Abstract
Multicellular organisms are similar to biological communities, consisting of various cell types; thus, inter-cell communication is critical for the functioning of the whole system that ultimately constitutes a living being. Conventional models of cellular exchange include signaling molecules and direct contact-mediated cell communications. Exosomes, small vesicles originating from an inward budding of the plasma membrane, represent a new avenue for signaling between cells. This interchange is achieved by packaging RNA species into exosomes endowed with specific cell surface-targeting motifs. The delivered RNA molecules are functional and mRNA can be translated into new proteins, while miRNAs target host mRNAs in the recipient cell. RNA involved in transmitting information or molecules between cells is called exosomal RNA (esRNA). This review summarizes the characteristics of exosomes, specifically focusing on their role in the horizontal transfer of cellular information.
Keywords: Cell-cell communication, exosomes, esRNA, miRNA
Exosomes, originally discovered in reticulocytes1, are multivesicular vacuoles and carriers of cellular cargo. Exosomes are the extracellular counterparts of endosomes, which are found in the cytoplasm. Endosomes were discovered in electron microscopic studies2 and range from 30–80 nM in diameter. They are formed from an inward budding of the plasma membrane, often containing vesicles inside the lumen. To clarify the terminology, ‘exosomes’ can also refer to multisubunit protein complexes involved in RNA degradation3, however, the exosome complexes discussed in this article refer to microvesicular exosomes4. Exosomes differ from ‘microparticles’ released by cells, which arise from a budding of the outer layers of the plasma membrane. Microparticles are much larger than exosomes and range in size from 100 nm to 1 µm in diameter. Exosomes, on the other hand, are formed from endosomes, which in turn arise from an inward budding of the plasma membrane into the cytoplasm. There are often target cell-specific receptor molecules to guide exosomes to their cell of influence. Fusion of the exosomes with the plasma membrane of the recipient cell allows for transfer of the internal components to the target cell and thus, the transfer of information. The molecules being transferred can include proteins such as receptors or enzymes and nucleic acid molecules such as RNA. The RNA included in exosomes is known as exosomal RNA (esRNA). The ability to influence gene expression in distant cells through exosomes presents a remarkable model for cell-to-cell signaling. The horizontal transfer of exosomal RNA offers a new perspective on intercellular communication and has potential therapeutic applications, such as in diagnosis and gene delivery.
Biogenesis of exosomes
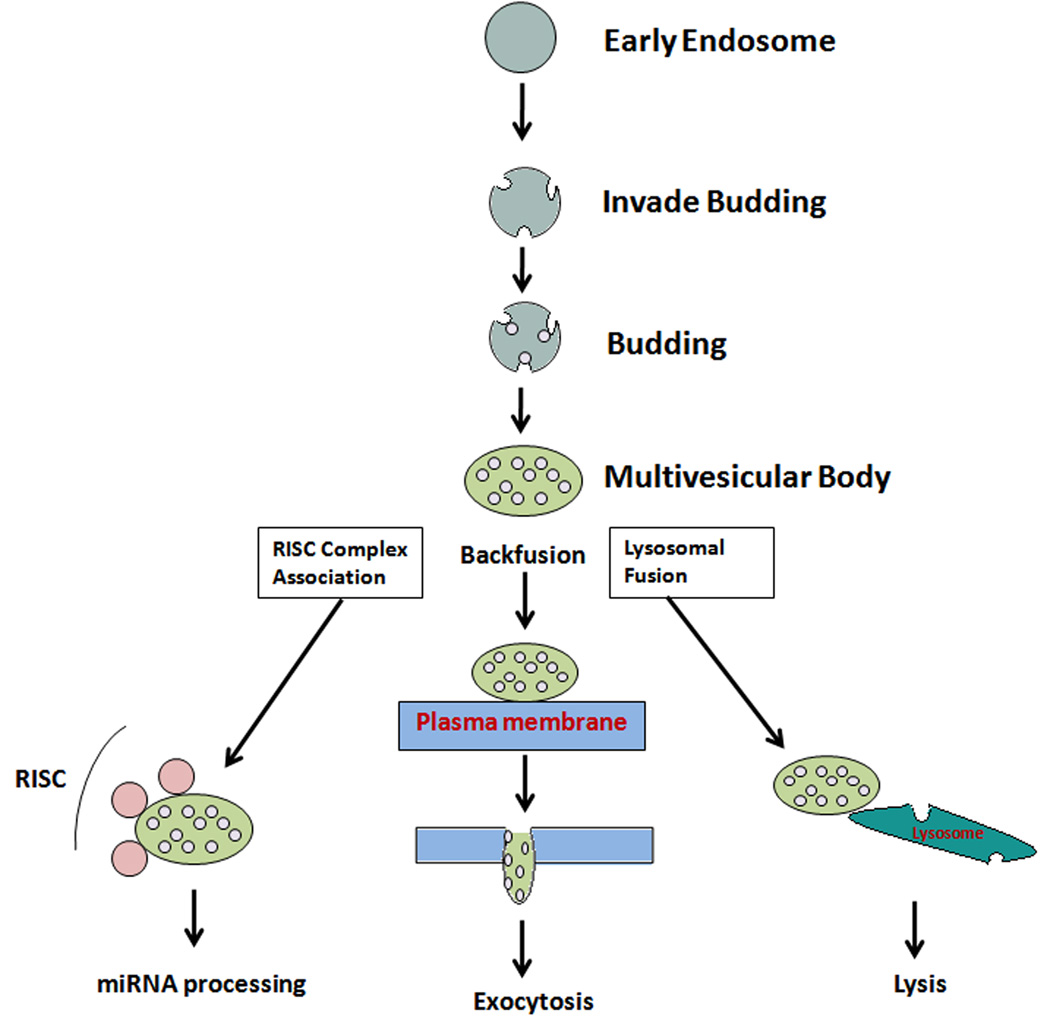
Exosomes are secretory products of endosomal origin and are classified into early, late, and recycling endosomes. Endosomes further develop vesicles inside their lumen, and there are several intermediates in the maturation process from late to early endosomes. These intermediates can be much larger than the average endosomes, ranging from 400–500 nm in diameter5. These intermediates are called multivesicular bodies (MVBs) or endosomal carrier vesicles. A MVB is often large and ‘irregularly’ shaped, depending on the number and morphology of the vesicles it contains. The MVB can bud off to release further ‘endosome-like particles’, which can fuse with the plasma membrane, get recycled to form fresh endosomes, or fuse with the plasma membrane to release vesicles into the extra-cellular milieu. Eventually, an endosome can also fuse with lysosomes. Endosomes are often linked to the Golgi apparatus. The role of endosomes in protein sorting and directing to lysosomes-mediated degradation was first documented in studies of the EGF receptor kinase from rat liver6. It appears that endosomes can consist of different regions or domains marked by various specific lipid or protein profiles. The formation of MVBs and their separation from the ‘parent’ endosome appears to be regulated by hepatocyte growth factor-regulated tyrosine-kinase substrate (HRS) and annexin II, respectively5, as studied in HeLa cells. Annexin II is also known to regulate the recycling of endosomes7, 8. Of all the possible outcomes of the endocytic pathway, back fusion with the plasma membrane leads to the release of multivesicular compartments, otherwise known as exosomes (Figure 1). The fate of the contents of an exosome depends on its target destination. It is expected that the sorting of various proteins to different cellular locations through exosomes is a regulated event. Once released out of the cell, exosomes, carrying various biomolecules (predominantly proteins and nucleic acids), are potential carriers of cargo and information between cells. It is understood that complex ‘cargo-sorting’ machinery for the packaging of protein, RNA, and other molecules contained in endosomes tightly regulates the exosome–mediated transfer of informational molecules. The targeting of proteins is facilitated by molecular ‘stamps’ in the form of signaling peptides, ubiquitination, glycosylation, phosphorylation, and other targeting markers on the protein surface9. Thus, exosomes function as transportation, sorting, and delivery agents of the cell.
Figure 1.
Schematic diagram illustrates the model for sorting of cargo into MVB and fate of the exosomes. Sorting of exosomes followed by the biosynthetic pathway leads to miRNA processing, exocytosis, or back fusion with the plasma membrane/within an endosomal compartment or lysosomal degradation.
The formation of exosomes is facilitated by endosomal sorting complexes required for transport (ESCRT) proteins, which are multi-subunit complexes consisting of the vacuolar protein sorting family of proteins. Specifically, the packaging of vesicles within endosomes is mediated by the ESCRT protein pathway. The ESCRT machinery consists of ESCRT 0, ESCRT I, ESCRT II, and ESCRT III10 protein complexes. An alternative pathway exists for the creation of exosomes, which includes the ceramide and sphingolipid pathway11, 12, where the enzyme sphingomyelinase 2 is involved in mediating exosomal release.
Components of Exosomes
Exosomes contain lipid, protein, and RNA components. As byproducts of the endosomal biogenesis, exosomes are surrounded by a lipid bilayer. Exosomal densities range between 1.13 and 1.19 g/ml as measured by sucrose-density gradient centrifugation. Because exosomes originate from the fusion of multivesicular late endosomes with the plasma membrane, cholesterol is a common component of exosomal lipids. However, exosomes contain higher levels of cholesterol and glycosphingolipids than do cell membranes13. The endosomic origin of exosomes accounts for the fact that they do not exhibit proteins originating in the nucleus, mitochondria, Golgi apparatus, or endoplasmic-reticulum. Instead they often contain cytoplasmic proteins, endosomal membrane proteins and proteins of the plasma membrane 14. The membranes of exosomes are organized differently than the cellular plasma membrane. Exosomal membranes exhibit a tight packing of lipids at neutral pH, a rapid flip-flop of lipids between the two leaflets losing lipid asymmetry, and have a random distribution of phosphotidylethanolamines15.
Exosomes display ‘inverse topology’, wherein a protein’s extracellular domain is displayed on the outer membrane of the exosomes16. Protein components of mammalian exosomes include heat shock proteins (Hsp70, Hsp90), cytoskeletal components like actin and tubulin, tetraspanins (CD9, CD81, and CD63), and other proteins, such as tumor susceptibility gene 1014. Proteins involved in cell-specific functions may be carried in exosomes. For example, major histocompatibility complex (MHC) molecules are known to be components of exosomes from antigen-presenting cells. Dendritic cell-derived exosomes are known as ‘dexosomes’17. Exosomes that facilitate the spread of morphogens during embryonic development are in a special class called ‘argosomes’18. Exosomes are known to be released by T-cells19, B cells20, mast cells21, dendritic cells22, and other cells of the immune system. They are also released by epithelial cells23, reticulocytes2, neuronal cells24, and cancerous cells25. Exosomes are secreted into body fluids such as saliva26, blood plasma27, breast milk28 and urine29. Urinary exosomes show the presence of carbonic anhydrase and acquaporin-229. The differences between exosomes from various cells suggest a specialized adaptation of exosomes to perform specific functions under varying contexts. In fact, exosomes have probably evolved to help the cell rid itself of useless proteins and other components that cannot be degraded in the lysozome14. However, recent studies show that exosomes might have other functions, such as the activation and facilitation of the immune response 30, 31. Other functions of exosomes include the release of cellular waste. For instance, in hematopoetic cells, such as reticulocytes, exosomes allow for release of discarded transferrin receptors32 and chemotherapeutic drugs33. The exciting and recently discovered role for exosomes in the horizontal transfer of information between cells is discussed below.
Exosomes as Mediators of Intercellular Communication
Cell communication is classified broadly as contact-dependent, paracrine, synaptic, or endocrine34. Traditional cell-cell communication occurs by several means, including chemical receptor-medicated events, direct cell-cell contact, and cell-cell synapses. Contact-dependent cellular communication involves a physical connection between the participating cells. Examples include gap junctions and synaptic connections. Contact-independent communication can occur between cells not directly linked and may be mediated through paracrine, endocrine, or similar mechanisms involving the extracellular transfer of information molecules. In this regard, exosomes have recently been shown to participate in a contact-independent mode of cell communication35, 36.
Recently, exosomes were shown to have the ability to establish communication between neighboring cells through RNA signal delivery via esRNA 37. This new mechanism of cell-cell communication by esRNA increases the complexity of cellular communication. Prion proteins are transported through exosomes38, 39. Exosomes are involved in the activation and functioning the immune system. Immature dendritic cells are known to transfer MHC peptide molecules to other dendritic cells for immune response activation40. miRNAs involved in intercellular communication are associated with exosomes41, 42. The profile of mRNAs observed in exosomes does not match the composition of the donor cells, suggesting that there is a selective loading of specific mRNA and miRNA molecules into exosomes21, 43. A study of the horizontal transfer of miRNA from activated T-cells to antigen-presenting cells (APCs) suggested the existence of immune synapse-mediated exosomal transport43. The results of this study provided evidence for the directed and unidirectional transfer of miRNA-carrying exosomes through the immune synapse between the donor T cells and the recipient APCs43. Likewise, the mRNAs contained within exosomes can be transcribed into cDNA or translated in the recipient cell37, 44. These features provide exosomes with an important potential to influence gene expression in target cells. Fluorescently labeled glioblastoma microvesicles have been shown to transfer Gaussia luciferase (Gluc) mRNA to human brain microvascular endothelial cells (HBMVECs), which then expressed the protein product44. Tannous et al. used a lentiviral vector encoding a secreted luciferase from Gaussia (Gluc)45 to study the trafficking of RNA in exosomes. Purified microvesicles containing Gluc mRNA were added to HBMVECs and Gluc activity released into the medium by these endothelial cells was monitored over time. Gluc activity produced by recipient cells showed a continuing increase over 24 h, suggesting the ongoing translation of the Gluc mRNA. This study showed that mRNA incorporated into tumor microvesicles can be delivered to recipient normal cells and generate functional protein, thus enabling horizontal transfer of genetic information. Incubation of human mast cells with exosomes derived from mouse cells resulted in the expression of the donor mouse proteins in the recipient human cells37. This established the capacity of exosomes to transfer biological information between cells across species. Exosomes can assist in the transfer of viral mediators. For example, Epstein Barr virus (EBV) miRNA is transferred from infected to uninfected cells through exosomes46. Exosomes released from cells infected with EBV contain viral oncoproteins, miRNA, as well as signal transduction factors47. The major oncogenic transforming factor of EBV, latent membrane protein (LMP-1) is enriched in cells secreted from infected nasopharyngeal carcinoma (NPC) cells. In addition, these exosomes contain protein members of signaling pathways (phosphoinositide 3-kinase and epidermal growth factor receptor) activated by LMP-147. Viral miRNA BART-1 transcripts (BamH1 A rightward transcripts) are incorporated in the exosomes from infected cells. Labeled exosomes transfer the miRNA from lymphoblast cells to uninfected monocyte-derived dendritic cells and repress the expression of target genes in the dendritic cells46. A similar study done with exosomes derived from NPC cells revealed the transfer of LMP-1-mediated activation of signaling to recipient human umbilical vein endothelial cells47. Even the conditioned media containing exosomes from donor cells, cleared of cell debris, was sufficient to transport LMP-1 and activate LMP-regulated signaling molecules, Erk and Akt47. These results highlight the functional influence of exosomes in the horizontal transfer of genetic information.
Purified exosome-like vesicles from cultured monocytes contain many miRNAs and components of RNA-induced silencing complex (RISC), such as AGO2 and its interacting partner GW18248. Importantly, mature miRNAs and their target mRNAs also co-localize to MVBs, but not to exosomes, which raises the possibility that MVBs are sites of miRNA-loaded RISC assembly49, 50. These observations suggest that miRNA induced silencing is intricately regulated with certain activities distributed over MVBs and others designated for exosomes.
An advantage of exosomes as mediators of extracellular communication is that the message can be targeted to multiple locations. miRNA transfer through exosomes allows for rapid alterations in gene expression in the targeted cells. The messages transmitted by intercellular communication may include those for survival, growth, division, differentiation, stress responses, apoptosis, etc. Exosomes have been studied as facilitators of the immune response31 and the role of exosomes in antigen presentation has been well-documented14. Roles for exosomes in programmed cell death, inflammation, angiogenesis51, and coagulation have also been established. Exosomes have been implicated in the creation of morphogen polarity during development and differentiation18. The possible evolution of exosomes into the role of mediators of ‘horizontal transfer’ seems quite plausible (Figure 2).
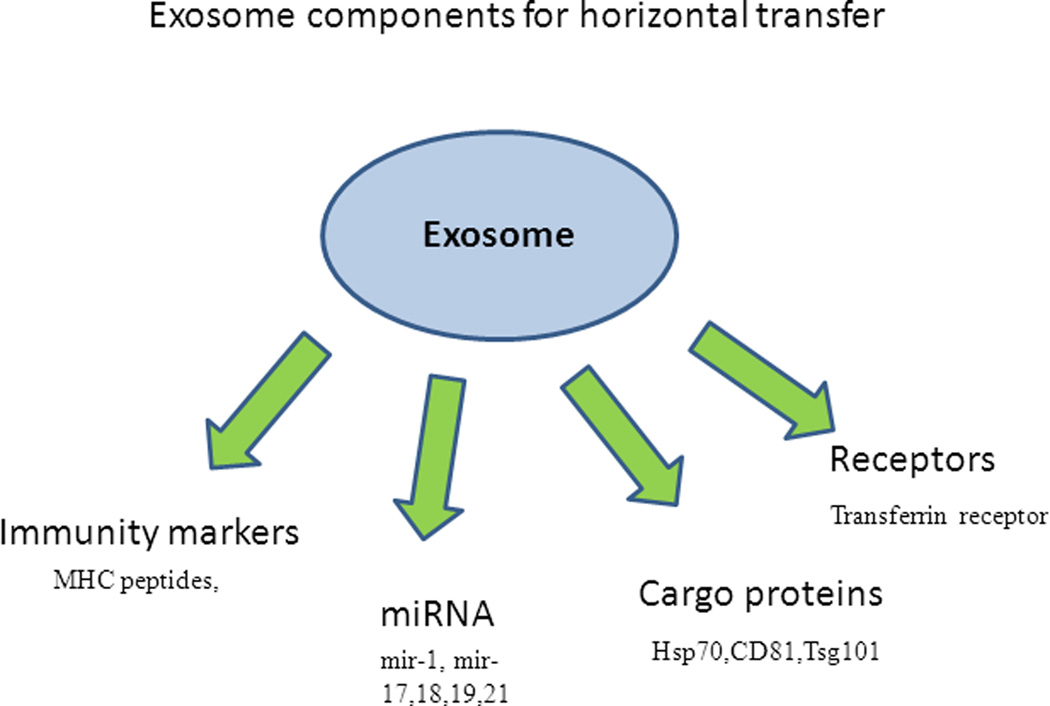
Figure 2.
A model for horizontal transfer of biological information through exosomes. Components transported via exosomes include proteins, including receptors, antigenic peptides and other cargo proteins. Nucleic acids transferred through exosomes include RNA, specifically microRNAs that can further regulate the gene expression in the recipient cells.
Active tumor cells may be the origin of extracellular RNAs found in plasma of cancer patients, which circulate in association with multiparticle complexes52. The particle-associated nature of these molecules was postulated by Ng et al.53 demonstrated that the RNA can be removed by passing the plasma through filters of various pore-sizes. This particle-associated RNA is enriched in mRNA. As an example, here is more LISCH7 mRNA outside the cell than inside it, relative to a housekeeping gene. This kind of an unbalanced distribution of the two gene-products can only be explained by a differential release mechanism52. Apart from studies describing miRNA transfer through exosomes, other reports have described the extracellular population of miRNA independent of exosome association54. miRNA bound to Ago-2 and other RNA-binding proteins, including components of the RISC machinery have been described 54, 55. The exact quantitative contribution of these mechanisms versus the functional role of exosomes in mediating intercellular communication remains to be delineated. Human saliva, breast milk, and blood plasma contain exosomes carrying functional RNAs28. In addition, fluorescent exosomes, labeled with PKH67 dye, can get taken up by macrophages28.
Clinical and Therapeutic Potential
The intercellular travels of exosomes as molecular messengers allow for a plethora of possible diagnostic and therapeutic applications. The function of exosomes as molecular cargo-carriers may allow for their use as diagnostic markers. This is because the surface markers on exosomes and their internal components are likely to reflect the physiological status of the cells and organs they originate from. Exosomes released in human kidney29 provide a great opportunity to use urinary exosomes as diagnostic tools. HIV 56–59 and hepatitis C virus 60 transmission have been shown to be facilitated through exosomes. The HIV accessory protein negative factor protein involved in the progression of AIDS has been shown to propagate via exosomes. This protein induces its own release through exocytosis and activates apoptosis in CD4+T cells, which is an important element of AIDS 61. The blood of cancer patients contains more microvesicles compared to those of normal individuals 62. In addition, exosomes allow invasive growth of tumor cells 63. The protein composition of exosomes is also known to change with infection of viruses or cancerous transformation.
Exosomes have been implicated in the expulsion of anti-cancer drugs from cancer cells, allowing resistance to chemotherapeutic drugs64. This gives exosomes a potentially important role in the prognosis of tumor development. The potential use of exosomes in gene therapy is promising. This is especially true in light of the immunological complementarity of exosomes obtained and modified from a host patient that could receive the modified exosomes containing an appropriate chemotherapeutic drug or nucleic acid molecule. It may also be possible to use exosomes for vaccination purposes 65, 66. The application of exosomes in developing cancer vaccines is a promising area of investigation 67.
The discovery of microRNA in human salivary samples suggests a promising use of salivary exosomes as biomarkers for disease diagnosis68. Skog et al. found that glioblastoma tumor cells release exosomes containing mRNA, miRNA, and angiogenic proteins 44. The glioma-related transcript epidermal growth factor receptor-VIII was detected in the serum microvesicles of glioblastoma patients, but not in samples from normal patients44. As a technology, exosome display allows for new therapeutic possibilities69. Recently, exosomes were used to target siRNA to brain cells including microglia, oligodendrocytes, and neurons70. The exosomes engineered to carry a neuron-specific peptide-fused with exosomal protein (Lamp2b) were electroporated with gene-specific siRNA. Intravenous injection of these exosomes led to a significant down-regulation of the gene targeted by the exosomal siRNA (BACE1)70. These results point to the potential application of exosomes in gene delivery, opening up avenues for therapeutic applications.
Conclusions and Perspectives
Exosomes represent a unique method of cell-cell communication. Although well established in certain cell types, including cells of the immune system, the role of exosomes in cellular physiology and signaling between cells is not yet clearly understood. The signals that regulate the packaging of specific miRNA and other informational molecules into exosomes, their targeting to various extracellular destinations, and other details of exosomes-mediated cellular signaling are topics inviting further study. The possible targeting of exosomes to specific extracellular locations and the mechanisms involved in incorporating exosomes into targeted cells remains mysterious. However, the involvement of exosomes in cell signaling adds yet another layer in the complexity of eukaryotic communication networks.
The mechanisms by which exosomes are targeted to destination cells, how their contents are assigned and what triggers exosome release in different cell types are some of the questions that invite exploration. Answers to these questions could allow for the design of strategies for delivery of drugs and gene therapy molecules using exosomes. As therapeutic delivery agents, exosomes offer an exciting tool that would potentially be better tolerated by the immune system, since they are natural transporters derived from cells. There is also the potential for use of exosomes in diagnosis. For instance, detection of viral miRNA in the plasma of patients could allow for detection of infections. Organ dysfunctions could be observed by changes in miRNA, protein, or mRNA profiles of organ-specific exosomes in the blood plasma or urine. These are avenues of future exploration.
Exosome biology provides many promising opportunities. However, a major challenge to future exosome research will be in extrapolating basic knowledge obtained from in vitro models to the more directly applicable areas of translational research. This will be especially interesting and useful in the context of human diseases, such as cancer.
Acknowledgements
This work was funded in part by National Institutes of Health grants P20RR017696 and R00DE018165 to VP. We apologize to authors whose work we were unable to reference due to space limitations.
References
- 1.Trams EG, Lauter CJ, Salem N, Jr, Heine U. Exfoliation of membrane ecto-enzymes in the form of micro-vesicles. Biochimica et biophysica acta. 1981;645:63–70. doi: 10.1016/0005-2736(81)90512-5. [DOI] [PubMed] [Google Scholar]
- 2.Pan BT, Johnstone RM. Fate of the transferrin receptor during maturation of sheep reticulocytes in vitro: selective externalization of the receptor. Cell. 1983;33:967–978. doi: 10.1016/0092-8674(83)90040-5. [DOI] [PubMed] [Google Scholar]
- 3.Kiss DL, Andrulis ED. The exozyme model: a continuum of functionally distinct complexes. RNA. 2011;17:1–13. doi: 10.1261/rna.2364811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Johnstone RM. Exosomes biological significance: A concise review. Blood Cells Mol Dis. 2006;36:315–321. doi: 10.1016/j.bcmd.2005.12.001. [DOI] [PubMed] [Google Scholar]
- 5.Gruenberg J, Stenmark H. The biogenesis of multivesicular endosomes. Nat Rev Mol Cell Biol. 2004;5:317–323. doi: 10.1038/nrm1360. [DOI] [PubMed] [Google Scholar]
- 6.Authier F, Chauvet G. In vitro endosome-lysosome transfer of dephosphorylated EGF receptor and Shc in rat liver. FEBS letters. 1999;461:25–31. doi: 10.1016/s0014-5793(99)01413-1. [DOI] [PubMed] [Google Scholar]
- 7.Mayran N, Parton RG, Gruenberg J. Annexin II regulates multivesicular endosome biogenesis in the degradation pathway of animal cells. The EMBO journal. 2003;22:3242–3253. doi: 10.1093/emboj/cdg321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Morel E, Gruenberg J. Annexin A2 binding to endosomes and functions in endosomal transport are regulated by tyrosine 23 phosphorylation. The Journal of biological chemistry. 2009;284:1604–1611. doi: 10.1074/jbc.M806499200. [DOI] [PubMed] [Google Scholar]
- 9.Piper RC, Katzmann DJ. Biogenesis and function of multivesicular bodies. Annual review of cell and developmental biology. 2007;23:519–547. doi: 10.1146/annurev.cellbio.23.090506.123319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Babst M. A protein's final ESCRT. Traffic. 2005;6:2–9. doi: 10.1111/j.1600-0854.2004.00246.x. [DOI] [PubMed] [Google Scholar]
- 11.Trajkovic K, Hsu C, Chiantia S, Rajendran L, Wenzel D, Wieland F, et al. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science. 2008;319:1244–1247. doi: 10.1126/science.1153124. [DOI] [PubMed] [Google Scholar]
- 12.Marsh M, van Meer G. Cell biology. No ESCRTs for exosomes. Science. 2008;319:1191–1192. doi: 10.1126/science.1155750. [DOI] [PubMed] [Google Scholar]
- 13.Wubbolts R, Leckie RS, Veenhuizen PT, Schwarzmann G, Mobius W, Hoernschemeyer J, et al. Proteomic and biochemical analyses of human B cell-derived exosomes. Potential implications for their function and multivesicular body formation. The Journal of biological chemistry. 2003;278:10963–10972. doi: 10.1074/jbc.M207550200. [DOI] [PubMed] [Google Scholar]
- 14.Thery C, Zitvogel L, Amigorena S. Exosomes: composition, biogenesis and function. Nature reviews Immunology. 2002;2:569–579. doi: 10.1038/nri855. [DOI] [PubMed] [Google Scholar]
- 15.Laulagnier K, Motta C, Hamdi S, Roy S, Fauvelle F, Pageaux JF, et al. Mast cell- and dendritic cell-derived exosomes display a specific lipid composition and an unusual membrane organization. The Biochemical journal. 2004;380:161–171. doi: 10.1042/BJ20031594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Anand PK. Exosomal membrane molecules are potent immune response modulators. Communicative & integrative biology. 2010;3:405–408. doi: 10.4161/cib.3.5.12474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morse MA, Garst J, Osada T, Khan S, Hobeika A, Clay TM, et al. A phase I study of dexosome immunotherapy in patients with advanced non-small cell lung cancer. Journal of translational medicine. 2005;3:9. doi: 10.1186/1479-5876-3-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lakkaraju A, Rodriguez-Boulan E. Itinerant exosomes: emerging roles in cell and tissue polarity. Trends in cell biology. 2008;18:199–209. doi: 10.1016/j.tcb.2008.03.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Blanchard N, Lankar D, Faure F, Regnault A, Dumont C, Raposo G, et al. TCR activation of human T cells induces the production of exosomes bearing the TCR/CD3/zeta complex. Journal of immunology. 2002;168:3235–3241. doi: 10.4049/jimmunol.168.7.3235. [DOI] [PubMed] [Google Scholar]
- 20.Raposo G, Nijman HW, Stoorvogel W, Liejendekker R, Harding CV, Melief CJ, et al. B lymphocytes secrete antigen-presenting vesicles. J Exp Med. 1996;183:1161–1172. doi: 10.1084/jem.183.3.1161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Raposo G, Tenza D, Mecheri S, Peronet R, Bonnerot C, Desaymard C. Accumulation of major histocompatibility complex class II molecules in mast cell secretory granules and their release upon degranulation. Molecular biology of the cell. 1997;8:2631–2645. doi: 10.1091/mbc.8.12.2631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Thery C, Regnault A, Garin J, Wolfers J, Zitvogel L, Ricciardi-Castagnoli P, et al. Molecular characterization of dendritic cell-derived exosomes. Selective accumulation of the heat shock protein hsc73. The Journal of cell biology. 1999;147:599–610. doi: 10.1083/jcb.147.3.599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.van Niel G, Raposo G, Candalh C, Boussac M, Hershberg R, Cerf-Bensussan N, et al. Intestinal epithelial cells secrete exosome-like vesicles. Gastroenterology. 2001;121:337–349. doi: 10.1053/gast.2001.26263. [DOI] [PubMed] [Google Scholar]
- 24.Faure J, Lachenal G, Court M, Hirrlinger J, Chatellard-Causse C, Blot B, et al. Exosomes are released by cultured cortical neurones. Mol Cell Neurosci. 2006;31:642–648. doi: 10.1016/j.mcn.2005.12.003. [DOI] [PubMed] [Google Scholar]
- 25.Mears R, Craven RA, Hanrahan S, Totty N, Upton C, Young SL, et al. Proteomic analysis of melanoma-derived exosomes by two-dimensional polyacrylamide gel electrophoresis and mass spectrometry. Proteomics. 2004;4:4019–4031. doi: 10.1002/pmic.200400876. [DOI] [PubMed] [Google Scholar]
- 26.Palanisamy V, Sharma S, Deshpande A, Zhou H, Gimzewski J, Wong DT. Nanostructural and transcriptomic analyses of human saliva derived exosomes. PLoS One. 2010;5:e8577. doi: 10.1371/journal.pone.0008577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Caby MP, Lankar D, Vincendeau-Scherrer C, Raposo G, Bonnerot C. Exosomal-like vesicles are present in human blood plasma. International immunology. 2005;17:879–887. doi: 10.1093/intimm/dxh267. [DOI] [PubMed] [Google Scholar]
- 28.Lasser C, Alikhani VS, Ekstrom K, Eldh M, Paredes PT, Bossios A, et al. Human saliva, plasma and breast milk exosomes contain RNA: uptake by macrophages. Journal of translational medicine. 2011;9:9. doi: 10.1186/1479-5876-9-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pisitkun T, Shen RF, Knepper MA. Identification and proteomic profiling of exosomes in human urine. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:13368–13373. doi: 10.1073/pnas.0403453101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Clayton A, Mason MD. Exosomes in tumour immunity. Current oncology. 2009;16:46–49. doi: 10.3747/co.v16i3.367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Thery C, Ostrowski M, Segura E. Membrane vesicles as conveyors of immune responses. Nature reviews Immunology. 2009;9:581–593. doi: 10.1038/nri2567. [DOI] [PubMed] [Google Scholar]
- 32.Johnstone RM, Adam M, Hammond JR, Orr L, Turbide C. Vesicle formation during reticulocyte maturation. Association of plasma membrane activities with released vesicles (exosomes) The Journal of biological chemistry. 1987;262:9412–9420. [PubMed] [Google Scholar]
- 33.Chen VY, Posada MM, Blazer LL, Zhao T, Rosania GR. The role of the VPS4A-exosome pathway in the intrinsic egress route of a DNA-binding anticancer drug. Pharmaceutical research. 2006;23:1687–1695. doi: 10.1007/s11095-006-9043-0. [DOI] [PubMed] [Google Scholar]
- 34.Ratajczak J, Wysoczynski M, Hayek F, Janowska-Wieczorek A, Ratajczak MZ. Membrane-derived microvesicles: important and underappreciated mediators of cell-to-cell communication. Leukemia : official journal of the Leukemia Society of America, Leukemia Research Fund, UK. 2006;20:1487–1495. doi: 10.1038/sj.leu.2404296. [DOI] [PubMed] [Google Scholar]
- 35.Sadowski L, Pilecka I, Miaczynska M. Signaling from endosomes: location makes a difference. Experimental cell research. 2009;315:1601–1609. doi: 10.1016/j.yexcr.2008.09.021. [DOI] [PubMed] [Google Scholar]
- 36.Platta HW, Stenmark H. Endocytosis and signaling. Curr Opin Cell Biol. 2011 doi: 10.1016/j.ceb.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 37.Lotvall J, Valadi H. Cell to cell signalling via exosomes through esRNA. Cell Adh Migr. 2007;1:156–158. doi: 10.4161/cam.1.3.5114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Porto-Carreiro I, Fevrier B, Paquet S, Vilette D, Raposo G. Prions and exosomes: from PrPc trafficking to PrPsc propagation. Blood cells, molecules & diseases. 2005;35:143–148. doi: 10.1016/j.bcmd.2005.06.013. [DOI] [PubMed] [Google Scholar]
- 39.Fevrier B, Vilette D, Archer F, Loew D, Faigle W, Vidal M, et al. Cells release prions in association with exosomes. Proceedings of the National Academy of Sciences of the United States of America. 2004;101:9683–9688. doi: 10.1073/pnas.0308413101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Segura E, Amigorena S, Thery C. Mature dendritic cells secrete exosomes with strong ability to induce antigen-specific effector immune responses. Blood cells, molecules & diseases. 2005;35:89–93. doi: 10.1016/j.bcmd.2005.05.003. [DOI] [PubMed] [Google Scholar]
- 41.Wang K, Zhang S, Weber J, Baxter D, Galas DJ. Export of microRNAs and microRNA-protective protein by mammalian cells. Nucleic acids research. 2010;38:7248–7259. doi: 10.1093/nar/gkq601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Valadi H, Ekstrom K, Bossios A, Sjostrand M, Lee JJ, Lotvall JO. Exosome-mediated transfer of mRNAs and microRNAs is a novel mechanism of genetic exchange between cells. Nat Cell Biol. 2007;9:654–659. doi: 10.1038/ncb1596. [DOI] [PubMed] [Google Scholar]
- 43.Mittelbrunn M, Gutierrez-Vazquez C, Villarroya-Beltri C, Gonzalez S, Sanchez-Cabo F, Gonzalez MA, et al. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nature communications. 2011;2:282. doi: 10.1038/ncomms1285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Skog J, Wurdinger T, van Rijn S, Meijer DH, Gainche L, Sena-Esteves M, et al. Glioblastoma microvesicles transport RNA and proteins that promote tumour growth and provide diagnostic biomarkers. Nature cell biology. 2008;10:1470–1476. doi: 10.1038/ncb1800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Tannous BA, Kim DE, Fernandez JL, Weissleder R, Breakefield XO. Codon-optimized Gaussia luciferase cDNA for mammalian gene expression in culture and in vivo. Mol Ther. 2005;11:435–443. doi: 10.1016/j.ymthe.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 46.Pegtel DM, Cosmopoulos K, Thorley-Lawson DA, van Eijndhoven MA, Hopmans ES, Lindenberg JL, et al. Functional delivery of viral miRNAs via exosomes. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:6328–6333. doi: 10.1073/pnas.0914843107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Meckes DG, Jr, Shair KH, Marquitz AR, Kung CP, Edwards RH, Raab-Traub N. Human tumor virus utilizes exosomes for intercellular communication. Proceedings of the National Academy of Sciences of the United States of America. 2010;107:20370–20375. doi: 10.1073/pnas.1014194107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Liu J, Rivas FV, Wohlschlegel J, Yates JR, 3rd, Parker R, Hannon GJ. A role for the P-body component GW182 in microRNA function. Nature cell biology. 2005;7:1261–1266. doi: 10.1038/ncb1333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Gibbings DJ, Ciaudo C, Erhardt M, Voinnet O. Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nature cell biology. 2009;11:1143–1149. doi: 10.1038/ncb1929. [DOI] [PubMed] [Google Scholar]
- 50.Siomi H, Siomi MC. RISC hitches onto endosome trafficking. Nature cell biology. 2009;11:1049–1051. doi: 10.1038/ncb0909-1049. [DOI] [PubMed] [Google Scholar]
- 51.Janowska-Wieczorek A, Wysoczynski M, Kijowski J, Marquez-Curtis L, Machalinski B, Ratajczak J, et al. Microvesicles derived from activated platelets induce metastasis and angiogenesis in lung cancer. International journal of cancer Journal international du cancer. 2005;113:752–760. doi: 10.1002/ijc.20657. [DOI] [PubMed] [Google Scholar]
- 52.Garcia JM, Garcia V, Pena C, Dominguez G, Silva J, Diaz R, et al. Extracellular plasma RNA from colon cancer patients is confined in a vesicle-like structure and is mRNA-enriched. RNA. 2008;14:1424–1432. doi: 10.1261/rna.755908. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ng EK, Tsui NB, Lam NY, Chiu RW, Yu SC, Wong SC, et al. Presence of filterable and nonfilterable mRNA in the plasma of cancer patients and healthy individuals. Clin Chem. 2002;48:1212–1217. [PubMed] [Google Scholar]
- 54.Arroyo JD, Chevillet JR, Kroh EM, Ruf IK, Pritchard CC, Gibson DF, et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proceedings of the National Academy of Sciences of the United States of America. 2011;108:5003–5008. doi: 10.1073/pnas.1019055108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Turchinovich A, Weiz L, Langheinz A, Burwinkel B. Characterization of extracellular circulating microRNA. Nucleic acids research. 2011 doi: 10.1093/nar/gkr254. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Jouve M, Sol-Foulon N, Watson S, Schwartz O, Benaroch P. HIV-1 buds and accumulates in "nonacidic" endosomes of macrophages. Cell Host Microbe. 2007;2:85–95. doi: 10.1016/j.chom.2007.06.011. [DOI] [PubMed] [Google Scholar]
- 57.Kramer B, Pelchen-Matthews A, Deneka M, Garcia E, Piguet V, Marsh M. HIV interaction with endosomes in macrophages and dendritic cells. Blood Cells Mol Dis. 2005;35:136–142. doi: 10.1016/j.bcmd.2005.06.006. [DOI] [PubMed] [Google Scholar]
- 58.von Schwedler UK, Stuchell M, Muller B, Ward DM, Chung HY, Morita E, et al. The protein network of HIV budding. Cell. 2003;114:701–713. doi: 10.1016/s0092-8674(03)00714-1. [DOI] [PubMed] [Google Scholar]
- 59.Wiley RD, Gummuluru S. Immature dendritic cell-derived exosomes can mediate HIV-1 trans infection. Proceedings of the National Academy of Sciences of the United States of America. 2006;103:738–743. doi: 10.1073/pnas.0507995103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Masciopinto F, Giovani C, Campagnoli S, Galli-Stampino L, Colombatto P, Brunetto M, et al. Association of hepatitis C virus envelope proteins with exosomes. European journal of immunology. 2004;34:2834–2842. doi: 10.1002/eji.200424887. [DOI] [PubMed] [Google Scholar]
- 61.Lenassi M, Cagney G, Liao M, Vaupotic T, Bartholomeeusen K, Cheng Y, et al. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic. 2010;11:110–122. doi: 10.1111/j.1600-0854.2009.01006.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.van Doormaal FF, Kleinjan A, Di Nisio M, Buller HR, Nieuwland R. Cell-derived microvesicles and cancer. The Netherlands journal of medicine. 2009;67:266–273. [PubMed] [Google Scholar]
- 63.Hendrix A, Westbroek W, Bracke M, De Wever O. An ex(o)citing machinery for invasive tumor growth. Cancer research. 2010;70:9533–9537. doi: 10.1158/0008-5472.CAN-10-3248. [DOI] [PubMed] [Google Scholar]
- 64.Safaei R, Larson BJ, Cheng TC, Gibson MA, Otani S, Naerdemann W, et al. Abnormal lysosomal trafficking and enhanced exosomal export of cisplatin in drug-resistant human ovarian carcinoma cells. Molecular cancer therapeutics. 2005;4:1595–1604. doi: 10.1158/1535-7163.MCT-05-0102. [DOI] [PubMed] [Google Scholar]
- 65.De La Pena H, Madrigal JA, Rusakiewicz S, Bencsik M, Cave GW, Selman A, et al. Artificial exosomes as tools for basic and clinical immunology. Journal of immunological methods. 2009;344:121–132. doi: 10.1016/j.jim.2009.03.011. [DOI] [PubMed] [Google Scholar]
- 66.Kuate S, Cinatl J, Doerr HW, Uberla K. Exosomal vaccines containing the S protein of the SARS coronavirus induce high levels of neutralizing antibodies. Virology. 2007;362:26–37. doi: 10.1016/j.virol.2006.12.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Tan A, De La Pena H, Seifalian AM. The application of exosomes as a nanoscale cancer vaccine. International journal of nanomedicine. 2010;5:889–900. doi: 10.2147/IJN.S13402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Michael A, Bajracharya SD, Yuen PS, Zhou H, Star RA, Illei GG, et al. Exosomes from human saliva as a source of microRNA biomarkers. Oral diseases. 2010;16:34–38. doi: 10.1111/j.1601-0825.2009.01604.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Delcayre A, Estelles A, Sperinde J, Roulon T, Paz P, Aguilar B, et al. Exosome Display technology: applications to the development of new diagnostics and therapeutics. Blood cells, molecules & diseases. 2005;35:158–168. doi: 10.1016/j.bcmd.2005.07.003. [DOI] [PubMed] [Google Scholar]
- 70.Alvarez-Erviti L, Seow Y, Yin H, Betts C, Lakhal S, Wood MJ. Delivery of siRNA to the mouse brain by systemic injection of targeted exosomes. Nature biotechnology. 2011;29:341–345. doi: 10.1038/nbt.1807. [DOI] [PubMed] [Google Scholar]