Abstract
Solid tumors often develop high interstitial fluid pressure (IFP) as a result of increased water leakage and impaired lymphatic drainage, as well as changes in the extracellular matrix composition and elasticity. This high fluid pressure forms a barrier to drug delivery and hence, resistance to therapy. We have developed techniques based on contrast enhanced magnetic resonance imaging for mapping in tumors the vascular and transport parameters determining the delivery efficiency of blood borne substances. Sequential images are recorded during continuous infusion of a Gd-based contrast agent and analyzed according to a new physiological model, yielding maps of microvascular transfer constants, as well as outward convective interstitial transfer constants and steady state interstitial contrast agent concentrations both reflecting IFP distribution. We further demonstrated in non small cell human lung cancer xenografts the capability of our techniques to monitor in vivo collagenase induced increase in contrast agent delivery as a result of decreased IFP. These techniques can be applied to test drugs that affect angiogenesis and modulate interstitial fluid pressure and has the potential to be extended to cancer patients for assessing resistance to drug delivery.
Keywords: Interstitial fluid pressure, drug delivery, magnetic resonance imaging, lung cancer xenografts, collagenase
Introduction
Resistance of solid tumors to anti-neoplastic agents is a complex phenomenon resulting from processes occurring both inside tumor cells and within their surrounding microenvironment: the microvascular network, stromal components and extracellular matrix. Intracellular mechanisms of resistance involve the activation of multidrug resistance genes and drug export pumps , as well as alterations in metabolic pathways which may prevent the activity of drugs in the tumor (Litman et al., 2001; Katragadda et al., 2005; Mimeault et al., 2008). Resistance to drug delivery, which develops in the tumor microenvironment, is mainly caused by impaired function of the microvasculature and lymphatic systems that leads to increased interstitial fluid pressure (IFP) (Fukumura and Jain, 2006). In healthy tissues with normal microvascular perfusion and lymphatic drainage, the IFP is close to zero mm Hg resulting in a positive transcapillary pressure gradient which favors extravasation of the drugs from the capillaries into the interstitial space. In tumors, however, the increase in the capillary permeability and impaired lymphatic drainage augment IFP to values ranging from 7 mm Hg to as high as 60 mm Hg thereby reducing, and at times even eliminating, the positive transcapillary pressure gradient that cause extravasation of solutes from the capillaries. Furthermore, the interstitial pressure gradient generated by the high IFP in the tumor center as compared to the tumor periphery, causes outward convection and reduced delivery to central regions (Baxter and Jain, 1989; Heldin et al., 2004).
In addition to the changes in the properties of blood and lymphatic vessels described above, the stroma of solid tumors also actively participates in increasing IFP (Reed et al., 2001). It has been proposed that interstitial fluid pressure is normally regulated through interactions between the extracellular matrix (ECM) and the fibroblasts (Reed et al., 2001).The extravasation of plasma macromolecules, such as fibrinogen, through the permeable vasculature of the progressing tumor and the high deposition of collagen lead to the formation of a very dense extracellular matrix in the tumor. Fibroblasts can easily proliferate in this specific microenvironment and eventually gain contractile function through the acquisition of smooth muscle cell properties. The binding of “activated” fibroblasts to interstitial fibers via a variety of integrins leads to increased pressure within the tumor ECM (Desmouliere et al., 2004).
Several studies performed on animal models showed that the abnormal distribution of pressure gradients in high IFP tumors attenuates drug delivery and may result in chemotherapy failure ((Jain, 1987; Heldin et al., 2004) and the references cited therein). High IFP has also been demonstrated in human tumors such as breast carcinoma (Less et al., 1992; Nathanson and Nelson, 1994), metastatic melanoma (Boucher et al., 1991; Less et al., 1992; Curti et al., 1993), head and neck carcinoma (Gutmann et al., 1992), colorectal carcinoma (Less et al., 1992), and cervical carcinoma (Fyles et al., 2006). The studies of cervical cancer demonstrated that the survival rate of patients with tumors exhibiting low IFP (IFP < 19 mm Hg) was significantly higher than that of patients bearing tumors with high IFP (Milosevic et al., 2001). Furthermore, IFP levels in cervical cancer also showed a negative correlation with transcapillary transfer constants derived from dynamic contrast enhanced magnetic resonance imaging (MRI) studies (Haider et al., 2007), suggesting impaired extravasation of blood borne molecules with increased IFP.
Different approaches for improving drug delivery to high IFP tumors were proposed and tested (Jirtle, 1981; Horsman et al., 1992; Quinn et al., 1992; Stone et al., 1992; Lee et al., 2000; Wildiers et al., 2003; Tong et al., 2004; Willett et al., 2004). Recent studies also showed that treatment with agents that modulate the tumors’ stromal tissue components can reduce IFP level. For example, collagenase, an enzyme that degrades collagen and modulates the assembly of collagen fibers and fibroblasts, reduced IFP within osteosarcoma xenoggrafts inducing a 2-fold increase in the tumor uptake and distribution of a monoclonal antibody (Eikenes et al., 2004).
A major drawback that inhibits detection and treatment of resistance stemming from impaired delivery is the lack of a non invasive imaging technique that identifies regions exhibiting interstitial hypertension. Current methods for determining IFP such as the perforated capsule (micropore chamber) method (Guyton, 1963; Wiig et al., 1986), the wick-in-needle technique (Jain, 1987; Griffon-Etienne et al., 1997), or the micropipettes and servo null device (Boucher et al., 1990) are invasive, limited to few external locations in a tissue and consequently not clinically practical.
Herein, we present non invasive MRI experiments that yielded maps of the parameters characterizing the transfer of a contrast agent across the capillary’s walls and due to interstitial pressure gradients in tumors. These experiments are based on recording images during slow infusion of the contrast agent, as well as at steady state infusion (Hassid et al., 2006). We demonstrate the performance of our techniques in non small cell lung cancer xenografts that exhibit high interstitial fluid pressure. Furthermore, we show their application to monitor response to collagenase treatment as a result of IFP reduction by detecting changes in pressure gradients and increased delivery into central regions of tumors .
Materials and Methods
Cells and tumors in animals
Human NCI-H460 non small cell lung cancer (NSCLC) cells were cultivated and implanted into the flanks of female CD1-NU immunodeficient mice, as previously described (Hassid et al., 2006). During the MRI experiments, mice were anaesthetized by inhalation of 1% isofluorane (Medeva Pharmaceuticals, Inc., Rochester, NY, USA) in an O2/N2O (3:7) mixture applied through a nose cone (Hassid et al., 2006). All animal protocols were approved by the Weizmann Institute’s Animal Care and Use Committee.
IFP measurements
IFP of the tumors was measured using the wick-in-needle method (Boucher et al., 1991). Briefly, a 23-gauge needle with a side hole located at about 3 mm from the needle tip was connected to a pressure monitor system (model 295-1 Pressure; Stryker, Kalamazoo, MI) specially designed for measuring tissue fluid pressures. The system was filled with saline. The needle was inserted into the center of the tumor, or into the flank muscle for reference, and 50μL of 0.9% sodium chloride was injected to ensure fluid communication between the tissue and the pressure monitor system.
Histology and immunostaining
At the end of the experiments tumors were removed, fixed in 4% zinc solution (0.5% zinc chloride and zinc acetate, 0.05% calcium acetate in 0.1 M tris buffer, pH 7.4) and maintained in paraffin blocks. Microsections from each tumor were processed using hematoxylin-eosin staining and CD-31 immunofluorescence staining of endothelial cells. For the immunofluorescence staining the sections were deparaffinized in xylene, hydrated in series of graded ethanol solutions and rinsed in double distilled water (DDW). The sections were then placed for 7 min in cold acetone (−20°C), rinsed in DDW, and blocked with 20% normal rabbit serum followed by overnight incubation with primary rat anti mouse CD31 (PECAM-1, monoclonal Rat anti mouse 1:100, PharMingen, San Diego, CA). The CD31 treated sections were further incubated for 1h in biotinilated anti rat CD31 (1:100, Vector lab, Burlingame, CA). Antibody distribution was then visualized using a fluorescence streptavidin-Cy3 conjugated complex (Jackson Imunoresearch Lbr.inc, Baltimore, USA). Nuclear staining was performed using 1:2000 diluted Hoechst solution (Molecular Probes, Oregon, USA).
The extracellular volume fraction was assessed by analyzing digital images of the hematoxylin-eosin slides assessing the fraction of eosin stained areas using photoshop 7.0 ME. CD31 immunostained sections were examined by a fluorescence microscope (E600; Nikon, Tokyo, Japan) equipped with Plan Fluor objective connected to a CCD camera (DMX1200F; Nikon). Digital images (3.2 mm2) of tumor sections were collected and analyzed using the Image-Pro plus 4.1 software. Quantification of endothelial staining area was performed by measuring positive staining for CD31, The mean capillary area fraction was determined by measuring the area of stained endothelial cells + area surrounded by stained endothelial cells relative to the total area of a tumor slice.
Treatment with collagenase and characterization of collagenase effects
Collagenase (Sigma-Aldrich, St Louis, MO, USA) treatment was applied by i.v. injection into the tail vein of 0.4 mg/ kg body-weigh of this enzyme diluted in PBS to 0.1% w/v (1 mg = 161 collagen digestion units).
The IFP of each tumor was measured twice, 24 h before and 5 h after i.v. injection of collagenase, by means of the wick-in-needle method (described above). MRI studies of the effect of collagenase on the tumors were performed by employing twice the MRI protocols of slow infusion: 24 hours before and from 3 to 5 hours after collagenase administration. Immediately after the MRI study the tumors were surgically removed for histological analysis.
MRI experiments
MRI scanning was performed on a 4.7-T Biospec spectrometer (Bruker Biospin, Rheinstetten, Germany) employing in all imaging protocols the same spatial resolution of 0.2 × 0.2 × 1 mm3. Delineation of the boundary of the tumors and determination of their volume was achieved by analyzing images recorded with a 2-dimensional fast spin echo T2 weighted sequence using echo-time (TE)/repetition-time (TR) =49/3000 ms as previously described (Furman-Haran et al., 1997).
Magnetic resonance images were then sequentially recorded before (5 images) and during infusion into the tail vein of the common gadolinium-based contrast agent – GdDTPA (gadopentate-dimeglumine, Schering, Berlin, Germany), at a rate of 0.011 mmol/min/kg body weight, for approximately 90 minutes. The recording protocol consisted of a 3-dimensional T1-weighted gradient echo sequence with TE/TR=2.1/18.3 ms, flip angle α = 45° and 47 s temporal resolution.
T1 measurements were performed using 2D-sequential inversion recovery snap shot fast low angle shot (FLASH) sequence with 11 inversion times (TI) ranging from 10 ms to 10 s, TE/TR=3.5 /15 ms and flip angle=100, and the same matrix size and FOV as the T2 weighted images.
MRI data analysis
For image processing the dynamic datasets and the T1 measurements datasets were loaded into matlab (version 7.0.1). workspace.
T1 relaxation rates were calculated at pixel resolution applying a non linear least square fit of the intensity per pixel (I) at varying inversion times (TI). The curves obtained from measurements before administration of the contrast agent and at steady state infusion conditions fitted to the following equation: I = Iinf[1-Aexp(−TI/T1)]; where IInf is the maximum intensity at 10s, A is a free parameter with a maximum value 2, and T1 is a free parameter. The R2 of the fit was > 0.9 suggesting a dominant single T1 relaxation rate. Steady state tissue GdDTPA concentration (CGd), defined as the amount of GdDTPA in mmol per tissue volume at steady state infusion, was obtained from the measured T1 relaxation rates according to: CGd = (1/T1ss-1/T10)/ r1 , where r1 is the water proton relaxivity of GdDTPA = 4.2 s−1mM−1 (Furman-Haran et al., 1997); 1/T1ss and 1/T10 are the relaxation rates at steady state concentration and before the infusion, respectively.
Maps of the steady state GdDTPA concentration in the interstitial space were calculated by dividing the tissue GdDTPA concentration by the estimated extracellular volume fraction of 0.2.
The dynamic datasets composed of T1 weighted gradient echo images recorded before and during the infusion of the contrast agent, as described above, were also analyzed pixel by pixel. The signal intensities per pixel were normalized to the pre-contrast intensity yielding enhancement datasets defined as [I(t) − I(0)] / I(0) where I(0) and I(t) are the signal intensity pre- and during-contrast infusion. The enhancement datasets were fitted to Equation 1 below:
| (1) |
Where TR and α are fixed and known experimental parameters, the relaxivity, r1, is a known fixed parameter (see above), T10 was measured, as described above, and determined for each tumor and Ct(t), the tissue concentration includes the unknown, searched for parameters, and is given according to Equation 2 below which is based on a two compartment pharmacokinetic model (Tofts, 1997):
| (2) |
where the unknown parameters are: kin - the influx transcapillary transfer constant, the ratio kout /νe termed the efflux rate constant kep (or kout- the outflux transcapillary transfer constant if νe, the extracellular volume fraction is independently estimated as in our case), and νp the intravascular volume fraction. The fixed parameters are: Dinf - the contrast agent infusion rate, and the parameters ai, and mi (i = 1,2) which define the plasma pharmacokinetics that were obtained by modeling the changes in the concentration of the GdDTPA in the plasma during infusion , Cpinf(t), according to equation 3 (Tofts and Berkowits, 1994):
| (3) |
The fitting of Equation 1 was further extended to include constrains based on a model proposed by Jain et al (Jain and Baxter, 1988; Baxter and Jain, 1989; Netti et al., 1995; Netti et al., 1996; Baish et al., 1997). According to this model, the change in the concentration of a blood borne tracer in the tissue interstitium is described by a differential equation that takes into account the transfer of solutes through the capillary walls and within the interstitial spaces by both diffusion in the direction of concentration gradients and convection in the direction of pressure gradients (eq H in ref (Jain and Baxter, 1988)).
We have simplified this model making several approximations based on the assumption that tumor tissue can be divided into two major regions (i) Regions with low IFP, where the dominant pressure gradient across the capillary walls is positive and leads to inward extravasation, whereas pressure-gradient dependent outward convection and diffusion in the interstitium is minimal and can be neglected. Hence in these regions there are concentration-gradient and inward extravasation pressure-gradient dependent transfer contrasts. Consequently, in such regions, kin = ktrans + kΔP(e) and kout= ktrans where ktrans is a concentration gradient dependent transcapillary transfer constant and kΔP(e) = kin − kout >0 is an extravasative pressure gradient dependent transfer constants.
(ii). Regions with high IFP where positive pressure gradient dependent extravasation is canceled and a pressure dependent gradient, opposite in direction to the inward extravasative pressure gradient in (i), causes outward convection into the capillaries and from high IFP tumor regions to low IFP outside the tumor. This outward convection reduces, and sometimes may completely abolish, the net amount of the contrast agent in the high IFP regions, despite the presence of concentration gradient dependent transfer across the capillaries. Consequently, in such regions, kin = ktrans and kout = ktrans + kΔP(c) where ktrans has the same definition as in (i) and kΔP(c)= kout − kin >0 is an outward convective pressure gradient dependent transfer constant.
It should be noted that in the absence of pressure gradients, when the only transcapillary transfer mechanism is concentration dependent such that kin = kout , νe can be calculated from the ratio kin/kep and hence the parameters kin, νe and νp are identifiable and distinguishable (Koh, 2008). In the presence of pressure gradients, νe needs to be independently estimated, and then the parameters kin, kout and νp are identifiable. We have estimated in from the histological stained slices of H460 xengrafts an approximated νe of 0.2 ranging from 0.15 to 0.3.
The plasma pharmacokinetic parameters, previously obtained in the same female CD-1 NU mice strain at the same age as in this study, were: a1=2.94 kg/l, a2=4.85 kg/l, m1=0.73 min−1, and m2=0.075 min−1 (Furman-Haran et al., 1997). These values were recently confirmed to apply to the slow GdDTPA infusion protocol by analyzing the changes in T1 weighted images (obtained at 6 sec temporal resolution using inversion recovery fast low angle shot sequence with a fixed inversion time) in the carotid arteries of mice (Hassid et al., 2006). Although under continuous slow infusion one does not observe as in a bolus injection the washout of the contrast agent, the concentration during the slow infusion is constantly affected by the clearance rates as was previously shown (Tofts and berkowitz, 1994).
For the fitting of Equation 1 we applied a nonlinear least square Levenberg-Marquardt algorithm. We fitted each enhancement dataset to Equation 1 using the constrain of condition (i): kin > kout , and then the constrain of condition (ii): kin.< kout The goodness of the fits for condition (i) or condition (ii) were assessed by calculating for all pixels within the ROI region of the tumor and its surrounding the proportion of variability, R2 , also termed the coefficient of determination or correlation index, (Lyman Ott and Longnecker, 2001), with R2 defined as:
| (3) |
The selection of the final fitted parameters for each pixel was based on choosing the fitted model parameters with the highest R2 among the two conditions. Thus, each final parametric map of ktrans, kΔP(e) and kΔP(c), and νp presented the best fitted values derived from the two conditions (i) and (ii). Note that in the final presentation of the parametric maps of the pressure gradient dependent transfer constants we assigned a negative value to kΔP(c) (kΔP(c) = −kΔp) to indicate the opposite direction of transfer with respect to kΔP(e), and presented the inward extravasative and the outward convective pressure gradient dependent transfer constants on the same parametric map using ± kΔP.
Statistical analysis of the results
Median and mean values of the vascular parameters and of the steady state tissue concentration of the contrast agent were calculated per tumor by analyzing the frequency histograms of each parameter. Since these histograms were frequently not symmetric around the mean the final results were presented as the mean of medians ± standard deviation (SD) of each parameter for all measured tumors.
The congruence between the interstitial GdDTPA concentration and the pressure gradient dependent transfer constants was evaluated using a Pearson correlation, which yielded a correlation coefficient r and its statistical significance p.
The collagenase induced changes in the vascular parameters derived from analyzing the slow infusion MRI data were calculated for the central region and peripheral region in each tumor. The regions were delineated using a Matlab program based on dividing to 3 identical lengths the diameter of the tumor throughout its entire area. Median values of each parameter were calculated in the central and peripheral regions of each tumor, before and after collagenase treatment. A paired t test between the parameters in the corresponding areas was then employed in order to compare the median of each parameter before collagenase and after collagenase treatment using p<0.05 to indicate significance. The significance (p<0.05) of the collagenase induced changes in the steady state tissue concentration were also evaluated in the corresponding inner and outer regions before and after collagenase treatment using a paired t-test .
Results
H460 human non-small-cell lung cancer cells were implanted ectopically into the flanks of female CD1 immunodeficient mice. Two weeks after implantation the tumors reached a median size of 270 mm3 (range: 96-1080) mm3 (n=11) as determined by standard T2 weighted MRI (see Methods). High interstitial fluid pressure with an average IFP of 31±7 mm Hg (n=11) was measured in the center of these tumors using the wick-in-needle method (Leander et al., 1996).
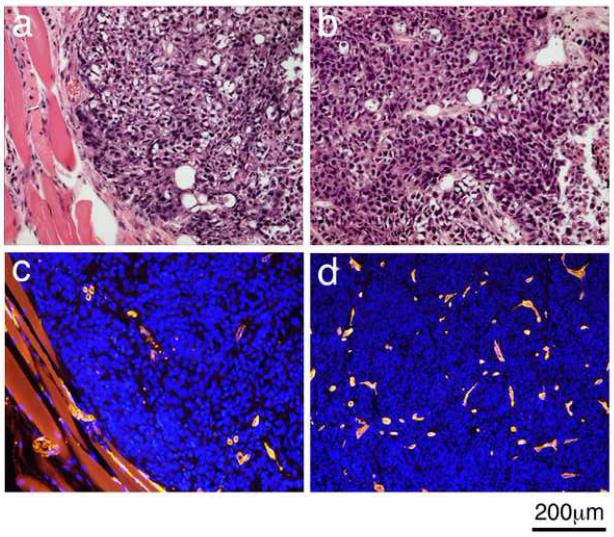
Histopathology analysis of tumors removed at the end of the MRI experiments revealed in all tumors the presence of mostly viable, homogeneous and densely-packed cancer cells. The high cellular density and low fraction of extracellular spaces, estimated at approximately νe = 0.2 (ranging between 0.15 to 0.3), was similar in the rim and the center of the tumors (Figure 1 a and b). CD31 immunostaining (Figure 1 c and d) showed the distribution of the blood capillaries in these tumors. Statistical analysis of the capillary staining did not show a significant difference between the density of capillaries in the rim and the center of the tumors (Two sample equal variance t-test n=7, p=0.40) and yielded a mean capillary area fraction of 6±3 % (n=11).
Figure 1. Microscopic views of the cells and capillaries in NSCLC H460 tumor implanted in the flank of nude mice.
a & b. Hematoxylin and eosin staining of a peripheral region and a central region, respectively. c & d. CD31 imunostaining of the blood capillaries in a peripheral and central region, respectively of the same tumor as in a&b.
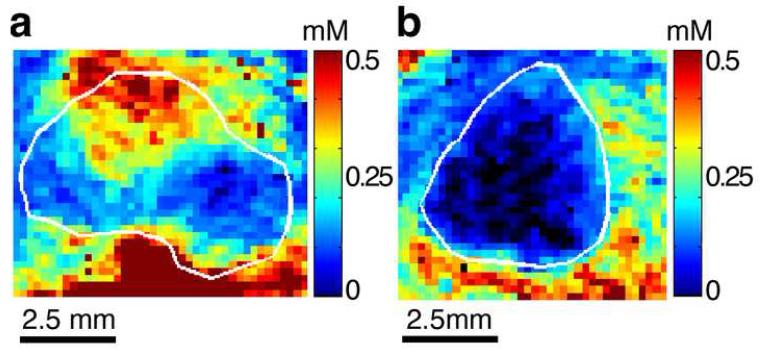
In vivo magnetic resonance imaging of H460 tumors was applied before, during intravascular infusion and at steady state intravascular infusion of a GdDTPA. Analysis of the images recorded during steady state revealed that the tissue contrast agent concentration at this state is distributed heterogeneously within tumors (Figure 2). In some tumors all pixels within the tumor boundary revealed delivery and accumulation of contrast agent at steady state, albeit, in varying concentrations (Figure 2a). In other tumors, no contrast agent was found in part of the pixels in the tumors’ interior even after 90 minutes of infusion (Figure 2b). Null or very low concentrations in the interior regions suggested rapid outward convection of the contrast agent due to the presence of high interstitial fluid pressure. Indeed, the lower contrast agent tissue concentration of the tumor in Figure 2b relative to that in Figure 2a is in accord with the difference in their IFP; 38 mm Hg as compared to 26 mm Hg, respectively.
Figure 2. Maps of steady-state tissue GdDTPA concentration in NSCLC H460 tumors.
a. A tumor in which GdDTPA at steady state reached all areas at varying concentrations. This tumor demonstrated IFP of 26 mmHg.
b. A tumor in which GdDTPA at steady state did not reach part of the tumor interior. This tumor demonstrated IFP of 38 mmHg
Maps were calculated from T1 relaxation rates measured before and during infusion of GdDTPA, when it reached steady state.
The boundary of each tumor, marked in white, was initially outlined on the T2 weighted image and transferred to the corresponding maps of the vascular parameters
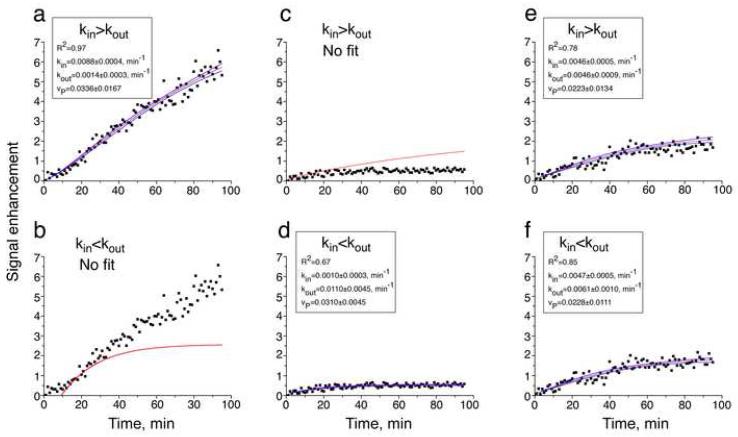
The enhancement datasets obtained from analyzing the images recorded before and during the infusion of the contrast agent served to calculate the vascular and transport parameters. Pixel by pixel fitting of these datasets to Equation 1 was performed under the two conditions: (i) kin>kout in low IFP regions with a transfer due to hydrostatic pressure gradient across the capillaries that causes inward extravasation, and (ii) kin<kout in high IFP regions with a transfer due to pressure gradients in the interstitium that cause outward convection away from the tumor tissue. In both conditions the equation included the concentration gradient dependent transfer constant across the walls of the capillaries and used an estimated extracellular volume fraction of 0.2 (for details see Methods). Figure 3. demonstrates typical examples of enhancement dataset per pixel and their fitting to Equation 1 under the two conditions described above. In the case of high inward extravasation (Figure 3a) it was not possible to fit Equation 3 under the outward convection condition (Figure 3b). Similarly, for high outward convection (Figure 3c) it was not possible to fit the data under the inward extravasation condition (Figure 3d). For relatively low pressure gradients both conditions yielded fitted parameters (Figure 3e and 3f), but with different R2, and hence, the parameters of the fitting with the higher value of R2 were chosen to represent such pixels (Figure 3c). Further evaluation of the effect of the approximated νe on the values of the transfer constants was obtained by fitting the enhancement datasets to the lower and higher limits of νe. Table 1 summarizes the results for the three pixels demonstrated in Figure 3 and indicates the possible over and under estimation of the concentration gradient dependent and pressure gradient dependent transfer constants.
Figure 3.
Examples of typical enhancement time curves and their best fit and 95% confidence interval. The enhancement curves were fitted to Equation 1 under condition (i) kin>kout and (ii)kin<kout. Note the high R2 in a and d and the lack of fitting in b and c. Also note the higher R2 in f as compared to e.
The enhancement datasets were analyzed using Origin version 6.1
Table 1.
The dependence of the fitted parameters on the estimation of the extracellular volume fraction, νe, for the data presented in Figure 3. The fitted νp values were not affected by variations in νe.
| Condition | Transfer Constants ×10−3, min−1 |
νe = 0.2 | 0.15 - 0.3 | |
|---|---|---|---|---|
| kin> kout (Fig 3a) | kin | 8.9 | 8.9 | 8.9 |
| kout (= ktrans) | 1.4 | 1.1 | 2.2 | |
| kΔp(e) | 7.5 | 7.8 | 6.7 | |
|
| ||||
| kin< kout (Fig 3d) | kin (= ktrans) | 1.0 | 1.0 | 1.0 |
| kout | 11.9 | 8.4 | 16.8 | |
| kΔp(c) | 10.9 | 7.4 | 15.8 | |
|
| ||||
| kin< kout (Fig 3f) | kin (= ktrans) | 4.7 | 4.7 | 4.7 |
| kout | 6.1 | 4.6 | 9.2 | |
| kΔp(c) | 1.4 | 0 | 4.5 | |
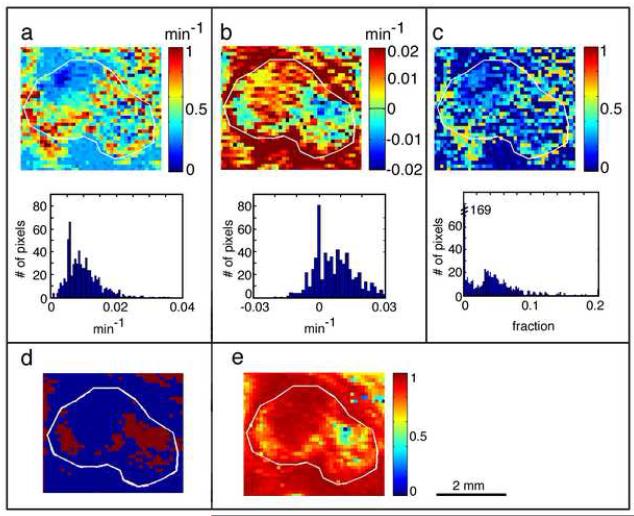
The final output of the pixel by pixel analysis of each tumor yielded the following parametric maps: concentration-gradient dependent transcapillary transfer constants (ktrans) (Figure 4a); inward extravasative pressure-gradient dependent transfer constants in regions with low IFP (kΔp(e) defined as +kΔp) and outward convective pressure-gradient dependent transfer constants in regions with high IFP (kΔp(c) defined as −kΔp), represented together on one map - ±kΔp (Figure 4b), as well as intravascular volume fraction, νp (Figure 4c). The frequency histograms of these parameters indicated that they are heterogeneous and frequently not symmetric around the mean (Figure 4a to 4c ). As described above, each pixel showed a better fit to either the inward extravasation or the outward convection condition as demonstrated in Figure 4d. For pixels with ±kΔp~0, the two conditions coincide and kin=kout=ktrans. An example of the final assessment of the goodness of the fit is presented in the combined R2 map shown in Figure 4e.
Figure 4. Maps and frequency distribution of the vascular parameters in NSCLC H460 tumor.
The parameters were calculated from contrast enhanced images scanned during GdDTPA slow infusion; IFP inside this tumor was 26 mm Hg.
a. Concentration gradients dependent transcapillary transfer constant (ktrans) map. b. extravasation from plasma (positive) and outward convection from the interstitial space (negative) pressure gradients dependent transfer constants (± kΔp) map. c. Intravascular volume fraction (νp) map. d. Distribution map of pixels showing a better fit to extravasative transfer (condition (i) kin>kout ) and those showing a better fit to convective transfer (condition (ii) kin<kout ) e. Combined proportion of variability (R2) map; presents the best fit to either condition.
The boundary of each tumor, marked in white, was initially outlined on the T2 weighted image and transferred to the corresponding maps of the vascular parameters
Statistical analysis of the values of the three fitted parameters in all tumors (n=11) showed that the concentration dependent transfer constants and the pressure-dependent transfer constants exhibited high inter tumoral heterogeneity and large variations between individual tumors (Table 2). The mean intravascular volume fraction of approximately 6.3 ± 3.0 %, obtained from the MRI image analysis is in accord with the mean area fraction of capillaries (6 ± 3) % determined from the immunostaining studies.
Table 2.
The vascular parameters of H460 tumors derived from slow infusion contrast enhanced MRI data
| Vascular and transport parameters | Mean ± SD* |
|---|---|
| Concentration gradient dependent transfer constant- ktrans , min−1 | (9.5± 8.4) ×10−3 |
| Extravasation pressure gradient dependent transfer constant , + kΔp, min−1 | (5.4 ± 4.4)×10−3 |
| Convective pressure gradient dependent transfer constant, − kΔp, min−1 | (5.5 ± 3.4)×10−3 |
| Intravascular volume fraction, νp | 0.063 ± 0.030 |
Mean of median ± standard deviation (n=12)
The association of the values of −kΔp ( kΔp(c)) with high IFP, and of +kΔp (kΔp(e)) with low IFP was further supported by correlating pixel by pixel the pressure-gradient dependent transfer constants with the interstitial concentrations of the contrast agent at steady state of infusion (Figure 5), which were shown to be inversely related to IFP (Hassid et al., 2006). Indeed, in regions with high positive pressure-gradient dependent transfer constants we found also high interstitial concentrations of the contrast agent, whereas in regions with negative pressure-gradient dependent transfer constants, the interstitial concentrations of the contrast agent were low (Figure 5). The Pearson linear correlation between ktrans , the pressure-dependent transfer constants and the steady-state concentrations (Figure 5c and 5g) yielded significant (p<0.0001) correlation factors with r > 0.5, whereas lower correlation factors and significance (or no significance) were found for the correlation between these two measured parameters (figure 5d and 5h). These results suggest a similar origin, predominantly the level of IFP, for the distribution of the pressure-gradient dependent transfer constants and the steady-state interstitial concentrations of the contrast agent.
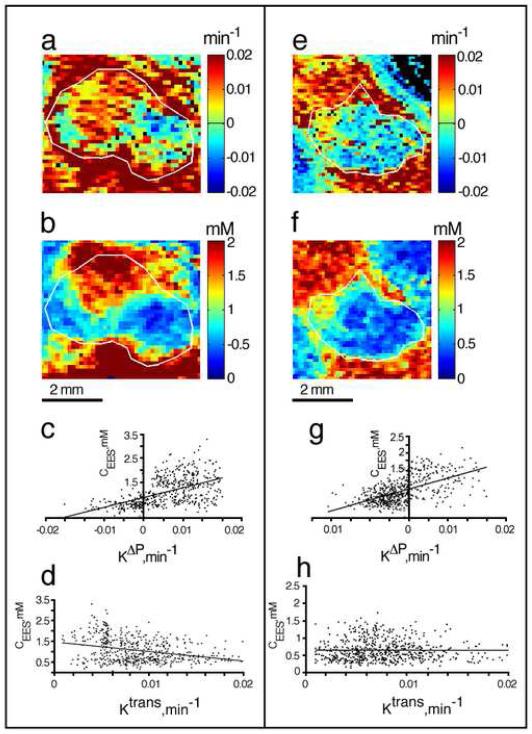
Figure 5. Correlation of ±kΔp and steady state interstitial GdDTPA concentration maps in two typical NSCLC H460 tumors.
a and e. Maps of pressure gradients dependent transfer constant (± kΔp)
b and f . Maps of steady state interstitial GdDTPA concentration.
c and g. Pearson linear correlation of ±kΔp versus the steady state interstitial GdDTPA concentration .( r= 0.54 and r=0.58 for c and g, respectively with p<0.0001)
d and h. Pearson linear correlation of ktrans versus the steady state interstitial GdDTPA concentration (r= −0.3 and r= −0.007 in d and h respectively)
The boundary of each tumor, marked in white, was initially outlined on the T2 weighted image and transferred to the corresponding maps of the vascular parameters.
In order to demonstrate the capability of our imaging methods to monitor changes in IFP and consequently, in delivery of blood-borne agents to tumors, we modulated the tumor’s interstitial pressure using collagenase treatment. IFP measurements using the wick-in-needle method showed that 5 hours after collgenase treatment the interstitial pressure of H460 xenografts was reduced from 26.1 ±3.3 mm Hg to 13.7 ± 3.0 mm Hg (n=8, p=0.016 - paired t test). Histological evaluation of hematoxylin-eosin stained slides of tumors removed 5 hours after administration of collagenase showed densely cellular and disorganized sheets of viable pleomorphic cells as was found in non treated tumors (Figure 1) with the extracellular volume fraction not affected by the short collagenase treatment.
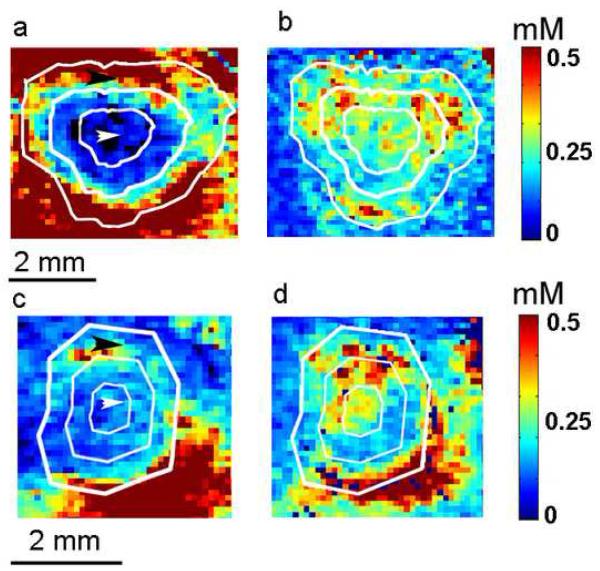
Employing our in vivo MRI techniques made it possible to monitor twice the vascular and transport parameters of the same tumor, 24 h before and 3 h after collagenase treatment. In all treated tumors collagenase elevated the steady state tissue contrast agent concentration in the tumors’ center and made the distribution of the contrast agent throughout the tumor more even. Figure 6 demonstrates examples of extreme and mild collagenase induced changes in the steady state tissue contrast agent concentration within tumors. In the extreme case, the contrast agent barely entered the central region of the tumor before treatment whereas after treatment, the transfer of the contrast agent into the center of the tumor markedly increased and nearly reached the level of transfer in the periphery of the tumor suggesting a reduction of the interior IFP to values similar to those at the periphery (Figure 6a and 6b). In the mild case the change was not dramatic but a clear increase of the contrast agent level in the center of the tumor could be observed as a result of collagenase treatment (Figure 6c and 6d). Statistical analysis of all tumors based on dividing each tumor to a central and a peripheral region (see Figure 6 for this division) indicated a significant increase in the contrast agent concentration in the central region (p<0.029; t-test) with no significant difference in the outer – peripheral regions (Table 3). Similarly, analysis of enhancement datasets showed an increase in the central regions of the tumors of the mean pressure-gradient dependent transfer constant yielding a faster inward extravasation transfer in response to collagenase treatmen. In contrast, in the peripheral regions, no significant changes in the pressure-gradient dependent transfer constant or the other parameters had occurred as a result of collagenase treatment (Table 4).
Figure 6. Collagenase induced changes in the steady-state tissue GdDTPA concentration of H460 tumors.
Example of a tumor exhibiting an extreme change (a and b) and a tumor exhibiting a mild change (c and d).
a and c. Maps of the steady state tissue GdDTPA concentration 24 hours before the administration of collagenase. The white arrows indicate the central region; black arrows represent the peripheral region.
b and d. Maps of the steady state tissue GdDTPA concentration 5 hours after the i.v. administration of collagenase to the tumors in a and c respectively.
Maps were calculated from T1 relaxation rates measured before and during steady state infusion of GdDTPA.
Note demarcation of each tumor boundary and of a central and peripheral region.
Table 3.
Collagenase induced changes in the steady state tissue contrast agent concentration in H460 tumors
| Steady state tissue contrast agent concentration, mM Mean of median ± SD |
|||
|---|---|---|---|
| Region | Before collagenase | After collagenase | p-value* |
| Central | 0.60±0.40 | 0.80±0.50 | 0.029 |
| Peripheral | 0.88±0.29 | 0.89±0.39 | 0.97 |
paired t-test (n=12).
Table 4.
Collagenase induced changes in the transfer constants of the contrast agent at the central region of H460 tumors
| Vascular and transport parameters |
Before collagenase Mean of median ± SD |
After collagenase Mean of median ± SD |
value* |
|---|---|---|---|
|
p Pressure gradient-dependent transfer constant, ±kΔp, min−1 |
(2.1±9.4) ×10−3 | (6.7 ±9.0)× 10−3 | 0.006 |
| Concentration gradient- dependent transfer constant, ktrans, min−1 |
(7.1±5.7)×10−3 | (4.7 ±4.5)×10−3 | 0.27 |
| Intravascular volume fraction, νp | 0.029 ±0.015 | 0.034 ± 0.017 | 0.51 |
paired t-test (n=12)
Discussion
We developed and tested high spatial resolution contrast enhanced MRI techniques that map the distribution of a soluble substance and its concentration and pressure dependent transfer constants across the capillary network and within the interstitium of tumors. Taken together, the results demonstrate the significant effect of pressure gradients on the delivery and distribution of soluble substances in tumors. Interestingly, in some tumors, the impact of interstitial hypertension may reduce or even completely eliminate delivery of agents to the tumor interior although small nutrients such as glucose and amino- acids, as well as oxygen may still reach these regions and maintain their viability.
The transfer constants that depend on internal pressure gradients between the vascular volume and the interstitium, and within different regions in the interstitium (to distinguish from induced external mechanical pressure detected through MRI elastography (Manduca et al., 2001)) are affected by hydrostatic and oncotic pressures (Heldin et al., 2004). In many tumors the large difference in the hydrostatic induced IFP between the rim and internal regions dominates transport in the tumor tissue (Jain and Baxter, 1988; Baxter and Jain, 1989). Namely, a marked increase in tumor IFP reduces the transcapillary pressure gradient that leads to extravasation, and creates an outward convective force either back to the capillaries or through the interstitial spaces to adjacent regions in the tumor periphery with low IFP. Hence, the distribution of the pressure-dependent transfer constants can be used to assess the distribution of IFP, which in turn determines the distribution of pressure-gradient dependent barriers to drug delivery.
In the past, several imaging methods such as computed tomography (CT), positron emission tomography (PET), single photon emission computed tomography (SPECT), and MRI were employed for mapping the distribution of a specific drug or contrast agent within tumors (Leander et al., 1996; Bhatnagar et al., 2000; Matteucci et al., 2000; Suga et al., 2001). However, these studies did not attempt to reveal the effects of pressure gradients and convection on drug delivery. Most model based analyses of dynamic contrast enhanced MRI data following a bolus injection took into account only concentration dependent diffusion across the capillary walls and did not include pressure gradients and outward convection (Tofts et al., 1999) although including a disparity between the inward and outward transcapillary transfer constants appeared to account for the presence of high interstitial fluid pressure (Dadiani et al., 2004). In a recent study of a single murine sarcoma tumor, Zhao et al (Zhao et al., 2007) have used dynamic contrast enhanced–MRI data and a physiological model that accounted for the presence of pressure gradients to simulate the IFP distribution and outward interstitial velocity. Our work herein demonstrates that analyzing the dynamic contrast enhanced changes during infusion of a contrast agent and at steady state infusion, makes it possible to map physiological parameters that reflect the distribution of IFP.
It should be pointed out that important general dilemmas arise when dynamic contrast enhanced time courses are fitted to complex non linear equations with several unknown parameters. For example, because of convergence to local minima the values of the resultant physiological parameters may not be the factually correct tissue parameters. Furthermore, the accuracy with which the tumor vascular function can be characterized using our model based approach is limited by the accuracy of the estimated fixed parameters.
It was previously shown that T10 relaxation rates may introduced the largest error propagation ratio and should therefore be measured as accurately as possible (Tofts et al., 1995). In our experiments the protocol included accurate T10 measurements that also revealed similar T10 values throughout the tumor prior to contrast agent administration (see for example Figure 5 in Hassid et al., 2006) (Hassid et al., 2006). Thus, the error due to this parameter was most likely very low.
The average plasma parameters a1 a2, m1 and m2 defined in Equation 3 could also introduce an error, albeit much lower than that of T10 (Tofts et al., 1995). Furthermore, as the mice in our study were of the same origin, as well as very close in age and weight, their body and renal clearance rates of the contrast agent were similar and hence, errors due to these parameters were most likely small.
The approximate estimation of νe is also a source for an error in the fitted parameters. Analysis of histological slices that stained the cancer cells provided an estimation of νe and confirmed its homogeneous distribution. The estimated range for this parameter caused deviations of the various fitted transfer constants ( Table 1) but did not affect the mechanism of transfer of either inward extravasation or outward convection.
In conclusion, the fitting to the model based equation cannot confirm the correctness of the model. The correctness of the fitted parameters can not be confirmed by independent measurements as no other method can provide such information at the spatial resolution we have employed. However, in this study we have shown that analysis of the slow infusion kinetics provides a means to delineate the regions with high IFP that present barriers to drug delivery. We also showed that it is possible to monitor local changes in IFP induced by specific treatment. The presence of high IFP in the center of the tumors (localization was not exactly defined), as well as the treatment induced reduction in IFP were indeed confirmed by the wick in needle method.
Another validation of the parameters derived from the dynamic MRI curves was obtained by demonstrating a significant correlation between the distribution of the contrast agent at steady state and the distribution of the pressure-dependent transfer constant. This correlation between the results of two independent MRI measurements indicated that the properties they have measured were determined by the same physiological mechanism. Overall, both MRI results suggested the presence of high interstitial fluid pressure in interior parts of the tumors which dropped precipitously at the tumor margins, as was previously predicted theoretically (Jain and Baxter, 1988; Baxter and Jain, 1989) and shown experimentally by other methods (Boucher et al., 1990; Rofstad et al., 2002; Eikenes et al., 2004).
The noninvasive nature of MRI methods enabled us to monitor drug induced changes in IFP. Attempts were previously made to reduce IFP by administrating drugs that influence blood pressure and flow, however, the results were not consistent and the agents were not specific and demonstrated cardiovascular side effects (Jirtle, 1981; Horsman et al., 1992; Quinn et al., 1992; Stone et al., 1992). Another approach to reduce IFP was based on degradation of the ECM (Brekken et al., 2000; Netti et al., 2000; Eikenes et al., 2004). For example, it was shown that collagenase and hyaluronidase reduce IFP and increase the uptake of antibodies (Brekken et al., 2000; Eikenes et al., 2004). We have also shown herein that collagenase reduces IFP and increases the uptake of a contrast agent within few hours after its administration. However, as collagenase activity is not selective to the tumor tissue and may affect other tissues and possibly also facilitate tumor metastasis it is not a clinically relevant agent. Future studies should focus on developing new drugs that can be targeted to tumors and specifically reduce their IFP. Interestingly, the mechanism by which anti-angiogenic agents, such as monoclonal antibodies to VEGF, exert their activity was associated with decreased IFP and improved delivery of chemotherapy ((Hurwitz et al., 2004; Cabebe and Wakelee, 2007) and references cited therein) presumably, as a result of normalizing the functional properties of the capillary network (Lee et al., 2000; Wildiers et al., 2003; Tong et al., 2004; Willett et al., 2004). Using our contrast enhanced techniques it is now possible to test and verify this mechanism.
In principle, our MRI techniques can be translated to humans, however, care must be taken using the Gd-based contrast agents as they must be excluded in renal failure patients who may develop nephritic systemic fibrosis (Thomsen, 2006; Thomsen et al., 2007). Such a translation requires adjusting the infusion protocol to the pharmacokinetics in humans and reducing the total infused amount to the allowed dose (0.2 mmol/kg weight). The utilization of the dynamic data alone enables shortening of the measurement time and reduction in the total infused amount of contrast agent as compared to the steady state method.
In summary, we have demonstrated the feasibility of using magnetic resonance imaging and a common gadolidium-based contrast agent infused at a slow rate, to provide information how a complex vascular network would deliver soluble substances throughout the tumor and its surrounding tissues. Our techniques provide a novel means for monitoring over time the capability of drugs to reduce IFP and increase the delivery of blood borne solutes. Adapting the protocol and contrast agent dose to the range approved for human studies could facilitate detection of barriers to drug delivery thereby helping design efficient administration of chemotherapy. It also can be used to test the efficiency of novel anti-angiogenic and IFP reducing drugs that are now being developed. Further studies in the clinic are required to ultimately reach these goals.
Acknowledgement
This work was supported by the National Institute of Health, USA CA 422238, the Israel Science foundation 801/04, and by Mario-Negri Institute - Weizmann Institute collaboration grant.
We wish to thank Tamar Kreizman for technical assistance, Barbara Morgenstern for skillful editing, and Drs. Ori Brenner and R. Eilam for their advice and support in the histology and immunochemistry studies. Hadassa Degani is the incumbent of the Fred and Andrea Fallek Professorial Chair for Breast Cancer Research.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baish JW, Netti PA, Jain RK. Transmural coupling of fluid flow in microcirculatory network and interstitium in tumors. Microvasc Res. 1997;53(2):128–141. doi: 10.1006/mvre.1996.2005. [DOI] [PubMed] [Google Scholar]
- Baxter LT, Jain RK. Transport of fluid and macromolecules in tumors. I. Role of interstitial pressure and convection. Microvasc Res. 1989;37(1):77–104. doi: 10.1016/0026-2862(89)90074-5. [DOI] [PubMed] [Google Scholar]
- Bhatnagar A, Hustinx R, Alavi A. Nuclear imaging methods for non-invasive drug monitoring. Adv Drug Deliv Rev. 2000;41(1):41–54. doi: 10.1016/s0169-409x(99)00055-1. [DOI] [PubMed] [Google Scholar]
- Boucher Y, Baxter LT, Jain RK. Interstitial pressure gradients in tissue-isolated and subcutaneous tumors: implications for therapy. Cancer Res. 1990;50(15):4478–4484. [PubMed] [Google Scholar]
- Boucher Y, Kirkwood JM, Opacic D, Desantis M, Jain RK. Interstitial hypertension in superficial metastatic melanomas in humans. Cancer Res. 1991;51(24):6691–6694. [PubMed] [Google Scholar]
- Brekken C, Bruland OS, de Lange Davies C. Interstitial fluid pressure in human osteosarcoma xenografts: significance of implantation site and the response to intratumoral injection of hyaluronidase. Anticancer Res. 2000;20(5B):3503–3512. [PubMed] [Google Scholar]
- Brekken C, Hjelstuen MH, Bruland OS, de Lange Davies C. Hyaluronidase-induced periodic modulation of the interstitial fluid pressure increases selective antibody uptake in human osteosarcoma xenografts. Anticancer Res. 2000;20(5B):3513–3519. [PubMed] [Google Scholar]
- Cabebe E, Wakelee H. Role of Anti-angiogenesis Agents in Treating NSCLC: Focus on Bevacizumab and VEGFR Tyrosine Kinase Inhibitors. Curr Treat Options Oncol. 2007;8(1):15–27. doi: 10.1007/s11864-007-0022-4. [DOI] [PubMed] [Google Scholar]
- Curti BD, Urba WJ, Alvord WG, Janik JE, Smith JW, 2nd, Madara K, Longo DL. Interstitial pressure of subcutaneous nodules in melanoma and lymphoma patients: changes during treatment. Cancer Res. 1993;53(10 Suppl):2204–2207. [PubMed] [Google Scholar]
- Dadiani M, Margalit R, Sela N, Degani H. High-resolution magnetic resonance imaging of disparities in the transcapillary transfer rates in orthotopically inoculated invasive breast tumors. Cancer Res. 2004;64(9):3155–3161. doi: 10.1158/0008-5472.can-03-2665. [DOI] [PubMed] [Google Scholar]
- Desmouliere A, Guyot C, Gabbiani G. The stroma reaction myofibroblast: a key player in the control of tumor cell behavior. Int J Dev Biol. 2004;48(5-6):509–517. doi: 10.1387/ijdb.041802ad. [DOI] [PubMed] [Google Scholar]
- Eikenes L, Bruland OS, Brekken C, Davies Cde L. Collagenase increases the transcapillary pressure gradient and improves the uptake and distribution of monoclonal antibodies in human osteosarcoma xenografts. Cancer Res. 2004;64(14):4768–4773. doi: 10.1158/0008-5472.CAN-03-1472. [DOI] [PubMed] [Google Scholar]
- Fukumura D, Jain RK. Tumor microenvironment abnormalities: Causes, consequences, and strategies to normalize. J Cell Biochem. 2006;101(4):937–949. doi: 10.1002/jcb.21187. [DOI] [PubMed] [Google Scholar]
- Furman-Haran E, Grobgeld D, Degani H. Dynamic contrast-enhanced imaging and analysis at high spatial resolution of MCF7 human breast tumors. J Magn Reson. 1997;128(2):161–171. doi: 10.1006/jmre.1997.1220. [DOI] [PubMed] [Google Scholar]
- Fyles A, Milosevic M, Pintilie M, Syed A, Levin W, Manchul L, Hill RP. Long-term performance of interstial fluid pressure and hypoxia as prognostic factors in cervix cancer. Radiother Oncol. 2006;80(2):132–137. doi: 10.1016/j.radonc.2006.07.014. [DOI] [PubMed] [Google Scholar]
- Griffon-Etienne G, Boucher Y, Jain RK, Suit HD. Effects of needle insertion in tumors on interstitial fluid pressure. Microvasc Res. 1997;54(2):174–177. doi: 10.1006/mvre.1997.2037. [DOI] [PubMed] [Google Scholar]
- Gutmann R, Leunig M, Feyh J, Goetz AE, Messmer K, Kastenbauer E, Jain RK. Interstitial hypertension in head and neck tumors in patients: correlation with tumor size. Cancer Res. 1992;52(7):1993–1995. [PubMed] [Google Scholar]
- Guyton AC. A concept of negative interstitial pressure based on pressures in implanted perforated capsules. Circ Res. 1963;12:399–414. doi: 10.1161/01.res.12.4.399. [DOI] [PubMed] [Google Scholar]
- Haider MA, Sitartchouk I, Roberts TP, Fyles A, Hashmi AT, Milosevic M. Correlations between dynamic contrast-enhanced magnetic resonance imaging-derived measures of tumor microvasculature and interstitial fluid pressure in patients with cervical cancer. J Magn Reson Imaging. 2007;25(1):153–159. doi: 10.1002/jmri.20795. [DOI] [PubMed] [Google Scholar]
- Hassid Y, Furman-Haran E, Margalit R, Eilam R, Degani H. Noninvasive magnetic resonance imaging of transport and interstitial fluid pressure in ectopic human lung tumors. Cancer Res. 2006;66(8):4159–4166. doi: 10.1158/0008-5472.CAN-05-3289. [DOI] [PubMed] [Google Scholar]
- Heldin CH, Rubin K, Pietras K, Ostman A. High interstitial fluid pressure - an obstacle in cancer therapy. Nat Rev Cancer. 2004;4(10):806–813. doi: 10.1038/nrc1456. [DOI] [PubMed] [Google Scholar]
- Horsman MR, Christensen KL, Overgaard J. Relationship between the hydralazine-induced changes in murine tumor blood supply and mouse blood pressure. Int J Radiat Oncol Biol Phys. 1992;22(3):455–458. doi: 10.1016/0360-3016(92)90852-9. [DOI] [PubMed] [Google Scholar]
- Hurwitz H, Fehrenbacher L, Novotny W, Cartwright T, Hainsworth J, Heim W, Berlin J, Baron A, Griffing S, Holmgren E, Ferrara N, Fyfe G, Rogers B, Ross R, Kabbinavar F. Bevacizumab plus irinotecan, fluorouracil, and leucovorin for metastatic colorectal cancer. N Engl J Med. 2004;350(23):2335–2342. doi: 10.1056/NEJMoa032691. [DOI] [PubMed] [Google Scholar]
- Jain RK. Transport of molecules in the tumor interstitium: a review. Cancer Res. 1987;47(12):3039–3051. [PubMed] [Google Scholar]
- Jain RK, Baxter LT. Mechanisms of heterogeneous distribution of monoclonal antibodies and other macromolecules in tumors: significance of elevated interstitial pressure. Cancer Res. 1988;48(24 Pt 1):7022–7032. [PubMed] [Google Scholar]
- Jirtle RL. Blood flow to lymphatic metastases in conscious rats. Eur J Cancer. 1981;17(1):53–60. doi: 10.1016/0014-2964(81)90211-5. [DOI] [PubMed] [Google Scholar]
- Katragadda S, Budda B, Anand BS, Mitra AK. Role of efflux pumps and metabolising enzymes in drug delivery. Expert Opin Drug Deliv. 2005;2(4):683–705. doi: 10.1517/17425247.2.4.683. [DOI] [PubMed] [Google Scholar]
- Koh TS. On the a priori identifiability of the two-compartment distributed parameter model from residual tracer data acquired by dynamic contrast-enhanced imaging. IEEE Trans Biomed Eng. 2008;55(1):340–344. doi: 10.1109/TBME.2007.910682. [DOI] [PubMed] [Google Scholar]
- Leander P, Mansson S, Ege T, Besjakov J. CT and MR imaging of the liver using liver-specific contrast media. A comparative study in a tumour model. Acta Radiol. 1996;37(3 Pt 1):242–249. doi: 10.1177/02841851960371P155. [DOI] [PubMed] [Google Scholar]
- Lee CG, Heijn M, di Tomaso E, Griffon-Etienne G, Ancukiewicz M, Koike C, Park KR, Ferrara N, Jain RK, Suit HD, Boucher Y. Anti-Vascular endothelial growth factor treatment augments tumor radiation response under normoxic or hypoxic conditions. Cancer Res. 2000;60(19):5565–5570. [PubMed] [Google Scholar]
- Less JR, Posner MC, Boucher Y, Borochovitz D, Wolmark N, Jain RK. Interstitial hypertension in human breast and colorectal tumors. Cancer Res. 1992;52(22):6371–6374. [PubMed] [Google Scholar]
- Litman T, Druley TE, Stein WD, Bates SE. From MDR to MXR: new understanding of multidrug resistance systems, their properties and clinical significance. Cell Mol Life Sci. 2001;58(7):931–959. doi: 10.1007/PL00000912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lyman Ott R, Longnecker M. Statitical Methods and Data Analysis. Duxbury; Pacific Grove: 2001. pp. 627–657. [Google Scholar]
- Manduca A, Oliphant TE, Dresner MA, Mahowald JL, Kruse SA, Amromin E, Felmlee JP, Greenleaf JF, Ehman RL. Magnetic resonance elastography: non-invasive mapping of tissue elasticity. Med Image Anal. 2001;5(4):237–254. doi: 10.1016/s1361-8415(00)00039-6. [DOI] [PubMed] [Google Scholar]
- Matteucci ML, Anyarambhatla G, Rosner G, Azuma C, Fisher PE, Dewhirst MW, Needham D, Thrall DE. Hyperthermia increases accumulation of technetium-99m-labeled liposomes in feline sarcomas. Clin Cancer Res. 2000;6(9):3748–3755. [PubMed] [Google Scholar]
- Milosevic M, Fyles A, Hedley D, Pintilie M, Levin W, Manchul L, Hill R. Interstitial fluid pressure predicts survival in patients with cervix cancer independent of clinical prognostic factors and tumor oxygen measurements. Cancer Res. 2001;61(17):6400–6405. [PubMed] [Google Scholar]
- Mimeault M, Hauke R, Batra SK. Recent advances on the molecular mechanisms involved in the drug resistance of cancer cells and novel targeting therapies. Clin Pharmacol Ther. 2008;83(5):673–691. doi: 10.1038/sj.clpt.6100296. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nathanson SD, Nelson L. Interstitial fluid pressure in breast cancer, benign breast conditions, and breast parenchyma. Ann Surg Oncol. 1994;1(4):333–338. doi: 10.1007/BF03187139. [DOI] [PubMed] [Google Scholar]
- Netti PA, Baxter LT, Boucher Y, Skalak R, Jain RK. Time-dependent behavior of interstitial fluid pressure in solid tumors: implications for drug delivery. Cancer Res. 1995;55(22):5451–5458. [PubMed] [Google Scholar]
- Netti PA, Berk DA, Swartz MA, Grodzinsky AJ, Jain RK. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000;60(9):2497–2503. [PubMed] [Google Scholar]
- Netti PA, Roberge S, Boucher Y, Baxter LT, Jain RK. Effect of transvascular fluid exchange on pressure-flow relationship in tumors: a proposed mechanism for tumor blood flow heterogeneity. Microvasc Res. 1996;52(1):27–46. doi: 10.1006/mvre.1996.0041. [DOI] [PubMed] [Google Scholar]
- Quinn PK, Bibby MC, Cox JA, Crawford SM. The influence of hydralazine on the vasculature, blood perfusion and chemosensitivity of MAC tumours. Br J Cancer. 1992;66(2):323–330. doi: 10.1038/bjc.1992.264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reed RK, Berg A, Gjerde EA, Rubin K. Control of interstitial fluid pressure: role of beta1-integrins. Semin Nephrol. 2001;21(3):222–230. doi: 10.1053/snep.2001.21646. [DOI] [PubMed] [Google Scholar]
- Rofstad EK, Tunheim SH, Mathiesen B, Graff BA, Halsor EF, Nilsen K, Galappathi K. Pulmonary and lymph node metastasis is associated with primary tumor interstitial fluid pressure in human melanoma xenografts. Cancer Res. 2002;62(3):661–664. [PubMed] [Google Scholar]
- Stone HB, Minchinton AI, Lemmon M, Menke D, Brown JM. Pharmacological modification of tumor blood flow: lack of correlation between alteration of mean arterial blood pressure and changes in tumor perfusion. Int J Radiat Oncol Biol Phys. 1992;22(1):79–86. doi: 10.1016/0360-3016(92)90985-q. [DOI] [PubMed] [Google Scholar]
- Suga K, Mikawa M, Ogasawara N, Okazaki H, Matsunaga N. Potential of Gd-DTPA-mannan liposome particles as a pulmonary perfusion MRI contrast agent: an initial animal study. Invest Radiol. 2001;36(3):136–145. doi: 10.1097/00004424-200103000-00002. [DOI] [PubMed] [Google Scholar]
- Thomsen HS. Nephrogenic systemic fibrosis: A serious late adverse reaction to gadodiamide. Eur Radiol. 2006;16(12):2619–2621. doi: 10.1007/s00330-006-0495-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomsen HS, Marckmann P, Logager VB. Nephrogenic systemic fibrosis (NSF): a late adverse reaction to some of the gadolinium based contrast agents. Cancer Imaging. 2007;7:130–137. doi: 10.1102/1470-7330.2007.0019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tofts PS. Modeling tracer kinetics in dynamic Gd-DTPA MR imaging. J Magn Reson Imaging. 1997;7(1):91–101. doi: 10.1002/jmri.1880070113. [DOI] [PubMed] [Google Scholar]
- Tofts PS, Berkowitz B, Schnall MD. Quantitative analysis of dynamic Gd-DTPA enhancement in breast tumors using a permeability model. Magn Reson Med. 1995;33(4):564–568. doi: 10.1002/mrm.1910330416. [DOI] [PubMed] [Google Scholar]
- Tofts PS, Berkowitz BA. Measurement of capillary permeability from the Gd enhancement curve: a comparison of bolus and constant infusion injection methods. Magn Reson Imaging. 1994;12(1):81–91. doi: 10.1016/0730-725x(94)92355-8. [DOI] [PubMed] [Google Scholar]
- Tofts PS, Brix G, Buckley DL, Evelhoch JL, Henderson E, Knopp MV, Larsson HB, Lee TY, Mayr NA, Parker GJ, Port RE, Taylor J, Weisskoff RM. Estimating kinetic parameters from dynamic contrast-enhanced T(1)-weighted MRI of a diffusable tracer: standardized quantities and symbols. J Magn Reson Imaging. 1999;10(3):223–232. doi: 10.1002/(sici)1522-2586(199909)10:3<223::aid-jmri2>3.0.co;2-s. [DOI] [PubMed] [Google Scholar]
- Tong RT, Boucher Y, Kozin SV, Winkler F, Hicklin DJ, Jain RK. Vascular normalization by vascular endothelial growth factor receptor 2 blockade induces a pressure gradient across the vasculature and improves drug penetration in tumors. Cancer Res. 2004;64(11):3731–3736. doi: 10.1158/0008-5472.CAN-04-0074. [DOI] [PubMed] [Google Scholar]
- Wiig H, Reed RK, Aukland K. Measurement of interstitial fluid pressure: comparison of methods. Ann Biomed Eng. 1986;14(2):139–151. doi: 10.1007/BF02584264. [DOI] [PubMed] [Google Scholar]
- Wildiers H, Guetens G, De Boeck G, Verbeken E, Landuyt B, Landuyt W, de Bruijn EA, van Oosterom AT. Effect of antivascular endothelial growth factor treatment on the intratumoral uptake of CPT-11. Br J Cancer. 2003;88(12):1979–1986. doi: 10.1038/sj.bjc.6601005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Willett CG, Boucher Y, di Tomaso E, Duda DG, Munn LL, Tong RT, Chung DC, Sahani DV, Kalva SP, Kozin SV, Mino M, Cohen KS, Scadden DT, Hartford AC, Fischman AJ, Clark JW, Ryan DP, Zhu AX, Blaszkowsky LS, Chen HX, Shellito PC, Lauwers GY, Jain RK. Direct evidence that the VEGF-specific antibody bevacizumab has antivascular effects in human rectal cancer. Nat Med. 2004;10(2):145–147. doi: 10.1038/nm988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J, Salmon H, Sarntinoranont M. Effect of heterogeneous vasculature on interstitial transport within a solid tumor. Microvasc Res. 2007;73(3):224–236. doi: 10.1016/j.mvr.2006.12.003. [DOI] [PubMed] [Google Scholar]