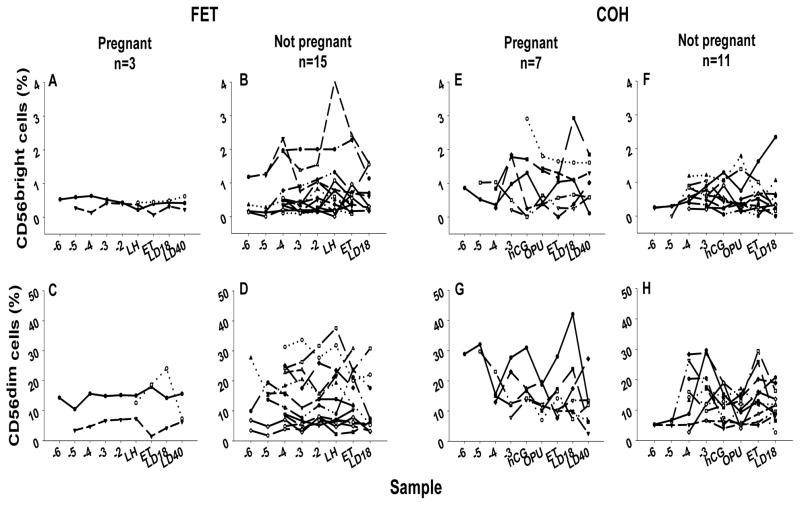
Figure 2 shows the percentage of the total lymphocyte population within the lymphocyte gate that expresses CD56dim and CD56bright at each sample point for each subject in each of the cohorts. There was no difference between the percentage of total lymphocytes occupied in the CD56bright subset in pregnant (0.432%) and non-pregnant (0.385%) women (p=0.7162) nor in the rates of change over time (p=0.0718). Thus the percentage of CD56bright cells was not diagnostic of pregnancy success. No difference was found in %CD56dim cells between the pregnant (13.43%) and non-pregnant (11.77%) groups (p=0.7546) or in rates of change over time (p=0.6069) despite large fluctuations in individual profiles. Moreover, there were no differences between CD4, CD8, CD19, CD33 or CD34 expressing lymphocytes amongst the groups (data not shown).
Figure 2.
Percentage of the total lymphocyte population that expresses CD56bright (A, B, E and F) or CD56dim (C, D, G and H) surface phenotype plotted against day of blood collection. Connected points represent individual samples through the course of the study.
COH cohort
Analyses of the CD56bright subset in pregnant and non-pregnant cohorts of the COH group indicated that there was a difference in the mean percentage of lymphocytes that were CD56bright cells between the pregnant (0.84%) and the non-pregnant (0.48%) cohorts (p=0.0320) but no difference in rate of change over time (p=0.4975). With regard to the CD56dim population, the mean percentage of CD56dim cells in the pregnant group decreased over time (slope = −0.06898, se.=0.03469, p =0.0495), while remaining constant in the non-pregnant group (slope=0.02879, se.=0.02948, p=0.3312). The difference between slopes was significant (p=0.0342), indicating that the response of the CD56dim subset to hormone stimulation differed between the groups. A positive association was found between the percentages of CD56dim and CD56bright cells within each group (pregnant, p=0.0074, non-pregnant, p=0.0001).
Analysis of hormone data
FET cohort
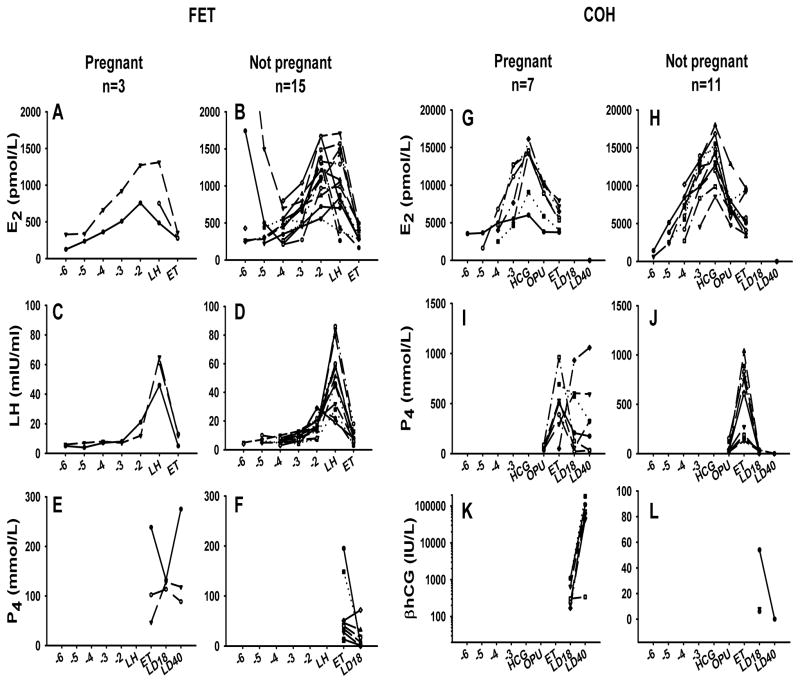
The plots of Figure 3 depict mean hormone measurements within each treatment group. Cubic functions were necessary to adequately model E2 and LH up to day of LH surge. There were no differences detected in E2 or LH concentrations between pregnant and non-pregnant groups (p=0.6842, p=0.5905 respectively). Furthermore, there were no differences in P4 values at ET (p=0.071, Mann-Whitney Rank Sum). Estradiol was not significantly associated with the percentage of CD56bright cells in either pregnant (p= 0.5618) or non-pregnant (p=0.5062) groups or with percentage of CD56dim cells in either pregnant (p=0.0884) or non-pregnant (p=0.5281) groups.
Figure 3.
Hormone concentrations from FET (A–F) and COH (G–L) cohorts, plotted against day of blood collection. Data are presented for individual subjects over the course of the study.
COH cohort
Hormone levels for the women who underwent COH are shown in Figure 3. Cubic and quadratic functions were necessary to adequately fit the E2 and P4 values over time. There were no differences in E2 levels (p=0.3894), or P4 values (p=0.1701) between those who became pregnant and those who did not. In addition, there was no correlation between E2 and percentage CD56bright in either the pregnant group (p=0.9675) or the non-pregnant group (p=0.1824). Similarly, there was no correlation between E2 and percentage CD56dim in either the pregnant group (p=0.2482) or the non-pregnant group (p=0.7556).
Discussion
We found significant differences in patterns of CD56bright cell adhesion between women who became pregnant and those who did not, with those who became pregnant displaying higher peri-ovulatory adhesion. As shown in Figure 1, there is considerable variability in the number of adherent cells between women. However, there is a consistent pattern over time for each individual woman who became pregnant, namely a rise in adhesion, followed by a decline to below baseline levels by LD18. This pattern was also seen in two of the 26 subjects who did not become pregnant (neither had a biochemical pregnancy). This finding reinforces our previous observations in mouse models, which indicated that parallel and co-ordinated changes in adhesive function occurred in both circulating lymphocytes and uterine endothelial cells during pregnancy (17). In this study, since we measure only the lymphocyte response, as it is not possible to assess uterine endothelial cell responses in women attempting pregnancy, we surmise that in these 2 women, endothelial cells did not respond adequately to hormonal signals. The changes in lymphocyte adhesiveness occur before an embryo is present, so are completely independent of a pregnancy, or embryonic factors. We speculate that these changes are associated with endometrial products involved in implantation and could be predictive of successful treatment outcome.
Since we had previously determined that NK cell homing potential increased at ovulation (21), the first objective of this study was to determine whether conception extended the interval for homing potential. Adhesion was studied in infertile women not receiving hormone treatment (FET) because their cycles are closely monitored clinically. We did not find that very early pregnancy was associated with a state of elevated adhesion. Indeed, a drop in adhesion occurred between embryo transfer and LD18 in pregnant women. This outcome is consistent with our previous experiments in which we observed that adhesion was reduced post-ovulation in fertile donors, and that culture of lymphocytes from male donors in the presence of P4 reduced adhesion (21). In contrast, consistent changes in adhesion profile were not detected in the women who did not become pregnant or support early implanting embryos and the predicted profile for this group was constant over the test period. At ET, the estimated mean adhesion was higher in the pregnant group by exp(1.09) or 2.78 cells per high power field.
The second objective of this study was to determine whether administration of exogenous hormones to infertile women modified the adhesive potential of their CD56+ blood cells. In the COH group, adhesion in the pregnant cohort peaked on the day of OPU (1 day post hCG), which differed from that of the pregnant outcome group of the FET cohort which peaked at ET (3 days post LH). The women in the COH group who did not become pregnant showed no consistent pattern in adhesion profiles, and the estimated mean profile in this group remained unchanged through the test period. Thus, the observed differences in lymphocyte behavior were between women who became pregnant and those who did not, rather than between women conceiving during a natural cycle versus those conceiving during an hormonally-medicated cycle. This suggests that circulating CD56+ cells and dNK cell progenitors are not significantly altered in function by current clinical protocols for COH.
Our definition of pregnancy is based on clinical pregnancy; that is, a β-hCG level of greater than 5 IU/L on LD18, followed by detection of a fetal heart beat or gestational sac on ultrasound at LD40. If the level was below 50 IU, the test was repeated 2 days later. In a viable early stage pregnancy, β-hCG values double every 2 days. A positive LD18 β-hCG value that cannot be confirmed by the presence of fetal heart beat or gestational sac on ultrasound at LD40 was classified as a biochemical pregnancy. The LD18 serum β-hCG concentrations in the pregnant groups ranged from 170 IU/L to 2604 IU/L with an average of 860 IU/L. All but four patients in the non-pregnant groups had a negative β-hCG test at LD18. Of the single FET patient and the 3 COH non-pregnant patients with positive LD18 β-hCG values, only one had what appeared to be a viable pregnancy (54 IU/L) which was lost by LD40. The adhesion data of these biochemical pregnancy patients is highlighted in Figure 1 by the presence of heavier lines. The data of the patient with a LD18 β-hCG level of 54 is represented by the solid line in Figure 1D, while the others are shown as dashed lines. Since each of these patients was defined as having a biochemical rather than a clinical pregnancy, they were grouped with the non-pregnant group. The adhesion data of these patients more closely resembles that of the non-pregnant cohorts than the pregnant groups, suggesting viable embryos but a non-sustaining maternal environment.
There were no differences in embryo quality as assessed by morphological criteria or in hormone levels between those who became pregnant and those who did not. Measurements of soluble HLA-G were not available. Further, we were not able to correlate etiology of infertility to outcome. For both groups of pregnant women, we observed lower levels of CD56+ cell adhesion at LD18 and higher levels at LD40. This suggests that the initial phase of homing to the uterus is triggered by peri-ovulatory conditions during the menstrual cycle (before embryo transfer) and that a second wave of homing occurs about week 6 of pregnancy. Since peak numbers of dNK cells are found 6–12 weeks of pregnancy (7;22), these data are consistent with a scenario in which embryo induced factors such as CXCL12 (SDF-1) attract a further contingent of CD56+ dNK precursor cells, over a longer period of elevated lymphocyte-endothelial cell interactions, to replenish the rapidly proliferating uterine population.
In both FET and COH groups, not only did the pattern of adhesive change seen in women who became pregnant differ from those who did not, but the mean number of adhering cells was higher. Yamamoto et al. reported that the ratio of peripheral CD56dim to CD56bright cells was higher in women with recurrent spontaneous abortion (RSA) and that these women had a reduced number of uterine CD56+ cells (24). This observation is related to earlier studies which show that proportions of CD56dim cells in peripheral blood are higher in infertile women and women with RSA than in fertile women and that cytotoxicity of NK cells is enhanced in women with RSA. However, the proportion of NK cell subtypes did not correlate to cytotoxic effector capability (25;26). It has also been suggested that successful pregnancy is associated with a peripheral CD56+ population of <12% (26). Our data differs from these reports. We found no difference in peripheral CD56dim or CD56bright cell proportions between the pregnant and non-pregnant groups of FET patients, but we did detect a decrease over time in percentage of CD56dim cells (from a mean of 30.89% at ET-6 to 11.61% at LD40) in COH patients who became pregnant. In addition, we found the percentage of CD56bright cells was significantly higher in the group that became pregnant in the COH cohort. Thus, neither the number of peripheral NK cells nor their relative proportions appear to be associated with pregnancy success in natural cycles, but in hormone-treated cycles, higher levels of CD56bright cells are associated with pregnancy success.
In fertile cycles, CD56bright cells responded to rising E2 and in the FET group to LH, by enhanced adhesiveness, but in non-fertile cycles this reaction did not occur. Increased adhesiveness could be either due to a direct hormonal effect on a subset of cells or other unidentified soluble factors up-regulated by E2 or LH, which then act on adhesion molecules expressed by NK cells. The latter seems more probable because we have been unable to detect hormone receptors (ERα, ERβ, PR or LHR) on CD56+ cells isolated from blood using quantitative PCR (manuscript in preparation).
We demonstrate here that peripheral blood CD56+ cells in fertile cycles differ in homing potential from those of infertile cycles. These results obtained indicate that alterations in NK cell adhesion during the ovulation/embryo transfer period is a mandatory, but not sufficient, prerequisite for establishing pregnancy. These studies provide a potential measure of the state of uterine readiness for implantation, however larger studies are required to rigorously evaluate the predictive value of this assay. These studies may also provide a rare measure of immune/uterine synchronization with conceptus development, since the study of peri-implantation uterine endothelium in women is difficult. More precise definition of the molecular basis of these phenomena, coordinated in blood NK cells, endothelium and decidua and perhaps trophoblast (27) is required to advance issues of patient classification and infertility diagnostics.
Acknowledgments
We thank all participants in this study for their co-operation and willingness to participate. We are grateful to the staff at Gamma Dynacare in London, ON for their teamwork in collecting blood samples. Our thanks to Julie Fisher for help with patient coordination and the REI physicians and nurses for patient recruitment.
2Supported by Awards from Natural Sciences and Engineering Council, Canada, Canadian Institutes for Health Research, Ontario Ministry of Agriculture, Food and Rural Affairs and Ontario Women’s Health Scholar Award (MvdH).
3Abbreviations used
- COH
controlled ovarian hyperstimulation
- dNK cell
decidual Natural Killer cell
- DB
decidua basalis
- ET
embryo transfer
- gd
gestation day
- FET
frozen embryo transfer
- LD
luteal day
- LH
luteinizing hormone
- E2
17-β estradiol
- OPU
oocyte pickup
- P4
progesterone
- uNK cell
uterine Natural Killer cell
References
- 1.King A. Uterine leukocytes and decidualization. Hum Reprod Update. 2000;6(1):28–36. doi: 10.1093/humupd/6.1.28. [DOI] [PubMed] [Google Scholar]
- 2.Chaouat G, Ledee-bataille N, Zourbas S, Dubanchet S, Sandra O, Martal J, Ostojojic S, Frydman R. Implantation: can immunological parameters of implantation failure be of interest for pre-eclampsia? J Reprod Immunol. 2003;59(2):205–217. doi: 10.1016/s0165-0378(03)00048-2. [DOI] [PubMed] [Google Scholar]
- 3.Kammerer U, Eggert AO, Kapp M, McLellan AD, Geijtenbeek TB, Dietl J, van Kooyk Y, Kampgen E. Unique appearance of proliferating antigen-presenting cells expressing DC-SIGN (CD209) in the decidua of early human pregnancy. Am J Pathol. 2003;162(3):887–896. doi: 10.1016/S0002-9440(10)63884-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Robertson SA, Redman CW, McCracken SA, Hunt JS, Dimitriadis E, Moffett-King A, Chamley L. Immune modulators of implantation and placental development-a workshop report. Placenta. 2003;24(Suppl A):S16–S20. doi: 10.1053/plac.2002.0937. [DOI] [PubMed] [Google Scholar]
- 5.Kitaya K, Nakayama T, Daikoku N, Fushiki S, Honjo H. Spatial and temporal expression of ligands for CXCR3 and CXCR4 in human endometrium. J Clin Endocrinol Metab. 2004;89(5):2470–2476. doi: 10.1210/jc.2003-031293. [DOI] [PubMed] [Google Scholar]
- 6.Henderson TA, Saunders PT, Moffett-King A, Groome NP, Critchley HO. Steroid receptor expression in uterine natural killer cells. J Clin Endocrinol Metab. 2003;88(1):440–449. doi: 10.1210/jc.2002-021174. [DOI] [PubMed] [Google Scholar]
- 7.Moffett-King A. Natural killer cells and pregnancy. Nat Rev Immunol. 2002;2(9):656–663. doi: 10.1038/nri886. [DOI] [PubMed] [Google Scholar]
- 8.Lidstrom C, Matthiesen L, Berg G, Sharma S, Ernerudh J, Ekerfelt C. Cytokine secretion patterns of NK cells and macrophages in early human pregnancy decidua and blood: implications for suppressor macrophages in decidua. Am J Reprod Immunol. 2003;50(6):444–452. doi: 10.1046/j.8755-8920.2003.00112.x. [DOI] [PubMed] [Google Scholar]
- 9.Ashkar AA, Croy BA. Interferon-gamma contributes to the normalcy of murine pregnancy. Biol Reprod. 1999;61(2):493–502. doi: 10.1095/biolreprod61.2.493. [DOI] [PubMed] [Google Scholar]
- 10.Ashkar AA, di Santo JP, Croy BA. Interferon gamma contributes to initiation of uterine vascular modification, decidual integrity, and uterine natural killer cell maturation during normal murine pregnancy. J Exp Med. 2000;192(2):259–270. doi: 10.1084/jem.192.2.259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Guimond MJ, Luross JA, Wang B, Terhorst C, Danial S, Croy BA. Absence of natural killer cells during murine pregnancy is associated with reproductive compromise in TgE26 mice. Biol Reprod. 1997;56(1):169–179. doi: 10.1095/biolreprod56.1.169. [DOI] [PubMed] [Google Scholar]
- 12.Croy BA, Ashkar AA, Foster RA, DiSanto JP, Magram J, Carson D, Gendler SJ, Grusby MJ, Wagner N, Muller W, Guimond MJ. Histological studies of gene-ablated mice support important functional roles for natural killer cells in the uterus during pregnancy. J Reprod Immunol. 1997;35(2):111–133. doi: 10.1016/s0165-0378(97)00054-5. [DOI] [PubMed] [Google Scholar]
- 13.Smith SK. Regulation of angiogenesis in the endometrium. Trends Endocrinol Metab. 2001;12(4):147–151. doi: 10.1016/s1043-2760(01)00379-4. [DOI] [PubMed] [Google Scholar]
- 14.Chantakru S, Miller C, Roach LE, Kuziel WA, Maeda N, Wang WC, Evans SS, Croy BA. Contributions from self-renewal and trafficking to the uterine NK cell population of early pregnancy. J Immunol. 2002;168(1):22–28. doi: 10.4049/jimmunol.168.1.22. [DOI] [PubMed] [Google Scholar]
- 15.Springer TA. Traffic signals on endothelium for lymphocyte recirculation and leukocyte emigration. Annu Rev Physiol. 1995;57:827–872. doi: 10.1146/annurev.ph.57.030195.004143. [DOI] [PubMed] [Google Scholar]
- 16.Butcher EC, Picker LJ. Lymphocyte homing and homeostasis. Science. 1996;272(5258):60–66. doi: 10.1126/science.272.5258.60. [DOI] [PubMed] [Google Scholar]
- 17.Chantakru S, Wang WC, van den Heuvel M, Bashar S, Simpson A, Chen Q, Croy BA, Evans SS. Coordinate Regulation of Lymphocyte-Endothelial Interactions by Pregnancy-Associated Hormones. J Immunol. 2003;171(8):1132–1145. doi: 10.4049/jimmunol.171.8.4011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Frey M, Packianathan NB, Fehniger TA, Ross ME, Wang WC, Stewart CC, Caligiuri MA, Evans SS. Differential expression and function of L-selectin on CD56bright and CD56dim natural killer cell subsets. J Immunol. 1998;161(1):400–408. [PubMed] [Google Scholar]
- 19.Kruse A, Merchant MJ, Hallmann R, Butcher EC. Evidence of specialized leukocyte-vascular homing interactions at the maternal/fetal interface. Eur J Immunol. 1999;29(4):1116–1126. doi: 10.1002/(SICI)1521-4141(199904)29:04<1116::AID-IMMU1116>3.0.CO;2-4. [DOI] [PubMed] [Google Scholar]
- 20.Campbell JJ, Qin S, Unutmaz D, Soler D, Murphy KE, Hodge MR, Wu L, Butcher EC. Unique subpopulations of CD56+ NK and NK-T peripheral blood lymphocytes identified by chemokine receptor expression repertoire. J Immunol. 2001;166(11):6477–6482. doi: 10.4049/jimmunol.166.11.6477. [DOI] [PubMed] [Google Scholar]
- 21.van den Heuvel M, Horrocks J, Bashar S, Taylor S, Burke S, Hatta K, Lewis JE, Croy BA. Menstrual Cycle Hormones Induce Changes in Functional Interactions Between Lymphocytes and Decidual Vascular Endothelial Cells. J Clin Endocrinol Metab. 2005 doi: 10.1210/jc.2004-1742. In press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bulmer JN, Lash GE. Human uterine natural killer cells: a reappraisal. Mol Immunol. 2005;42(4):511–521. doi: 10.1016/j.molimm.2004.07.035. [DOI] [PubMed] [Google Scholar]
- 23.Tesarik J, Hazout A, Mendoza C. Luteinizing hormone affects uterine receptivity independently of ovarian function. Reprod Biomed Online. 2003;7(1):59–64. doi: 10.1016/s1472-6483(10)61729-4. [DOI] [PubMed] [Google Scholar]
- 24.Yamamoto T, Takahashi Y, Kase N, Mori H. Decidual natural killer cells in recurrent spontaneous abortion with normal chromosomal content. Am J Reprod Immunol. 1999;41(5):337–342. doi: 10.1111/j.1600-0897.1999.tb00447.x. [DOI] [PubMed] [Google Scholar]
- 25.Morikawa M, Yamada H, Kato EH, Shimada S, Ebina Y, Yamada T, Sagawa T, Kobashi G, Fujimoto S. NK cell activity and subsets in women with a history of spontaneous abortion. Gynecol Obstet Invest. 2001;52(3):163–167. doi: 10.1159/000052966. [DOI] [PubMed] [Google Scholar]
- 26.Gilman-Sachs A, DuChateau BK, Aslakson CJ, Wohlgemuth GP, Kwak JY, Beer AE, Beaman KD. Natural killer (NK) cell subsets and NK cell cytotoxicity in women with histories of recurrent spontaneous abortions. Am J Reprod Immunol. 1999;41(1):99–105. doi: 10.1111/j.1600-0897.1999.tb00081.x. [DOI] [PubMed] [Google Scholar]
- 27.Genbacev OD, Prakobphol A, Foulk RA, Krtolica AR, Ilic D, Singer MS, Yang ZQ, Kiessling LL, Rosen SD, Fisher SJ. Trophoblast L-selectin-mediated adhesion at the maternal-fetal interface. Science. 2003;299(5605):405–408. doi: 10.1126/science.1079546. [DOI] [PubMed] [Google Scholar]