Abstract
Immunizations that target specific types of immune responses are used commonly to prevent microbial infections. However, a range of immune responses may prove necessary to combat the ravages of neurodegenerative diseases. The goal is to eliminate the ‘root’ cause of neurodegenerative disorders, misfolded aggregated proteins, while harnessing adaptive immune responses to promote neural repair. However, immunization strategies used to elicit humoral immune responses against aberrant brain proteins have yielded mixed success. While specific proteins can be cleared, the failures in halting disease progression revolve, in measure, around adaptive immune responses that promote autoreactive T cells and, as such, induce a meningoencephalitis, accelerating neurodegeneration. Thus, alternative approaches for protein clearance and neural repair are desired. To this end, our laboratories have sought to transform autoreactive adaptive immune responses into regulatory neuroprotective cells in Parkinson’s disease. In this context, induction of immune responses against modified brain proteins serves to break immunological tolerance, while eliciting adaptive immunity to facilitate neuronal repair. How to harness the immune response in the setting of Parkinson’s disease requires a thorough understanding of the role of immunity in human disease and the ways to modify such immune responses to elicit therapeutic gain. These are discussed in this review.
Keywords: immunization, immunotherapy, neurodegeneration, Parkinson’s disease, regulatory T cells, T cells
Next to Alzheimer’s disease (AD), Parkinson’s disease (PD) is the second most common neurodegenerative proteopathy. They share the common pathologic signature of proteinaceous aggregates comprised of mutated or post-translationally modified proteins, which affect misfolding and increase aggregation. The accumulation of such protein aggregates alters cell function, contributes to neuronal death and apoptosis, and initiates inflammatory responses that contribute to the underlying disease process. In PD and other synucleinopathies, intracytoplasmic protein aggregates, called Lewy bodies (LBs) accumulate both in the CNS and in the periphery [1]. Within the CNS, LBs are found in the substantia nigra pars compacta (SNc), medullary and pontine nuclei, locus coeruleus, amygdala, allocortex, cingulate area and isocortex [2].
PD is also characterized by the loss of dopaminergic neurons and dopamine [3,4]. Thus, current treatments for PD are specifically aimed at improving motor dysfunction by restoring the loss of dopamine. While levodopa is considered to be the ‘gold standard’ for the treatment of PD [5], patients usually begin treatment with ‘levodopa-sparing strategies’ [6]. After prolonged levodopa treatment, patients commonly develop fluctuations in motor control [7]. While these symptoms can be reduced with carbidopa [8], patients eventually become refractory to treatments [9]. Thus, therapies designed to halt neurodegeneration have been sought during past decades. Such neural repair modalities included growth factors, neural grafts, dopaminergic neuronal replacement via stem cells and immune modulation. All modalities target neural repair in PD, as well as other neurodegenerative proteopathies, yet none of these therapies have been fully realized owing to, in part, significant hurdles.
For example, neurorestorative therapies involving human fetal mesencephalic grafts show modest improvements in motor function and reduce the need for levodopa in most individuals [10,11], with benefits more evident in younger recipients or mild disease [12]. However, up to half of transplant recipients may suffer increased dyskinesia [13,14]. Furthermore, grafts may eventually develop LB inclusions and inflammation [14,15]. These studies suggest that α-synuclein (α-syn) aggregation, LB formation and loss of dopaminergic neurons have underlying degenerative processes that, at least in part, are driven by death mechanisms not yet realized [16]. Additional studies have aimed to improve disease by administering recombinant growth factors or increasing neurotrophins. Although the use of neurotrophic factors to promote neuronal survival and repair has had many successes in vitro [17–21] and in animal models of PD [22–24], growth factor therapies for PD have so far been met with limited success. Of the growth factors utilized, GDNF has been the most widely investigated [25]. Experimental observations demonstrated that GDNF positively affects the regeneration of dopaminergic neurons and, as such, is considered to be a realistic therapeutic option for advanced PD. In clinical trials performed thus far, severe adverse events were limited, but disease outcomes were often not changed substantially [26–28]. However, studies delivering GDNF to the putamen demonstrated improvements in clinical sores and decreases in dyskinesia, suggesting that the target area of the brain can significantly affect the outcome of treatments [29,30].
Common among these neuroregenerative therapies, are failures to clear misfolded proteins and to directly address inflammation in the brain and the effects of the innate and adaptive immune systems on neurodegeneration. To these ends, our laboratories have focused on neurorestorative research, utilizing control of the adaptive immune system for dopaminergic neuronal repair. The perils and promise of this approach are outlined in this review.
The immune system & neurodegeneration
Cells of the innate immune system that affect neuronal function include mononuclear phagocytes (MPs; macrophages, microglia and dendritic cells), neutrophils, mast cells, eosinophils, basophils and natural killer (NK) cells [31–33]. MP phagocytose aberrant proteins and cellular debris, secrete both proinflammatory neurotoxic molecules and neurotrophic molecules, and release chemokines that recruit cells of the adaptive immune system to the CNS. The cells use conserved pattern recognition receptors (PRRs), called toll-like receptors (TLRs), which are encoded in the germline and recognize broad pathogen-associated molecular patterns (PAMPs) on pathogens and danger-associated molecular patterns (DAMPs). These are self-molecules released following tissue injuries including those in the brain [34,35]. In neurodegenerative diseases, cells of the innate immune system are activated by DAMPs such as DNA, ATP, hyaluronan aggregates and fibrinogen, as well as modified or misfolded proteins [36]. Unlike the innate immune system, the adaptive immune system is highly specific. Membrane-bound receptors (T-cell receptors [TCRs] and B-cell receptors [BCRs]) recognize cognate foreign antigens. Recognition of antigen by TCRs in the context of the correct major histocompatibility complex allows for target effector responses via cell-to-cell contact or through soluble factors. B cells can secrete their BCRs as soluble antibodies (immunoglobulin [Ig]), which recognize and bind the pathogen or foreign debris and further aid in their removal by opsonization and activation of complement, increasing phagocytosis and uptake by antigen presenting cells (APCs) that include microglia.
MP neuroinflammation is now accepted as a characteristic of PD and other neurodegenerative diseases [37–42]. Moreover, systemic inflammation is linked to chronic neurodegeneration [43]. Risk factors associated with PD are also associated with inflammation and include aging, rural residence, pesticides, brain injury or encephalitic infection [44]. These elicit reactive oxygen species that are readily linked to nigrostriatal degeneration in PD [41,45]. Thus, initial immunotherapies have targeted inflammation. For example, in vitro, NSAIDs were shown to reduce levels of the proinflammatory enzymes cyclooxygenase 1 and 2 (COX-1 and COX-2) [46], and reduce secretion of neurotoxins by microglia in vitro [47]. However, while some epidemiological studies suggest that chronic use of some NSAIDs decrease risk for AD and PD, other studies have failed to reproduce these results [48–54]. These reports demonstrate that NSAIDs alone are not sufficient to prevent neurodegeneration, although reduction of inflammation is beneficial. The questions remaining are when to treat, how long and at what dose. These demand further investigation.
Innate immune responses
Microglia are the resident macrophages of the CNS and are continually sampling the local environment [55]. While normally in a quiescent state, they respond quickly to disturbances in their microenvironment and can migrate throughout the brain to areas of insult or injury [56,57]. The engagement of PRRs activates signaling pathways that lead to translocation of NF-kB and AP-1 to the nucleus where they induce transcription of innate immune proteins. Once activated, they secrete both neurotoxic and neurotrophic factors [58,59], such as the cytokines IL-1α, IL-1β and TNF-α [60,61], and neurotrophins, such as NGF and neurotrophin-3 [62,63]. Macrophages clearly have divergent effects [32], and their responses to specific stimuli and cytokines have led to the recent classification of macrophages as ‘classically activated’ (M1), which are more proinflammatory compared with the antiinflammatory ‘alternatively activated’ macrophages (M2) [64]. During chronic inflammation and CNS injury, the neurotoxic effects of microglia overcome the neurotrophic effects [65] and the M1 phenotype predominates [64]. A recent study investigating the polarization of macrophages in spinal cord injury demonstrated that both subsets of macrohpages are present, but only M1 macrophages are neurotoxic, while the M2 macrophages promote neuoronal repair [66]. These data suggest that modulation of the microglia or macrophage phenotype represents a candidate target for immunotherapies to combat neurodegeneration.
Proteopathies, inflammation & neurodegeneration
Neuroinflammation is seen in many neurodegenerative diseases, while activated microglia have long been recognized to exacerbate disease [31,32,57]. In neurodegenerative diseases, M1 microglia expressing human leukocyte antigen (HLA)-DR and -DQ are associated with the accumulation of aberrant proteins, oxidative stress and neuronal cell death. Reactive microglia have been found in AD, PD, Huntington’s disease and amyotrophic lateral sclerosis, as well as Creutzfeldt–Jakob disease, among other neurodegenerative disease [67–70], and are associated with amyloid plaques in AD brains and LB aggregates in PD brains [71–74].
In PD, the association of LBs and dopaminergic neuron cell death led to the α-syn burden hypothesis [41]. LBs are cytoplasmic inclusions consisting of aggregated and misfolded proteins, such as α-syn, ubiquitin and neurofilament, as well as many other proteins [75–78]. The α-syn present in LBs is often post-translationally modified; thus it may be ubiquitinated [79], phosphorylated [80] and/or oxidized or nitrated [81]. Post-translationally modified forms of α-syn have an increased propensity to aggregate into LBs [82,83], but are also found extra-neuronally in PD brains [84,85] or in the periphery [86]. As demonstrated in animal models and in vitro assays, overexpression of α-syn also increases the protein’s propensity to aggregate [87,88]. Furthermore, point mutations in the gene encoding α-syn (SNCA; OMIM 163890) are linked to parkinsonism [89–92], as are duplications and triplications of the SNCA gene [93,94]; all of which increase aggregation of α-syn [95–97]. Taken together, these observations support the α-syn burden hypothesis, which posits that sporadic PD results from the inability to clear α-syn, while familial PD results from the overproduction of normal α-syn or mutated α-syn that prevents or slows clearance. Alternatively, mutations occur in other proteins that normally assist in α-syn clearance, but become defective in this function [41]. McGeer and McGeer further hypothesized that disease can be eliminated with a reduction in α-syn production or prevention of α-syn aggregation [41]. Thus, it is not surprising that vaccines currently being developed for PD target α-syn with the aim to clear aggregated and aberrant forms of the protein. However, there is also much evidence to support a non-autonomous cell death theory, in which cells other than neurons contribute to PD [16].
The substantia nigra (SN) has the highest density of microglia of any brain region [98], and post-mortem studies consistently demonstrate microglial activation in this region in PD. Furthermore, modified and aggregated forms of α-syn present in the PD patient SNc activate microglia. Chronic microglial activation has been seen in mice that over-express human α-syn [99], and in vitro, microglia are activated by aggregated human α-syn, leading to increased dopaminergic cell death and increased levels of reactive oxygen species and NADPH oxidase production [100]. In the acute 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine (MPTP) mouse model of nigrostriatal degeneration, nitrated α-syn (N-α-syn) enhances the neurotoxic activities of microglia, contributing to neuronal death [101]. The elimination of microglia-derived nitric oxide and superoxide are neuroprotective, suggesting that oxidative mediators from activated microglia play a key role in neuroinflammation, neurodegeneration and the accumulation of aberrant forms of α-syn [102]. Indeed, microglia in PD brains are positive for inducible nitric oxide synthase [103], from which nitric oxide-derived peroxynitrite is formed and is available to nitrate α-syn, which in turn and in part, induces neuronal cell death [104]. In addition to reactive oxygen species, activated microglia secrete many other soluble factors, such as chemokines and cytokines. COX-2 is present, as well as increased levels of TNF-α and NFκB1 in the SN, and IL-15, RANTES and IL-10 levels are significantly elevated in PD brains and in peripheral circulation [105–109]. Cytokines such as IL-1α and TNF-α secreted by activated glia or endothelial cells increase blood–brain barrier (BBB) permeability [110], which upregulates the expression of cellular adhesion molecules (such as E-selectin) on microvascular endothelial cells [111]. Increased BBB permeability allows activated T cells and B cells to enter the CNS more readily and migrate to the site of neuronal injury [112–114]. Indeed, increased BBB permeability is found in both AD and PD, allowing increased lymphocyte ingress [110,115–117]. In this way, activated innate immune cells of the CNS can affect the adaptive immune system in the periphery and recruit cells to the CNS. In addition, aberrant species of disease-specific proteins, including phosphorylated α-syn, are detectable in tissues outside the CNS in PD and dementia with Lewy bodies patients [86]. The presence of aberrant forms of α-syn in the periphery, such as within the GI tract and draining cervical lymph nodes, presents a possible means for exposure to the protein as a neoantigen and subsequent activation of the adaptive immune system.
Adaptive immune responses
It was once thought that the CNS was an ‘immune privileged’ site, in which immune cells of the periphery could not enter or rarely entered, and thus the two systems had little to no interaction. This hypothesis was supported by the early observation that tissue grafts in the eye or brain survived longer than grafts in other areas of the body [118]. However, evidence for the interaction of the adaptive immune system and the CNS is now well-recognized, and researchers are beginning to harness the neurotrophic effects of the immune system to aid in repair and regeneration in the CNS.
In neurodegenerative disease, CNS proteins may drain to the lymphatic system where the protein is processed and presented by dendritic cells and other APCs to lymphocytes. As proteins associated with neurodegenerative disease are often modified, the immune system may recognize them as non-self, leading to an immune response. An influx of antigen-specific lymphocytes into the brain may exacerbate and perpetuate the activation of microglia near dopaminergic neurons and increase the concentration of neurotoxic molecules within the nigra. Indeed, innate immune system abnormalities have been found in PD and models of PD. Overexpression of human α-syn in a mouse model of PD induces an influx of T and B cells into the CNS with increased proinflammatory cytokines [99]. In MPTP-intoxicated mice, N-α-syn is found in both the brain and in cervical lymph nodes, and immunization with N-α-syn induces N-α-syn specific proliferation of T cells [119]. CD4+ and CD8+ T cells can be found near dopaminergic neurons in both MPTP-treated mice and in PD patients [120]. Moreover, plasma B cells produce autoantibodies against glia and neuron antigens, and these antibodies are present in the cerebrospinal fluid and serum of PD patients [121,122]. Studies of the peripheral blood from PD patients have also shown many immunological disparities such as decreased naive (CD4+CD45RA+) T cells and increased memory (CD4+ CD45RO+) T cells [123], increased activated CD4+ T cells expressing Fas [124], increased IFN-γ-producing Th1 cells, decreased IL-4-producing Th2 cells and a decrease in CD4+CD25+ T cells [125]. Altogether, these data suggest that preferential activation of immune cells in PD and immune responses to aberrant forms of α-syn, as well as oxidative stress, affect neuronal cell death, further suggesting the utility of targeted immunotherapies in the treatment of PD [33,41,126].
Immunotherapy for neurodegenerative disease
Immunotherapies are designed to induce, enhance or suppress the immune system for the benefit of the host in the treatment of disease and more recently in neurodegenerative disorders [127–131]. Vaccine therapies have been used for centuries to modulate the immune system’s response to antigens, with the goal of either producing immunity against an infectious disease or attenuating the disease. Vaccines in the traditional sense consist of attenuated or killed pathogens, viral proteins or toxoids to protect against infectious agents. However, in most neurodegenerative proteopathies, no infectious agent is present to target, with the exception of prions. Thus, therapeutic vaccines against neurodegenerative diseases would consist of the self-proteins or peptides derived from the proteins that characterize the disease. Vaccine therapy may evoke active or passive immunity. Active vaccines contain epitopes that elicit immunity or partial protection against the agent from which the epitope is derived. Immunity to the pathogenic agent is achieved upon immunization with the ensuing induction of an immune response and subsequent immunologic memory formation that is specific for the pathogen. For years thereafter, memory immune cells and antibodies specific to the pathogenic agent circulate in the host and quickly respond to the antigen upon reencounter. The response time of immune cells that are primed to the antigen is greatly increased over that of naive cells and is one mechanism by which vaccine-mediated protection is afforded. A therapy targeting the humoral response could aid in the clearance of α-syn or amyloid-β (Aβ) by inducing B cells and plasma cells to secrete antigen-specific antibodies. In addition to antibodies that can cross an already permeable BBB, the accumulation of antibodies outside of the CNS and within the peripheral circulation could act as a peripheral sink, reducing the protein load in the CNS by driving the aberrant proteins into the periphery by mass action. The concept of a peripheral sink also makes the infusion of preformed antibodies (passive immunization) against rogue proteins an attractive option for both AD and PD. Indeed, two humanized monoclonal anti-Aβ antibodies, bapineuzumab and solanezumab, are currently in clinical trials [132]. Other immunotherapies include adoptive transfer and immunosuppression. Adoptive transfer involves the transfer of lymphoid cells from an immune donor to a naive recipient, while immunosuppression simply decreases the number or function of effector immune cells.
As previously stated, most immunotherapies are designed to protect the host from recent or potential exposures to foreign invaders. However, for neurodegenerative proteopathies, the target is self-proteins or modified self-proteins. Thus, the extent and the type of the immune system’s response to the vaccine become critical. Excessive inflammation, especially in the CNS, can lead to tissue damage, and chronic inflammation can develop into aberrant autoimmune responses as was seen in the AN1792 clinical trial for AD. This was due in part by the inherent plasticity of CD4+ T cells, which can differentiate into Th1, Th2, Th17 or Tregs [133] depending on the microenvironment in which the antigen is recognized and the nature of the major histocompatibility complex–peptide complex. Thus, the response to the antigen can play an important role in the outcome of disease, and most new vaccines are developed with this in mind. Differentiation of naive T cells into Th1 effectors contributes to cell-mediated immunity (activation of macrophages), while Th2 effectors contribute to humoral immunity (activation of B cells). According to the Th1/Th2 paradigm, Th1 cells, which produce IL-2, IFN-γ and TNF-α, are proinflammatory and induce release of reactive oxygen species and nitric oxide by microglia, while Th2 cells that produce IL-4, IL-5 and IL-13 enhance microglial-mediated neuroprotective functions, and thus are considered anti-inflammatory [134]. Both responses may have beneficial effects for neurodegenerative proteopathies, therefore, a balance between the two responses is an important consideration in the design of immunotherapies for neurodegenerative disease.
In preclinical studies for the treatment of AD, using PDAPP mice that are transgenic for human amyloid precursor protein (APP) driven by the human PDGF-β promoter, active immunization with synthetic human Aβ42 before the onset of neuropathology prevented the development of plaque formation and gliosis, and vaccination after the onset of neuropathology, slowed progression of disease and as concomitant with the increase in serum antibody titers against Aβ42 [135]. Furthermore, intranasal administration of Aβ peptide lowered Aβ plaque burden and Aβ42 levels in PDAPP mice and was associated with reduced reactive microglia and increased levels of in the anti-inflammatory cytokines IL-4, IL-10 and TGF-β [136]. Additional studies with TgCRND8 and Tg2576 mice demonstrated that vaccination against Aβ reduced cognitive dysfunction with reduced deposition of cerebral fibrillar Aβ and protected against deficits of learning and age-related memory, respectively [137,138]. Similarly, passive immunization against Aβ reduced Aβ deposition in the CNS and Aβ plasma levels [139,140]. These studies suggested a therapeutic effect of Aβ42 vaccination, and after a Phase I clinical trial confirmed its safety, a Phase II clinical trial ensued (AN1792). However, dosing in the Phase II trial was terminated early owing to the occurrence of autoimmune meningoencephalitis in 6% of patients, which was found to be due to Th1-type immune responses [129,141]. Comparing the cytokine profile of peripheral mononuclear cells from patients of the Phase I trial to those of the Phase II trial, it became evident that responses to the vaccine shifted from a Th2 to a Th1 phenotype, which was thought to be due to the addition of polysorbate 80 to the vaccine between the Phase I and Phase II trials [129]. These data stress the importance of well-controlled immune responses in immunotherapies.
Expert commentary
Immunotherapies for Parkinson’s disease
To date, no PD vaccines have been to clinical trial, and relatively few immunotherapies have been developed for preclinical testing. However, of those in preclinical testing, clearance of α-syn and diminution of neuroinflammation represent the major therapeutic strategies. These therapies may improve neuronal survival by decreasing protein aggregates within dopaminergic neurons and by decreasing activation of microglia. In 2005, a preclinical study tested a vaccine against human α-syn (hα-syn) in a mouse model of LB disease [142]. Transgenic mice overexpressing hα-syn that model LB disease were immunized with recombinant hα-syn to increase antibody production and elicit clearance of aggregated hα-syn in the CNS. Immunized mice had elevated hα-syn antibody titers, and while all immunized mice displayed reduced hα-syn load compared with controls, immunized mice producing antibodies with high relative affinity to hα-syn had more pronounced reduction than those with low affinity antibodies. This study also suggested that antibodies against hα-syn are taken up by neurons, bind intracellular hα-syn and increase hα-syn degradation via lysosomal pathways [142]. The work by Masliah et al. [142], as well as the studies with AD, suggest that clearance of α-syn represent a viable target, and in 2011 the first clinical trial of a PD vaccine is said to begin. The Michael J Fox Foundation funded the preclinical efficacy testing of the vaccine, PD01, in 2010 by AFFIRiS. PD01 is a mimotope that elicits a highly specific humoral immune response to clear an aberrant form of α-syn [201]. PD01 targets the phosphorylated form of the protein, which activates microglia and induces neurotoxic proinflammatory responses. AFFIRiS claims that PD01 exhibits disease-modifying activity, but the company has yet to publish the preclinical data in a peer-reviewed format [143].
Our laboratory is developing an immunization strategy to clear aberrant forms of α-syn while concurrently controlling neurotoxic inflammatory responses by inducing Treg populations. Tregs are a specialized subset of CD4+ T cells that maintain self-tolerance, prevent autoimmunity and maintain immune homeostasis by attenuating inflammation caused by pathogens, injury or autoimmunity [144–151]. In mice, Tregs are identified by CD4 and CD25 cell surface markers and by the transcription factor forkhead box P3 (FOXP3) [152–154]. In humans, they are identified as CD4+, CD25+, CD39+, CD49d+, FOXP3+ and CD127− [155,156]. While naturally occurring Tregs (nTregs) mature in the thymus, naive CD4+ T cells in the periphery can be polarized into the inducible Treg phenotype under certain conditions. The presence of TGF-β, IL-2, IL-10 and all-trans retinoic acid polarize T cells to the inducible Treg phenotype [157–160], and histone deacetylase inhibitors induce proliferation and increase the suppressive function of Tregs [161–164]. Tregs can also be induced by vasoactive intestinal peptide (VIP) [165–167]. Once induced, Tregs promote neurotrophic support by inducing astrocytes to increase expression of BDNF and GDNF [168,169] and may promote glutamate clearance [170]. This is significant considering that astrocyte dysfunction is a known contributor to neurodegeneration [171]. Furthermore, in in vitro studies, Tregs suppress effector T-cell (Teff) responses via cell-to-cell contact [150] and with the secretion of soluble factors [172]. Tregs also inhibit the adaptive immune system indirectly by affecting antigen presentation by APCs [173]. Dysfunctional and reduced frequencies of Tregs are associated with several diseases [174] and are being investigated for therapeutic use [167,175–178]. Tregs may also be attractive therapeutic targets in neurodegenerative diseases, as their induction could modulate microglia phenotypes and control adaptive immune responses to CNS proteins. Indeed, works from our laboratory support the utility of Tregs in neuroprotection.
Early studies in our laboratory tested the ability of copolymer-1 (Cop-1; Copaxone®, glatiramer acetate) as an immunomodulatory agent in a model of PD. Cop-1 is a random polymer composed of four amino acids that induces Th2/Th3/Tr1 cells [179]. The immune response induced by Cop-1 cross-reacts with myelin basic protein [180,181]. Thus, Cop-1 is used as an immunomodulator in the treatment of relapsing–remitting multiple sclerosis, and is thought to work largely by changing the proinflammatory immune response to a more anti-inflammatory response, in part by the induction of Tregs. In relapsing–remitting multiple sclerosis patients, Cop-1 increases Treg frequencies [177], and in mice with experimental autoimmune encephalomyelitis, reduces Th17 frequencies, while increasing Tregs [182]. We tested the immunomodulatory effects of Cop-1 in the acute MPTP-mouse model of nigrostriatal degeneration. Adoptive transfer of splenocytes from Cop-1-immunized mice to MPTP-intoxicated recipients led to T-cell infiltration in the SN, a reduction in microgliosis, an increase in neurotrophic factors and an increase in neuronal survival [169]. The protection provided by the transferred splenocytes was found to be dose dependent, with CD4+ T cells providing the greatest degree of neuroprotection [183]. Further investigation provided evidence for the role of CD4+CD25+ Tregs in the neuroprotection provided by Cop-1-splenocytes transferred to MPTP recipients. Adoptive transfer of CD3-activated Tregs to MPTP-intoxicated mice reduced microgliosis, increased neurotrophic factors and provided greater than 90% protection of dopaminergic neurons in a dose-dependant manner, while in vitro assays demonstrated that Tregs modulate the phenotype of microglia and control microglial responses to N-α-syn, lipopolysaccharide and TNF-α with or without phorbol myristate acetate [168]. N-α-syn is immunogenic and vaccination with nitrated-4YSyn (N-4YSyn) elicits an adaptive immune response; 4YSyn is the C-terminal portion of recombinant mouse α-syn that contains four out of five of the nitratable tyrosine residues. Adoptive transfer of T cells from mice immunized with N-4YSyn to MPTP-intoxicated recipients increased dopaminergic neuronal loss [119]. However, when splenocytes from VIP-treated mice were coadoptively transferred with splenocytes from N-4YSyn-immunized mice, a decrease in microgliosis and an increase in dopaminergic neuron survival were observed, while adoptive transfer of splenocytes from mice treated with VIP alone showed only a small increase in neuronal survival, and adoptive transfer of splenocytes from N-4YSyn-immunized mice alone significantly decreased neuronal survival and exacerbated microgliosis. To elucidate which cell types are involved in this process, CD4+ T cells were polarized before adoptive transfer, and demonstrated that Th1 and Th17 cells exacerbated the MPTP-induced neurodegeneration. Furthermore, when Tregs from VIP-treated mice were adoptively transferred with splenocytes from N-4YSyn-immunized mice, dopaminergic neuronal survival increased to 96% [184]. Together, these data implicate Th1 and Th17 cells in neurodegeneration and suggest that CD4+CD25+ Tregs have increased suppressive function in the presence of N-4YSyn-specific T cells.
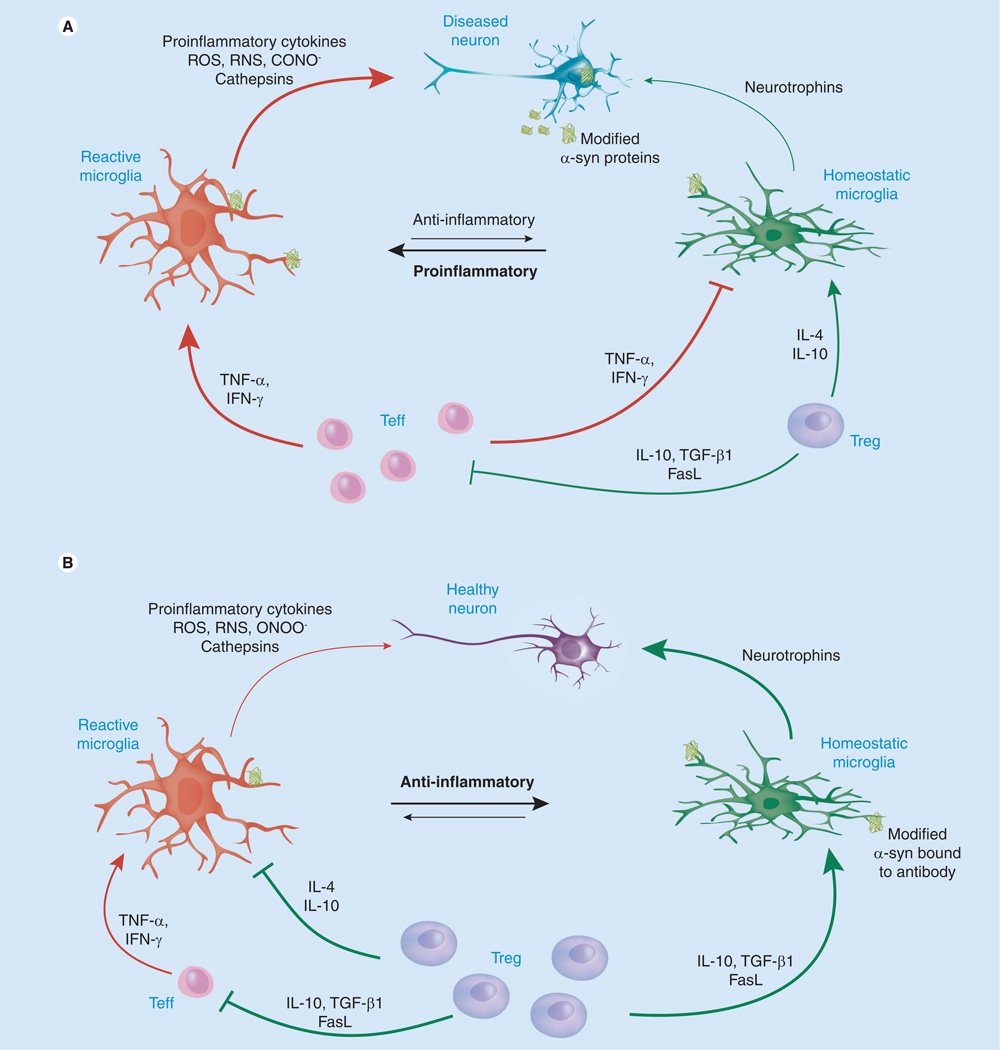
The abovementioned studies support the utility of Tregs to reduce microgliosis and modulate the adaptive immune response to a neurotrophic phenotype, while reinforcing the therapeutic efficacy of an α-syn vaccine for the clearance of LBs via humoral responses (Figure 1). In disease, proinflammatory M1 microglia predominate, and potentially autoreactive Th1 or Th17 cells exacerbate this phenotype. While M2 microglia may still be present in the diseased brain, the release of reactive oxygen species by M1 microglia and infiltrating macrophages is linked to the accumulation of modified α-syn proteins that contribute to neurotoxicity. An α-syn vaccine designed to elicit a predominately humoral response could induce the production of antibodies specific for modified species of α-syn, which would increase clearance of LBs. The induction of Tregs may ameliorate disease further by modulating the innate and adaptive immune responses as demonstrated in mouse models. Tregs would act by inducing phenotypic switching in Teffs, microglia and macrophages or by killing autoreactive T cells, thus supporting a neurotrophic and anti-inflammatory microenvironment. All together, these strategies may decrease the α-syn load in the CNS and modulate microglia towards a more homeostatic neuroprotective M2 phenotype, thereby increasing neuronal survival and function, and slowing disease progression.
Figure 1. Immunomodulation in Parkinson’s disease.
In Parkinson’s disease, modified α-syn affect microglial immune responses. Reactive microglia secrete neurotoxins, chemokines, peroxynitrite ONOO− and other ROS and RNS, which increase the formation of oxidized proteins. Chemokines attract cells of the adaptive immune system, and infiltrating Teffs exacerbate a proinflammatory neurotoxic phenotype by secreting TNF-α and IFN-γ. Tregs may be present in low numbers or are dysfunctional, and thus have a limited affect on disease progression (A). Tregs promote neurotrophic support, suppress Teff responses and secrete anti-inflammatory cytokines, such as IL-10, IL-4 and TGF-β. In the brain of the immunotherapy-treated patient, Treg induction could decrease microglial responses and control adaptive immune activities while supporting the clearance of misfolded proteins though induction of humoral responses. Thus, the α-syn load in the CNS would be decreased and microglia would switch to a more homeostatic neuroprotective phenotype, thereby increasing neuronal survival and function and slowing disease progression (B) [184].
α-syn: α-synuclein; FasL: Fas ligand; RNS: Reactive nitrogen species; ROS: Reactive oxygen species; Teff: Effector T-cell.
Adapted with permission from [33].
Five-year view
PD is manifest, in part, as a consequence of progressive nigrostriatal degeneration with parallel losses in dopamine and communication links between the SNc and the striatum. Genetic environmental exposure, toxins and aging all contribute. To date, there are only symptomatic treatments available with dopamine replacement therapy or dopamine agonists. A real treatment for disease would involve replacement of damaged neurons and its connections (stem cell therapy), reversal of neural damage (through growth or other neuroregenerative factors) or by engaging the host’s immune system to exact repair through natural means. As for the latter, the realization of a neuroprotective immunization strategy remains the lifeblood of our laboratories. How best to control the production of neurotoxic aggregated, misfolded and oxidized proteins that affect free radical formation and tissue damage is difficult. Such proteins are, for the better part, intracellular and released in limited bursts to the extracellular and extravascular compartments. However, its devastating effects on neural connections and neuroinflammation cannot be understated. We posit that effective clearance can be realized through specific antibody and cellular immune responses that can also transform autoreactive neurotoxic Teffs into neurotrophic ones. It is our belief that the mechanism by which the immune system can be controlled for therapeutic benefit will be realized in the next decade, and when combined with other treatment modalities, such as neuronal replacement by stem cells and/or growth factors, will change the course of human disease from devastating to controlled and usher in an era where the ravishes of PD are no longer seen.
Key issues.
Aberrant forms of proteins can be immunogenic.
Immunization against aberrant forms of α-synuclein could affect clearance of Lewy bodies.
Immunotherapies may be used to modulate or transform the immune system and lead to neuroprotection in Parkinson’s disease and other neurodegenerative diseases.
Immunotherapies for Parkinson’s disease are being developed in preclinical studies.
Acknowledgments
This work was supported in part by the Michael J. Fox Foundation and by NIH grants P20 DA026146, 5P01 DA028555-02, R01 NS36126, P01 NS31492, 2R01 NS034239, P20 RR15635, P01 MH64570, P01 NS43985 and 5R01 NS070190.
Footnotes
Financial & competing interests disclosure
The authors have no other relevant affiliations or financial involvement with any organization or entity with a financial interest in or financial conflict with the subject matter or materials discussed in the manuscript apart from those disclosed.
No writing assistance was utilized in the production of this manuscript.
References
Papers of special note have been highlighted as:
• of interest
•• of considerable interest
- 1.Jellinger KA. Formation and development of Lewy pathology: a critical update. J. Neurol. 2009;256 suppl. 3:270–279. doi: 10.1007/s00415-009-5243-y. [DOI] [PubMed] [Google Scholar]
- 2. Jellinger KA. α-synuclein pathology in Parkinson's and Alzheimer's disease brain: incidence and topographic distribution – a pilot study. Acta Neuropathol. 2003;106(3):191–201. doi: 10.1007/s00401-003-0725-y.. •• Investigates the topographic distribution of α-synuclein (α-syn) inclusions in Parkinson’s disease (PD), dementia with Lewy bodies, and Alzheimer’s disease and demonstrates the pathological similarities among the diseases.
- 3.Bernheimer H, Birkmayer W, Hornykiewicz O, Jellinger K, Seitelberger F. Brain dopamine and the syndromes of Parkinson and Huntington. Clinical, morphological and neurochemical correlations. J. Neurol. Sci. 1973;20(4):415–455. doi: 10.1016/0022-510x(73)90175-5. [DOI] [PubMed] [Google Scholar]
- 4. Lees AJ, Hardy J, Revesz T. Parkinson’s disease. Lancet. 2009;373(9680):2055–2066. doi: 10.1016/S0140-6736(09)60492-X.. •• Comprehensive literature review of PD pathology, clinical symptoms, diagnosis, current and prospective treatments.
- 5. Mercuri NB, Bernardi G. The ‘magic’ of l-dopa: why is it the gold standard Parkinson’s disease therapy? Trends Pharmacol. Sci. 2005;26(7):341–344. doi: 10.1016/j.tips.2005.05.002.. • Reviews evidence suggesting that dopamine produced from l-dopa may act on adrenoceptors, as well as other sites that make it superior to dopamine receptor agonists.
- 6.Silver DE, Ruggieri S. Initiating therapy for Parkinson’s disease. Neurology. 1998;50 suppl. 6:S18–S22. doi: 10.1212/wnl.50.6_suppl_6.s18. [DOI] [PubMed] [Google Scholar]
- 7.Fahn S. ‘On-off ’ phenomenon with levodopa therapy in Parkinsonism. Clinical and pharmacologic correlations and the effect of intramuscular pyridoxine. Neurology. 1974;24(5):431–441. doi: 10.1212/wnl.24.5.431. [DOI] [PubMed] [Google Scholar]
- 8.Hutton JT, Morris JL. Long-acting carbidopa–levodopa in the management of moderate and advanced Parkinson’s disease. Neurology. 1992;42 1 Suppl. 1:51–56. [PubMed] [Google Scholar]
- 9. Rascol O, Lozano A, Stern M, Poewe W. Milestones in Parkinson’s disease therapeutics. Mov. Disord. 2011;26(6):1072–1082. doi: 10.1002/mds.23714.. • Provides a comprehensive history of PD therapeutics from the introduction of dopamine replacement therapies to surgical interventions and cell-based and gene therapy.
- 10.Clarkson ED. Fetal tissue transplantation for patients with Parkinson’s disease: a database of published clinical results. Drugs Aging. 2001;18(10):773–785. doi: 10.2165/00002512-200118100-00006. [DOI] [PubMed] [Google Scholar]
- 11.Lindvall O, Rehncrona S, Brundin P, et al. Human fetal dopamine neurons grafted into the striatum in two patients with severe Parkinson’s disease. A detailed account of methodology and a 6-month follow-up. Arch. Neurol. 1989;46(6):615–631. doi: 10.1001/archneur.1989.00520420033021. [DOI] [PubMed] [Google Scholar]
- 12.Freed CR, Greene PE, Breeze RE, et al. Transplantation of embryonic dopamine neurons for severe Parkinson’s disease. N. Engl. J. Med. 2001;(10):710–719. doi: 10.1056/NEJM200103083441002. [DOI] [PubMed] [Google Scholar]
- 13.Olanow CW, Goetz CG, Kordower JH, et al. A double-blind controlled trial of bilateral fetal nigral transplantation in Parkinson’s disease. Ann. Neurol. 2003;54(3):403–414. doi: 10.1002/ana.10720. [DOI] [PubMed] [Google Scholar]
- 14.Olanow CW, Kordower JH, Lang AE, Obeso JA. Dopaminergic transplantation for Parkinson’s disease: current status and future prospects. Ann. Neurol. 2009;66(5):591–596. doi: 10.1002/ana.21778. [DOI] [PubMed] [Google Scholar]
- 15. Kordower JH, Chu Y, Hauser RA, Freeman TB, Olanow CW. Lewy body-like pathology in long-term embryonic nigral transplants in Parkinson’s disease. Nat. Med. 2008;14(5):504–506. doi: 10.1038/nm1747.. • Shows that grafted neurons become affected by disease progression.
- 16. Dawson TM. Non-autonomous cell death in Parkinson’s disease. Lancet Neurol. 2008;7(6):474–475. doi: 10.1016/S1474-4422(08)70099-1.. • Suggests that non-neuronal cells contribute to neurodegeneration in PD.
- 17.Ferrari G, Minozzi MC, Toffano G, Leon A, Skaper SD. Basic fibroblast growth factor promotes the survival and development of mesencephalic neurons in culture. Dev. Biol. 1989;133(1):140–147. doi: 10.1016/0012-1606(89)90305-9. [DOI] [PubMed] [Google Scholar]
- 18.Knusel B, Michel PP, Schwaber JS, Hefti F. Selective and nonselective stimulation of central cholinergic and dopaminergic development in vitro by nerve growth factor, basic fibroblast growth factor, epidermal growth factor, insulin and the insulin-like growth factors I and II. J. Neurosci. 1990;10(2):558–570. doi: 10.1523/JNEUROSCI.10-02-00558.1990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Park TH, Mytilineou C. Protection from 1-methyl-4-phenylpyridinium (MPP+) toxicity and stimulation of regrowth of MPP(+)-damaged dopaminergic fibers by treatment of mesencephalic cultures with EGF and basic FGF. Brain Res. 1992;599(1):83–97. doi: 10.1016/0006-8993(92)90855-4. [DOI] [PubMed] [Google Scholar]
- 20.Hyman C, Hofer M, Barde YA, et al. BDNF is a neurotrophic factor for dopaminergic neurons of the substantia nigra. Nature. 1991;350(6315):230–232. doi: 10.1038/350230a0. [DOI] [PubMed] [Google Scholar]
- 21.Lin LF, Doherty DH, Lile JD, Bektesh S, Collins F. GDNF: a glial cell line-derived neurotrophic factor for midbrain dopaminergic neurons. Science. 1993;260(5111):1130–1132. doi: 10.1126/science.8493557. [DOI] [PubMed] [Google Scholar]
- 22.Beck KD, Valverde J, Alexi T, et al. Mesencephalic dopaminergic neurons protected by GDNF from axotomy-induced degeneration in the adult brain. Nature. 1995;373(6512):339–341. doi: 10.1038/373339a0. [DOI] [PubMed] [Google Scholar]
- 23.Gash DM, Zhang Z, Ovadia A, et al. Functional recovery in parkinsonian monkeys treated with GDNF. Nature. 1996;380(6571):252–255. doi: 10.1038/380252a0. [DOI] [PubMed] [Google Scholar]
- 24.Kordower JH, Emborg ME, Bloch J, et al. Neurodegeneration prevented by lentiviral vector delivery of GDNF in primate models of Parkinson’s disease. Science. 2000;290(5492):767–773. doi: 10.1126/science.290.5492.767. [DOI] [PubMed] [Google Scholar]
- 25.Hong M, Mukhida K, Mendez I. GDNF therapy for Parkinson’s disease. Expert Rev. Neurother. 2008;8(7):1125–1139. doi: 10.1586/14737175.8.7.1125. [DOI] [PubMed] [Google Scholar]
- 26.Rangasamy SB, Soderstrom K, Bakay RA, Kordower JH. Neurotrophic factor therapy for Parkinson’s disease. Prog. Brain Res. 2010;184:237–264. doi: 10.1016/S0079-6123(10)84013-0. [DOI] [PubMed] [Google Scholar]
- 27.Kordower JH, Palfi S, Chen EY, et al. Clinicopathological findings following intraventricular glial-derived neurotrophic factor treatment in a patient with Parkinson’s disease. Ann. Neurol. 1999;46(3):419–424. doi: 10.1002/1531-8249(199909)46:3<419::aid-ana21>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 28.Nutt JG, Burchiel KJ, Comella CL, et al. Randomized, double-blind trial of glial cell line-derived neurotrophic factor (GDNF) in PD. Neurology. 2003;60(1):69–73. doi: 10.1212/wnl.60.1.69. [DOI] [PubMed] [Google Scholar]
- 29.Slevin JT, Gerhardt GA, Smith CD, Gash DM, Kryscio R, Young B. Improvement of bilateral motor functions in patients with Parkinson disease through the unilateral intraputaminal infusion of glial cell line-derived neurotrophic factor. J. Neurosurg. 2005;102(2):216–222. doi: 10.3171/jns.2005.102.2.0216. [DOI] [PubMed] [Google Scholar]
- 30.Gill SS, Patel NK, Hotton GR, et al. Direct brain infusion of glial cell line-derived neurotrophic factor in Parkinson disease. Nat. Med. 2003;9(5):589–595. doi: 10.1038/nm850. [DOI] [PubMed] [Google Scholar]
- 31. Luo XG, Ding JQ, Chen SD. Microglia in the aging brain: relevance to neurodegeneration. Mol. Neurodegener. 2010;5:12. doi: 10.1186/1750-1326-5-12.. • Provides evidence that age-induced microglial senescence occurs in the brain and leads to neurotoxic activities during disease.
- 32.Graeber MB, Streit WJ. Microglia: biology and pathology. Acta Neuropathol. 2010;119(1):89–105. doi: 10.1007/s00401-009-0622-0. [DOI] [PubMed] [Google Scholar]
- 33.Kosloski LM, Ha DM, Hutter JA, et al. Adaptive immune regulation of glial homeostasis as an immunization strategy for neurodegenerative diseases. J. Neurochem. 2010;114(5):1261–1276. doi: 10.1111/j.1471-4159.2010.06834.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Janeway CA., Jr Approaching the asymptote? Evolution and revolution in immunology. Cold Spring Harb. Symp. Quant. Biol. 1989;54(Pt 1):1–13. doi: 10.1101/sqb.1989.054.01.003. [DOI] [PubMed] [Google Scholar]
- 35.Janeway CA, Jr, Medzhitov R. Innate immune recognition. Annu. Rev. Immunol. 2002;20:197–216. doi: 10.1146/annurev.immunol.20.083001.084359. [DOI] [PubMed] [Google Scholar]
- 36. Amor S, Puentes F, Baker D, Van Der Valk P. Inflammation in neurodegenerative diseases. Immunology. 2010;129(2):154–169. doi: 10.1111/j.1365-2567.2009.03225.x.. • Comprehensive review of evidence supporting the role of innate and adaptive immunity in the pathobiology of neurodegenerative diseases.
- 37.McGeer PL, Yasojima K, McGeer EG. Inflammation in Parkinson’s disease. Adv. Neurol. 2001;86:83–89. [PubMed] [Google Scholar]
- 38.Smith PF. Inflammation in Parkinson’s disease: an update. Curr. Opin. Investig. Drugs. 2008;9(5):478–484. [PubMed] [Google Scholar]
- 39. Tansey MG, Goldberg MS. Neuroinflammation in Parkinson’s disease: its role in neuronal death and implications for therapeutic intervention. Neurobiol. Dis. 2010;37(3):510–518. doi: 10.1016/j.nbd.2009.11.004.. • Comprehenisve review of data suggesting that chronic neuroinflammation plays a role in PD; includes epidemiological evidence supporting the use of anti-inflammatory drugs.
- 40.Siffrin V, Brandt AU, Herz J, Zipp F. New insights into adaptive immunity in chronic neuroinflammation. Adv. Immunol. 2007;96:1–40. doi: 10.1016/S0065-2776(07)96001-0. [DOI] [PubMed] [Google Scholar]
- 41. McGeer PL, McGeer EG. Glial reactions in Parkinson’s disease. Mov. Dis. 2008;23(4):474–483. doi: 10.1002/mds.21751.. • Describes the neurotrophic and neurotoxic activities of glia in PD.
- 42.Hirsch EC, Hunot S. Neuroinflammation in Parkinson’s disease: a target for neuroprotection? Lancet Neurol. 2009;8(4):382–397. doi: 10.1016/S1474-4422(09)70062-6. [DOI] [PubMed] [Google Scholar]
- 43.Perry VH. Contribution of systemic inflammation to chronic neurodegeneration. Acta Neuropathol. 2010;120(3):277–286. doi: 10.1007/s00401-010-0722-x. [DOI] [PubMed] [Google Scholar]
- 44.Liu B, Gao HM, Hong JS. Parkinson’s disease and exposure to infectious agents and pesticides and the occurrence of brain injuries: role of neuroinflammation. Environ. Health Perspect. 2003;111(8):1065–1073. doi: 10.1289/ehp.6361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Andersen JK. Oxidative stress in neurodegeneration: cause or consequence? Nat. Med. 2004;10 suppl.:S18–S25. doi: 10.1038/nrn1434. [DOI] [PubMed] [Google Scholar]
- 46.Blasko I, Grubeck-Loebenstein B. Role of the immune system in the pathogenesis, prevention and treatment of Alzheimer’s disease. Drugs Aging. 2003;20(2):101–113. doi: 10.2165/00002512-200320020-00002. [DOI] [PubMed] [Google Scholar]
- 47.Klegeris A, McGeer PL. Non-steroidal anti-inflammatory drugs (NSAIDs) and other anti-inflammatory agents in the treatment of neurodegenerative disease. Curr. Alzheimer Res. 2005;2(3):355–365. doi: 10.2174/1567205054367883. [DOI] [PubMed] [Google Scholar]
- 48.Rich JB, Rasmusson DX, Folstein MF, Carson KA, Kawas C, Brandt J. Nonsteroidal anti-inflammatory drugs in Alzheimer’s disease. Neurology. 1995;45(1):51–55. doi: 10.1212/wnl.45.1.51. [DOI] [PubMed] [Google Scholar]
- 49.in t’ Veld BA, Ruitenberg A, Hofman A, et al. Nonsteroidal antiinflammatory drugs and the risk of Alzheimer’s disease. N. Engl. J. Med. 2001:1515–1521. doi: 10.1056/NEJMoa010178. [DOI] [PubMed] [Google Scholar]
- 50.Zandi PP, Anthony JC, Hayden KM, Mehta K, Mayer L, Breitner JC. Reduced incidence of AD with NSAID but not H2 receptor antagonists: the Cache County Study. Neurology. 2002;59(6):880–886. doi: 10.1212/wnl.59.6.880. [DOI] [PubMed] [Google Scholar]
- 51.Becker C, Jick SS, Meier CR. NSAID use and risk of Parkinson disease: a population-based case–control study. Eur. J. Neurol. 2011;(11):1336–1342. doi: 10.1111/j.1468-1331.2011.03399.x. [DOI] [PubMed] [Google Scholar]
- 52.Gao X, Chen H, Schwarzschild MA, Ascherio A. Use of ibuprofen and risk of Parkinson disease. Neurology. 2011;76(10):863–869. doi: 10.1212/WNL.0b013e31820f2d79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Manthripragada AD, Schernhammer ES, Qiu J, et al. Non-steroidal anti-inflammatory drug use and the risk of Parkinson’s disease. Neuroepidemiology. 2011;36(3):155–161. doi: 10.1159/000325653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Cunningham C, Skelly DT. Non-steroidal anti-inflammatory drugs and cognitive function: are prostaglandins at the heart of cognitive impairment in dementia and delirium? J. Neuroimmune Pharmacol. 2011 doi: 10.1007/s11481-011-9312-5. (Epub ahead of print). •• Discusses the potential role of anti-inflammatory drugs and their effects on prostaglandins and cognitive function in neurodegeneration.
- 55.Nimmerjahn A, Kirchhoff F, Helmchen F. Resting microglial cells are highly dynamic surveillants of brain parenchyma in vivo. Science. 2005;308(5726):1314–1318. doi: 10.1126/science.1110647. [DOI] [PubMed] [Google Scholar]
- 56.Davalos D, Grutzendler J, Yang G, et al. ATP mediates rapid microglial response to local brain injury in vivo. Nat. Neurosci. 2005;8(6):752–758. doi: 10.1038/nn1472. [DOI] [PubMed] [Google Scholar]
- 57.Lull ME, Block ML. Microglial activation and chronic neurodegeneration. Neurotherapeutics. 2010;7(4):354–365. doi: 10.1016/j.nurt.2010.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang SC, Fedoroff S. Neuron-microglia interactions in vitro. Acta Neuropathol. 1996;91(4):385–395. doi: 10.1007/s004010050440. [DOI] [PubMed] [Google Scholar]
- 59.Glezer I, Simard AR, Rivest S. Neuroprotective role of the innate immune system by microglia. Neuroscience. 2007;147(4):867–883. doi: 10.1016/j.neuroscience.2007.02.055. [DOI] [PubMed] [Google Scholar]
- 60.Giulian D, Baker TJ, Shih LC, Lachman LB. Interleukin 1 of the central nervous system is produced by ameboid microglia. J. Exp. Med. 1986;164(2):594–604. doi: 10.1084/jem.164.2.594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Sawada M, Kondo N, Suzumura A, Marunouchi T. Production of tumor necrosis factor-α by microglia and astrocytes in culture. Brain Res. 1989;491(2):394–397. doi: 10.1016/0006-8993(89)90078-4. [DOI] [PubMed] [Google Scholar]
- 62.Elkabes S, Dicicco-Bloom EM, Black IB. Brain microglia/macrophages express neurotrophins that selectively regulate microglial proliferation and function. J. Neurosci. 1996;16(8):2508–2521. doi: 10.1523/JNEUROSCI.16-08-02508.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Heese K, Hock C, Otten U. Inflammatory signals induce neurotrophin expression in human microglial cells. J. Neurochem. 1998;70(2):699–707. doi: 10.1046/j.1471-4159.1998.70020699.x. [DOI] [PubMed] [Google Scholar]
- 64.Gordon S, Martinez FO. Alternative activation of macrophages: mechanism and functions. Immunity. 2010;32(5):593–604. doi: 10.1016/j.immuni.2010.05.007. [DOI] [PubMed] [Google Scholar]
- 65.Rock RB, Gekker G, Hu S, et al. Role of microglia in central nervous system infections. Clin. Microbiol. Rev. 2004;17(4):942–964. doi: 10.1128/CMR.17.4.942-964.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kigerl KA, Gensel JC, Ankeny DP, Alexander JK, Donnelly DJ, Popovich PG. Identification of two distinct macrophage subsets with divergent effects causing either neurotoxicity or regeneration in the injured mouse spinal cord. J. Neurosci. 2009;29(43):13435–13444. doi: 10.1523/JNEUROSCI.3257-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.McGeer PL, Itagaki S, McGeer EG. Expression of the histocompatibility glycoprotein HLA-DR in neurological disease. Acta Neuropathol. 1988;76(6):550–557. doi: 10.1007/BF00689592. [DOI] [PubMed] [Google Scholar]
- 68.Muhleisen H, Gehrmann J, Meyermann R. Reactive microglia in Creutzfeldt–Jakob disease. Neuropathol. Appl. Neurobiol. 1995;21(6):505–517. doi: 10.1111/j.1365-2990.1995.tb01097.x. [DOI] [PubMed] [Google Scholar]
- 69.Gonzalez-Scarano F, Baltuch G. Microglia as mediators of inflammatory and degenerative diseases. Annu. Rev. Neurosci. 1999;22:219–240. doi: 10.1146/annurev.neuro.22.1.219. [DOI] [PubMed] [Google Scholar]
- 70.Engelhardt JI, Appel SH. IgG reactivity in the spinal cord and motor cortex in amyotrophic lateral sclerosis. Arch. Neurol. 1990;47(11):1210–1216. doi: 10.1001/archneur.1990.00530110068019. [DOI] [PubMed] [Google Scholar]
- 71.Dickson DW, Farlo J, Davies P, Crystal H, Fuld P, Yen SH. Alzheimer’s disease. A double-labeling immunohistochemical study of senile plaques. Am. J. Pathol. 1988;132(1):86–101. [PMC free article] [PubMed] [Google Scholar]
- 72.Mattiace LA, Davies P, Yen SH, Dickson DW. Microglia in cerebellar plaques in Alzheimer’s disease. Acta Neuropathol. 1990;80(5):493–498. doi: 10.1007/BF00294609. [DOI] [PubMed] [Google Scholar]
- 73.McGeer PL, Itagaki S, Boyes BE, McGeer EG. Reactive microglia are positive for HLA-DR in the substantia nigra of Parkinson’s and Alzheimer’s disease brains. Neurology. 1988;38(8):1285–1291. doi: 10.1212/wnl.38.8.1285. [DOI] [PubMed] [Google Scholar]
- 74.Crystal H, Dickson D, Fuld P, et al. Clinico-pathologic studies in dementia: nondemented subjects with pathologically confirmed Alzheimer’s disease. Neurology. 1988;38(11):1682–1687. doi: 10.1212/wnl.38.11.1682. [DOI] [PubMed] [Google Scholar]
- 75.Spillantini MG, Schmidt ML, Lee VM, Trojanowski JQ, Jakes R, Goedert M. α-synuclein in Lewy bodies. Nature. 1997;388(6645):839–840. doi: 10.1038/42166. [DOI] [PubMed] [Google Scholar]
- 76.Spillantini MG, Crowther RA, Jakes R, Hasegawa M, Goedert M. α-Synuclein in filamentous inclusions of Lewy bodies from Parkinson’s disease and dementia with lewy bodies. Proc. Natl Acad. Sci. USA. 1998;95(11):6469–6473. doi: 10.1073/pnas.95.11.6469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Goldman JE, Yen SH, Chiu FC, Peress NS. Lewy bodies of Parkinson’s disease contain neurofilament antigens. Science. 1983;221(4615):1082–1084. doi: 10.1126/science.6308771. [DOI] [PubMed] [Google Scholar]
- 78.Jellinger KA. Lewy body disorders. In: Youdim MBH, editor. Degenerative Diseases of the Nervous System. New York, NY, USA: Springer Science; 2007. pp. 270–343. [Google Scholar]
- 79.Shimura H, Schlossmacher MG, Hattori N, et al. Ubiquitination of a new form of α-synuclein by parkin from human brain: implications for Parkinson’s disease. Science. 2001;293(5528):263–269. doi: 10.1126/science.1060627. [DOI] [PubMed] [Google Scholar]
- 80.Fujiwara H, Hasegawa M, Dohmae N, et al. α-Synuclein is phosphorylated in synucleinopathy lesions. Nat. Cell Biol. 2002;4(2):160–164. doi: 10.1038/ncb748. [DOI] [PubMed] [Google Scholar]
- 81.Giasson BI, Duda JE, Murray IV, et al. Oxidative damage linked to neurodegeneration by selective α-synuclein nitration in synucleinopathy lesions. Science. 2000;290(5493):985–989. doi: 10.1126/science.290.5493.985. [DOI] [PubMed] [Google Scholar]
- 82.Uversky VN, Yamin G, Munishkina LA, et al. Effects of nitration on the structure and aggregation of α-synuclein. Brain Res. Mol. Brain Res. 2005;134(1):84. 84–102. doi: 10.1016/j.molbrainres.2004.11.014. [DOI] [PubMed] [Google Scholar]
- 83.Cavallarin N, Vicario M, Negro A. The role of phosphorylation in synucleinopathies: focus on Parkinson’s disease. CNS Neurol. Disord. Drug Targets. 2010;9(4):471–481. doi: 10.2174/187152710791556140. [DOI] [PubMed] [Google Scholar]
- 84.Lee SJ. Origins and effects of extracellular α-synuclein: implications in Parkinson’s disease. J. Mol. Neurosci. 2008;34(1):17–22. doi: 10.1007/s12031-007-0012-9. [DOI] [PubMed] [Google Scholar]
- 85.Good PF, Hsu A, Werner P, Perl DP, Olanow CW. Protein nitration in Parkinson’s disease. J. Neuropathol. Exp. Neurol. 1998;57(4):338–342. doi: 10.1097/00005072-199804000-00006. [DOI] [PubMed] [Google Scholar]
- 86.Beach TG, Adler CH, Sue LI, et al. Multi-organ distribution of phosphorylated α-synuclein histopathology in subjects with Lewy body disorders. Acta Neuropathol. 2010;119(6):689–702. doi: 10.1007/s00401-010-0664-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Koprich JB, Johnston TH, Reyes MG, Sun X, Brotchie JM. Expression of human A53T α-synuclein in the rat substantia nigra using a novel AAV1/2 vector produces a rapidly evolving pathology with protein aggregation, dystrophic neurite architecture and nigrostriatal degeneration with potential to model the pathology of Parkinson’s disease. Mol. Neurodegener. 2010;5:43. doi: 10.1186/1750-1326-5-43. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Parihar MS, Parihar A, Fujita M, Hashimoto M, Ghafourifar P. α-synuclein overexpression and aggregation exacerbates impairment of mitochondrial functions by augmenting oxidative stress in human neuroblastoma cells. Int. J. Biochem. Cell Biol. 2009;41(10):2015–2024. doi: 10.1016/j.biocel.2009.05.008. [DOI] [PubMed] [Google Scholar]
- 89.Polymeropoulos MH, Higgins JJ, Golbe LI, et al. Mapping of a gene for Parkinson’s disease to chromosome 4q21-q23. Science. 1996;274(5290):1197–1199. doi: 10.1126/science.274.5290.1197. [DOI] [PubMed] [Google Scholar]
- 90.Polymeropoulos MH, Lavedan C, Leroy E, et al. Mutation in the α-synuclein gene identified in families with Parkinson’s disease. Science. 1997;276(5321):2045–2047. doi: 10.1126/science.276.5321.2045. [DOI] [PubMed] [Google Scholar]
- 91.Kruger R, Kuhn W, Muller T, et al. Ala30Pro mutation in the gene encoding α-synuclein in Parkinson’s disease. Nat. Genet. 1998;18(2):106–108. doi: 10.1038/ng0298-106. [DOI] [PubMed] [Google Scholar]
- 92.Zarranz JJ, Alegre J, Gomez-Esteban JC, et al. The new mutation, E46K, of α-synuclein causes Parkinson and Lewy body dementia. Ann. Neurol. 2004;55(2):164–173. doi: 10.1002/ana.10795. [DOI] [PubMed] [Google Scholar]
- 93.Chartier-Harlin MC, Kachergus J, Roumier C, et al. α-synuclein locus duplication as a cause of familial Parkinson’s disease. Lancet. 2004;364(9440):1167–1169. doi: 10.1016/S0140-6736(04)17103-1. [DOI] [PubMed] [Google Scholar]
- 94.Singleton AB, Farrer M, Johnson J, et al. α-Synuclein locus triplication causes Parkinson’s disease. Science. 2003;302(5646):841. doi: 10.1126/science.1090278. [DOI] [PubMed] [Google Scholar]
- 95.Uversky VN. Neuropathology, biochemistry, and biophysics of α-synuclein aggregation. J. Neurochem. 2007;103(1):17–37. doi: 10.1111/j.1471-4159.2007.04764.x. [DOI] [PubMed] [Google Scholar]
- 96.Narhi L, Wood SJ, Steavenson S, et al. Both familial Parkinson’s disease mutations accelerate α-synuclein aggregation. J. Biol. Chem. 1999;274(14):9843–9846. doi: 10.1074/jbc.274.14.9843. [DOI] [PubMed] [Google Scholar]
- 97.Li J, Uversky VN, Fink AL. Effect of familial Parkinson’s disease point mutations A30P and A53T on the structural properties, aggregation, and fibrillation of human α-synuclein. Biochemistry. 2001;40(38):11604–11613. doi: 10.1021/bi010616g. [DOI] [PubMed] [Google Scholar]
- 98.Kim WG, Mohney RP, Wilson B, Jeohn GH, Liu B, Hong JS. Regional difference in susceptibility to lipopolysaccharide-induced neurotoxicity in the rat brain: role of microglia. J. Neurosci. 2000;20(16):6309–6316. doi: 10.1523/JNEUROSCI.20-16-06309.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99. Theodore S, Cao S, McLean PJ, Standaert DG. Targeted overexpression of human α-synuclein triggers microglial activation and an adaptive immune response in a mouse model of Parkinson disease. J. Neuropathol. Exp. Neurol. 2008;67(12):1149–1158. doi: 10.1097/NEN.0b013e31818e5e99.. • Demonstrates that overexpression of human α-syn can trigger neuroinflammation though activation of microglia and engagement of the adaptive immune system.
- 100.Zhang W, Wang T, Pei Z, et al. Aggregated α-synuclein activates microglia: a process leading to disease progression in Parkinson’s disease. FASEB J. 2005;19(6):533–542. doi: 10.1096/fj.04-2751com. [DOI] [PubMed] [Google Scholar]
- 101.Reynolds AD, Kadiu I, Garg SK, et al. Nitrated α-synuclein and microglial neuroregulatory activities. J. Neuroimmune Pharm. 2008;3(2):59–74. doi: 10.1007/s11481-008-9100-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Gao HM, Kotzbauer PT, Uryu K, Leight S, Trojanowski JQ, Lee VM. Neuroinflammation and oxidation/nitration of α-synuclein linked to dopaminergic neurodegeneration. Neuroscience. 2008;28(30):7687–7698. doi: 10.1523/JNEUROSCI.0143-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Hunot S, Boissiere F, Faucheux B, et al. Nitric oxide synthase and neuronal vulnerability in Parkinson’s disease. Neuroscience. 1996;72(2):355–363. doi: 10.1016/0306-4522(95)00578-1. [DOI] [PubMed] [Google Scholar]
- 104.Shavali S, Combs CK, Ebadi M. Reactive macrophages increase oxidative stress and α-synuclein nitration during death of dopaminergic neuronal cells in co-culture: relevance to Parkinson’s disease. Neurochem. Res. 2006;31(1):85–94. doi: 10.1007/s11064-005-9233-x. [DOI] [PubMed] [Google Scholar]
- 105.Rentzos M, Nikolaou C, Andreadou E, et al. Circulating interleukin-15 and RANTES chemokine in Parkinson’s disease. Acta Neurol. Scand. 2007;116(6):374–379. doi: 10.1111/j.1600-0404.2007.00894.x. [DOI] [PubMed] [Google Scholar]
- 106.Rentzos M, Nikolaou C, Andreadou E, et al. Circulating interleukin-10 and interleukin-12 in Parkinson’s disease. Acta Neurol. Scand. 2009;119(5):332–337. doi: 10.1111/j.1600-0404.2008.01103.x. [DOI] [PubMed] [Google Scholar]
- 107.Teismann P, Tieu K, Choi DK, et al. Cyclooxygenase-2 is instrumental in Parkinson’s disease neurodegeneration. Proc. Natl Acad. Sci. USA. 2003;100(9):5473–5478. doi: 10.1073/pnas.0837397100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Blum-Degen D, Muller T, Kuhn W, Gerlach M, Przuntek H, Riederer P. Interleukin-1 β and interleukin-6 are elevated in the cerebrospinal fluid of Alzheimer’s and de novo Parkinson’s disease patients. Neurosci. Lett. 1995;202(1–2):17–20. doi: 10.1016/0304-3940(95)12192-7. [DOI] [PubMed] [Google Scholar]
- 109.Reynolds AD, Glanzer JG, Kadiu I, et al. Nitrated α-synuclein-activated microglial profiling for Parkinson’s disease. J. Neurochem. 2008;104(6):1504–1525. doi: 10.1111/j.1471-4159.2007.05087.x. [DOI] [PubMed] [Google Scholar]
- 110.Desai BS, Monahan AJ, Carvey PM, Hendey B. Blood–brain barrier pathology in Alzheimer’s and Parkinson’s disease: implications for drug therapy. Cell Transplant. 2007;16(3):285–299. doi: 10.3727/000000007783464731. [DOI] [PubMed] [Google Scholar]
- 111.Wong D, Prameya R, Dorovini-Zis K. In vitro adhesion and migration of T lymphocytes across monolayers of human brain microvessel endothelial cells: regulation by ICAM-1, VCAM-1, E-selectin and PECAM-1. J. Neuropathol. Exp. Neurol. 1999;58(2):138–152. doi: 10.1097/00005072-199902000-00004. [DOI] [PubMed] [Google Scholar]
- 112.Olson JK, Miller SD. Microglia initiate central nervous system innate and adaptive immune responses through multiple TLRs. J. Immunol. 2004;173(6):3916–3924. doi: 10.4049/jimmunol.173.6.3916. [DOI] [PubMed] [Google Scholar]
- 113.Aloisi F, Ria F, Columba-Cabezas S, Hess H, Penna G, Adorini L. Relative efficiency of microglia, astrocytes, dendritic cells and B cells in naive CD4+ T cell priming and Th1/Th2 cell restimulation. Eur. J. Immunol. 1999;29(9):2705–2714. doi: 10.1002/(SICI)1521-4141(199909)29:09<2705::AID-IMMU2705>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 114.McGeer EG, McGeer PL. Inflammatory processes in Alzheimer’s disease. Prog. Neuropsychopharmacol. Biol. Psychiatry. 2003;27(5):741–749. doi: 10.1016/S0278-5846(03)00124-6. [DOI] [PubMed] [Google Scholar]
- 115.Rogers J, Luber-Narod J, Styren SD, Civin WH. Expression of immune system-associated antigens by cells of the human central nervous system: relationship to the pathology of Alzheimer’s disease. Neurobiol. Aging. 1988;9(4):339–349. doi: 10.1016/s0197-4580(88)80079-4. [DOI] [PubMed] [Google Scholar]
- 116.Togo T, Akiyama H, Iseki E, et al. Occurrence of T cells in the brain of Alzheimer’s disease and other neurological diseases. J. Neuroimmunol. 2002;124(1–2):83–92. doi: 10.1016/s0165-5728(01)00496-9. [DOI] [PubMed] [Google Scholar]
- 117.Farkas E, De Jong GI, Apro E, De Vos RA, Steur EN, Luiten PG. Similar ultrastructural breakdown of cerebrocortical capillaries in Alzheimer’s disease, Parkinson’s disease, and experimental hypertension. What is the functional link? Ann. NY Acad. Sci. 2000;903:72–82. doi: 10.1111/j.1749-6632.2000.tb06352.x. [DOI] [PubMed] [Google Scholar]
- 118.Medawar PB. Immunity to homologous grafted skin; the fate of skin homografts transplanted to the brain, to subcutaneous tissue, and to the anterior chamber of the eye. Br. J. Exp. Pathol. 1948;29(1):58–69. [PMC free article] [PubMed] [Google Scholar]
- 119. Benner EJ, Banerjee R, Reynolds AD, et al. Nitrated α-synuclein immunity accelerates degeneration of nigral dopaminergic neurons. PLoS ONE. 2008;3(1):e1376. doi: 10.1371/journal.pone.0001376.. •• Demonstrates that post-translational modifications to α-syn can break immune tolerance and induce an adaptive neurotoxic immune response in an animal model of PD.
- 120.Brochard V, Combadiere B, Prigent A, et al. Infiltration of CD4+ lymphocytes into the brain contributes to neurodegeneration in a mouse model of Parkinson disease. J. Clin. Invest. 2009;119(1):182–192. doi: 10.1172/JCI36470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 121.McRae-Degueurce A, Rosengren L, Haglid K, et al. Immunocytochemical investigations on the presence of neuron-specific antibodies in the CSF of Parkinson’s disease cases. Neurochem. Res. 1988;13(7):679–684. doi: 10.1007/BF00973287. [DOI] [PubMed] [Google Scholar]
- 122.Maetzler W, Berg D, Synofzik M, et al. Autoantibodies against amyloid and glial-derived antigens are increased in serum and cerebrospinal fluid of lewy body-associated dementias. J. Alzheimers Dis. 2011;26(1):171–179. doi: 10.3233/JAD-2011-110221. [DOI] [PubMed] [Google Scholar]
- 123.Fiszer U, Mix E, Fredrikson S, Kostulas V, Link H. Parkinson’s disease and immunological abnormalities: increase of HLA-DR expression on monocytes in cerebrospinal fluid and of CD45RO+ T cells in peripheral blood. Acta Neurol. Scand. 1994;90(3):160–166. doi: 10.1111/j.1600-0404.1994.tb02699.x. [DOI] [PubMed] [Google Scholar]
- 124.Hisanaga K, Asagi M, Itoyama Y, Iwasaki Y. Increase in peripheral CD4 bright+ CD8 dull+ T cells in Parkinson disease. Arch. Neurol. 2001;58(10):1580–1583. doi: 10.1001/archneur.58.10.1580. [DOI] [PubMed] [Google Scholar]
- 125.Baba Y, Kuroiwa A, Uitti RJ, Wszolek ZK, Yamada T. Alterations of T-lymphocyte populations in Parkinson disease. Parkinsonism Relat. Disord. 2005;11(8):493–498. doi: 10.1016/j.parkreldis.2005.07.005. [DOI] [PubMed] [Google Scholar]
- 126.Fiszer U. Does Parkinson’s disease have an immunological basis? The evidence and its therapeutic implications. Biodrugs. 2001;15(6):351–355. doi: 10.2165/00063030-200115060-00001. [DOI] [PubMed] [Google Scholar]
- 127.Bradbury J. Immunotherapy for Parkinson’s disease: a developing therapeutic strategy. Drug Discov. Today. 2005;10(16):1075–1076. doi: 10.1016/S1359-6446(05)03562-2. [DOI] [PubMed] [Google Scholar]
- 128.Klegeris A, McGeer EG, McGeer PL. Therapeutic approaches to inflammation in neurodegenerative disease. Curr. Opin. Neurol. 2007;20(3):351–357. doi: 10.1097/WCO.0b013e3280adc943. [DOI] [PubMed] [Google Scholar]
- 129.Pride M, Seubert P, Grundman M, Hagen M, Eldridge J, Black RS. Progress in the active immunotherapeutic approach to Alzheimer’s disease: clinical investigations into AN1792-associated meningoencephalitis. Neurodegener. Dis. 2008;5(3–4):194–196. doi: 10.1159/000113700. [DOI] [PubMed] [Google Scholar]
- 130.Villoslada P, Moreno B, Melero I, et al. Immunotherapy for neurological diseases. Clin. Immunol. 2008;128(3):294–305. doi: 10.1016/j.clim.2008.04.003. [DOI] [PubMed] [Google Scholar]
- 131.Agbo DB, Neff F, Seitz F, et al. Immunization as treatment for Parkinson’s disease. J. Neural. Transm. Suppl. 2009;73:311–315. doi: 10.1007/978-3-211-92660-4_26. [DOI] [PubMed] [Google Scholar]
- 132.Reichert JM. Antibody-based therapeutics to watch in 2011. MAbs. 2011;3(1):76–99. doi: 10.4161/mabs.3.1.13895. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Janeway CA, Jr, Medzhitov R. Introduction: the role of innate immunity in the adaptive immune response. Semin. Immunol. 1998;10(5):349–350. doi: 10.1006/smim.1998.0142. [DOI] [PubMed] [Google Scholar]
- 134.Weiner HL. A shift from adaptive to innate immunity: a potential mechanism of disease progression in multiple sclerosis. J. Neurol. 2008;255 Suppl. 1:3–11. doi: 10.1007/s00415-008-1002-8. [DOI] [PubMed] [Google Scholar]
- 135.Schenk D, Barbour R, Dunn W, et al. Immunization with amyloid-β attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400(6740):173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 136.Weiner HL, Lemere CA, Maron R, et al. Nasal administration of amyloid-β peptide decreases cerebral amyloid burden in a mouse model of Alzheimer’s disease. Ann. Neurol. 2000;48(4):567–579. [PubMed] [Google Scholar]
- 137.Janus C, Pearson J, McLaurin J, et al. A β peptide immunization reduces behavioural impairment and plaques in a model of Alzheimer’s disease. Nature. 2000;408(6815):979–982. doi: 10.1038/35050110. [DOI] [PubMed] [Google Scholar]
- 138.Morgan D, Diamond DM, Gottschall PE, et al. A β peptide vaccination prevents memory loss in an animal model of Alzheimer’s disease. Nature. 2000;408(6815):982–985. doi: 10.1038/35050116. [DOI] [PubMed] [Google Scholar]
- 139.Bard F, Cannon C, Barbour R, et al. Peripherally administered antibodies against amyloid β-peptide enter the central nervous system and reduce pathology in a mouse model of Alzheimer disease. Nat. Med. 2000;6(8):916–919. doi: 10.1038/78682. [DOI] [PubMed] [Google Scholar]
- 140.Demattos RB, Bales KR, Cummins DJ, Dodart JC, Paul SM, Holtzman DM. Peripheral anti-A β antibody alters CNS and plasma A β clearance and decreases brain A β burden in a mouse model of Alzheimer’s disease. Proc. Natl Acad. Sci. USA. 2001;98(15):8850–8855. doi: 10.1073/pnas.151261398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 141.Senior K. Dosing in Phase II trial of Alzheimer’s vaccine suspended. Lancet Neurol. 2002;1(1):3. doi: 10.1016/s1474-4422(02)00023-6. [DOI] [PubMed] [Google Scholar]
- 142.Masliah E, Rockenstein E, Adame A, et al. Effects of α-synuclein immunization in a mouse model of Parkinson’s disease. Neuron. 2005;46(6):857–868. doi: 10.1016/j.neuron.2005.05.010. [DOI] [PubMed] [Google Scholar]
- 143.Schneeberger A, Mandler M, Mattner F, Schmidt W. AFFITOME(R) technology in neurodegenerative diseases: the doubling advantage. Hum. Vaccin. 2010;6(11):64–68. doi: 10.4161/hv.6.11.13217. [DOI] [PubMed] [Google Scholar]
- 144.Sakaguchi S, Sakaguchi N, Asano M, Itoh M, Toda M. Immunologic self-tolerance maintained by activated T cells expressing IL-2 receptor α-chains (CD25). Breakdown of a single mechanism of self-tolerance causes various autoimmune diseases. J. Immunol (Balt.) 1995;155(3):1151–1164. [PubMed] [Google Scholar]
- 145.Sakaguchi S, Hori S, Fukui Y, Sasazuki T, Sakaguchi N, Takahashi T. Thymic generation and selection of CD25+CD4+ regulatory T cells: implications of their broad repertoire and high self-reactivity for the maintenance of immunological self-tolerance. Novartis Found. Symp. 2003;252:106–114. [PubMed] [Google Scholar]
- 146.Sakaguchi S. Naturally arising CD4+ regulatory T cells for immunologic self-tolerance and negative control of immune responses. Ann. Rev. Immunol. 2004;22:531–562. doi: 10.1146/annurev.immunol.21.120601.141122. [DOI] [PubMed] [Google Scholar]
- 147.Kim JM, Rasmussen JP, Rudensky AY. Regulatory T cells prevent catastrophic autoimmunity throughout the lifespan of mice. Nature Immunol. 2007;8(2):191–197. doi: 10.1038/ni1428. [DOI] [PubMed] [Google Scholar]
- 148.Coombes JL, Robinson NJ, Maloy KJ, Uhlig HH, Powrie F. Regulatory T cells and intestinal homeostasis. Immunol. Rev. 2005;204:184–194. doi: 10.1111/j.0105-2896.2005.00250.x. [DOI] [PubMed] [Google Scholar]
- 149.Bourreau E, Ronet C, Darcissac E, et al. Intralesional regulatory T-cell suppressive function during human acute and chronic cutaneous leishmaniasis due to Leishmania guyanensis. Infect. Immun. 2009;77(4):1465–1474. doi: 10.1128/IAI.01398-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 150.Cederbom L, Hall H, Ivars F. CD4+CD25+ regulatory T cells down-regulate costimulatory molecules on antigen-presenting cells. Eur. J. Immunol. 2000;30(6):1538–1543. doi: 10.1002/1521-4141(200006)30:6<1538::AID-IMMU1538>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 151.Kipnis J, Mizrahi T, Hauben E, Shaked I, Shevach E, Schwartz M. Neuroprotective autoimmunity: naturally occurring CD4+CD25+ regulatory T cells suppress the ability to withstand injury to the central nervous system. Proc. Natl Acad. Sci. USA. 2002;99(24):15620–15625. doi: 10.1073/pnas.232565399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 152.Fontenot JD, Gavin MA, Rudensky AY. Foxp3 programs the development and function of CD4+CD25+ regulatory T cells. Nature Immunol. 2003;4(4):330–336. doi: 10.1038/ni904. [DOI] [PubMed] [Google Scholar]
- 153.Hall BM, Pearce NW, Gurley KE, Dorsch SE. Specific unresponsiveness in rats with prolonged cardiac allograft survival after treatment with cyclosporine. III. Further characterization of the CD4+ suppressor cell and its mechanisms of action. J. Exp. Med. 1990;171(1):141–157. doi: 10.1084/jem.171.1.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 154.Hori S, Nomura T, Sakaguchi S. Control of regulatory T cell development by the transcription factor Foxp3. Science. 2003;299(5609):1057–1061. doi: 10.1126/science.1079490. [DOI] [PubMed] [Google Scholar]
- 155.Fletcher JM, Lonergan R, Costelloe L, et al. CD39+Foxp3+ regulatory T Cells suppress pathogenic Th17 cells and are impaired in multiple sclerosis. J. Immunol. (Balt.) 2009;183(11):7602–7610. doi: 10.4049/jimmunol.0901881. [DOI] [PubMed] [Google Scholar]
- 156.Kleinewietfeld M, Starke M, Di Mitri D, et al. CD49d provides access to ‘untouched’human Foxp3+ Treg free of contaminating effector cells. Blood. 2009;113(4):827–836. doi: 10.1182/blood-2008-04-150524. [DOI] [PubMed] [Google Scholar]
- 157.Lee YK, Mukasa R, Hatton RD, Weaver CT. Developmental plasticity of Th17 and Treg cells. Curr. Opin. Immunol. 2009;21(3):274–280. doi: 10.1016/j.coi.2009.05.021. [DOI] [PubMed] [Google Scholar]
- 158. Khattar M, Chen W, Stepkowski SM. Expanding and converting regulatory T cells: a horizon for immunotherapy. Arch. Immunol. Ther. Exp. (Warsz.) 2009;57(3):199–204. doi: 10.1007/s00005-009-0021-1.. • Tregs may be of use in immunotherapy.
- 159.Zheng SG, Wang J, Horwitz DA. Cutting edge: Foxp3+CD4+CD25+ regulatory T cells induced by IL-2 and TGF-β are resistant to Th17 conversion by IL-6. J. Immunol. (Balt.) 2008;180(11):7112–7116. doi: 10.4049/jimmunol.180.11.7112. [DOI] [PubMed] [Google Scholar]
- 160.Zheng SG, Wang J, Wang P, Gray JD, Horwitz DA. IL-2 is essential for TGF-β to convert naive CD4+CD25− cells to CD25+Foxp3+ regulatory T cells and for expansion of these cells. J. Immunol. (Balt.) 2007;178(4):2018–2027. doi: 10.4049/jimmunol.178.4.2018. [DOI] [PubMed] [Google Scholar]
- 161.Johnson J, Pahuja A, Graham M, Hering B, Hancock WW, Bansal-Pakala P. Effects of histone deacetylase inhibitor SAHA on effector and FOXP3+regulatory T cells in rhesus macaques. Transplant. Proc. 2008;40(2):459–461. doi: 10.1016/j.transproceed.2008.01.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Lucas JL, Mirshahpanah P, Haas-Stapleton E, Asadullah K, Zollner TM, Numerof RP. Induction of Foxp3+ regulatory T cells with histone deacetylase inhibitors. Cell. Immunol. 2009;257(1–2):97–104. doi: 10.1016/j.cellimm.2009.03.004. [DOI] [PubMed] [Google Scholar]
- 163.Saouaf SJ, Li B, Zhang G, et al. Deacetylase inhibition increases regulatory T cell function and decreases incidence and severity of collagen-induced arthritis. Exp. Mol. Pathol. 2009;87(2):99–104. doi: 10.1016/j.yexmp.2009.06.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 164.Tao R, De Zoeten EF, Ozkaynak E, et al. Histone deacetylase inhibitors and transplantation. Curr. Opin. Immunol. 2007;19(5):589–595. doi: 10.1016/j.coi.2007.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 165.Delgado M, Chorny A, Gonzalez-Rey E, Ganea D. Vasoactive intestinal peptide generates CD4+CD25+ regulatory T cells in vivo. J. Leukoc. Biol. 2005;78(6):1327–1338. doi: 10.1189/jlb.0605299. [DOI] [PubMed] [Google Scholar]
- 166.Gonzalez-Rey E, Chorny A, Fernandez-Martin A, Ganea D, Delgado M. Vasoactive intestinal peptide generates human tolerogenic dendritic cells that induce CD4 and CD8 regulatory T cells. Blood. 2006;107(9):3632–3638. doi: 10.1182/blood-2005-11-4497. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 167.Gonzalez-Rey E, Fernandez-Martin A, Chorny A, Delgado M. Vasoactive intestinal peptide induces CD4+,CD25+ T regulatory cells with therapeutic effect in collagen-induced arthritis. Arthritis Rheum. 2006;54(3):864–876. doi: 10.1002/art.21652. [DOI] [PubMed] [Google Scholar]
- 168.Reynolds AD, Banerjee R, Liu J, Gendelman HE, Mosley RL. Neuroprotective activities of CD4+CD25+ regulatory T cells in an animal model of Parkinson’s disease. J. Leukoc. Biol. 2007;82(5):1083–1094. doi: 10.1189/jlb.0507296. [DOI] [PubMed] [Google Scholar]
- 169. Benner EJ, Mosley RL, Destache CJ, et al. Therapeutic immunization protects dopaminergic neurons in a mouse model of Parkinson’s disease. Proc. Natl Acad. Sci. USA. 2004;101(25):9435–9440. doi: 10.1073/pnas.0400569101.. • Demonstrates that lymphocytes primed with Cop-1 are neuroprotective and reduce microgliosis in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine mouse model.
- 170.Garg SK, Banerjee R, Kipnis J. Neuroprotective immunity: T cell-derived glutamate endows astrocytes with a neuroprotective phenotype. J. Immunol. (Balt.) 2008;180(6):3866–3873. doi: 10.4049/jimmunol.180.6.3866. [DOI] [PubMed] [Google Scholar]
- 171.Rossi D, Volterra A. Astrocytic dysfunction: insights on the role in neurodegeneration. Brain Res. Bull. 2009;80(4–5):224–232. doi: 10.1016/j.brainresbull.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 172.Wahl SM, Swisher J, McCartney-Francis N, Chen W. TGF-β: the perpetrator of immune suppression by regulatory T cells and suicidal T cells. J. Leukoc. Biol. 2004;76(1):15–24. doi: 10.1189/jlb.1103539. [DOI] [PubMed] [Google Scholar]
- 173.Maloy KJ, Salaun L, Cahill R, Dougan G, Saunders NJ, Powrie F. CD4+CD25+ T(R) cells suppress innate immune pathology through cytokine-dependent mechanisms. J. Exp. Med. 2003;197(1):111–119. doi: 10.1084/jem.20021345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 174.Costantino CM, Baecher-Allan CM, Hafler DA. Human regulatory T cells and autoimmunity. Eur. J. Immunol. 2008;38(4):921–924. doi: 10.1002/eji.200738104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 175.Putnam AL, Brusko TM, Lee MR, et al. Expansion of human regulatory T-cells from patients with Type 1 diabetes. Diabetes. 2009;58(3):652–662. doi: 10.2337/db08-1168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 176. Brusko TM, Putnam AL, Bluestone JA. Human regulatory T cells: role in autoimmune disease and therapeutic opportunities. Immunol. Rev. 2008;223:371–390. doi: 10.1111/j.1600-065X.2008.00637.x.. • Presents data supporting the efficacy in vitro and ex vivo-expanded Treg in immunotherapy.
- 177.Haas J, Korporal M, Balint B, Fritzsching B, Schwarz A, Wildemann B. Glatiramer acetate improves regulatory T-cell function by expansion of naive CD4(+)CD25(+) FOXP3(+)CD31(+) T-cells in patients with multiple sclerosis. J. Neuroimmunol. 2009;216(1–2):113–117. doi: 10.1016/j.jneuroim.2009.06.011. [DOI] [PubMed] [Google Scholar]
- 178.Vandenbark AA, Huan J, Agotsch M, et al. Interferon-β-1a treatment increases CD56bright natural killer cells and CD4+CD25+ Foxp3 expression in subjects with multiple sclerosis. J. Neuroimmunol. 2009;215(1–2):125–128. doi: 10.1016/j.jneuroim.2009.08.007. [DOI] [PubMed] [Google Scholar]
- 179.Aharoni R, Teitelbaum D, Leitner O, Meshorer A, Sela M, Arnon R. Specific Th2 cells accumulate in the central nervous system of mice protected against experimental autoimmune encephalomyelitis by copolymer 1. Proc. Natl Acad. Sci. USA. 2000;97(21):11472–11477. doi: 10.1073/pnas.97.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 180.Chen M, Gran B, Costello K, Johnson K, Martin R, Dhib-Jalbut S. Glatiramer acetate induces a Th2-biased response and crossreactivity with myelin basic protein in patients with MS. Mult. Scler. 2001;7(4):209–219. doi: 10.1177/135245850100700401. [DOI] [PubMed] [Google Scholar]
- 181.Aharoni R, Teitelbaum D, Sela M, Arnon R. Copolymer 1 induces T cells of the T helper type 2 that crossreact with myelin basic protein and suppress experimental autoimmune encephalomyelitis. Proc. Natl Acad. Sci. USA. 1997;94(20):10821–10826. doi: 10.1073/pnas.94.20.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 182.Aharoni R, Eilam R, Stock A, et al. Glatiramer acetate reduces Th-17 inflammation and induces regulatory T-cells in the CNS of mice with relapsing–remitting or chronic EAE. J. Neuroimmunol. 2010;225(1–2):100–111. doi: 10.1016/j.jneuroim.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 183.Laurie C, Reynolds A, Coskun O, Bowman E, Gendelman HE, Mosley RL. CD4+ T cells from Copolymer-1 immunized mice protect dopaminergic neurons in the 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine model of Parkinson’s disease. J. Neuroimmunol. 2007;183(1–2):60–68. doi: 10.1016/j.jneuroim.2006.11.009. [DOI] [PubMed] [Google Scholar]
- 184. Reynolds AD, Stone DK, Hutter JA, Benner EJ, Mosley RL, Gendelman HE. Regulatory T cells attenuate TH17 cell-mediated nigrostriatal dopaminergic neurodegeneration in a model of Parkinson’s disease. J. Immunol. (Balt.) 2010;184(5):2261–2271. doi: 10.4049/jimmunol.0901852.. •• Demonstrates that Tregs can attenuate microgliosis and 1-methyl-4-phenyl-1,2,3,6-tetrahydropyridine-induced neurodegeneration that is exacerbated by Th17 cells.
Patent
- 201.Affiris AG. WO2009103105. 2009