SUMMARY
In obesity, anorectic responses to leptin are diminished, giving rise to the concept of ‘leptin resistance’. Increased expression of protein tyrosine phosphatase 1B (PTP1B) has been associated with the attenuation of leptin signaling and development of cellular leptin resistance. Here we report that hypothalamic levels of the tyrosine phosphatase TCPTP are also elevated in obesity to attenuate the leptin response. We show that mice that lack TCPTP in neuronal cells have enhanced leptin sensitivity and are resistant to high fat diet-induced weight gain and the development of leptin resistance. Also, intracerebroventricular administration of a TCPTP inhibitor enhances leptin signaling and responses in mice. Moreover, the combined deletion of TCPTP and PTP1B in neuronal cells has additive effects in the prevention of diet-induced obesity. Our results identify TCPTP as a critical negative regulator of hypothalamic leptin signaling and causally link elevated TCPTP to the development of cellular leptin resistance in obesity.
INTRODUCTION
Leptin is an important hormone that integrates peripheral energy stores with the central adaptive control of energy expenditure for survival and reproductive fitness. Leptin acutely decreases food intake and body weight, increases energy expenditure in lean humans and animals and reverses obesity and the associated pathologies in leptin-deficient rodents and humans (Myers et al., 2008). However, leptin responsiveness decreases with increasing adiposity, and obese rodents and humans are resistant to the effects of leptin on body weight (Myers et al., 2008). The decreased leptin sensitivity in obesity is associated with alterations in varied cellular and molecular processes that attenuate the leptin signal (Myers et al., 2008). Therefore, approaches that enhance leptin signalling may be effective in overcoming cellular leptin resistance and combating obesity.
Leptin acts in several regions of the hypothalamus, including the arcuate nucleus (ARC), the ventromedial hypothalamus (VMH) and the dorsomedial hypothalamus (DMH) to regulate body weight and glucose homeostasis (Myers et al., 2008). In the ARC leptin acts on anorexigenic proopiomelanocortin (POMC; the precursor of α-melanocyte-stimulating hormone, α-MSH) and orexigenic neuropeptide Y (NPY) and agouti-related peptide (AgRP) expressing neurons. POMC expression is increased by leptin, whereas AgRP (antagonizes α-MSH binding to melanocortin receptors) and NPY levels are decreased (Cowley et al., 2001; Elias et al., 1999). These changes in neuropeptide expression serve to decrease food intake and increase locomotor activity and metabolic rate to enhance weight loss and glucose homeostasis (Myers et al., 2008).
Leptin signals by binding to the leptin receptor (LEPR-B) to activate the tyrosine kinase JAK2 (Janus activated kinase 2), which in turn phosphorylates LEPR-B and promotes signaling via several effector cascades, including the Ras/mitogen-activated protein kinase (MAPK), phosphatidylinositol 3-kinase (PI3K)/Akt and STAT3 (signal transducer and activator of transcription 3) pathways (Myers et al., 2008). The STAT3 pathway is particularly critical and necessary for the effects of leptin on food intake and body weight (Bates et al., 2003; Myers et al., 2008). STAT3 is recruited to the LEPR-B and is phosphorylated by JAK2 on Tyr-705, allowing for STAT3 dimerisation and translocation to the nucleus to mediate gene transcription. The activation of STAT3 stimulates POMC expression and suppresses AgRP expression (Myers et al., 2008); other leptin-activated pathways regulate the expression of NPY (Bates et al., 2003).
Two important negative regulators of leptin signaling have been implicated in the development of cellular leptin resistance, protein tyrosine phosphatase (PTP) PTP1B (encoded by Ptpn1) and suppressor of cytokine signaling-3 (SOCS3). PTP1B is localised on the cytoplasmic face of the endoplasmic reticulum (ER) and attenuates leptin signaling by dephosphorylating and inactivating JAK2 (Myers et al., 2001), but not STAT3 (Cheng et al., 2002). Whole body- (Ptpn−/−), brain- (Ptpn1loxlox; Nestin-Cre), or POMC neuron-specific (Ptpn1loxlox; Pomc-Cre)-PTP1B knockout mice exhibit leptin hypersensitivity, increased energy expenditure and insulin sensitivity, and resistance to diet-induced obesity (DIO) (Banno et al., 2010; Bence et al., 2006; Cheng et al., 2002; Elchebly et al., 1999; Klaman et al., 2000; Zabolotny et al., 2002). Furthermore, hypothalamic PTP1B knockdown with antisense oligonucleotides or intracerebroventricular (icv) administration of PTP1B inhibitors enhance leptin sensitivity, decrease weight gain and improve insulin sensitivity in rodent models of obesity (Morrison et al., 2007; Picardi et al., 2008), validating hypothalamic PTP1B as a therapeutic target for the treatment of the disease. SOCS3 attenuates leptin signaling by binding and inhibiting JAK2 and by targeting the LEPR-B complex, or components thereof for proteasomal degradation (Myers et al., 2008). Socs3 is a transcriptional target for STAT3 and hypothalamic Socs3 levels are increased after leptin administration, consistent with SOCS3 acting in a negative feedback loop (Bjorbaek et al., 1998). As for PTP1B, whole brain- or neuronal cell-specific Socs3 knockout mice exhibit enhanced leptin sensitivity and resistance to DIO (Kievit et al., 2006; Mori et al., 2004). PTP1B and SOCS3 levels are elevated in obesity, driven in part by the hyperleptinemia, which is characteristic of the obese state, as well as by the inflammation and ER stress that are important in the aetiology of cellular leptin resistance and the pathology of obesity (Bjorbaek et al., 1998; Morrison et al., 2007; Ozcan et al., 2009; Zabolotny et al., 2008; Zhang et al., 2008).
Although PTP1B and SOCS3 are important negative regulators of hypothalamic leptin signaling and increases in PTP1B and SOCS3 expression contribute to the development of cellular leptin resistance, deletion of either (Bence et al., 2006; Mori et al., 2004), or both (Briancon et al., 2010), in neuronal cells reduces, but does not prevent DIO in mice. Thus, additional molecular factors might contribute to the development of cellular leptin-resistance and DIO. T cell PTP (TCPTP; encoded by Ptpn2) is closely related to PTP1B, sharing a high degree of primary (72% identity) and tertiary structural similarity (Shields et al., 2008; Tiganis and Bennett, 2007; Yamamoto et al., 2002). Despite this, PTP1B and TCPTP exhibit exquisite substrate selectivity in vivo; for example, PTP1B dephosphorylates JAK2, but not JAK1/3, and TCPTP dephosphorylates JAK1/3, but not JAK2 (Myers et al., 2001; Simoncic et al., 2002; Zabolotny et al., 2002). Moreover, alternative splicing of Ptpn2 message can give rise to two TCPTP variants: a 48 kDa TCPTP (TC48) that is targeted to the ER and a 45 kDa variant (TC45) that shuttles in and out of the nucleus; nuclear substrates of TCPTP include STAT3 (Shields et al., 2008; Tiganis and Bennett, 2007; Yamamoto et al., 2002). Here we identify TCPTP as an integral negative regulator of leptin-induced STAT3 signaling. We report that hypothalamic TCPTP levels are increased in obesity acting together with elevated PTP1B in the attenuation of the leptin signal and the development of cellular leptin resistance.
RESULTS
Hypothalamic TCPTP is increased in obesity
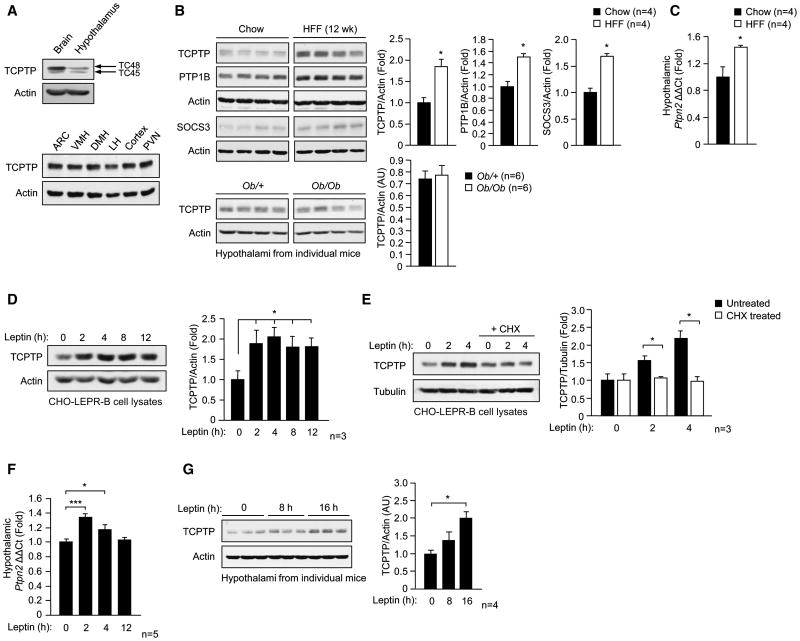
As a first step towards elucidating TCPTP’s role in the central control of body weight and energy homeostasis we assessed TCPTP expression in the hypothalami of lean versus high fat-fed [HFF; 23% fat (45% energy from fat)] obese C57BL/6 mice. TCPTP was expressed in the hypothalami of mice and detected in the ARC, VMH, DMH, lateral hypothalamus (LH) and the paraventricular nucleus (PVN) of the hypothalamus (Fig. 1a). Hypothalamic TCPTP (TC48 and TC45) protein levels were increased by as much as 2-fold in HFF obese mice (Fig. 1b). Consistent with previous studies, we noted similar increases in hypothalamic PTP1B and SOCS3 levels (Fig. 1b). The increase in TCPTP protein coincided with a 1.4-fold increase in Ptpn2 message as assessed by quantitative real time PCR (Fig. 1c). By contrast, TCPTP protein levels were not altered in skeletal muscle, liver or fat from obese mice (Supplementary Fig. 1). Moreover, TCPTP expression was not elevated in the hypothalami of chow-fed leptin-deficient (Ob/Ob) obese mice when compared to lean (Ob/+) controls (Fig. 1b). Therefore, the hyperleptinemia in DIO might be responsible for the elevated hypothalamic TCPTP protein. To test this directly, we determined whether leptin administration could promote TCPTP expression in CHO cells stably overexpressing the leptin receptor (CHO-LEPR-B). TCPTP expression was increased by approximately 2-fold in response to leptin, and this could be prevented if CHO-LEPR-B cells were pre-treated with the protein synthesis inhibitor cycloheximide (Fig. 1d–e). We also determined if TCPTP expression could be induced by leptin in vivo. Leptin was administered to 8 week-old C57BL/6 mice, and hypothalamic Ptpn2 message and TCPTP protein levels were determined. Leptin treatment resulted in a transient increase in Ptpn2 message within 2 h of administration, and repeated dosing increased TCPTP protein in the hypothalamus (Fig. 1f–g). Therefore, these results indicate that TCPTP expression can be induced by leptin and that the elevated hypothalamic TCPTP in the HFF obese state is driven by the accompanying hyperleptinemia.
Fig. 1. TCPTP expression is elevated in obesity and is induced by leptin.
(a) Extracts of whole brain, hypothalamus and microdissected ARC, VMH, DMH, LH, cortex and PVN were processed for immunoblot analysis. (b) Hypothalamic extracts from 8 week old chow-fed versus 12 week HFF C57BL/6 male mice, or chow-fed Ob/+ lean versus leptin-deficient Ob/Ob obese mice were processed for immunoblot analysis. (c) Hypothalami were extracted from chow-fed versus 12 week HFF C57BL/6 mice and processed for quantitative real time PCR to measure Ptpn2 expression. (d–e) CHO-LEPR-B cells were serum starved for 12 h and left untreated or pre-treated with 10 μg/ml cycloheximide (CHX) for 4 h and stimulated with 10 ng/ml murine leptin for the indicated times and processed for immunoblot analysis. 8 week old C57BL/6 mice were fasted for 18 h and (f) administered leptin [5 μg/g intraperitoneal (IP)] and hypothalami extracted at the indicated times and processed for real time PCR to measure ptpn2 expression, or (g) administered leptin (every 2 h for 16 h) and hypothalami extracted for immunoblot analysis. Results in a are representative of two and b–g of at least three independent experiments; quantified results are means ± SEM.
Neuronal TCPTP-deficient mice
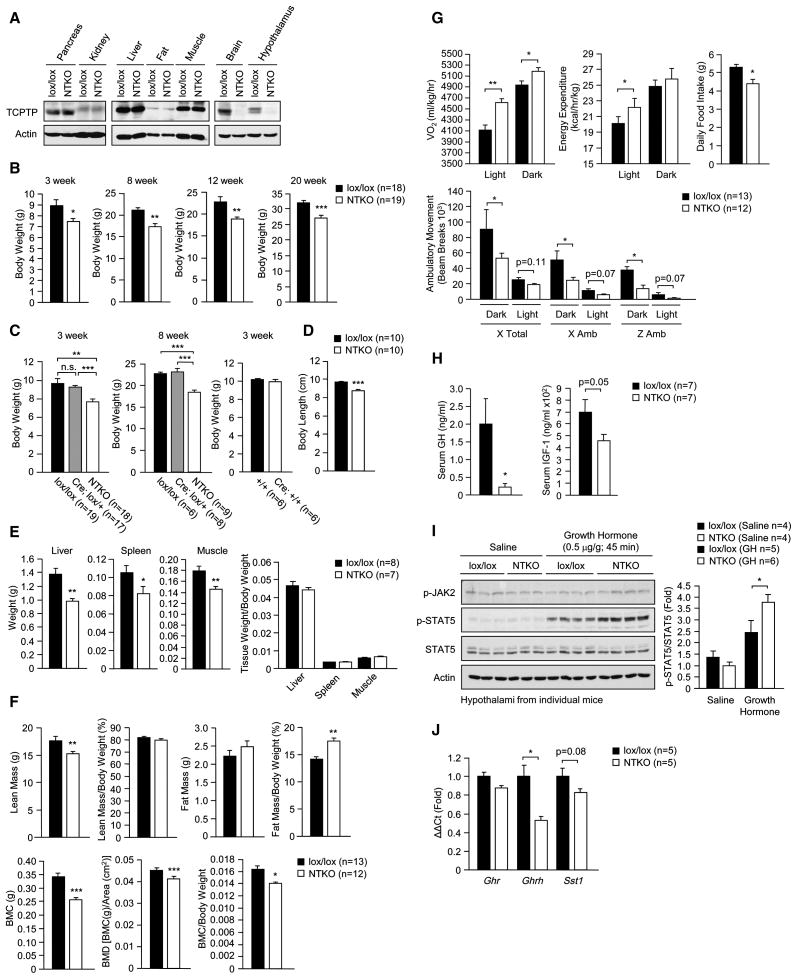
To determine if TCPTP has a role in the central control of body weight and glucose homeostasis in vivo we generated a floxed allele of Ptpn2 (loxP sites flanking exons 5 and 6 encoding the core of the catalytic domain including the catalytically essential Asp-182 and Cys-216 residues; Supplementary Fig. 2). Ptpn2lox/lox (C57BL/6) mice were crossed with mice expressing the Cre recombinase under the control of the Nestin promoter (Nes-Cre; C57BL/6) to excise Ptpn2 in neuronal cells. TCPTP expression, as assessed by immunoblot analysis, was ablated in brain in Nes-Cre; Ptpn2lox/lox mice, but not in pancreas, kidney, liver, fat, muscle or bone marrow (Fig. 2a; data not shown). TCPTP deletion did not result in a compensatory increase in PTP1B in the hypothalami of chow-fed mice (data not shown).
Fig. 2. Generation of neuronal cell TCPTP-deficient mice.
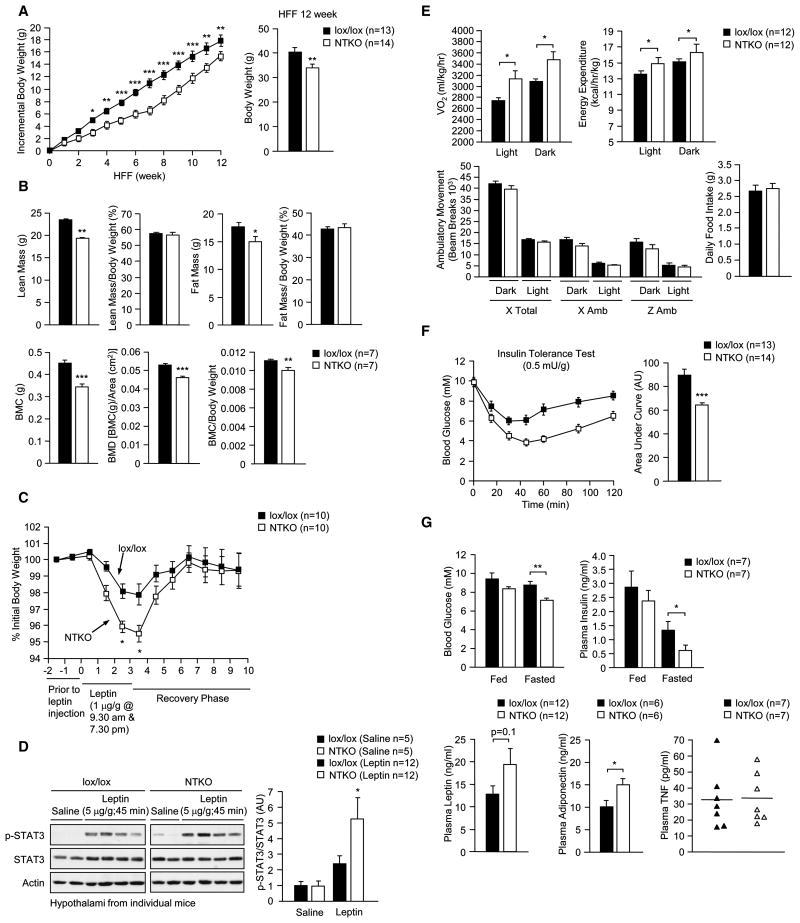
(a) TCPTP protein levels in Ptpn2lox/lox control (lox/lox) and Nes-Cre;Ptpn2lox/lox neuronal cell TCPTP knockout (NTKO) mice. (b–c) Body weights in 3–20 week old male lox/lox, Nes-Cre;Ptpn2lox/+ (Cre;lox/+), NTKO, Ptpn2+/+ (+/+) and Nes-Cre;Ptpn2+/+ (Cre;+/+) mice. (d) Nose to tail body lengths in 8 week old and (e) tissue weights in 8–10 week old male lox/lox and NTKO mice. (f) Body composition [lean, fat, bone mineral content (BMC) and bone mineral density (BMD)] measured by DEXA and normalised to total body weight and (g) light and dark cycle oxygen consumption, energy expenditure, ambulatory activity (x and z axes) and daily food intake in 8–10 week old lox/lox and NTKO mice. (h) Fed serum GH and IGF-1 levels (collected at 8 am) from 8 week old male lox/lox and NTKO mice. (i) 8 week old male lox/lox and NTKO mice were fasted for 18 h and injected with saline or GH (0.5 μg/g IP) and hypothalami extracted and processed for immunoblot analysis with antibodies to Y1007/Y1008 phosphorylated JAK2 (p-JAK2) and phosphorylated (Y694) STAT5 (p-STAT5) and reprobed for STAT5 and actin. (j) Hypothalami were extracted from 8 week old male lox/lox and NTKO mice (at 8 am) and processed for real time PCR to measure Ghrh, Ghr and Sst1 expression. Results are representative of at least three independent experiments; quantified results are means ± SEM for the indicated number of mice.
Body weights in 3 week old Nes-Cre; Ptpn2lox/lox mice were approximately 20% lower that those of Ptpn2lox/lox control littermates (Fig. 2b). No overt difference in body weight was evident in heterozygous Nes-Cre; Ptpn2lox/+ compared to Ptpn2lox/lox male mice, and no difference was noted between Nes-Cre; Ptpn2+/+ and Ptpn2+/+ littermate controls (Fig. 2c), consistent with the difference in size being due to TCPTP deficiency rather than the Nes-Cre transgene. This difference in body weight was evident in male and female mice and persisted in older mice, coinciding with significant decreased body length (Fig. 2b,d; Supplementary Fig. 3a). Corresponding tissue weights also were lower in Nes-Cre; Ptpn2lox/lox mice, but normalised when corrected for total body weight, consistent with Nes-Cre; Ptpn2lox/lox mice being proportionately smaller (Fig. 2e). Dual energy X-ray absorptiometry (DEXA) in 8 week-old mice revealed that lean mass (tissues other than bone and fat) was reduced, but normal when corrected for body weight (Fig. 2f). By contrast, bone mineral density was reduced and remained lower when normalised for body weight (Fig. 2f). The decrease in bone mineral density was associated with a decrease in trabecular bone volume and decreased bone marrow cellularity (Supplementary Fig. 3b–c). A decrease in bone mineral content has been noted previously in neuronal cell-specific (Nes-Cre; Ptpn1lox/lox) PTP1B knockout mice (Bence et al., 2006) and is consistent with leptin hypersensitivity and the established potential of hypothalamic leptin signaling to inhibit bone formation via the sympathetic control of osteoblasts (Takeda, 2005). We found that fat mass as determined by DEXA was unaltered in male Nes-Cre; Ptpn2lox/lox versus Ptpn2lox/lox mice and decreased in female Nes-Cre; Ptpn2lox/lox mice, but relative adiposity in Nes-Cre; Ptpn2lox/lox mice was increased irrespective of gender (Fig. 2f; Supplementary Fig. 3d). The increased relative adiposity coincided with decreased ambulatory activity, but increased oxygen consumption and energy expenditure and decreased daily food intake (Fig. 2g; Supplementary Fig. 3e–f).
Altered GH/IGF-1 axis in neuronal TCPTP-deficient mice
Growth hormone (GH) regulates important physiological processes acting directly and indirectly, through insulin-like growth factor (IGF)-1, to regulate growth and development as well as carbohydrate and lipid metabolism (Lichanska and Waters, 2008). GH is released from the anterior pituitary and signals via JAK2 and STAT5 to promote lipolysis in fat and IGF-1 production in the liver (Lichanska and Waters, 2008). Importantly, GH and IGF-1 feed back onto somatotrophs as well as hypothalamic neurons to repress GH-releasing hormone (GHRH) and stimulate somatostatin expression, thereby inhibiting GH release (Becker et al., 1995; Burton et al., 1992; Minami et al., 1993; Romero et al., 2010). Somatostatin levels are decreased in STAT5b-deficient mice (Bennett et al., 2005) consistent with STAT5 being important in the feedback regulation of GH production. Cell-based studies have shown that Y694 phosphorylated STAT5 can serve as a TCPTP substrate (Aoki and Matsuda, 2002; Simoncic et al., 2002). As GH deficiency in rodents and humans is associated with dwarfism and increased adiposity (Lichanska and Waters, 2008), we asked whether the GH/IGF-1 axis was perturbed in Nes-Cre; Ptpn2lox/lox mice. We found that circulating GH and IGF-1 levels (from fed mice at 8 am) were reduced in Nes-Cre; Ptpn2lox/lox mice (Fig. 2h) coinciding with elevated hypothalamic GH-induced STAT5 Y694 phosphorylation (Fig. 2i) and reduced Ghrh expression (Fig. 2j). These results are consistent with neuronal TCPTP-deficiency affecting the GH/IGF-1 axis, and the decreased size and the increased relative adiposity in Nes-Cre; Ptpn2lox/lox mice being due to lower circulating GH.
Enhanced leptin sensitivity in neuronal TCPTP-deficient mice
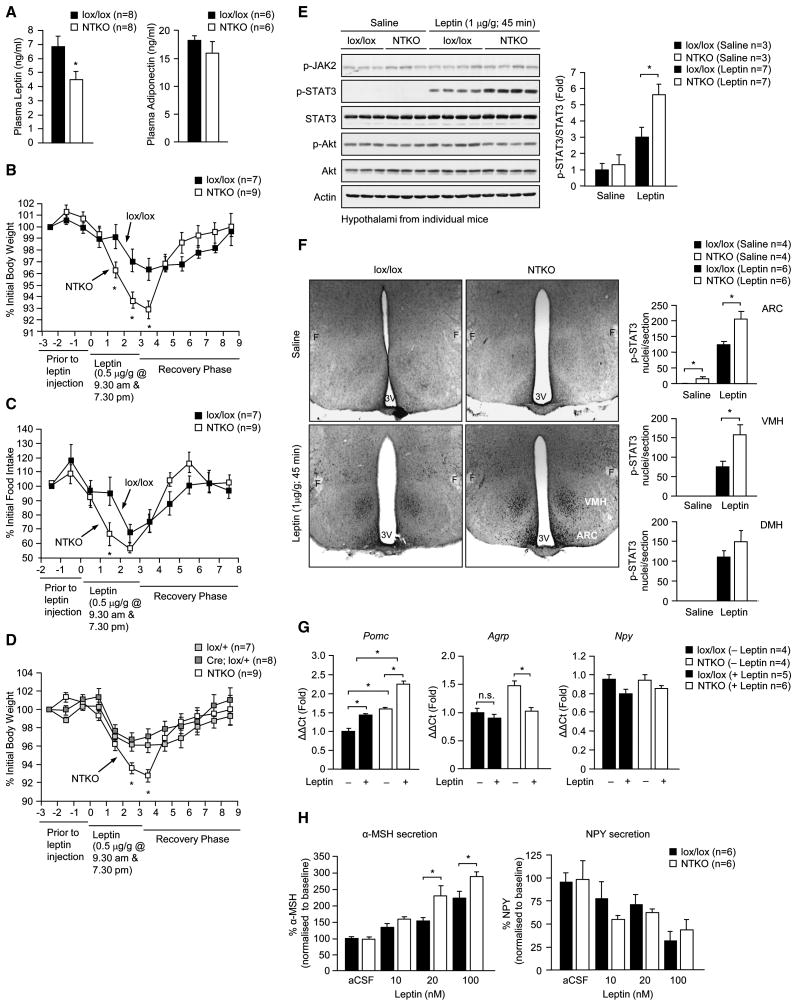
Central leptin signaling lowers plasma leptin by decreasing fat mass through the control of appetite and energy expenditure and by increasing sympathetic activity to adipose tissue to both promote lipolysis and inhibit leptin gene expression (Bartness et al., 2010; Commins et al., 2000) (Myers et al., 2008). Despite the increased relative adiposity, chow-fed 8 week-old male and female Nes-Cre; Ptpn2lox/lox mice had decreased fed plasma leptin levels compared with controls (Fig. 3a; Supplementary Fig. 4a). Plasma adiponectin, which is produced by fat, inversely correlates with fat mass and promotes insulin sensitivity (Shetty et al., 2009), was unaltered by TCPTP deficiency in chow-fed mice (Fig. 3a) indicating that adipokine production was not in general altered. The lower leptin levels in the face of increased relative adiposity, in conjunction with decreased food intake and increased energy expenditure are consistent with a significant enhancement in central leptin sensitivity. To directly examine leptin sensitivity we administered leptin to 8–10 week old Nes-Cre; Ptpn2lox/lox and Ptpn2lox/lox littermates in the mornings and evenings for 3 consecutive days and recorded body weights and food intake daily. The effects of leptin on body weight and food intake were enhanced in male and female Nes-Cre; Ptpn2lox/lox mice (Fig. 3b–c; Supplementary Fig. 4b). By contrast, leptin-induced changes in body weight were not altered in Nes-Cre; Ptpn2lox/+ compared to Ptpn2lox/+ heterozygous mice (Fig. 3d). The elevated leptin sensitivity in Nes-Cre; Ptpn2lox/lox mice coincided with enhanced hypothalamic leptin signaling [as assessed by immunoblot (Fig. 3e) or immunohistochemical (Fig. 3f) analysis]. Hypothalamic STAT3 Y705 phosphorylation, but not JAK2 Y1007/Y1008 or Akt Ser-473 phosphorylation, was increased in Nes-Cre; Ptpn2lox/lox male and female mice after bolus leptin administration (Fig. 3e; Supplementary Fig. 4c). Basal STAT3 phosphorylation was increased significantly in the ARC, whereas leptin-induced phosphorylation was elevated in the ARC and VMH, but not the DMH (Fig. 3f). These results are consistent with TCPTP selectively regulating leptin signaling in hypothalamic nuclei and demonstrate that TCPTP-deficiency enhances leptin signaling in nuclei that are essential for the control of body weight and metabolism.
Fig. 3. Neuronal TCPTP deficiency enhances leptin sensitivity.
(a) Fed plasma leptin and adiponectin in 8–10 week old male lox/lox and NTKO mice. (b–d) 8–10 week old male lox/lox, NTKO, Ptpn2lox/+ (lox/+) and Nes-Cre;Ptpn2lox/+ (Cre;lox/+) mice were administered leptin and body weight and food intake monitored as indicated; b and d were undertaken at the same time and the NTKO data set is the same in both. (e–f) 8–10 week old male lox/lox and NTKO mice were fasted for 18 h and injected with saline or leptin and (e) hypothalami extracted and processed for immunoblot analysis with antibodies to p-STAT3, p-JAK2, or Ser-473 phosphorylated Akt (p-Akt) and reprobed as indicated, or (f) paraformaldehyde (PFA)-perfused brains extracted and processed for immunohistochemistry with antibodies to p-STAT3, or (g) hypothalami extracted after 2 h and processed for real time PCR to measure Pomc, Agrp and Npy expression. In (f) p-STAT3 positive nuclei in the ARC, DMH or VMH were counted in serial sections. (h) Hypothalamic slices from 8–10 week old male lox/lox and NTKO mice were incubated in aCSF for 45 min (basal) and then stimulated with leptin (20–100 nM) for 45 min. Secreted NPY and a-MSH were measured by radioimmunoassay. Results in a–e and h are representative of three independent experiments; quantified results are means ± SEM for the indicated number of mice.
Selective regulation of STAT3 signaling
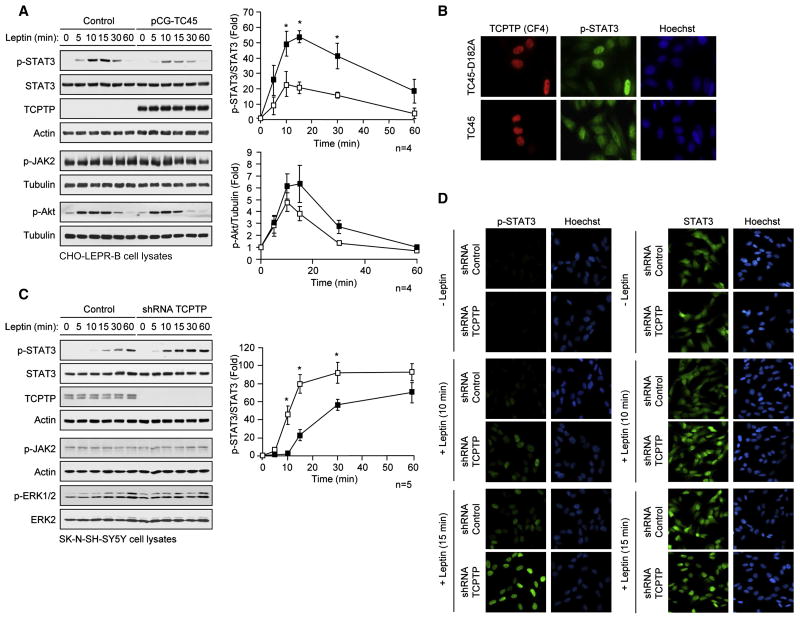
To determine how TCPTP affects leptin sensitivity in vivo we examined TCPTP’s capacity to regulate leptin signaling in vitro. Overexpression of TC45 in LEPR-B-expressing CHO cells suppressed leptin-induced STAT3 Y705 phosphorylation (Fig. 4a), but did not significantly alter JAK2 Y1007/Y1008 phosphorylation, or downstream PI3K/Akt signaling, as assessed by Akt Ser-473 phosphorylation (Fig. 4a), consistent with TC45 acting selectively on STAT3. To determine the subcellular compartment in which TC45 dephosphorylates STAT3 in response to leptin, we took advantage of the TC45-D182A ‘substrate-trapping’ mutant, which forms stable complexes with tyrosine phosphorylated substrates in a cellular context and prevents their dephosphorylation from endogenous phosphatases (Tiganis and Bennett, 2007; Tiganis et al., 1998). Expression of TC45-D182A in LEPR-B-expressing CHO cells resulted in the nuclear accumulation of Y705 phosphorylated STAT3 (Fig. 4b), consistent with TC45 dephosphorylating STAT3 in the nucleus. In addition, TC45-D182A was not evident in the cytoplasm (Fig. 4b) consistent with TC45 acting exclusively on nuclear substrates. To complement the overexpression studies we stably knocked down TCPTP by RNA interference in SK-N-SH-SY5Y neuroblastoma cells. TCPTP knockdown resulted in elevated leptin-induced STAT3 phosphorylation but importantly, did not affect the activation of the Ras/MAPK pathway (ERK1/2 phosphorylation; Fig. 4c), which occurs downstream of JAK2 and in parallel to the STAT3 pathway; high basal JAK2 and Akt phosphorylation precluded any assessment of leptin-induced JAK2/Akt signaling in SK-N-SH-SY5Y cells (Fig. 4c). Finally, TCPTP knockdown resulted in enhanced nuclear (but not cytoplasmic) accumulation of phosphorylated STAT3 (Fig. 4d). Taken together, these results are consistent with TCPTP attenuating leptin-induced STAT3 signaling through the selective dephosphorylation of STAT3.
Fig. 4. Selective regulation leptin-induced STAT3 signaling.
(a) CHO-LEPR-B cells transfected with vector control or constructs for the expression of 45 kDa TCPTP (TC45) were serum starved and stimulated with murine leptin and processed for immunoblot analysis. (b) CHO-LEPR-B cells transfected with constructs for the expression of wildtype TC45, or TC45-D182A ‘substrate-trapping’ mutant, were serum starved and stimulated with leptin for the indicated times and processed for immunofluorescence microscopy with antibodies to p-STAT3 and TCPTP and stained with Hoechst to visualise nuclei. SK-N-SH-SY5Y cells transduced with control or TCPTP shRNA lentiviral particles were serum starved, stimulated with leptin and processed for (c) immunoblot analysis, or (d) immunofluorescence microscopy. Results are representative of at least three independent experiments; quantified results are means ± SEM.
TCPTP-deficiency affects Pomc and Agrp, but not Npy expression
Leptin suppresses the expression of the orexigenic neuropeptides NPY and AgRP and promotes the expression of the anorexigenic neuropeptide POMC (Myers et al., 2008). Mice were fasted for 18 h and hypothalamic Pomc, Agrp and Npy expression assessed by quantitative real time PCR, before and 2 h after bolus leptin (5μg/g IP) administration. Basal hypothalamic Pomc levels were elevated in Nes-Cre; Ptpn2lox/lox mice consistent with enhanced leptin sensitivity (Fig. 3g). Fasted levels of hypothalamic Agrp were also increased, similar to the effects of neuronal PTP1B deficiency (Bence et al., 2006), possibly representing a compensatory response to neuronal TCPTP deficiency. Nevertheless, neuronal TCPTP deficiency exaggerated the effects of leptin on Pomc and Agrp expression consistent with enhanced leptin sensitivity; Npy expression was not altered by TCPTP deficiency (Fig. 3g). To exclude any extrinsic influences on leptin sensitivity and neuropeptide expression we also measured the secretion of α-MSH and NPY ex vivo using hypothalamic blocks prepared from Nes-Cre;Ptpn2lox/lox and Ptpn2lox/lox mice (Fig. 3h). Leptin-induced α-MSH secretion ex vivo was increased significantly by TCPTP deficiency, whereas NPY secretion was decreased comparably by leptin with or without TCPTP (Fig. 3h). Taken together, these results are consistent with neuronal cell TCPTP deficiency enhancing the effects of leptin on neuropeptide expression to alter food intake and body weight. Moreover, given the specific alterations in POMC/α-MSH and AgRP but not NPY, these results are consistent with TCPTP exerting its effects via STAT3 (Bates et al., 2003).
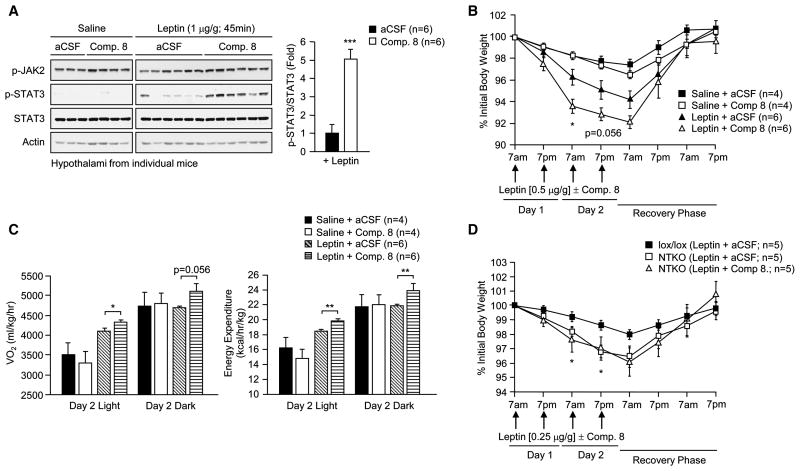
Inhibition of neuronal TCPTP enhances leptin sensitivity
We sought to establish an independent model by which to assess the role of TCPTP in the central control of body weight. We took advantage of the highly specific and cell permeable TCPTP inhibitor, compound 8 (Zhang et al., 2009). This pharmacological inhibitor is 8-fold more selective for TCPTP over PTP1B and exhibits greater than 200-fold selectivity for TCPTP over other PTPs (Zhang et al.,2009). Compound 8 (1.5 μl, 0.2 nmol) or vehicle control [artificial cerebrospinal fluid (aCSF)] was administered (icv) into 12 week-old fasted C57BL/6 mice, and mice then injected with leptin (1 μg/g IP). Hypothalami were extracted and processed for immunoblot analysis. Administration of TCPTP inhibitor increased leptin-induced STAT3 phosphorylation by more than 3-fold (Fig. 5a). Next we assessed the effects of compound 8 on body weight, oxygen consumption and energy expenditure. C57BL/6 mice were administered compound 8 (icv) followed by leptin (0.5 μg/g IP) at 7 am and 7 pm for two consecutive days and body weights, oxygen consumption and energy expenditure recorded. Compound 8 significantly enhanced the effects of leptin on body weight (Fig. 5b) and increased oxygen consumption and energy expenditure (Fig. 5c). Importantly, compound 8 did not enhance the effects of leptin (0.25–0.5 μg/g IP) on body weight in Nes-Cre;Ptpn2lox/lox mice (Fig. 5d; Supplementary Fig. 4d), consistent with the inhibitor acting via the specific inhibition of TCPTP in neuronal cells. Taken together these results confirm that TCPTP has a key role in the control of leptin sensitivity.
Fig. 5. Inhibition of neuronal TCPTP enhances leptin sensitivity.
(a) 12 week old C57BL/6 male mice were fasted for 18 h and aCSF or TCPTP inhibitor compound 8 (Comp. 8) administered icv and then injected with saline or leptin as indicated. (a) Hypothalami were extracted and processed for immunoblotting, or (b–c) body weights, oxygen consumption and energy expenditure monitored. (d) 12 week old male lox/lox and NTKO mice were icv administered aCSF or Comp. 8 and injected with leptin as indicated and body weights monitored. In (b) significance values correspond to aCSF + Leptin versus Comp. 8 + Leptin treated mice and in (d) lox/lox (Leptin + aCSF) versus NTKO (Leptin + aCSF) mice. Results are representative of three independent experiments; quantified results are means ± SEM for the indicated number of mice.
Glucose homeostasis in neuronal TCPTP-deficient mice
We found that insulin sensitivity and whole body glucose utilisation, as assessed in insulin tolerance tests (ITTs) and glucose tolerance tests respectively, were increased in male Nes-Cre;Ptpn2lox/lox mice (Supplementary Fig. 5a); insulin sensitivity also was increased in female Nes-Cre;Ptpn2lox/lox compared with control mice (Supplementary Fig. 5b). The enhanced insulin sensitivity in male and female Nes-Cre;Ptpn2lox/lox mice coincided with significantly reduced fasted blood glucose levels and reduced fasted plasma insulin (Supplementary Fig. 5c–d). No differences in insulin sensitivity, were noted in Nes-Cre;Ptpn2lox/+ versus Ptpn2lox/+ mice (Supplementary Fig. 5e). Therefore, TCPTP deficiency promotes leptin sensitivity and enhances peripheral insulin sensitivity despite the increased relative adiposity.
Obesity resistance in neuronal TCPTP-deficient mice
Next, we assessed the impact of neuronal TCPTP deficiency on DIO and the development of leptin and insulin resistance. Eight week-old male Nes-Cre;Ptpn2lox/lox and Ptpn2lox/lox mice were fed a high fat diet (23% fat) for 12 weeks. The incremental increase in body weight over the 12 weeks of high fat feeding was reduced significantly by TCPTP deficiency (Fig. 6a). DEXA analysis revealed that this was associated with a decrease in fat accumulation (Fig. 6b). In contrast to 8 week-old (Fig. 2f) or similarly aged chow-fed mice (data not shown), where the % fat mass was increased by TCPTP deficiency, total fat mass was reduced in HFF Nes-Cre;Ptpn2lox/lox versus Ptpn2lox/lox mice, so that their relative adiposity was identical (Fig. 6b); relative lean mass remained unaltered whereas bone density was reduced (Fig. 6b), as seen in chow-fed mice (Fig. 2f). Although Nes-Cre;Ptpn2lox/lox mice on a high fat diet developed cellular leptin resistance (when compared to similarly aged chow-fed mice; data not shown), the effects of leptin on body weight continued to be enhanced by TCPTP deficiency (Fig. 6c) and this was accompanied by increased leptin-induced STAT3 phosphorylation (Fig. 6d). The enhanced leptin sensitivity coincided with increased oxygen consumption and energy expenditure; no difference was noted in ambulatory movement or food intake (Fig. 6e). Somewhat surprisingly, leptin levels trended higher in Nes-Cre;Ptpn2lox/lox mice (Fig. 6g) despite their enhanced leptin sensitivity. Nonetheless, insulin sensitivity (ITTs) in neuronal TCPTP-deficient mice was enhanced (Fig. 6f), and this coincided with reduced fasted blood glucose levels and insulin levels (Fig. 6g). Consistent with this, adiponectin levels remained elevated in HFF Nes-Cre;Ptpn2lox/lox mice (Fig. 6g); TNF which is also produced by adipose tissue in obesity was unaltered indicating that adipokines were not in general increased by TCPTP deficiency (Fig. 6g). Taken together these results indicate that TCPTP-deficiency enhances leptin sensitivity, decreases weight gain and prevents the development of insulin resistance induced by high fat feeding.
Fig. 6. TCPTP-deficient mice are resistant to DIO.
8–10 week old male lox/lox and NTKO mice were HFF for 12 wk. (a) Body weights were measured on a weekly basis and the incremental increase in body weight determined. (b) Body composition measured by DEXA. (c) Mice were administered leptin as indicated and body weights monitored. (d) Mice were fasted for 18 h and injected with saline or leptin and hypothalami extracted and processed for immunoblot analysis. (e) Oxygen consumption, energy expenditure and ambulatory activity were determined and daily food intake measured. (f) Mice were fasted and ITTs performed; areas under ITT curves were determined. (g) Fed and fasted blood glucose and plasma insulin levels and fed plasma leptin, adiponectin and TNF levels were measured. Results are representative of three independent experiments; quantified results are means ± SEM for the indicated number of mice.
Hypothalamic PTP1B, SOCS3 and TCPTP expression
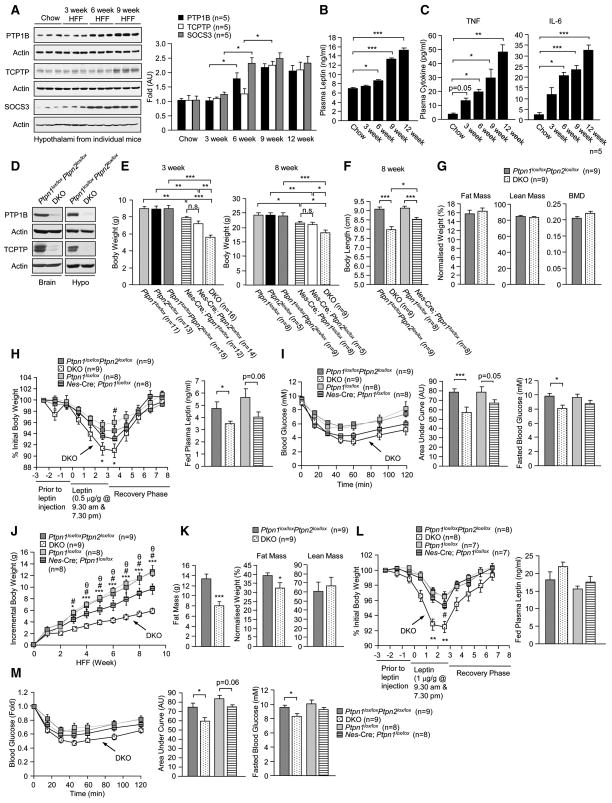
PTP1B and SOCS3 exert their effects on the leptin pathway through the dephosphorylation and inactivation of JAK2, and would thus act upstream of TCPTP, which can dephosphorylate STAT3 in the nucleus. In HFF Nes-Cre;Ptpn2lox/lox mice we noted a point of inflection at approximately 7 weeks on diet, so that the benefit of TCPTP deficiency in the prevention of DIO was in fact diminished by 12 weeks (Fig. 6a). We reasoned that an increase in hypothalamic PTP1B and/or SOCS3 expression might account for this phenomenon. To examine this we compared TCPTP, PTP1B and SOCS3 expression in the hypothalami of C57BL/6 mice fed a high fat diet for 0,3, 6, 9 and 12 weeks (Fig. 7a). We noted that increases in SOCS3 and PTP1B expression first occurred at 6 weeks, followed by increases in TCPTP at 9 weeks of high fat feeding (Fig. 7a). The increase in hypothalamic TCPTP coincided with a significant rise in plasma leptin levels at 9 weeks of high fat feeding (Fig. 7b), whereas the increase in PTP1B and SOCS3 coincided with a rise in circulating pro-inflammatory cytokines IL-6 and TNF at 3–6 weeks of high fat feeding (Fig. 7c). These results are consistent with increases in PTP1B, SOCS3 and TCPTP coordinately contributing to the high fat diet-induced development of cellular leptin resistance, with SOCS3 and PTP1B acting early, driven by the developing inflammation and TCPTP later, driven by the hyperleptinemia to exacerbate disease progression.
Fig. 7. Increases in hypothalamic PTP1B and TCPTP contribute to DIO.
8 week old C57BL/6 male mice were HFF for 0–9 weeks and (a) hypothalami isolated and processed for immunoblot analysis for an assessment of PTP1B, TCPTP and SOCS3 levels, or (b–c) mice were bled to measure plasma leptin, TNF and IL-6 levels. (d) Ptpn1lox/loxPtpn2loxlox and neuronal cell TCPTP and PTP1B double KO mice (Nes-Cre;Ptpn1lox/loxPtpn2loxlox; DKO) were generated and whole brains or hypothalami (Hypo) extracted for an assessment of PTP1B and TCPTP protein levels. (e) Body weights (f) or body lengths (nose to tail) in male Ptpn1lox/lox, Ptpn2loxlox, Ptpn1lox/loxPtpn2loxlox, Nes-Cre;Ptpn1lox/lox, Nes-Cre;Ptpn2loxlox and DKO mice. (g) Body composition in 8–10 week old male Ptpn1lox/loxPtpn2loxlox and DKO measured by DEXA and normalised to total body weight. (h) 8 week old male mice were administered leptin as indicated and body weights monitored. Fed plasma leptin levels were determined. (i) ITTs in 8 week old male mice; areas under ITT curves were determined. Fed and fasted blood glucose levels were measured. (j) The indicated 8–10 week old male mice were HFF and the incremental increase in body weight determined. (k) Relative lean and fat masses in HFF Ptpn1lox/loxPtpn2loxlox and DKO measured by DEXA. (l) Leptin sensitivity was assessed and fed plasma leptin levels determined in HFF mice. (m) ITTs were performed and fed and fasted blood glucose levels were determined in HFF mice. Results are representative of three independent experiments; quantified results are means ± SEM for the indicated number of mice; n.s.: not significant. In (j and l) * corresponds to Ptpn1lox/loxPtpn2loxlox versus DKO mice, # to Ptpn1loxlox versus Nes-Cre;Ptpn1lox/lox mice and θ Nes-Cre;Ptpn1lox/lox versus DKO mice.
Neuronal PTP1B and TCPTP double knockout mice
To test whether increases in hypothalamic PTP1B and TCPTP might additively contribute to the development of cellular leptin resistance in obesity, we generated mice that lacked PTP1B alone (data not shown), or PTP1B and TCPTP in neuronal cells (Fig. 7d; Supplemental methods). At 3 weeks of age, the combined deficiency in PTP1B and TCPTP led to additive effects on body weight and length that persisted at 8 weeks of age (Fig. 7e–f). At 8 weeks, male PTP1B and TCPTP double knockout (DKO) mice were proportionately smaller with no overt effect on bone density, lean mass or fat mass (assessed by DEXA) when normalised for total body weight (Fig. 7g). DKO had enhanced leptin sensitivity and lower plasma leptin levels (Fig. 7h), improved insulin sensitivity and lower fasted blood glucose levels (Fig. 7i), significantly enhanced oxygen consumption and energy expenditure and decreased food intake, but unaltered ambulatory activity (Supplementary Fig. 6). Interestingly, the respiratory exchange ratios (RERs; a measure of carbohydrate and fat oxidation) in DKO mice were decreased in the light cycle and increased in the dark cycle (Supplementary Fig. 6), consistent with DKO mice utilising more fat during the day and carbohydrate at night when the mice are more active. Mice that lacked PTP1B alone (Nes-Cre;Ptpn1lox/lox) had reduced plasma leptin, enhanced leptin sensitivity (Fig. 7h) and improved insulin sensitivity (Fig. 7i), but these effects were not as pronounced as in DKO mice; Nes-Cre;Ptpn1lox/lox mice also exhibited trends for reduced food intake and increased energy expenditure and activity, but had unaltered RERs (data not shown). Double floxed and DKO mice were placed on a high fat diet and the incremental increase in body weight was monitored for up to 9 weeks (Fig. 7j). The combined deletion of TCPTP and PTP1B led to a significantly greater reduction in high fat diet-induced weight gain than deletion of PTP1B alone (Fig. 7j), and this was associated with a decrease in fat accumulation in DKO mice (Fig. 7k). HFF DKO mice had significantly enhanced leptin sensitivity (Fig. 7l) and improved glucose homeostasis (Fig. 7m). In contrast, mice lacking PTP1B alone exhibited only a modest increase in leptin sensitivity and a trend for enhanced insulin sensitivity (Fig. 7l-m); leptin levels trended higher in both HFF DKO and Nes-Cre;Ptpn1lox/lox mice (Fig. 7m), as seen neuronal cell-specific TCPTP-deficient mice (Fig. 6g). Importantly, the inflection in weight gain noted in neuronal cell-specific TCPTP-deficient mice at 6–7 weeks of age (Fig. 6a) was not evident in HFF DKO mice (Fig. 7j). These results are consistent with increases in hypothalamic PTP1B and TCPTP in obesity additively attenuating the leptin response for the promotion of cellular leptin resistance and obesity.
DISCUSSION
Leptin integrates the status of peripheral fat stores with the central control of energy expenditure and food intake for the homeostatic control of body weight. In normal-weight individuals, complex biological mechanisms protect against both weight gain and weight loss to stabilise fat stores and ensure survival and reproductive fitness. Specific feedback mechanisms exist to protect against excessive weight loss that may otherwise be associated with prolonged leptin signaling (Myers et al., 2008). In this study, we demonstrate that the tyrosine-specific phosphatase TCPTP serves in a negative feedback loop for the inhibition of leptin signaling. We report that hypothalamic TCPTP expression is induced by leptin and that elevated leptin levels in obesity result in increased TCPTP to exacerbate the development of cellular resistance and progression towards morbid obesity. Therefore, our studies identify TCPTP as a member of regulatory triad that includes PTP1B and SOCS3 and functions to comprehensively attenuate the leptin response in obesity by inhibiting signaling at the receptor, JAK2 and STAT3.
Leptin exerts its effects on body weight via the activation of the tyrosine kinase JAK2 that phosphorylates STAT3 and in parallel activates the Ras/MAPK and PI3K/Akt pathways (Myers et al., 2008). Importantly, STAT3 can elicit changes in Pomc and Agrp, but not Npy expression (Bates et al., 2003). In this study, we found that TCPTP attenuated leptin-induced STAT3, but not Akt or ERK1/2 signaling in cell culture systems in vitro, and that TCPTP dephosphorylated STAT3 in the nucleus. Moreover, we found that leptin-induced hypothalamic STAT3 signaling and responses were elevated in neuronal cell-specific TCPTP-deficient mice in the ARC and VMH and that this was associated with changes in Pomc and Agrp, but not Npy expression/secretion. Hence, our studies are consistent with TCPTP selectively regulating leptin-induced STAT3 signaling in vivo. Previous studies have established that PTP1B, but not TCPTP dephosphorylates and inactivates JAK2 (Myers et al., 2001; Simoncic et al., 2002). Moreover, PTP1B and TCPTP can function cooperatively in cells in vitro to regulate the phosphorylation of JAKs and STATs in the cytoplasm & nucleus respectively (Lu et al., 2007; Lu et al., 2008; Sharma et al., 2008; Simoncic et al., 2006). Accordingly, we propose that the two phosphatases act to co-ordinately regulate leptin signaling in vivo: PTP1B attenuating JAK2 phosphorylation and possibly STAT3 in the cytoplasm, and TCPTP dephosphorylating STAT3 in the nucleus.
As reported previously for Nes-Cre;Ptpn1lox/lox mice (Bence et al., 2006), neuronal cell-specific TCPTP-deficient mice were proportionately smaller than their floxed littermates. Although recent studies by Briancon et al. (Briancon et al., 2010) have reported that some strains of Nes-Cre mice exhibit a phenotype (being smaller than wild type mice), this was not evident in our studies. We noted no overt difference in body weight, leptin sensitivity or glucose homeostasis in hemizygous Nes-Cre;Ptpn2lox/+ mice or Nes-Cre;Ptpn2+/+ mice, consistent with previous studies using the same Nes-Cre strain (Bence et al., 2006). Moreover, we found that the combined deficiency of PTP1B and TCPTP had additive effects on body weight and size. Our studies suggest that the size difference in Nes-Cre;Ptpn1lox/lox mice might be due to perturbations in the GH/IGF-1 axis. Previous studies have shown that, PTP1B can dephosphorylate JAK2, STAT5 and the IGF-1 receptor (IGF-1R) in cells to attenuate GH and IGF-1 signaling (Aoki and Matsuda, 2000; Gu et al., 2003; Myers et al., 2001), whereas TCPTP has the capacity to dephosphorylate STAT5 (but not JAK2 or IGF-1R) (Aoki and Matsuda, 2002; Buckley et al., 2002; Simoncic et al., 2002). We found that GH and IGF-1 levels were decreased in TCPTP knockout (Nes-Cre;Ptpn2lox/lox) mice and that hypothalamic STAT5 phosphorylation was enhanced in response to bolus GH administration. Increased STAT5 phosphorylation in the hypothalamus would inhibit GH release from the pituitary, thus perturbing the GH/IGF-1 axis (Becker et al., 1995; Burton et al., 1992; Minami et al., 1993; Romero et al., 2010). We suggest that the lower circulating GH and IGF-1 levels account for the decreased size and the increased relative adiposity in chow-fed Nes-Cre;Ptpn2lox/lox mice, as GH promotes postnatal skeletal growth and lipolysis (Lichanska and Waters, 2008). Recent studies have also reported that neuronal cell-specific PTP1B knockout mice have reduced circulating IGF-1 (Briancon et al., 2010). We expect that the combined deficiency of PTP1B and TCPTP (in DKO mice) probably further decreases GH and IGF-1 levels, compounding the effects on body size.
Several lines of evidence support the conclusion that TCPTP acts as a key regulator of hypothalamic leptin signaling. First, TCPTP expression was increased transiently in the hypothalami of C57BL/6 mice in response bolus leptin administration and in LEPR-B expressing cells treated with leptin, consistent with TCPTP serving in a feedback loop. Second, despite the decreased ambulatory activity and increased relative adiposity, Nes-Cre;Ptpn2lox/lox mice had significantly lower circulating leptin levels and lower fasting blood glucose and insulin levels, consistent with enhanced leptin and insulin sensitivity. This is particularly striking and suggests that the impact of TCPTP deficiency on leptin sensitivity and the central control of glucose homeostasis overrides the increase in leptin production and the development of peripheral insulin resistance that would otherwise be expected with increased adiposity. Third, despite the decreased ambulatory activity, Nes-Cre;Ptpn2lox/lox mice exhibited increased oxygen consumption and energy expenditure (corrected for body weight) and decreased food intake as might be expected for enhanced leptin sensitivity. Fourth, the reduction in food intake and body weight after leptin administration was significantly enhanced by TCPTP deficiency in both chow and HFF mice. Fifth, hypothalamic STAT3 phosphorylation was significantly enhanced in the ARC and VMH after leptin administration coinciding with significant changes in Pomc and Agrp expression in Nes-Cre;Ptpn2lox/lox mice. The increase in leptin-induced signaling could be recapitulated in vitro in cells of neuronal origin after TCPTP knockdown. Sixth, TCPTP deficiency enhanced the leptin-induced α-MSH secretion from hypothalamic slices ex vivo, making any extrinsic influences that may be associated with the differences in body weight in the whole animals unlikely. Finally, the effects of TCPTP deficiency could be recapitulated by the icv administration of a specific TCPTP inhibitor. Inhibition of TCPTP in C57BL/6 mice enhanced leptin-induced hypothalamic STAT3 signaling and increased the effects of leptin on body weight and energy expenditure. Importantly, the inhibitor did not have any overt effect on leptin-induced responses in Nes-Cre;Ptpn2lox/lox mice, consistent with the inhibitor being specific for TCPTP. Taken together, these studies provide compelling evidence for TCPTP being a key regulator of central leptin sensitivity.
Our studies indicate that increased hypothalamic TCPTP contributes to cellular leptin resistance in obesity. In HFF C57BL/6 mice, increased hypothalamic TCPTP coincided with increased circulating leptin. The increase in TCPTP was preceded by increases in hypothalamic PTP1B and SOCS3 that coincided with elevated circulating TNF and IL-6. Zabolotny et al. (Zabolotny et al., 2008) have reported that hypothalamic PTP1B expression can be driven by TNF in vivo, whereas hypothalamic SOCS3 expression can be induced by IKKβ/NFκB signaling (Zhang et al., 2008), an effector pathway of TNF. Accordingly, we suggest that cellular leptin resistance and obesity develop along a continuum, with inflammation associated with high fat feeding at first promoting hypothalamic PTP1B and SOCS3 to attenuate JAK2 and reduce leptin sensitivity, to consequently increase adiposity and circulating leptin to promote hypothalamic TCPTP expression. The increased TCPTP would in turn dephosphorylate STAT3 to further attenuate the leptin response and contribute to the development of overt cellular leptin resistance and progression to morbid obesity. Although one might expect that the hyperleptinemia should solely compensate for the developing leptin resistance, it is possible that leptin signaling pathways and/or leptin responsive neurons may be differentially sensitive to leptin. In keeping with this possibility, some leptin functions, such as leptin’s cardiovascular effects, remain intact in the leptin-resistant state (Correia et al., 2002; Rahmouni et al., 2005). In the case of TCPTP, it is important to note that TCPTP has a long protein half-life (Bukczynska et al., 2004) so that the sustained hyperleptinemia in obesity may be sufficient to promote and maintain TCPTP levels. However, we cannot exclude the possibility that in the obese state additional molecular factors, which themselves have no effect, contribute to the promotion of TCPTP expression. Nevertheless, consistent with increases in hypothalamic TCPTP and PTP1B coordinately contributing to the onset and progression of cellular leptin resistance, we found that the combined ablation of TCPTP and PTP1B had additive effects in protecting mice from high fat diet-induced weight gain. Briancon et al. (Briancon et al., 2010) have reported that the combined inactivation of PTP1B and SOCS3 in neuronal cells decreases adiposity and improves glucose tolerance, with minimal if any impact on high fat diet-induced, or age-associated weight gain. In our studies we found that the combined deletion of PTP1B and TCPTP had a pronounced effect on high fat diet-induced weight gain, but did not completely protect mice. The latter may be attributable to varied factors, among which include increases in SOCS3, the hedonistic attractiveness of a high fat diet, and/or gliosis and changes in the blood brain barrier that may impair leptin’s access to neurons in the ARC (El-Haschimi et al., 2000; Horvath et al., 2010).
The results of this study define the role of TCPTP in the central control of leptin signaling and delineate a negative feedback loop that functions together with PTP1B and SOCS3 for the attenuation of the leptin response. Importantly, our results indicate that increases in hypothalamic TCPTP may be causally linked to the attenuation of leptin sensitivity working in conjunction with PTP1B for the coordinated suppression of JAK2/STAT3 signaling and the promotion of cellular leptin resistance. Our studies underscore the highly specific nature of phosphatases such as PTP1B and TCPTP in vivo and highlight their capacity to work in concert for the comprehensive regulation of signaling networks and biological responses. Moreover, our findings indicate that the combined inhibition of PTP1B and TCPTP might be required for the effective alleviation of cellular leptin resistance in obesity, and therapeutic approaches currently aimed at targeting PTP1B should take this into consideration.
EXPERIMENTAL PROCEDURES
Mice
We maintained mice on a 12 h light-dark cycle in a temperature-controlled high barrier facility (Monash ARL) with free access to food and water according to NHMRC Australian Code of Practice for the Care and Use of Animals. The Nes-Cre (originating from R. Klein, Max Planck Institute of Neurobiology) backcrossed for six generations onto the C57BL/6J background have been described previously (Bence et al., 2006). Ptpn2lox/+ (C57BL/6) were generated as described in Supplemental Methods and mated with Nes-Cre (C57BL/6) for the conditional deletion of Ptpn2. Mice were fed a standard chow (19% protein, 4.6% fat and 4.8% crude fibre) or a high fat diet (23% fat; 45% of total energy from fat; SF04-027; Specialty Feeds) as indicated.
Lateral ventricle cannulation
Cannulas were stereotaxically inserted (1.0 mm lateral and 0.3 mm rostral to bregma) as described in Supplemental Methods. After recovery, mice were fasted and aCSF or Compound 8 (1.5 μl, 0.2 nmol) administered icv prior to saline or leptin injection (1 μg/g, IP) for 45 min. Mice were sacrificed by cervical dislocation and hypothalami isolated. For leptin sensitivity assays, mice were icv injected with either aCSF or TCPTP inhibitor and saline or leptin at 7 am and 7 pm for 2 consecutive days and body weight, oxygen consumption and energy expenditure recorded.
Hypothalamic peptide secretion
Mice were decapitated and whole brains removed for the isolation of hypothalami and 2 mm thick slices of mediobasal forebrain including the paraventricular and arcuate nuclei prepared using a vibrating microtome and processed for peptide secretion assays as described in Supplemental Methods.
ARC and VMH microdissection
Isolated brains were frozen and sectioned at 300 μm in a cyrostat and collected on microscope slides. The sections were briefly warmed and refrozen to adhere to slides. The ARC, VMH, DMH, PVN, LH and cortex were identified using a surgical microscope. A blunted 21G needle was used to punch out frozen brain regions. The ARC was collected from 3 consecutive sections, the VMH from 4 consecutive sections and the DMH, PVN and LH punched out of 2 consecutive sections each.
Immunohistochemistry
Animals were anesthetized with isoflurane and perfused with PBS and then 4% PFA. Brains were post-fixed in 4% PFA overnight, then placed in sucrose and cut at 40 μm on a cryostat. We performed immunohistochemistry to monitor for p-STAT3 and POMC neurons as described previously (Andrews et al., 2008). STAT3 phosphorylation was quantified in neurons in the ARC (Br −1.22 to −2.54), the VMH (Br −1.22 to −2.06) and DMH (Br −1.46 to −2.18). p-STAT3 positive cells were visualized on a Zeiss microscope (Carl Zeiss) and counted in serial sections throughout the hypothalamus using a grid eye piece.
Metabolic measurements and body composition
Insulin and glucose tolerance tests and blood glucose and plasma insulin and adipokine analyses were performed as described previously (Loh et al., 2009). For leptin sensitivity assays, 12 week old mice were administered recombinant mouse leptin (IP) at 9.30 am and 7.30 pm for 3 days in a row and body weights and food intake recorded daily at 1 pm for up to 9 days. Body weight and baseline food intake for the 3 days prior to the start of the experiment were averaged and used to calculate the percent change. Activity, food intake and energy expenditure were assessed using a Comprehensive Lab Animal Monitoring System (Columbus Instruments) and body composition determined by DEXA (Lunar PIXImus2; GE Healthcare) as described in Supplemental Methods.
Cell culture and Immunofluorescence microscopy
SK-N-SH-5Y5Y neuroblastoma cells and CHO-LEPR-B cells were cultured as described in Supplemental Methods. Control SK-N-SH-5Y5Y cells and those expressing TCPTP-specific shRNAs were generated as described previously (Shields et al., 2008). CHO-LEPR-B cells were transfected with pCG, pCG-TC45 or pCG-TC45-D182A using Lipofectamine 2000 (Invitrogen). SK-N-SH-5Y5Y and CHO-LEPR-B cells were serum starved for 6 h and then stimulated with 100 ng/ml human and 0.1 ng/ml murine leptin respectively for the indicated times. Where indicated SK-N-SH-5Y5Y and CHO-LEPR-B were fixed with 3.2% PFA and processed for immunofluorescence microscopy as described previously (Tiganis et al., 1998).
Biochemical analyses
Mouse tissues were dissected and immediately frozen in liquid N2. Tissues were mechanically homogenized in ice-cold RIPA lysis buffer and processed for immunoblot analysis as described in Supplemental Methods.
Real time PCR
Hypothalami were dissected and immediately frozen in liquid N2 and RNA extracted using Trizol reagent and processed for quantitative real-time PCR using TaqMan™ Gene Expression Assays (Applied Biosystems) as described in Supplemental Methods. Reactions were performed in triplicate and relative quantification achieved using the ΔΔCt method.
Statistical Analyses
All data was presented as means ± SEM and statistical significance determined using the Student’s t test or two-way ANOVA; p < 0.05 was considered significant.
Supplementary Material
Acknowledgments
This work was supported by the National Health and Medical Research Council (NHMRC) of Australia (to TT, MAC, NAS, & ZBA), the National Institutes of Health [to Z-YZ (RO1 CA126937), KKB (RO1-DK082417), BBK (P01 DK56116) and BGN (R37 CA49152)] and funds from the Ontario Ministry of Health and Long Term Care and the Princess Margaret Hospital Foundation (BGN); ZBA is an ARC Future Fellow, MAC a Pfizer Senior Research Fellow, NAS and TT NHMRC Research Fellows and BGN a Canada Research Chair (Tier I).
References
- Andrews ZB, Liu ZW, Walllingford N, Erion DM, Borok E, Friedman JM, Tschop MH, Shanabrough M, Cline G, Shulman GI, Coppola A, Gao XB, Horvath TL, Diano S. UCP2 mediates ghrelin’s action on NPY/AgRP neurons by lowering free radicals. Nature. 2008;454:846–851. doi: 10.1038/nature07181. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aoki N, Matsuda T. A cytosolic protein-tyrosine phosphatase PTP1B specifically dephosphorylates and deactivates prolactin-activated STAT5a and STAT5b. J Biol Chem. 2000;275:39718–39726. doi: 10.1074/jbc.M005615200. [DOI] [PubMed] [Google Scholar]
- Aoki N, Matsuda T. A nuclear protein tyrosine phosphatase TC-PTP is a potential negative regulator of the PRL-mediated signaling pathway: dephosphorylation and deactivation of signal transducer and activator of transcription 5a and 5b by TC-PTP in nucleus. Mol Endocrinol. 2002;16:58–69. doi: 10.1210/mend.16.1.0761. [DOI] [PubMed] [Google Scholar]
- Banno R, Zimmer D, De Jonghe BC, Atienza M, Rak K, Yang W, Bence KK. PTP1B and SHP2 in POMC neurons reciprocally regulate energy balance in mice. J Clin Invest. 2010;120:720–734. doi: 10.1172/JCI39620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bartness TJ, Shrestha YB, Vaughan CH, Schwartz GJ, Song CK. Sensory and sympathetic nervous system control of white adipose tissue lipolysis. Mol Cell Endocrinol. 2010;318:34–43. doi: 10.1016/j.mce.2009.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates SH, Stearns WH, Dundon TA, Schubert M, Tso AW, Wang Y, Banks AS, Lavery HJ, Haq AK, Maratos-Flier E, Neel BG, Schwartz MW, Myers MG., Jr STAT3 signalling is required for leptin regulation of energy balance but not reproduction. Nature. 2003;421:856–859. doi: 10.1038/nature01388. [DOI] [PubMed] [Google Scholar]
- Becker K, Stegenga S, Conway S. Role of insulin-like growth factor I in regulating growth hormone release and feedback in the male rat. Neuroendocrinology. 1995;61:573–583. doi: 10.1159/000126882. [DOI] [PubMed] [Google Scholar]
- Bence KK, Delibegovic M, Xue B, Gorgun CZ, Hotamisligil GS, Neel BG, Kahn BB. Neuronal PTP1B regulates body weight, adiposity and leptin action. Nat Med. 2006;12:917–924. doi: 10.1038/nm1435. [DOI] [PubMed] [Google Scholar]
- Bennett E, McGuinness L, Gevers EF, Thomas GB, Robinson IC, Davey HW, Luckman SM. Hypothalamic STAT proteins: regulation of somatostatin neurones by growth hormone via STAT5b. J Neuroendocrinol. 2005;17:186–194. doi: 10.1111/j.1365-2826.2005.01296.x. [DOI] [PubMed] [Google Scholar]
- Bjorbaek C, Elmquist JK, Frantz JD, Shoelson SE, Flier JS. Identification of SOCS-3 as a potential mediator of central leptin resistance. Mol Cell. 1998;1:619–625. doi: 10.1016/s1097-2765(00)80062-3. [DOI] [PubMed] [Google Scholar]
- Briancon N, McNay DE, Maratos-Flier E, Flier JS. Combined neural inactivation of SOCS-3 and PTP-1B reveal additive, synergistic, and factor-specific roles in the regulation of body energy balance. Diabetes. 2010;59:3074–3084. doi: 10.2337/db10-0481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buckley DA, Cheng A, Kiely PA, Tremblay ML, O’Connor R. Regulation of insulin-like growth factor type I (IGF-I) receptor kinase activity by protein tyrosine phosphatase 1B (PTP-1B) and enhanced IGF-I-mediated suppression of apoptosis and motility in PTP-1B-deficient fibroblasts. Mol Cell Biol. 2002;22:1998–2010. doi: 10.1128/MCB.22.7.1998-2010.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bukczynska P, Klingler-Hoffmann M, Mitchelhill KI, Lam MH, Ciccomancini M, Tonks NK, Sarcevic B, Kemp BE, Tiganis T. The T-cell protein tyrosine phosphatase is phosphorylated on Ser-304 by cyclin-dependent protein kinases in mitosis. Biochem J. 2004;380:939–949. doi: 10.1042/BJ20031780. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton KA, Kabigting EB, Clifton DK, Steiner RA. Growth hormone receptor messenger ribonucleic acid distribution in the adult male rat brain and its colocalization in hypothalamic somatostatin neurons. Endocrinology. 1992;131:958–963. doi: 10.1210/endo.131.2.1353444. [DOI] [PubMed] [Google Scholar]
- Cheng A, Uetani N, Simoncic PD, Chaubey VP, Lee-Loy A, McGlade CJ, Kennedy BP, Tremblay ML. Attenuation of leptin action and regulation of obesity by protein tyrosine phosphatase 1B. Dev Cell. 2002;2:497–503. doi: 10.1016/s1534-5807(02)00149-1. [DOI] [PubMed] [Google Scholar]
- Commins SP, Watson PM, Levin N, Beiler RJ, Gettys TW. Central leptin regulates the UCP1 and ob genes in brown and white adipose tissue via different beta-adrenoceptor subtypes. J Biol Chem. 2000;275:33059–33067. doi: 10.1074/jbc.M006328200. [DOI] [PubMed] [Google Scholar]
- Correia ML, Haynes WG, Rahmouni K, Morgan DA, Sivitz WI, Mark AL. The concept of selective leptin resistance: evidence from agouti yellow obese mice. Diabetes. 2002;51:439–442. doi: 10.2337/diabetes.51.2.439. [DOI] [PubMed] [Google Scholar]
- Cowley MA, Smart JL, Rubinstein M, Cerdan MG, Diano S, Horvath TL, Cone RD, Low MJ. Leptin activates anorexigenic POMC neurons through a neural network in the arcuate nucleus. Nature. 2001;411:480–484. doi: 10.1038/35078085. [DOI] [PubMed] [Google Scholar]
- El-Haschimi K, Pierroz DD, Hileman SM, Bjorbaek C, Flier JS. Two defects contribute to hypothalamic leptin resistance in mice with diet-induced obesity. J Clin Invest. 2000;105:1827–1832. doi: 10.1172/JCI9842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elchebly M, Payette P, Michaliszyn E, Cromlish W, Collins S, Loy AL, Normandin D, Cheng A, Himms-Hagen J, Chan CC, Ramachandran C, Gresser MJ, Tremblay ML, Kennedy BP. Increased insulin sensitivity and obesity resistance in mice lacking the protein tyrosine phosphatase-1B gene. Science. 1999;283:1544–1548. doi: 10.1126/science.283.5407.1544. [DOI] [PubMed] [Google Scholar]
- Elias CF, Aschkenasi C, Lee C, Kelly J, Ahima RS, Bjorbaek C, Flier JS, Saper CB, Elmquist JK. Leptin differentially regulates NPY and POMC neurons projecting to the lateral hypothalamic area. Neuron. 1999;23:775–786. doi: 10.1016/s0896-6273(01)80035-0. [DOI] [PubMed] [Google Scholar]
- Gu F, Dube N, Kim JW, Cheng A, Ibarra-Sanchez Mde J, Tremblay ML, Boisclair YR. Protein tyrosine phosphatase 1B attenuates growth hormone-mediated JAK2-STAT signaling. Mol Cell Biol. 2003;23:3753–3762. doi: 10.1128/MCB.23.11.3753-3762.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horvath TL, Sarman B, Garcia-Caceres C, Enriori PJ, Sotonyi P, Shanabrough M, Borok E, Argente J, Chowen JA, Perez-Tilve D, Pfluger PT, Bronneke HS, Levin BE, Diano S, Cowley MA, Tschop MH. Synaptic input organization of the melanocortin system predicts diet-induced hypothalamic reactive gliosis and obesity. Proc Natl Acad Sci U S A. 2010;107:14875–14880. doi: 10.1073/pnas.1004282107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kievit P, Howard JK, Badman MK, Balthasar N, Coppari R, Mori H, Lee CE, Elmquist JK, Yoshimura A, Flier JS. Enhanced leptin sensitivity and improved glucose homeostasis in mice lacking suppressor of cytokine signaling-3 in POMC-expressing cells. Cell Metab. 2006;4:123–132. doi: 10.1016/j.cmet.2006.06.010. [DOI] [PubMed] [Google Scholar]
- Klaman LD, Boss O, Peroni OD, Kim JK, Martino JL, Zabolotny JM, Moghal N, Lubkin M, Kim YB, Sharpe AH, Stricker-Krongrad A, Shulman GI, Neel BG, Kahn BB. Increased energy expenditure, decreased adiposity, and tissue-specific insulin sensitivity in protein-tyrosine phosphatase 1B-deficient mice. Mol Cell Biol. 2000;20:5479–5489. doi: 10.1128/mcb.20.15.5479-5489.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lichanska AM, Waters MJ. How growth hormone controls growth, obesity and sexual dimorphism. Trends Genet. 2008;24:41–47. doi: 10.1016/j.tig.2007.10.006. [DOI] [PubMed] [Google Scholar]
- Loh K, Deng H, Fukushima A, Cai X, Boivin B, Galic S, Bruce C, Shields BJ, Skiba B, Ooms LM, Stepto N, Wu B, Mitchell CA, Tonks NK, Watt MJ, Febbraio MA, Crack PJ, Andrikopoulos S, Tiganis T. Reactive oxygen species enhance insulin sensitivity. Cell Metab. 2009;10:260–272. doi: 10.1016/j.cmet.2009.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Chen J, Sasmono RT, Hsi ED, Sarosiek KA, Tiganis T, Lossos IS. T-cell protein tyrosine phosphatase, distinctively expressed in activated-B-cell-like diffuse large B-cell lymphomas, is the nuclear phosphatase of STAT6. Mol Cell Biol. 2007;27:2166–2179. doi: 10.1128/MCB.01234-06. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu X, Malumbres R, Shields B, Jiang X, Sarosiek KA, Natkunam Y, Tiganis T, Lossos IS. PTP1B is a negative regulator of interleukin 4-induced STAT6 signaling. Blood. 2008;112:4098–4108. doi: 10.1182/blood-2008-03-148726. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minami S, Kamegai J, Hasegawa O, Sugihara H, Okada K, Wakabayashi I. Expression of growth hormone receptor gene in rat hypothalamus. J Neuroendocrinol. 1993;5:691–696. doi: 10.1111/j.1365-2826.1993.tb00541.x. [DOI] [PubMed] [Google Scholar]
- Mori H, Hanada R, Hanada T, Aki D, Mashima R, Nishinakamura H, Torisu T, Chien KR, Yasukawa H, Yoshimura A. Socs3 deficiency in the brain elevates leptin sensitivity and confers resistance to diet-induced obesity. Nat Med. 2004;10:739–743. doi: 10.1038/nm1071. [DOI] [PubMed] [Google Scholar]
- Morrison CD, White CL, Wang Z, Lee SY, Lawrence DS, Cefalu WT, Zhang ZY, Gettys TW. Increased hypothalamic protein tyrosine phosphatase 1B contributes to leptin resistance with age. Endocrinology. 2007;148:433–440. doi: 10.1210/en.2006-0672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Myers MG, Cowley MA, Munzberg H. Mechanisms of leptin action and leptin resistance. Annu Rev Physiol. 2008;70:537–556. doi: 10.1146/annurev.physiol.70.113006.100707. [DOI] [PubMed] [Google Scholar]
- Myers MP, Andersen JN, Cheng A, Tremblay ML, Horvath CM, Parisien JP, Salmeen A, Barford D, Tonks NK. TYK2 and JAK2 are substrates of protein-tyrosine phosphatase 1B. J Biol Chem. 2001;276:47771–47774. doi: 10.1074/jbc.C100583200. [DOI] [PubMed] [Google Scholar]
- Ozcan L, Ergin AS, Lu A, Chung J, Sarkar S, Nie D, Myers MG, Jr, Ozcan U. Endoplasmic reticulum stress plays a central role in development of leptin resistance. Cell Metab. 2009;9:35–51. doi: 10.1016/j.cmet.2008.12.004. [DOI] [PubMed] [Google Scholar]
- Picardi PK, Calegari VC, Prada Pde O, Moraes JC, Araujo E, Marcondes MC, Ueno M, Carvalheira JB, Velloso LA, Saad MJ. Reduction of hypothalamic protein tyrosine phosphatase improves insulin and leptin resistance in diet-induced obese rats. Endocrinology. 2008;149:3870–3880. doi: 10.1210/en.2007-1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rahmouni K, Morgan DA, Morgan GM, Mark AL, Haynes WG. Role of selective leptin resistance in diet-induced obesity hypertension. Diabetes. 2005;54:2012–2018. doi: 10.2337/diabetes.54.7.2012. [DOI] [PubMed] [Google Scholar]
- Romero CJ, Ng Y, Luque RM, Kineman RD, Koch L, Bruning JC, Radovick S. Targeted deletion of somatotroph insulin-like growth factor-I signaling in a cell-specific knockout mouse model. Mol Endocrinol. 2010;24:1077–1089. doi: 10.1210/me.2009-0393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sharma P, Chakraborty R, Wang L, Min B, Tremblay ML, Kawahara T, Lambeth JD, Haque SJ. Redox regulation of interleukin-4 signaling. Immunity. 2008;29:551–564. doi: 10.1016/j.immuni.2008.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shetty S, Kusminski CM, Scherer PE. Adiponectin in health and disease: evaluation of adiponectin-targeted drug development strategies. Trends Pharmacol Sci. 2009;30:234–239. doi: 10.1016/j.tips.2009.02.004. [DOI] [PubMed] [Google Scholar]
- Shields BJ, Hauser C, Bukczynska PE, Court NW, Tiganis T. DNA replication stalling attenuates tyrosine kinase signaling to suppress S phase progression. Cancer Cell. 2008;14:166–179. doi: 10.1016/j.ccr.2008.06.003. [DOI] [PubMed] [Google Scholar]
- Simoncic PD, Bourdeau A, Lee-Loy A, Rohrschneider LR, Tremblay ML, Stanley ER, McGlade CJ. T-cell protein tyrosine phosphatase (Tcptp) is a negative regulator of colony-stimulating factor 1 signaling and macrophage differentiation. Mol Cell Biol. 2006;26:4149–4160. doi: 10.1128/MCB.01932-05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simoncic PD, Lee-Loy A, Barber DL, Tremblay ML, McGlade CJ. The T cell protein tyrosine phosphatase is a negative regulator of janus family kinases 1 and 3. Curr Biol. 2002;12:446–453. doi: 10.1016/s0960-9822(02)00697-8. [DOI] [PubMed] [Google Scholar]
- Takeda S. Central control of bone remodeling. Biochem Biophys Res Commun. 2005;328:697–699. doi: 10.1016/j.bbrc.2004.11.071. [DOI] [PubMed] [Google Scholar]
- Tiganis T, Bennett AM. Protein tyrosine phosphatase function: the substrate perspective. Biochem J. 2007;402:1–15. doi: 10.1042/BJ20061548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tiganis T, Bennett AM, Ravichandran KS, Tonks NK. Epidermal growth factor receptor and the adaptor protein p52Shc are specific substrates of T-cell protein tyrosine phosphatase. Mol Cell Biol. 1998;18:1622–1634. doi: 10.1128/mcb.18.3.1622. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Sekine Y, Kashima K, Kubota A, Sato N, Aoki N, Matsuda T. The nuclear isoform of protein-tyrosine phosphatase TC-PTP regulates interleukin-6-mediated signaling pathway through STAT3 dephosphorylation. Biochem Biophys Res Commun. 2002;297:811–817. doi: 10.1016/s0006-291x(02)02291-x. [DOI] [PubMed] [Google Scholar]
- Zabolotny JM, Bence-Hanulec KK, Stricker-Krongrad A, Haj F, Wang Y, Minokoshi Y, Kim YB, Elmquist JK, Tartaglia LA, Kahn BB, Neel BG. PTP1B regulates leptin signal transduction in vivo. Dev Cell. 2002;2:489–495. doi: 10.1016/s1534-5807(02)00148-x. [DOI] [PubMed] [Google Scholar]
- Zabolotny JM, Kim YB, Welsh LA, Kershaw EE, Neel BG, Kahn BB. Protein-tyrosine phosphatase 1B expression is induced by inflammation in vivo. J Biol Chem. 2008;283:14230–14241. doi: 10.1074/jbc.M800061200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang S, Chen L, Luo Y, Gunawan A, Lawrence DS, Zhang ZY. Acquisition of a potent and selective TC-PTP inhibitor via a stepwise fluorophore-tagged combinatorial synthesis and screening strategy. J Am Chem Soc. 2009;131:13072–13079. doi: 10.1021/ja903733z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X, Zhang G, Zhang H, Karin M, Bai H, Cai D. Hypothalamic IKKbeta/NF-kappaB and ER stress link overnutrition to energy imbalance and obesity. Cell. 2008;135:61–73. doi: 10.1016/j.cell.2008.07.043. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.