Abstract
Background
Probe-based confocal laser endomicroscopy (pCLE) of the common bile duct (CBD) is a new procedure that can be used for assessing indeterminate billiary strictures. The CBD has been examined using the CholangioFlex miniprobe (Mauna Kea Technologies, Paris, France) which has a lateral resolution of 3.5μm and diameter of <1.0 mm. However, larger-diameter higher-resolution confocal probes are available. We aimed to determine if pCLE of the CBD with the high-definition GastroFlexUHD miniprobe (UHDp) was feasible. This probe has a lateral resolution of 1μm and an outer diameter of 2.6mm.
Methods
Eleven consecutive patients undergoing ERCP for various indications at a single, large, academic center were included in the study. Examination of the CBD was attempted with the UHDp after injection of 2.5mL of 10% fluorescein. A 0.035 inch guidewire was first placed into the CBD and the confocal probe was subsequently inserted adjacent to the guidewire. Position of the miniprobe was identified fluoroscopically.
Results
The GastroFlexUHD miniprobe was successfully introduced into the CBD in 10 of 11 patients. Cellular structures and individual cell morphology seemed to be more clearly visualized with the UHDp as compared to the CholangioFlex probe. No significant side effects except one case of mild pancreatitis.
Conclusions
We demonstrate that high-definition pCLE of the CBD via the GastroFlexUHD miniprobe is feasible and may offer improved image quality over the standard CholangioFlex probe. Further studies are needed to see if this improves the diagnostic accuracy of bile duct lesions.
Keywords: confocal, endomicroscopy, biliary, stricture
Background
The differentiation between benign and malignant tissue within the biliary system has been difficult. Although multiple imaging modalities are available, lesions seen via ultrasound, CT, EUS or MRI may raise suspicion that disease is present, but often are unable to provide definitive diagnosis1–4. The most common technique to verify suspicion of malignancy is tissue sampling, either via brush cytology or forceps biopsy. However, sensitivity and negative predictive value are often low, with the sensitivity of brush cytology for the diagnosis of malignant biliary structures between 30–60%, and between 43–81% for forceps biopsy5. Per oral cholangioscopy has been considered a good alternative, offering sensitivities as high as 78–89% and a negative predictive value of 58–95% for the diagnosis of pancreaticobiliary malignancy in patients with indeterminate strictures6, 7. However, adoption of this technology has been limited due to several factors, including scope fragility, high acquisition and maintenance costs, the lack of therapeutic capability, and often the requirement for two endoscopists and light sources. Furthermore, tissue sampling is still required for diagnosis of malignancy.
Probe-based confocal laser endomicroscopy (pCLE) is a new modality used to evaluate in vivo histopathology of the gastrointestinal tract. A laser scanning unit emits light and illuminates the tissue with a low-power laser. The light is locally absorbed by fluorophores, either applied to the tissue or naturally present within the tissue, and the reflected fluorescence is detected by the probe and transferred via optic fibers back to the scanning unit. A true microscopic image can then be seen in real time by the endoscopist. This can be performed either via a dedicated endoscope8, or a probe passing through the accessory channel of a standard endoscope9.
Recent technological advances have made it possible for the probe to be miniaturized to the point where it is able to be passed directly into the common bile duct directly via a standard duodenoscope, usually either through a catheter through the working channel of the duodenoscope or through a cholangioscope’s working channel9. Currently, evaluation of the common bile duct (CBD) is performed via the CholangioFlex miniprobe (Cellvizio, Mauna Kea Technologies, Paris, France) which has an outer diameter of less than 1.0 mm, a lateral resolution of 3.5 μm, a focal plane 55 μm beyond the probe tip, and 400-fold magnification10. Other miniprobe models such as the GastroFlexUHD miniprobe (Cellvizio, Mauna Kea Technologies, Paris, France) offer higher magnification and improved resolution, but these probes are larger in diameter in order to accommodate an increased number of fiber optics (Table 1). It is unclear whether these larger probes could be used to visualize the common bile duct, given the potential increased difficulty with passing a probe of larger size.
Table 1.
Specifications of the GastroFlexUHD and CholangioFlex miniprobe
| GastroFlexUHD miniprobe | Cholangioflex miniprobe | |
|---|---|---|
| Resolution | 1.03 μm | 3.5 μm |
| Field of View | 240 μm | 320 μm |
| Depth of Focus | 60 μm | 55 μm |
| Catheter Thickness | 2.6 mm | 1.0 mm |
Therefore, we aimed to determine if ERCP-guided pCLE of the CBD with the use of the high-definition GastroFlexUHD miniprobe would be feasible, with an outer diameter of 2.6 mm (compared to 1.0 mm in the CholangioFlex miniprobe), and a lateral resolution of 1 μm, a depth of focus of 60 μm, and 1000-fold magnification.
Methods
In a series of 11 patients undergoing ERCP for various indications, examination of the CBD was attempted with the GastroFlexUHD miniprobe. Indications included CBD strictures, biliary obstruction not due to CBD stricturing, and ampullary adenomas. IRB approval had been obtained to use this device in patients with both normal and abnormal pathology, as well as tissue sampling as deemed necessary to verify the diagnosis. Patients were sedated with either a combination of midazolam, fentanyl and meperidine, or with propofol under the supervision of an anesthesiologist. Glucagon was used as an antispasmodic agent whenever necessary to reduce intestinal motility to aid in image acquisition. The miniprobe was introduced via a standard duodenoscope (Olympus TJF-160VF). A 0.035 inch guidewire was first placed into the CBD and after injection of 2.5mL (250mg) of 10% fluorescein, the confocal probe was subsequently inserted freehand adjacent to the guidewire. The probe was placed in light contact against the wall of the CBD, and optical ‘biopsy’ images were obtained. The radio-opaque tip of the probe allowed for positioning of the miniprobe via fluoroscopy (Figure 1). Depending on the indication, imaged areas were subsequently sampled via either brushing and/or biopsy forceps (performed in 5 of 10 patients), and sent for evaluation by light microscopy.
Figure 1.
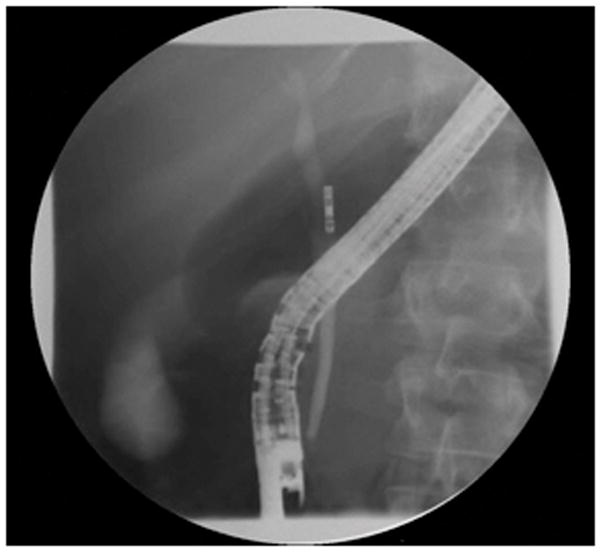
Fluoroscopic image of confocal probe demonstrating how the radio-opaque tip of the miniprobe can be positioned via fluoroscopy.
The confocal images were reviewed during the procedure by the endoscopist, as well as after each procedure by a co-investigator familiar with confocal endomicroscopy who was blinded to the status of each patient. Each set of images were graded as either normal or abnormal, and then compared to the pathology results.
Results
Feasibility
The GastroFlexUHD miniprobe (UHDp) was able to be introduced into the CBD in 10 of 11 consecutive patients. One patient with biliary obstruction due to retained CBD stone was unable to be cannulated. Although the UHDp is larger in size than the Cholangioflex miniprobe with an outer diameter of 2.6 mm vs. 1.0 mm (Figure 2), we did not subjectively find the UHDp to be particularly difficult to pass across the ampulla or to obtain good images. Although severe stricturing or acute angulation of the CBD might be expected to make the larger probe more difficult to pass, we did not experience this in our patient population.
Figure 2.
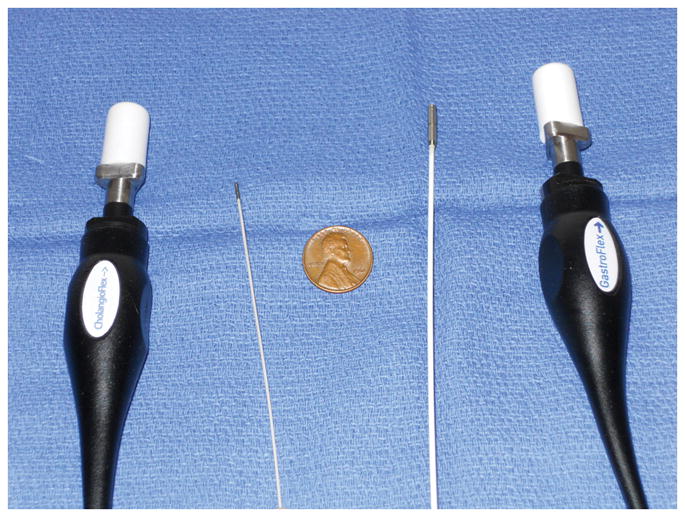
Image demonstrating the relative size of the Cholangioflex probe (left) compared to the GastroflexUHD probe (right).
Targeting of the probe was performed through fluoroscopy. A cholangiogram was initially performed, and this imaging was used in directing the probe into a particular area of interest, for example a stricture. The probe was then used to scan up and down the length of the stricture, and resulting images were recorded for review. Slight torqueing of the endoscope is often required in order to achieve the optimal perpendicular plane between the probe and the bile duct wall. Although we did not attempt this, it may be reasonable to perform dilation of a CBD stricture if good images are unable to be obtained.
We did not attempt to cannulate the pancreatic duct in every patient, but in one patient who had pancreatic duct stricturing, we were able to cannulate the pancreatic duct with the UHDp without difficulty. However, the images we obtained were suboptimal, perhaps due to our unwillingness to overmanipulate the pancreatic duct. We did not attempt to cannulate the hepatic ducts due to lack of indication in our patient population, although this would likely be feasible if the situation warranted. No fiber strand breaks compromising image quality were experienced.
Image Quality
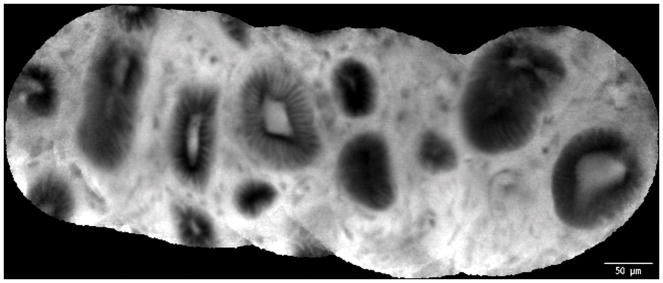
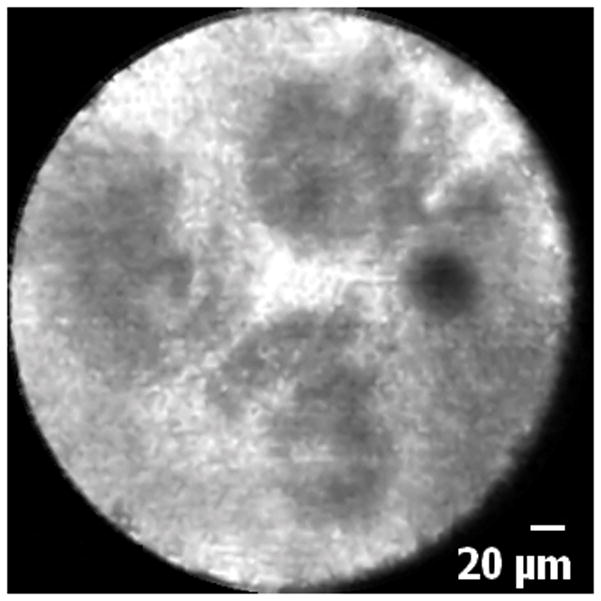
Biliary epithelia, crypts, glands, reticular network and blood vessels were clearly visualized with the UHDp. Although this study was not designed to directly compare the UHDp to the Cholangioflex miniprobe, the authors subjectively felt that images obtained with the UHDp enabled superior visualization of cellular structures as compared to other images previously obtained on different patients utilizing the CholangioFlex probe. Representative optical images using the Cholangioflex probe show that although crypts, epithelium, and vasculature can be grossly identified, it is difficult to visualize individual cells, which limits the endoscopist’s ability to describe cellular morphology (Figures 3 and 4). When images obtained via the Cholangioflex probe are compared to ones obtained via the UHDp, individual structures are more easily identified using the UHDp. This improved image quality is due to the increased resolution of the UHDp, which has a lateral resolution of 1μm, compared to 3.5μm for the Cholangioflex probe.
Figure 3.
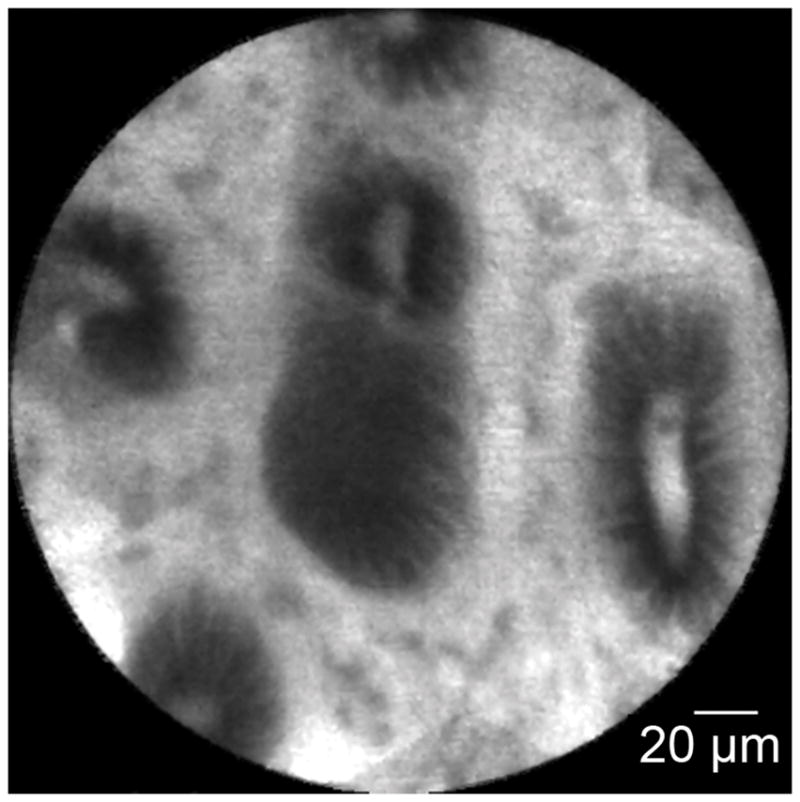
Normal common bile duct epithelium and crypts: A. Image obtained via CholangioFlex probe. B. Image obtained via GastroflexUHD probe. C. Mosaic rendering demonstrating normal common bile duct epithelium and crypts, image obtained via GastroflexUHD probe.
Figure 4.
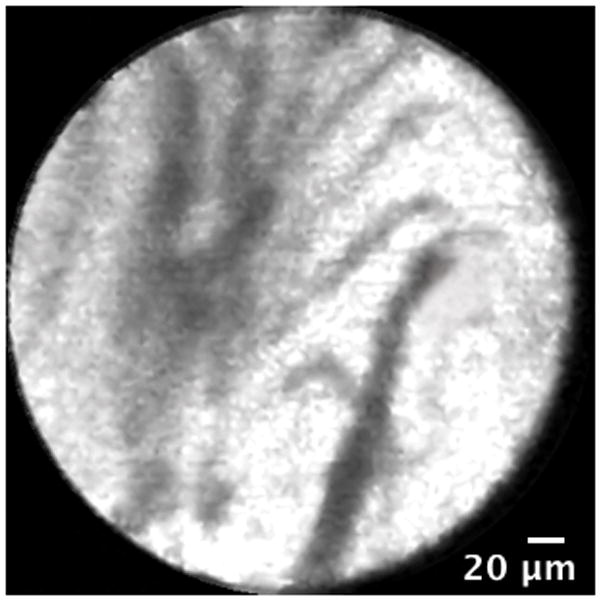
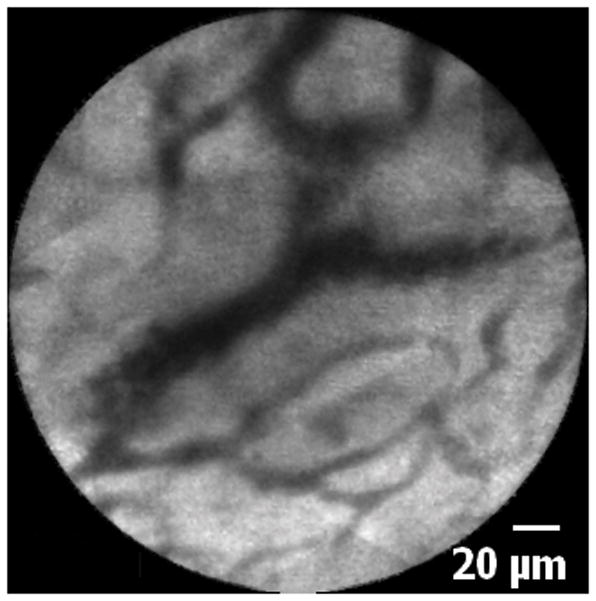
Normal common bile duct blood vessels and reticular network: A. Image obtained via CholangioFlex probe. B. Image obtained via GastroflexUHD probe.
Diagnostic Accuracy
In 9 patients, the bile duct was felt to be normal in appearance via pCLE; this was later confirmed in each patient via a combination of histology and/or clinical follow-up of at least one year (Table 2). In one patient with a biliary stricture, confocal endomicroscopy of this area revealed the presence of dark clumping, and markedly abnormal-appearing cellular architecture. Biopsies revealed that this area contained moderately-differentiated adenocarcinoma. Dedicated studies are needed to see whether diagnostic accuracy is improved using the UHDp as compared to the Cholangioflex probe.
Table 2.
Patient Findings
| Case | Age | Gender | Indication | Confocal Images | CBD Pathology | Complications | Final Diagnosis |
|---|---|---|---|---|---|---|---|
| 1 | 67 | Female | Ampullary Adenoma | Normal CBD | Not obtained | None | Ampullary Adenoma |
| 2 | 29 | Female | Ampullary Adenoma | Normal CBD | Not obtained | Mild pancreatitis | Normal Ampulla |
| 3 | 53 | Female | CBD Stricture | Normal CBD | Brushing – normal | None | Benign Stricture |
| 4 | 54 | Female | CBD Stricture | Normal CBD | Brushing – normal | None | Benign Stricture |
| 5 | 46 | Male | Biliary Obstruction | Normal CBD | Brushing & Biopsy – normal | None | Normal CBD |
| 6 | 61 | Male | CBD Stricture | Normal CBD & pancreatic duct | Brushing & Biopsy – normal | None | Benign Stricture |
| 7 | 44 | Female | Ampullary Adenoma | Normal CBD | Not obtained | None | Ampullary Adenoma |
| 8 | 61 | Male | Ampullary Adenoma | Normal CBD | Not obtained | None | Ampullary Adenoma |
| 9 | 86 | Female | Biliary Obstruction | Normal CBD | Not obtained | None | Normal CBD |
| 10 | 68 | Male | CBD Stricture | Abnormal CBD with presence of dark clumps and abnormal cellular architecture | Adenocarcinoma | None | Adenocarcinoma |
CBD = common bile duct
Complications
No significant side effects were noted as a direct consequence of the confocal procedure, aside from one episode of mild pancreatitis, which resolved within one day. The patient in whom we cannulated the pancreatic duct did not experience pancreatitis. Given that patients who undergo standard ERCP without confocal imaging can have a pancreatitis rate of up to 10%, our findings suggest that use of the UHDp does not significantly increase the incidence rate of pancreatitis, although larger prospective studies are needed to investigate this further.
Discussion
Confocal fluorescence microscopy is a type of microscopy in which the fluorescence signal from cells and other structures can be detected from a thin optical plane of focus within a thick tissue specimen. Confocal endomicroscopy permits confocal microscopic images of the gastrointestinal mucosa to be collected during the course of an endoscopic procedure. This technology thus has the potential to enable histological diagnoses to be made in real time. The most common fluorophore used in confocal endomicroscopy is flourescein, and the safety of this fluorescent dye in endoscopy has recently been demonstrated11. The clinical utility of confocal endomicroscopy has been examined in a range of clinical conditions, including colorectal neoplasia, collagenous colitis, H. pylori infection, and Barrett’s esophagus12–15. Confocal endomicroscopy generally employs one of two technological approaches. First, endoscope-based confocal laser endomicroscopy (eCLE) uses a specially designed endoscope in which the confocal optics are incorporated into the tip of the endoscope. Second, probe-based confocal laser endomicroscopy (pCLE) uses a confocal imaging system incorporated into a probe that is passed through the working channel of a standard endoscope. This latter approach makes pCLE uniquely suited for confocal microscopic examination of the biliary tract.
Indeterminate biliary strictures is a group of disorders that may benefit in particular from the ability to examine histology in real time. These strictures may be caused by a host of benign (inflammation, pancreatitis, ischemia, iatrogenic) or malignant causes16. Despite multiple available methods to investigate the causes for these strictures, such as tissue sampling, fluorescence in-situ hybridization, and digital image analysis, a definitive diagnosis can be elusive given the low sensitivity of these methods5, 7, 17–19. Because many of these techniques involve random samplings of tissue, a technique such as confocal endomicroscopy may provide unique benefits because it allows histopathological examination of several areas of interest within the biliary tree in vivo and in real time. More experience using the UHDp will allow us to determine whether more challenging biliary anatomy, e.g. structuring or tortuous ducts will preclude use of the larger probe, although we did not experience difficulty in the two patients with CBD strictures included in this study. Imaging criteria have been proposed for the confocal endomicroscopic diagnosis of malignancy in indeterminate biliary strictures20. These criteria include loss of reticular pattern of less than 20μm, detection of irregular epithelial lining, villi or gland-like structures, loss of mucosal structures, tortuous, dilated, saccular vessels with inconsistent branching, and presence of “black areas” thought to represent decreased uptake of fluorescein20. It has been reported that the presence of irregular vessels and loss of identifiable mucosal structures predicted neoplasia with an accuracy of 86%, sensitivity of 83% and specificity of 88%, compared to standard histopathology with respective numbers of 79%, 50%, and 100%20. Given the limited number of patients in this study and the fact that the procedures were performed at a single institution, these criteria need to be examined prospectively and on a larger scale. Because that study was performed using the CholangioFlex miniprobe, it remains to be seen if accuracy could be improved through the use of the higher definition GastroflexUHD probe. Furthermore, the diagnostic criteria for pCLE in the biliary tree is still evolving and operating characteristics still need to be defined after these diagnostic criteria are settled upon.
The smallest commercially available confocal probe is the CholangioFlex miniprobe, with an outer diameter of 0.9mm. There are several advantages of a thin miniprobe. First, a smaller miniprobe can be inserted into the CBD via an ERCP catheter, standard cholangioscope or Spyglass™ system. There was not a decreased cannulation rate using the GastroflexUHD probe in this study, but success with this larger diameter probe may be operator-dependent. Second, the tip of the miniprobe should be perpendicular to the tissue surface in order to obtain the best image, and so a smaller probe may be easier to manipulate within the limited space of the biliary system. It would be particularly difficult to manipulate a larger probe into smaller intrahepatic radicles, and may also be difficult to obtain the appropriate angle to fully investigate indeterminate strictures. Although we did not experience any difficulty with obtaining good imaging in our cases involving biliary strictures, larger numbers are needed to investigate whether the larger size of the GastroflexUHD probe will hinder its ability to provide adequate imaging of strictures, which unfortunately may be the very indication where better imaging would be most useful. Third, a probe <1mm enables insertion into the instrumentation channel of a cholangioscope, which allows direct visualization of the area upon which the probe is resting9. This may offer improved maneuverability and perhaps consequently improved diagnostic yield in certain cases; however, the easier manipulation of the smaller CholangioFlex miniprobe comes at the expense of image quality and spatial resolution. Whereas the GastroflexUHD miniprobe has a lateral resolution of 1μm, the CholangioFlex miniprobe has a resolution of only 3.5μm. Comparing images using both probes reveals that this decreased image resolution limits the ability to differentiate individual cells and to evaluate their morphology. Although our study was not designed to directly compare the two types of probes, the findings suggest that improved magnification and resolution may improve diagnostic capability, although this needs to be investigated in a prospective fashion. If the GastroflexUHD miniprobe does indeed provide this improvement, new diagnostic criteria will need to be established in the future.
Conclusions
In this pilot study, we demonstrate that high-definition probe-based confocal laser endomicroscopy of the CBD via the GastroFlexUHD miniprobe is feasible. This probe seems to offer significantly improved spatial resolution relative to the standard CholangioFlex miniprobe, allowing for easier identification of individual cellular structures, although this study was not designed to compare these two devices directly. Improved image quality may have the potential to increase the diagnostic accuracy of confocal imaging within the biliary tree, although prospective studies are necessary to prove this.
Figure 5.
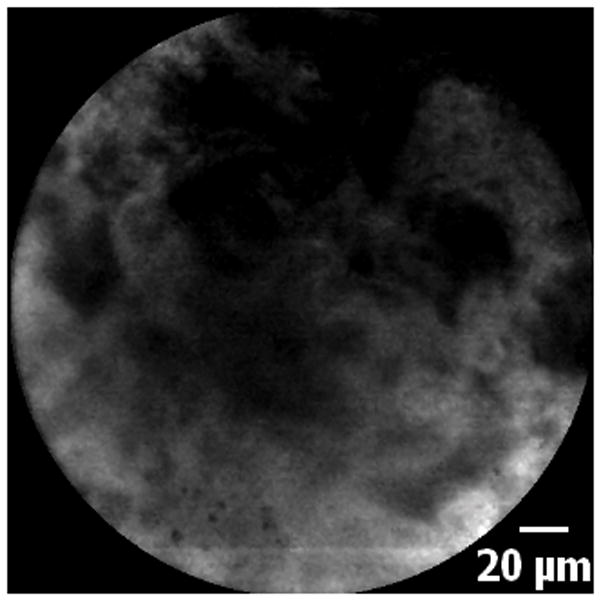
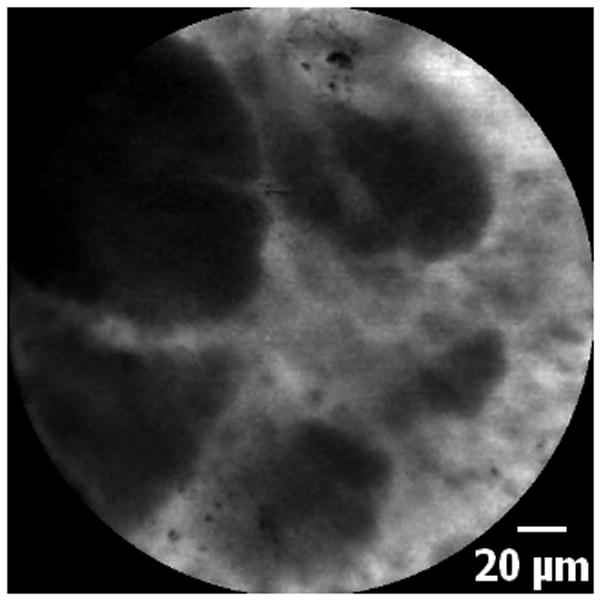
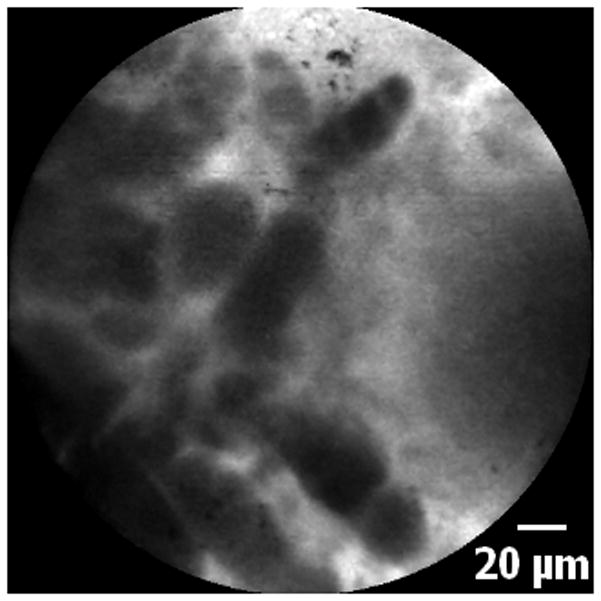
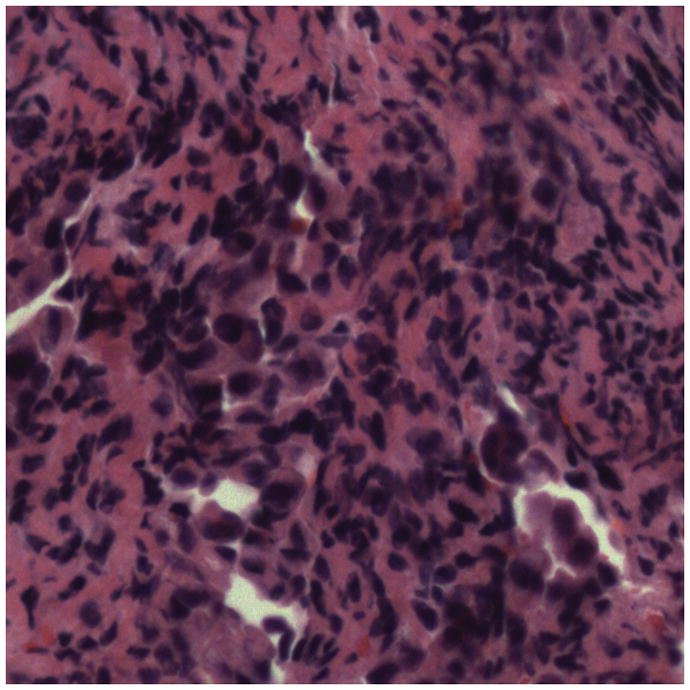
5A–5C. Images of adenocarcinoma seen within the CBD stricture as obtained via the GastroflexUHD probe. 5D. Biopsy from the CBD stricture demonstrating adenocarcinoma.
Acknowledgments
Grant support: The project was partially funded by NIH grants DK45710, DK34989, DK57751, and DK61747.
Abbreviations
- CBD
common bile duct
- pCLE
probe-based confocal laser endomicroscopy
- UHDp
GastroflexUHD miniprobe
Footnotes
Conflicts of Interest
Frederick K. Shieh – None
Hillary Drumm – None
Michael H. Nathanson – None
Priya A. Jamidar – Consultant for Cellvizio, Olympus America and Boston Scientific
Reference List
- 1.Saifuku Y, Yamagata M, Koike T, Hitomi G, Kanke K, Watanabe H, Murohisa T, Tamano M, Iijima M, Kubota K, Hiraishi H. Endoscopic ultrasonography can diagnose distal biliary strictures without a mass on computed tomography. World J Gastroenterol. 2010;16:237–244. doi: 10.3748/wjg.v16.i2.237. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Lee JH, Salem R, Aslanian H, Chacho M, Topazian M. Endoscopic ultrasound and fine-needle aspiration of unexplained bile duct strictures. Am J Gastroenterol. 2004;99:1069–1073. doi: 10.1111/j.1572-0241.2004.30223.x. [DOI] [PubMed] [Google Scholar]
- 3.Yeh BM, Liu PS, Soto JA, Corvera CA, Hussain HK. MR imaging and CT of the biliary tract. Radiographics. 2009;29:1669–1688. doi: 10.1148/rg.296095514. [DOI] [PubMed] [Google Scholar]
- 4.Kim JY, Lee JM, Han JK, Kim SH, Lee JY, Choi JY, Kim SJ, Kim HJ, Kim KH, Choi BI. Contrast-enhanced MRI combined with MR cholangiopancreatography for the evaluation of patients with biliary strictures: differentiation of malignant from benign bile duct strictures. J Magn Reson Imaging. 2007;26:304–312. doi: 10.1002/jmri.20973. [DOI] [PubMed] [Google Scholar]
- 5.Weber A, von WC, Fend F, Schneider J, Neu B, Meining A, Weidenbach H, Schmid RM, Prinz C. Endoscopic transpapillary brush cytology and forceps biopsy in patients with hilar cholangiocarcinoma. World J Gastroenterol. 2008;14:1097–1101. doi: 10.3748/wjg.14.1097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen YK, Pleskow DK. SpyGlass single-operator peroral cholangiopancreatoscopy system for the diagnosis and therapy of bile-duct disorders: a clinical feasibility study (with video) Gastrointest Endosc. 2007;65:832–841. doi: 10.1016/j.gie.2007.01.025. [DOI] [PubMed] [Google Scholar]
- 7.Shah RJ, Langer DA, Antillon MR, Chen YK. Cholangioscopy and cholangioscopic forceps biopsy in patients with indeterminate pancreaticobiliary pathology. Clin Gastroenterol Hepatol. 2006;4:219–225. doi: 10.1016/s1542-3565(05)00979-1. [DOI] [PubMed] [Google Scholar]
- 8.Polglase AL, McLaren WJ, Delaney PM. Pentax confocal endomicroscope: a novel imaging device for in vivo histology of the upper and lower gastrointestinal tract. Expert Rev Med Devices. 2006;3:549–556. doi: 10.1586/17434440.3.5.549. [DOI] [PubMed] [Google Scholar]
- 9.Meining A. Confocal endomicroscopy. Gastrointest Endosc Clin N Am. 2009;19:629–635. doi: 10.1016/j.giec.2009.07.005. [DOI] [PubMed] [Google Scholar]
- 10.Loeser C, Robert ME, Mennone A, Nathanson MH, Jamidar P. Histological basis for confocal endomicroscopic diagnosis of biliary malignancies. Journal of Clinical Gastroenterology. 2010 doi: 10.1097/MCG.0b013e3181fbdc38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace MB, Meining A, Canto MI, Fockens P, Miehlke S, Roesch T, Lightdale CJ, Pohl H, Carr-Locke D, Lohr M, Coron E, Filoche B, Giovannini M, Moreau J, Schmidt C, Kiesslich R. The safety of intravenous fluorescein for confocal laser endomicroscopy in the gastrointestinal tract. Aliment Pharmacol Ther. 2010;31:548–552. doi: 10.1111/j.1365-2036.2009.04207.x. [DOI] [PubMed] [Google Scholar]
- 12.Kiesslich R, Goetz M, Burg J, Stolte M, Siegel E, Maeurer MJ, Thomas S, Strand D, Galle PR, Neurath MF. Diagnosing Helicobacter pylori in vivo by confocal laser endoscopy. Gastroenterology. 2005;128:2119–2123. doi: 10.1053/j.gastro.2004.12.035. [DOI] [PubMed] [Google Scholar]
- 13.Kiesslich R, Goetz M, Hoffman A, Lanimersdorf K, Schneider C, Vieth M, Stoltle M, Galle PR, Neurath MF. Screening colonciscopy with confocal laser endomicroscopy (CLE) for in vivo diagnosis of colorectal neoplasias. Gastroenterology. 2006;130:A102–A102. [Google Scholar]
- 14.Kiesslich R, Gossner L, Goetz M, Dahlmann A, Vieth M, Stolte M, Hoffman A, Jung M, Nafe B, Galle PR, Neurath MF. In vivo histology of Barrett’s esophagus and associated neoplasia by confocal laser endomicroscopy. Clinical Gastroenterology and Hepatology. 2006;4:979–987. doi: 10.1016/j.cgh.2006.05.010. [DOI] [PubMed] [Google Scholar]
- 15.Kiesslich R, Hoffman A, Goetz M, Biesterfeld S, Vieth M, Galle PR, Neurath MF. In vivo diagnosis of collagenous colitis by confocal endomicroscopy. Gut. 2006;55:591–592. doi: 10.1136/gut.2005.084970. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Bennett JJ, Green RH. Malignant masquerade: dilemmas in diagnosing biliary obstruction. Surg Oncol Clin N Am. 2009;18:207–14. vii. doi: 10.1016/j.soc.2008.12.005. [DOI] [PubMed] [Google Scholar]
- 17.Harewood GC. Endoscopic tissue diagnosis of cholangiocarcinoma. Curr Opin Gastroenterol. 2008;24:627–630. doi: 10.1097/MOG.0b013e32830bf7e1. [DOI] [PubMed] [Google Scholar]
- 18.Iqbal S, Stevens PD. Cholangiopancreatoscopy for targeted biopsies of the bile and pancreatic ducts. Gastrointest Endosc Clin N Am. 2009;19:567–577. doi: 10.1016/j.giec.2009.06.005. [DOI] [PubMed] [Google Scholar]
- 19.Moreno Luna LE, Kipp B, Halling KC, Sebo TJ, Kremers WK, Roberts LR, Barr Fritcher EG, Levy MJ, Gores GJ. Advanced cytologic techniques for the detection of malignant pancreatobiliary strictures. Gastroenterology. 2006;131:1064–1072. doi: 10.1053/j.gastro.2006.08.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meining A, Frimberger E, Becker V, Von DS, Von Weyhern CH, Schmid RM, Prinz C. Detection of cholangiocarcinoma in vivo using miniprobe-based confocal fluorescence microscopy. Clin Gastroenterol Hepatol. 2008;6:1057–1060. doi: 10.1016/j.cgh.2008.04.014. [DOI] [PubMed] [Google Scholar]