Abstract
Lrp5/6 co-receptor is known to play a role in bone formation and lipid metabolism. This gene encodes a member of the low density lipoprotein (LDL) receptor gene family. This study tests the hypothesis that Lrp5/6 is necessary for the development of valve calcification in experimental hypercholesterolemia. Experimental hypercholesterolemia mouse models were tested: Lrp5−/−/ ApoE−/− :Lrp5−/−/ApoE−/− mice (n=180). Group I (n=60) normal diet, Group II (n=60) 0.25% chol diet (w/w), and Group III (n=60) 0.25% (w/w) chol diet+atorv for the development of calcification by MicroCT and Synchrotron MicroCT Scan and by Masson trichrome stain. Finally gene expression for Lrp5, Lrp6 and Runx2 PCR was performed to evaluate the expression in the control and the cholesterol valves. The ApoE−/− cholesterol treated mice developed calcification and increase in Lrp5, Runx2 (p<0.05) as compared to control. The Lrp5−/− mice developed no calcification by MicroCT and Synchrotron and there was no gene expression for Lrp5/6 or Runx2. The double knockout ApoE−/−:Lrp5−/− developed mild mineralization in the cholesterol treated valves with an increase in Lrp6 and Runx2 expression(p<0.05). There was no mineralization in the right sided hearts valves. In conclusion Lrp5/6 is necessary for calcification in the aortic valve in the presence of experimental hypercholesterolemia. These data present the mouse genetic evidence for the LDL-Density-Pressure theory in cardiac valves.
Keywords: Lipids, Lrp5, Wnt, Osteoblastogenesis
Introduction
The low-density lipoprotein-related receptor 5 and 6 (Lrp5 and Lrp6) genes were cloned in 1998 based on their homology with the low-density lipoprotein receptor (LDLR)[Dong et al., 1998] [Brown et al., 1998; Hey et al., 1998; Kim et al., 1998]. Mutations in either LRP5 or LRP6, proteins have caused a number of disease processed in the field of bone[Gong et al., 2001; Little et al., 2002], and have been associated with cardiovascular disease[Caira et al., 2006; Fujino et al., 2003a; Kim et al., 1998; Rajamannan et al., 2005a]. The LDL-Density-Pressure theory[Rajamannan, 2011] combines the structure, function analysis of these co-receptors with the results from the genetic studies to provide a unique hypothesis for the role of these receptors in the heart. This study provides the genetic mouse evidence to demonstrate that in the presence of experimental hypercholesterolemia, Lrp5/6 receptors, Runx2 genes are up-regulated in the presence of cholesterol and mediate calcification by MicroCT analysis.
Methods
ApoE−/− /Lrp5−/− experimental hypercholesterolemia mouse model
ApoE−/− mice were purchased from Jackson Laboratories (Bar Harbor, Maine) and Lrp5−/− were purchased from Taconic laboratories(Germantown, NY). ApoE−/−:Lrp5−/− were produced by cross breeding. Mice aged 6–8 weeks(male and female mice) were assigned to a control (N=60), a 0.2% cholesterol (w/w) diet (Harlan Teklad 88137), (N=60) and a 0.2% cholesterol (w/w) diet (Harlan Teklad 88137). All animals were fed ad libitum for 23 weeks. Control mice were fed a standard diet. Following this 23-week period, the mice were euthanized with inhalation CO2. All experiments were performed in an animal facility accredited by the Association for Assessment and Accreditation of Laboratory Animal Care, Inc. (ACUC- A3283-01, 1-08-382). Immediately after dissection from the heart one leaflet from each aortic valve was fixed in 10% buffered formalin for 24 hours and then embedded in paraffin. Valves were also snap frozen in liquid nitrogen and stored in −80 degree freezer for gene expression experiments and MicroCT Paraffin embedded sections (6µm) were cut and prepped for histopathology exam for Masson Trichrome Stain in the valves. Semi-quantitative PCR[Rajamannan et al., 2005a; Rajamannan et al., 2002; Rajamannan et al., 2005b] and Realtime PCR[Hawse et al., 2008] was performed to measure Lrp5/6 and Runx2 in the cardiac valves. The ApoE−/− mice were not tested for the Lrp6 receptor as the study was completed prior to the data from the double knockout were available.
Micro-CT
After fixing in formalin, the valves were examined using a Scanco MicroCT-40 system operated at 45 kV. Sampling was with ~8 µm voxels (volume elements) and maximum sensitivity (1000 projections, 2048 samples and 0.3 sec/projection integration [Rajamannan et al., 2003]) N=60 valves. Valve calcification will be quantified using the Scanco MicroCT-40 system. MicroCT (micro Computed Tomography) is essentially a high resolution version of clinical CT for smaller samples. After recording a set of slices of the valve tissue, the total amount of mineralized tissue and the fraction of tissue mineralized in the sample will be determined using techniques employed in earlier valve studies [Caira et al., 2006; Rajamannan et al., 2005a]. One threshold will be used for the total tissue and a second for mineralized tissue, and the necessary computations will be performed using the Scanco system’s software suite. The microstructure of mineralized valve tissue will also be characterized; if qualitative differences are noted, relationships with the different treatment groups will be sought.
MicroCT Synchrotron
MIcroComputed Tomography (MicroCT) was performed at station 2-BM of the Advanced Photon Source (APS, Argonne National Laboratory) for higher resolution when calcification was not detected by Scanco MicroCT 40 system. Each heart was removed from fluid and positioned securely within a sealed plastic tube. Data were collected using the following parameters: 15 keV photons and rotation over 180° in 0.12° increments. The sample-detector separation was ~50 mm (in order to produce sufficient x-ray phase contrast to differentiate different soft tissue types), and reconstruction was on a (2K)2 grid with ~2.8 µm isotropic volume elements (voxels).
Results
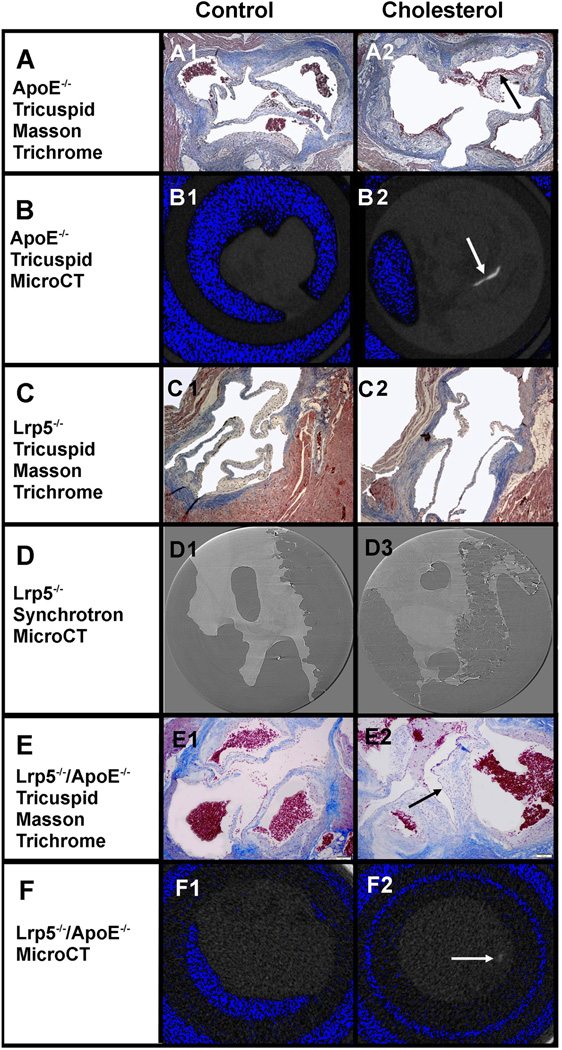
To understand if Lrp5−/−/ ApoE−/−:Lrp5−/−/ApoE−/−, mice develops atherosclerosis in the cholesterol valve inducing calcification via increase in Lrp5/6 receptors. Figure 1, demonstrates the characterization of the aortic valve phenotype as defined by histology and MicroCT. In Figure 1, Panel A1, A2, B1 and B2 is the histology and MicroCT scan for the ApoE−/− aortic valve in the control and cholesterol demonstrating atherosclerosis and calcification in the cholesterol treated valves and none in the controls. Figure 1, Panel C1, C2, D1 and D2 demonstrates that there is no atherosclerosis or calcification in the Lrp5−/− mice by MicroCT (Data not shown) MicroCT synchrotron scan. Figure 1, Panel E1, E2, F1 and F2 is the ApoE−/−:Lrp5−/− histology and MicroCT for the aortic valves from these mice demonstrating a mild increase in calcification in the cholesterol treated valves but to a lesser degree than the ApoE−/− cholesterol treated valves in Figure 1 Panel A2. Table 1, demonstrates the echocardiography, serum cholesterol and the gene expression for the valves. Table 1 is the quantification of the gene expression for the ApoE−/− valves and the ApoE−/−:Lrp5−/− valves the Lrp5−/− for Lrp5, Lrp6 and Runx2. The echocardiography demonstrated mild but not statistically significant increases in flow across the m cholesterol treated valves. The MicroCT demonstrates calcification in the ApoE−/− to a greater extent than the ApoE−/−:Lrp5−/−.
Figure 1. Experimental Hypercholesterolemia genetic Mouse Model: Control versus Cholesterol Diet.
| Panel A and B: ApoE−/− | A. Masson Trichrome | B. MicroCT |
| Panel C and D: Lrp5−/− | C. Masson Trichrome | D. Synchrotron MicroCT |
| Panel E and F: ApoE−/−:Lrp5−/− | E. Masson Trichrome | F. MicroCT |
Table 1. Quantification of the Echocardiography, Serum Cholesterol levels and the Gene Expression for Lrp5, Lrp6 and Runx2 in the ApoE−/− valves and ApoE−/−:Lrp5−/− valves:
Lrp5 and Runx2 is upregulated in the ApoE−/− valves, Lrp6 and Runx2 is upregulated in the ApoE−/−:Lrp5−/− valves, no Runx 2 or Lrp6 was present in the Lrp5−/− valves.
| Control | Cholesterol | |
|---|---|---|
| ApoE−/− | ||
| A. Echocardiographic | ||
| Peak Jet Velocity | 1.6 ± 0.1 | 1.7 ± 0.2 |
| Ejection Fraction (%) | 59 ± 7 | 55 ± 8* |
| B. Cholesterol Serum Level | 540 ± 63.9 | 1761 ± 510.4* |
| C. Gene Expression | ||
| Lrp5 | 0.34 | 2.38* |
| Runx2 | 0.05 | 0.86* |
| Lrp6 | - | - |
|
| ||
| Lrp5−/− | ||
| A. Echocardiographic | ||
| Peak Jet Velocity | 1.5 ± 0.082 | 1.6 ± 0.141 |
| Ejection Fraction (%) | 56 ± 8.29 | 49.0 ± 4.08* |
| B. Cholesterol | 90.5 ± 28.8 | 304.7 ± 78.4* |
| C. Gene Expression | ||
| Lrp5 | 0.11 | 0.14 |
| Runx2 | 0.43 | 0.90* |
| Lrp6 | 1.10 | 1.75* |
|
| ||
| ApoE−/−Lrp5−/− | ||
| A. Echocardiographic | ||
| Peak Jet Velocity | 1.8 ± 0.32 | 2.0 ± 0.40 |
| Ejection Fraction (%) | 50.1 ± 9.57 | 42.5 ± 1.24* |
| B. Cholesterol | 694.5 ± 70.9 | 1063.6 ± 620.0 |
| C. Gene Expression | ||
| Lrp5 | - | - |
| Runx2 | 0.80 | 1.36* |
| Lrp6 | 0.97 | 1.13 |
- Not performed
p < 0.05 Cholesterol compared to control.
Discussion
The low density lipoprotein co-receptor Lrp5/6 is a member of the family of structurally closely related cell surface low density lipoprotein receptors that have diverse biological functions in different organs, tissues and cell types which are important in development and disease mechanisms. The most prominent role in this evolutionary ancient family is cholesterol homeostasis. The LRP5 pathway regulates bone formation in different diseases of bone[Boyden et al., 2002; Gong et al., 2001]. The discovery of the LRP5 receptor in the gain of function[Boyden et al., 2002] and loss of function[Gong et al., 2001] mutations in the development of bone diseases, resulted in a number of studies which have shown that activation of the canonical Wnt pathway is important in osteoblastogenesis[Babij et al., 2003; Fujino et al., 2003b; Holmen et al., 2004; Westendorf et al., 2004]. Three studies to date have confirmed the regulation of the LRP5/Wnt pathway for cardiovascular calcification in vivo and ex vivo[Rajamannan et al., 2005a],[Caira et al., 2006],[Shao et al., 2005]. Lrp5 has been shown to have an effect on bone mass via the mechanostat effect on regulating bone formation. The findings in the human of the high bone mass gain of function mutation[Little et al., 2002], led to a series of discoveries that Lrp5 regulates bone mass via the mechanical force effect on the receptor. [Akhter et al., 2004; Johnson et al., 2004; Johnson and Summerfield, 2005], Lrp6 also regulates bone but has been found to have a low bone mass effect in patients in which a putative partial loss-of-function mutation in LRP6 was identified to early cardiovascular-related death associated with increased plasma LDL, triglycerides, hypertension, diabetes and osteoporosis[Mani et al., 2007]. This data is the first to demonstrate in the genetic mice to demonstrate that experimental cholesterol diet can upregulate Lrp5 and Lrp6 with varying degrees of calcification. The ApoE−/− demonstrated marked increase in the calcification which is consistent with the lipid and pressure effect of Lrp5 on the aortic valves. The Lrp5−/− had no calcification in the valves. The Lrp5−/− single gene KO demonstrates the role of Lrp5 for calcification and the ApoE−/− single gene knockout to demonstrate the role cholesterol to activate the Lrp5/6 receptors. The double knockout mice ApoE−/−:Lrp5−/− were tested to show that in the elevated lipids secondary to the lack of the ApoE receptor as compared to the Lrp5−/− mice caused some mild calcification via the upregulation of the Lrp6 gene expression in the mice.
The right sided valves in all of the specific mice did not develop any calcification, which further demonstrates the role of the higher pressures in the left side of the heart to activate the Lrp5/6 receptor in the valve. LRP5 binds apoE-containing lipoproteins in vitro and is widely expressed in many tissues including hepatocytes, adrenal gland and pancreas[Kim et al., 1998]. The production of mice lacking LRP5 revealed that LRP5 deficiency led to increased plasma cholesterol levels in mice fed a high-fat diet, secondary to decreased hepatic clearance of chylomicron remnants and also marked impaired glucose tolerance[Fujino et al., 2003b]. In the LRP5 mice that were not fed the high cholesterol diet, the mice did not develop high cholesterol levels[Magoori et al., 2003]. The investigators went on to define the role of LRP5 in the lipoprotein metabolism by developing a double knockout mouse for ApoE:LRP5. They found that the double KO mouse had approx 60% higher cholesterol levels compared with the age matched apoE knockout mice. High performance liquid chromatography analysis of plasma lipoproteins revealed that no difference in the apoproteins but the cholesterol levels in the very low density and low density lipoprotein fractions were markedly increased in the apoE:Lrp5 double KO mice. There was 3 fold increase in the atherosclerosis indicating that the Lrp5 mediates both apoE-dependent and apoE-independent catabolism of lipoproteins. In this current study performed the serum cholesterol levels and demonstrated marked increase in the cholesterol in both of the ApoE−/− and the ApoE:Lrp5 double KO mice further confirming the association of elevated cholesterol and the mineralization process. In 1994, studies demonstrated that the plasma cholesterol levels in the double KO mice lacking both ApoE and LDLR were not significantly different from the levels in the ApoE knockout mice[Ishibashi et al., 1994]. These results help to further demonstrate in a mouse model the LDL-Density-Pressure theory[Rajamannan, 2011] to indicate a biologic-hemodynamic foundation for the mechanism of Lrp5/6 activation in the heart.
Acknowledgements
This work was completed with the support of an American Heart Association Grant-in-Aid (0555714Z) and a grant from the National Institute of Health (5K08HL073927-04, 1R01HL085591-01A1). Nalini M. Rajamannan is an inventor on a patent for the use of statins in degeneration of aortic valve disease. This patent is owned by the Mayo Clinic and Dr. Rajamannan does not receive any royalties from this patent. Use of the Advanced Photon Source was supported by the U. S. Department of Energy, Office of Science, Office of Basic Energy Sciences, under Contract No. DEAC02-06CH11357. The author thanks Xianghui Xiao (Advanced Photon Source) along with Dr. Stuart Stock in for the synchrotron hearts analysis, Dr. Muzaffer Cicek for his help with the real time PCR and Amy Flores for her support of the genetic mouse breeding.
References
- Akhter MP, Wells DJ, Short SJ, Cullen DM, Johnson ML, Haynatzki GR, Babij P, Allen KM, Yaworsky PJ, Bex F, Recker RR. Bone biomechanical properties in LRP5 mutant mice. Bone. 2004;35:162–169. doi: 10.1016/j.bone.2004.02.018. [DOI] [PubMed] [Google Scholar]
- Babij P, Zhao W, Small C, Kharode Y, Yaworsky PJ, Bouxsein ML, Reddy PS, Bodine PV, Robinson JA, Bhat B, Marzolf J, Moran RA, Bex F. High bone mass in mice expressing a mutant LRP5 gene. Journal of Bone & Mineral Research. 2003;18:960–974. doi: 10.1359/jbmr.2003.18.6.960. [DOI] [PubMed] [Google Scholar]
- Boyden LM, Mao J, Belsky J, Mitzner L, Farhi A, Mitnick MA, Wu D, Insogna K, Lifton RP. High bone density due to a mutation in LDL-receptor-related protein 5. N Engl J Med. 2002;346:1513–1521. doi: 10.1056/NEJMoa013444. [DOI] [PubMed] [Google Scholar]
- Brown SD, Twells RC, Hey PJ, Cox RD, Levy ER, Soderman AR, Metzker ML, Caskey CT, Todd JA, Hess JF. Isolation and characterization of LRP6, a novel member of the low density lipoprotein receptor gene family. Biochem Biophys Res Commun. 1998;248:879–888. doi: 10.1006/bbrc.1998.9061. [DOI] [PubMed] [Google Scholar]
- Caira FC, Stock SR, Gleason TG, McGee EC, Huang J, Bonow RO, Spelsberg TC, McCarthy PM, Rahimtoola SH, Rajamannan NM. Human degenerative valve disease is associated with up-regulation of low-density lipoprotein receptor-related protein 5 receptor-mediated bone formation. J Am Coll Cardiol. 2006;47:1707–1712. doi: 10.1016/j.jacc.2006.02.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Y, Lathrop W, Weaver D, Qiu Q, Cini J, Bertolini D, Chen D. Molecular cloning and characterization of LR3, a novel LDL receptor family protein with mitogenic activity. Biochem Biophys Res Commun. 1998;251:784–790. doi: 10.1006/bbrc.1998.9545. [DOI] [PubMed] [Google Scholar]
- Fujino T, Asaba H, Kang MJ, Ikeda Y, Sone H, Takada S, Kim DH, Ioka RX, Ono M, Tomoyori H, Okubo M, Murase T, Kamataki A, Yamamoto J, Magoori K, Takahashi S, Miyamoto Y, Oishi H, Nose M, Okazaki M, Usui S, Imaizumi K, Yanagisawa M, Sakai J, Yamamoto TT. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proc Natl Acad Sci U S A. 2003a;100:229–234. doi: 10.1073/pnas.0133792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujino T, Asaba H, Kang MJ, Ikeda Y, Sone H, Takada S, Kim DH, Ioka RX, Ono M, Tomoyori H, Okubo M, Murase T, Kamataki A, Yamamoto J, Magoori K, Takahashi S, Miyamoto Y, Oishi H, Nose M, Okazaki M, Usui S, Imaizumi K, Yanagisawa M, Sakai J, Yamamoto TT. Low-density lipoprotein receptor-related protein 5 (LRP5) is essential for normal cholesterol metabolism and glucose-induced insulin secretion. Proceedings of the National Academy of Sciences of the United States of America. 2003b;100:229–234. doi: 10.1073/pnas.0133792100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gong Y, Slee RB, Fukai N, Rawadi G, Roman-Roman S, Reginato AM, Wang H, Cundy T, Glorieux FH, Lev D, Zacharin M, Oexle K, Marcelino J, Suwairi W, Heeger S, Sabatakos G, Apte S, Adkins WN, Allgrove J, Arslan-Kirchner M, Batch JA, Beighton P, Black GC, Boles RG, Boon LM, Borrone C, Brunner HG, Carle GF, Dallapiccola B, De Paepe A, Floege B, Halfhide ML, Hall B, Hennekam RC, Hirose T, Jans A, Juppner H, Kim CA, Keppler-Noreuil K, Kohlschuetter A, LaCombe D, Lambert M, Lemyre E, Letteboer T, Peltonen L, Ramesar RS, Romanengo M, Somer H, Steichen-Gersdorf E, Steinmann B, Sullivan B, Superti-Furga A, Swoboda W, van den Boogaard MJ, Van Hul W, Vikkula M, Votruba M, Zabel B, Garcia T, Baron R, Olsen BR, Warman ML Osteoporosis-Pseudoglioma Syndrome Collaborative G. LDL receptor-related protein 5 (LRP5) affects bone accrual and eye development. Cell. 2001;107:513–523. doi: 10.1016/s0092-8674(01)00571-2. [DOI] [PubMed] [Google Scholar]
- Hawse JR, Iwaniec UT, Bensamoun SF, Monroe DG, Peters KD, Ilharreborde B, Rajamannan NM, Oursler MJ, Turner RT, Spelsberg TC, Subramaniam M. TIEG-null mice display an osteopenic gender-specific phenotype. Bone. 2008;42:1025–1031. doi: 10.1016/j.bone.2008.02.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hey PJ, Twells RC, Phillips MS, Yusuke N, Brown SD, Kawaguchi Y, Cox R, Guochun X, Dugan V, Hammond H, Metzker ML, Todd JA, Hess JF. Cloning of a novel member of the low-density lipoprotein receptor family. Gene. 1998;216:103–111. doi: 10.1016/s0378-1119(98)00311-4. [DOI] [PubMed] [Google Scholar]
- Holmen SL, Giambernardi TA, Zylstra CR, Buckner-Berghuis BD, Resau JH, Hess JF, Glatt V, Bouxsein ML, Ai M, Warman ML, Williams BO. Decreased BMD and limb deformities in mice carrying mutations in both Lrp5 and Lrp6. J Bone Miner Res. 2004;19:2033–2040. doi: 10.1359/JBMR.040907. [DOI] [PubMed] [Google Scholar]
- Ishibashi S, Goldstein JL, Brown MS, Herz J, Burns DK. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J Clin Invest. 1994;93:1885–1893. doi: 10.1172/JCI117179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johnson ML, Harnish K, Nusse R, Van Hul W. LRP5 and Wnt signaling: a union made for bone. J Bone Miner Res. 2004;19:1749–1757. doi: 10.1359/JBMR.040816. [DOI] [PubMed] [Google Scholar]
- Johnson ML, Summerfield DT. Parameters of LRP5 from a structural and molecular perspective. Crit Rev Eukaryot Gene Expr. 2005;15:229–242. doi: 10.1615/critreveukargeneexpr.v15.i3.50. [DOI] [PubMed] [Google Scholar]
- Kim DH, Inagaki Y, Suzuki T, Ioka RX, Yoshioka SZ, Magoori K, Kang MJ, Cho Y, Nakano AZ, Liu Q, Fujino T, Suzuki H, Sasano H, Yamamoto TT. A new low density lipoprotein receptor related protein, LRP5, is expressed in hepatocytes and adrenal cortex, and recognizes apolipoprotein E. Journal of Biochemistry. 1998;124:1072–1076. doi: 10.1093/oxfordjournals.jbchem.a022223. [DOI] [PubMed] [Google Scholar]
- Little RD, Carulli JP, Del Mastro RG, Dupuis J, Osborne M, Folz C, Manning SP, Swain PM, Zhao SC, Eustace B, Lappe MM, Spitzer L, Zweier S, Braunschweiger K, Benchekroun Y, Hu X, Adair R, Chee L, FitzGerald MG, Tulig C, Caruso A, Tzellas N, Bawa A, Franklin B, McGuire S, Nogues X, Gong G, Allen KM, Anisowicz A, Morales AJ, Lomedico PT, Recker SM, Van Eerdewegh P, Recker RR, Johnson ML. A mutation in the LDL receptor-related protein 5 gene results in the autosomal dominant high-bone-mass trait. Am J Hum Genet. 2002;70:11–19. doi: 10.1086/338450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magoori K, Kang MJ, Ito MR, Kakuuchi H, Ioka RX, Kamataki A, Kim DH, Asaba H, Iwasaki S, Takei YA, Sasaki M, Usui S, Okazaki M, Takahashi S, Ono M, Nose M, Sakai J, Fujino T, Yamamoto TT. Severe hypercholesterolemia, impaired fat tolerance, and advanced atherosclerosis in mice lacking both low density lipoprotein receptor-related protein 5 and apolipoprotein E. J Biol Chem. 2003;278:11331–11336. doi: 10.1074/jbc.M211987200. [DOI] [PubMed] [Google Scholar]
- Mani A, Radhakrishnan J, Wang H, Mani A, Mani MA, Nelson-Williams C, Carew KS, Mane S, Najmabadi H, Wu D, Lifton RP. LRP6 mutation in a family with early coronary disease and metabolic risk factors. Science. 2007;315:1278–1282. doi: 10.1126/science.1136370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM. The Role of Lrp5/6 in cardiac valve disease: LDL-density-pressure theory. J Cell Biochem. 2011 doi: 10.1002/jcb.23182. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Caira F, Stock SR, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced calcification in the aortic valves via the Lrp5 receptor pathway. Circulation. 2005a;112:I229–I234. doi: 10.1161/01.CIRCULATIONAHA.104.524306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Rickard D, Stock SR, Donovan J, Springett M, Orszulak T, Fullerton DA, Tajik AJ, Bonow RO, Spelsberg T. Human aortic valve calcification is associated with an osteoblast phenotype. Circulation. 2003;107:2181–2184. doi: 10.1161/01.CIR.0000070591.21548.69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Springett M, Sebo TC, Niekrasz M, McConnell JP, Singh RJ, Stone NJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits hypercholesterolemia-induced cellular proliferation and bone matrix production in the rabbit aortic valve. Circulation. 2002;105:2260–2265. doi: 10.1161/01.cir.0000017435.87463.72. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajamannan NM, Subramaniam M, Stock SR, Stone NJ, Springett M, Ignatiev KI, McConnell JP, Singh RJ, Bonow RO, Spelsberg TC. Atorvastatin inhibits calcification and enhances nitric oxide synthase production in the hypercholesterolaemic aortic valve. Heart. 2005b;91:806–810. doi: 10.1136/hrt.2003.029785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shao JS, Cheng SL, Pingsterhaus JM, Charlton-Kachigian N, Loewy AP, Towler DA. Msx2 promotes cardiovascular calcification by activating paracrine Wnt signals. J Clin Invest. 2005;115:1210–1220. doi: 10.1172/JCI24140. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Westendorf JJ, Kahler RA, Schroeder TM. Wnt signaling in osteoblasts and bone diseases. Gene. 2004;341:19–39. doi: 10.1016/j.gene.2004.06.044. [DOI] [PubMed] [Google Scholar]