Abstract
Rationale
The concern that adjuvant cancer chemotherapy agents cause cognitive impairment in a significant number of patients has been expressed by patients and healthcare providers, but clinical studies have yielded conflicting results to date.
Objective
We directly tested two commonly used chemotherapeutic agents in a mouse model of learning and memory.
Materials and methods
In the present study, mice were conditioned to respond for a liquid reinforcer (Ensure solution) in the presence of an audible tone on day 1 as a measure of acquisition and were then required to perform the same response on day 2 as a measure of retrieval and retention. Methotrexate and 5-fluorouracil were administered prior to the day 1 session.
Results
Methotrexate (1.0–32 mg/kg) alone failed to alter mean latency acquisition, retrieval, or reinforced response rates. Similar to scopolamine, a known amnesic in this assay, 5-fluorouracil (3–75 mg/kg) failed to alter response rates or acquisition latency on day 1 but significantly altered latency to retrieve a previously learned response on day 2. In combination, 3.2 mg/kg methotrexate plus 75 mg/kg 5-fluorouracil significantly increased day 1 and day 2 acquisition and retrieval latencies without altering responserates or motivation to respond as measured by progressive ratio responding.
Conclusion
Taken together, these data demonstrate that 5-fluorouracil causes increased latencies for retrieval of previously learned behavioral responses and that combination of chemotherapeutic agents may produce greater delays than either agent alone, including when neither agent alone does so.
Keywords: Acquisition, Autoshaping-operant, Chemotherapy, Cognition, 5-Fluorouracil, Methotrexate, Methylscopolamine, Progressive ratio, Retention, Scopolamine
Impairments in attention, mental flexibility, concentration, visual memory, and speed of information processing are estimated in some studies to occur in 4–75% of breast cancer patients who receive adjuvant chemotherapy and to persist even years after completion of the therapy (Ahles and Saykin 2002; Kingma et al. 2001; O’Shaughnessy 2002; Schagen et al. 1999, 2001, 2002a, b, 2006; Silberfarb et al. 1980; Tchen et al. 2003; van Dam et al. 1998; Wieneke and Dienst 1995). In these studies, the cognitive impairments are attributed to the chemotherapy but not to anxiety, depression, fatigue, self-reported complaints of cognitive dysfunction (Schagen et al. 2006; Schagen et al. 1999), patient age, or menopausal status (Brezden et al. 2000). However, other studies have not detected impairments (Donovan et al. 2005; Jenkins et al. 2005) or have not attributed observed impairments to the chemotherapeutic drugs (Ahles and Saykin 2007; Hermelink et al. 2007). Studies using neuroimaging techniques in patients receiving chemotherapy have found structural and functional changes in brain regions associated with cognitive functioning (Inagaki et al. 2007; Saykin and Wishart 2003). For example, positron emission tomography of breast cancer survivors revealed significant alterations in cerebral blood flow and in resting glucose metabolism in the frontal cortex and the cerebellum that were correlated with impairments in short-term memory recall tasks (Silverman et al. 2007). Similarly, magnetic resonance imaging scans of breast cancer survivors showed reduced volume in various brain regions associated with cognitive function, and these changes were correlated with impaired performance on the attention/concentration and visual memory indices of the Wechsler Memory Scale-Revised or in working memory tasks (Inagaki et al. 2007; Saykin et al. 2006; Stemmer et al. 1994).
Despite the obvious importance of this question, only a few studies have been performed that test the effects of chemotherapeutic agents on learning and memory behavior using established rodent models, and the results of these studies have been somewhat mixed. For example, high doses of methotrexate impaired conditioned taste aversion and conditioned emotional response assays in male and female neonatal rats independent of sensory deficits, motor impairment, or histopathology (Yanovski et al. 1989), unless the conditioned taste aversion test included a feature-negative discrimination task (Stock et al. 1995). Recent studies have implicated hippocampal deficits in rodents treated with chemotherapeutic agents, although the results of these studies are also mixed. In the Morris water maze and the novel object recognition task, rats treated with methotrexate showed deficits correlated with decreased hippocampal cell proliferation, suggesting impairments of spatial memory and comparator functions of the hippocampus, respectively (Seigers et al. 2008). However, repeated injections of cyclophosphamide or 5-fluorouracil caused transient enhancements of both memory and hippocampal synaptic plasticity in spatial learning tasks (Lee et al. 2006) but produced transient memory deficits in a mouse step-down inhibitory avoidance-conditioning task (Reiriz et al. 2006). The susceptibility of neural cells to chemotherapeutic agents is supported by in vitro studies that demonstrate increased cell death and decreased cell division in the subventricular zone, in the dentate gyrus of the hippocampus, and in the corpus callosum of mice and rats even at doses below those used in standard chemotherapeutic clinical regimens (Dietrich et al. 2006; Mignone and Weber 2006; Seigers et al. 2008). The observation that chemotherapeutic agents are more toxic to hippocampal cells than to cancer cell lines suggests that learning and memory deficits might be detected at doses below those normally used in chemotherapeutic regimens.
The recently published studies of a possible cellular basis for chemotherapy-induced learning and memory deficits and the inconsistent preclinical and clinical data establish a compelling need for continued in vivo evaluation of these agents in additional types of learning and memory assays. In the present study, two widely used antimetabolite chemotherapeutic agents, 5-fluorouracil (pyrimidine antagonist) and methotrexate (folic acid antagonist), were tested in an operant variation (Davenport 1974) of the autoshaping procedure in mice, which can provide a rapid and objective measure of a drug’s effect on several aspects of learning and memory (Barrett and Vanover 2003; Vanover and Barrett 1998). Specifically, mice are conditioned to respond for a liquid reinforcer in the presence of an audible tone. Mean-adjusted latencies, dipper response rates, and non-reinforced activity rates on day 1 are measures of acquisition, and the same parameters on day 2 are measures of retrieval and/or retention of the previously learned response on day 1. Agents such as the centrally acting muscarinic antagonist scopolamine (Mundy and Iwamoto 1987) and the N-methyl-d-aspartate receptor antagonist dizocilpine (Coveney and Sparber 1982), which have previously been shown to alter acquisition and retention in rats, produce deficits in a similar procedure (Barrett and Vanover 2003; Vanover and Barrett 1998). In the present study, scopolamine and methylscopolamine were tested as reference compounds in the autoshaping-operant procedure. Methylscopolamine, a muscarinic acetylcholine antagonist similar to scopolamine but with limited capacity to cross the blood–brain barrier and exert central nervous system effects, can be used to assess the peripheral contributions of muscarinic blockade to learning and memory deficits (Richmond et al. 1997; Shannon and Eberle 2006). The doses of methotrexate and 5-fluorouracil examined in the present autoshaping-operant procedure were similar to the doses examined in other preclinical rodent studies using spatial learning, conditioned taste aversion, and conditioned avoidance or fear tasks (Lee et al. 2006; Madhyastha et al. 2002; Stock et al. 1995; Winocur et al. 2006; Yanovski et al. 1989).
Methotrexate and 5-fluorouracil were also tested in a series of dose combinations for their effects on learning and memory. Drug combination regimens are used clinically to target asynchronously dividing tumor cells, reduce the degree of drug resistance, and allow each agent to be administered at its highest tolerable dose in order to maximize efficacy without excessive toxicity (Davis and Lindley 2005). Indeed, some chemotherapeutic drug combinations take advantage of known drug synergisms. For example, 5-fluorouracil and methotrexate are used in conjunction for the treatment of breast, colon, and prostate cancers because methotrexate enhances the activity of 5-fluorouracil by elevating levels of 5-phosphoribosyl-1-pyrophosphate, which favors the conversion of 5-fluorouracil to its active form (Albritton et al. 2005). In rodents, certain combinations of chemotherapeutic drugs can cause behavioral effects even when the individual drugs do not. For example, combinations of methotrexate and prednisolone produced greater behavioral effects that were dose dependent: Some dose combinations were antagonistic, whereas others were neutral or synergistic (Mullenix et al. 1994).
Both methotrexate and 5-fluorouracil can produce anorexia, nausea, and emesis in patients (Chabner and Ratain 2007), raising the concern that the changes in retention and retrieval observed in the autoshaping-operant procedure in mice may be a result of conditioned taste aversion or altered motivation for the food reward. To examine altered motivation or changes in response rates produced by methotrexate or 5-fluorouracil alone or in combination, we trained mice to respond for the same Ensure solution in a progressive ratio procedure. In the progressive ratio procedure, the effects of drugs can be evaluated on two measures of reinforcing efficacy: breakpoint and response rate. The breakpoint is defined as the ratio at which the subject stops responding or the highest ratio completed if a time-constrained session is used (Hodos 1961; Hodos and Kalman 1963), thus, lower breakpoint values can indicate lower reinforcing value for a reward. Performance under PR schedules of food delivery is considered to reflect the efficacy or motivational strength of food because increases in either deprivation level or reinforcer magnitude increase break points (Hodos and Kalman 1963), and effects on motivation can be separated from overall suppression of response rate (see Stafford et al. 1998 for review). Any dose of drug that produced changes in the autoshaping-operant procedure was also examined in the progressive ratio procedure. In addition, m-chlorophenylpiperazine (mCPP), a serotonin agonist that produces hypophagia in many assays (e.g., Kennett and Curzon 1988; Kennett et al. 1996) and decreases the breakpoint value for Ensure in mice (Ward et al. 2008), was tested as a positive control of a compound that decreases breakpoint responding.
Materials and methods
Subjects
Male, Swiss–Webster mice (N=170) weighing 20–35 g were purchased from ACE Animals, Inc., (Philadelphia, PA, USA). Mice arrived group-housed in plastic cages and were allowed to acclimate to the temperature-and-humidity-controlled facility for 7 days before experimental sessions began. Mice had access to food and water ad libitum during this time. All mice were maintained in accordance with the guidelines of the Institutional Animal Care and Use Committee of Temple University and the “Guide for the Care and Use of Laboratory Animals” (Institute of Laboratory Animal Resources, National Academy Press 1996; NIH publication No. 85–23, revised 1996).
Apparatus
Six experimental chambers (21.6 cm×17.8 cm×12.7 cm, Model ENV-307W, MED Associates, St. Albans, VT, USA) were used for autoshaping-operant and progressive ratio procedures. Each chamber was located within a sound-attenuating enclosure and connected to a computer-driven interface (Model SG-502, MED Associates, St. Albans, VT, USA) programmed to control the experimental conditions and collect the data. One wall of the chamber contained three receptacles: one large dipper hole in the center (ENV 302W) and two smaller nose-poke holes on the left and right (ENV 313W). The opposite wall featured a house light that illuminated the chamber during the session. Each chamber was also equipped with an audible tone device (Sonalert, 2,900 Hz) that emitted a tone on a variable-interval schedule. Nose-pokes into each hole were detected by a photocell head entry detector (ENV 302HD) and recorded. A dipper lever and dipper well were located behind the center dipper hole.
Autoshaping-operant procedure
The autoshaping-operant procedure was modified from the method previously described for mice by Vanover and Barrett (1998) by removing the noncontingent dipper presentation (Davenport 1974). Specifically, after a 1-week acclimation period, mice (n=4–12/group) were separated into individual cages, weighed, and food-restricted for 24 h prior to injection. Water remained available ad libitum. During each session, the house light illuminated the chamber, and the mouse was presented a tone on a variable-interval schedule (mean of 45 s, range 4–132 s). The tone remained on for 6 s or until a nose-poke response occurred. If the mouse made a center-hole nose-poke during the tone, a 0.01-cc dipper filled with a vanilla-flavored liquid nutritional drink Ensure Plus/water (50:50) solution was presented for 3 s, and the tone was turned off. Nose-poke responses in the absence of the tone were counted but had no programmed consequences. Each session lasted for 2 h or until 20 reinforced nose-pokes were recorded. Mice were fed 1.5 g of food and returned to their cages. On day 2, the procedure was repeated. On day 1 of the autoshaping-operant procedure, mice were weighed and injected intraperitoneally with methotrexate, 5-fluorouracil, saline, or a combination of methotrexate and 5-fluorouracil under a fume hood. After a 15-min pretreatment period in the home cage, the mice were placed inside the experimental chambers for an additional 15 min before the session started. Scopolamine and methylscopolamine injections were followed by only the 15-min, in-chamber pretreatment. In this between-groups design, each group of mice was injected only once and tested on day 1 and day 2.
Progressive ratio procedure
A group of mice (N=12) was trained to nose-poke into an illuminated nose-poke hole under a fixed ratio 1 (FR1) schedule wherein each correct nose-poke resulted in the delivery of a 0.01 cc Ensure solution for 3 s in the dipper well. During the Ensure solution delivery, the house light and the active nose-poke hole light were turned off, and the dipper receptacle was illuminated. After achieving stable levels of responding under the FR1 schedule, mice were trained to respond for the Ensure solution under a progressive ratio (PR) schedule of reinforcement, wherein the ratio requirement to obtain the Ensure solution increased throughout a test session. The following modified log progression of response requirements was used in the present study: 1, 2, 4, 6, 9, 12, 15, 20, 25, 32, 40, 50, 62, 77, 95, 118, 145, etc. (Richardson and Roberts 1996). For example, a final log ratio of 12 would indicate that a mouse completed a response requirement of 50 nose-poke responses to receive a single presentation of Ensure solution. ‘Break point’ was defined as the final ratio completed by the end of the 1-h test session or before 10 min elapsed without a reinforced response. This progression of response requirement, coupled with the time constraint, was chosen because vehicle-treated mice routinely achieved break point within 20–30 min, well within the 1-h session time constraint. Stable responding under the PR schedule was defined as 2 days of baseline responding where the number of reinforcers earned differed by no more than three. Once stable responding was achieved, the drugs that produced retrieval deficits on day 2 in the autoshaping-operant procedure described above were tested in the progressive ratio procedure. Mice were pretreated with saline prior to baseline sessions and pretreated with 10 mg/kg scopolamine, 75 mg/kg 5-fluorouracil, 3.2 mg/kg mCPP, or a combination of 3.2 mg/kg methotrexate and 75 mg/kg 5-fluorouracil prior to the test sessions. Pretreatment times were as described above. The effects of the drugs on breakpoint and response rate on day 1 and then 24 h after the injections were compared to responding on the previous saline baseline sessions. Each test followed at least 2 days of stable baseline responding and occurred once per week. The effect of each dose of drug or drug combination was determined in seven to 12 mice using a within-subjects design.
Data and statistical analysis
Each nose-poke into the center nose-poke hole in the presence of the tone elicited the presentation of the dipper of Ensure solution and was recorded as a reinforced response (up to a maximum of 20 in each 2-h session). The main measure of acquisition and retrieval of the nose-poke behavior was the mean-adjusted latency. Mean-adjusted latency was the elapsed time, in seconds, to the tenth reinforced response minus the latency to the first reinforcer (L10–L1). With this adjustment, we corrected for the unequal opportunity for each mouse to achieve the first reinforcer due to the variable-interval schedule of the tone (Barrett and Vanover 2003; Vanover and Barrett 1998). To calculate the rate of dipper nose-poke hole responding, the total number of dipper nose-pokes made during the session—regardless of the presence or absence of the tone—was recorded and divided by the total session time in seconds for each mouse. This dipper rate measure served as a guide to whether differences in adjusted latency were dependent or independent of overall rate of responding. Left and right nose-poke holes were not reinforced but were counted and divided by total session time as a measure of response discrimination. All mice were included in acquisition measures from the day 1 sessions. However, mice that failed to achieve at least ten reinforcers on day 1 of testing were excluded from the latency measures on day 2. The rationale for this exclusion was that it was not appropriate to evaluate day 2 retrieval of a response that was insufficiently reinforced, or not reinforced at all, on day 1 (Barrett and Vanover 2003; Vanover and Barrett 1998).
For the autoshaping-operant procedure, the effects of each drug on mean-adjusted latency, dipper response rates, or non-reinforced nose-poke response rates were compared to saline using a one-way analysis of variance (ANOVA) and, if p<0.05, followed by Dunnett’s multiple comparison post hoc test. In the combination experiments, the effects of 5-fluorouracil in the presence of methotrexate on mean-adjusted latency, dipper response rates, or non-reinforced nose-poke response rates were compared to methotrexate alone using one-way ANOVA with Dunnett’s multiple comparison post hoc tests. In addition, unpaired t tests were used to compare the effects of a single dose of 5-fluorouracil alone to the effects of a dose of 5-fluorouracil in combination with methotrexate. Significance was set at p<0.05.
For the progressive ratio studies, breakpoint was defined as the final ratio completed by the end of the 1-h test session or before 10 min elapsed without a reinforced response for each individual mouse, reported as a group mean (±SEM). The rate of responding was calculated by dividing the total number of responses by the total session time for each mouse and reported as a group mean (±SEM). The final ratios completed on the day prior to the test (saline), on the day of injection (day 1), and on the day following injection (day 2) were compared using repeated measures ANOVA and Dunnett’s multiple comparisons post hoc tests where appropriate for each drug tested. Significance was set at p<0.05.
Drugs
Scopolamine hydrobromide (TOCRIS Bioscience, Ellsville, MD), (−)-scopolamine methylbromide, methotrexate, mCPP, and 5-fluorouracil (Sigma-Aldrich, St. Louis, MO, USA) were dissolved in sterile water. Injections were administered in a volume of 0.1 mL/10 g, i.p., prior to the day 1 autoshaping-operant or progressive ratio session. Each dose of drug or saline was administered in a separate group of mice in the autoshaping-operant procedure (between-groups design) and in the same group of mice in the progressive ratio procedure (within-group design). Chemotherapeutic agents were stored and handled in accordance with guidelines set forth by the Temple University Department of Environmental Health and Radiation Safety. Due to their limited solubility in sterile water, the highest doses of methotrexate and 5-fluorouracil administered were 32 and 75 mg/kg, respectively.
Results
Saline control
On day 1, mice treated with saline acquired the nose-poke response into the dipper well in the presence of the tone with a mean-adjusted latency of 2,550±608 s (Fig. 1, upper left panel) and the rate of responding into the dipper well overall was 0.14±0.021 resp/s (Fig. 1, upper middle panel). The rate of responding into the non-reinforced, left and right nose-pokes was 0.0092±0.0029 resp/s during the day 1 session (Fig. 1, upper right panel). On day 2, the mice were placed back into the experimental chambers without receiving injections, and the experimental conditions proceeded exactly as on day 1. The mice previously treated with saline (on day 1) responded into the dipper well in the presence of the tone with a mean-adjusted latency of 621±117 s on day 2, which was significantly shorter than the mean-adjusted latency on day 1 (p<0.01; Fig. 1, upper left panel). The dipper response rate increased to 0.41±0.062 resp/s (Fig. 1, upper middle panel), which was faster than day 1 (p<0.0001), and the non-reinforced, nose-poke rate dropped to 0.0017±0.00063 resp/s on day 2, which was slower (p<0.02) than day 1 (Fig. 1, upper right panel). In summary, the mice responded with a faster latency and dipper response rate and directed fewer of their responses to the non-reinforced nose-poke holes on day 2, demonstrating that the dipper nose-poke response had been effectively learned on day 1 and retrieved on day 2.
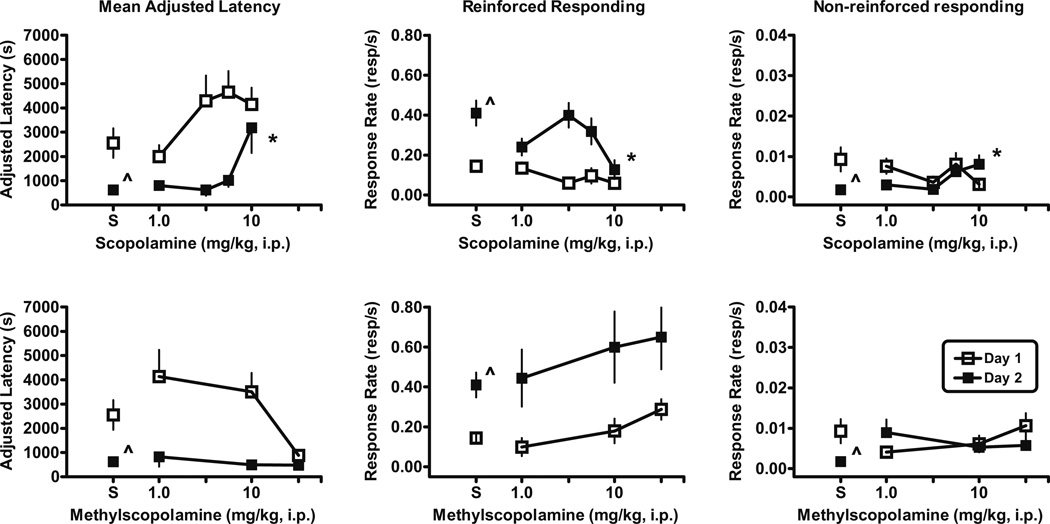
Fig. 1.
Effects of scopolamine (upper panels) and methylscopolamine (lower panels) on autoshaped-operant responding on day 1 (open squares) and day 2 (filled squares) in male, Swiss–Webster mice. Left panels The adjusted latency (latency to the tenth reinforcer minus the latency to the first reinforcer). Previous administration of scopolamine significantly increased adjusted latency on day 2 (F(4, 32)=6.7, p<0.0006) compared to saline. Middle panels Rate of nose-poke responses per second in the dipper well. Dipper response rate was slower (F(4, 41)=3.6, p<0.01) on day 2 in mice treated with scopolamine compared to saline-treated mice. Right panels Rate of nose-pokes in either the right or left holes per second as a measure of general activity. Non-reinforced nose-poke rate was higher (F(4, 41)=3.5, p<0.02) on day 2 after previous administration of scopolamine compared to saline. Total number of mice tested on day 1/number of mice completing ten reinforcers on day 1 and therefore included on day 2: scopolamine [1 mg/kg (6/6); 3.2 mg/kg (6/5); 5.6 mg/kg (10/6); 10 mg/kg (8/5)] and methylscopolamine [1 mg/kg (6/4); 10 mg/kg (6/5); 32 mg/kg (4/4)]. Abscissa Dose in milligram per kilogram administered i.p. Points above S are the effects in two groups of saline-treated mice tested 5 months apart and averaged into one group mean represented in Figs. 1 and 2. Vertical lines represent SEM. Asterisk Significantly different than saline control by Dunnett’s multiple comparison tests, p<0.05. Caret Significantly different than day 1 as determined by paired t tests: mean-adjusted latency, p<0.01; dipper response rates, p<0.0001; non-reinforced nose-poke rate, p<0.02
Scopolamine and methylscopolamine
Mice were injected with either scopolamine or methylscopolamine prior to the day 1 session. Although increasing doses of scopolamine tended toward increased mean-adjusted latencies, these latencies were not significantly different than saline values on day 1 (Fig. 1, upper left panel). Similarly, the dipper response rates (Fig. 1, upper middle panel) and the non-reinforced, nose-poke rates (Fig. 1, upper right panel) were not different than saline control response rates. However, on day 2, the mean-adjusted latency for mice treated with 10 mg/kg scopolamine on the prior day was significantly longer (p<0.01), dipper response rate was slower (p<0.01), and non-reinforced nose-poke rate was higher (p<0.05) relative to the saline control values (Fig. 1, upper panels). Unlike the saline control data described above, the adjusted latency values and the dipper response rates were not significantly different from day 1 to day 2, but non-reinforced, nose-poke response rates were different (p<0.04) on day 1 from day 2 after the injection of 10 mg/kg scopolamine. These results suggest that scopolamine disrupted the consolidation or retrieval of the dipper nosepoke response on day 2. Like scopolamine, methylscopolamine administered prior to the day 1 session did not alter day-1-adjusted latency, dipper response rates, or non-reinforced, nose-poke response rates relative to the saline control mice. In contrast to scopolamine, methylscopolamine failed to alter day-2-adjusted latency, dipper response rates, or non-reinforced, nose-poke response rates relative to the vehicle control mice, suggesting that CNS penetration is required to disrupt the consolidation or retrieval of the reinforced nose-poke response.
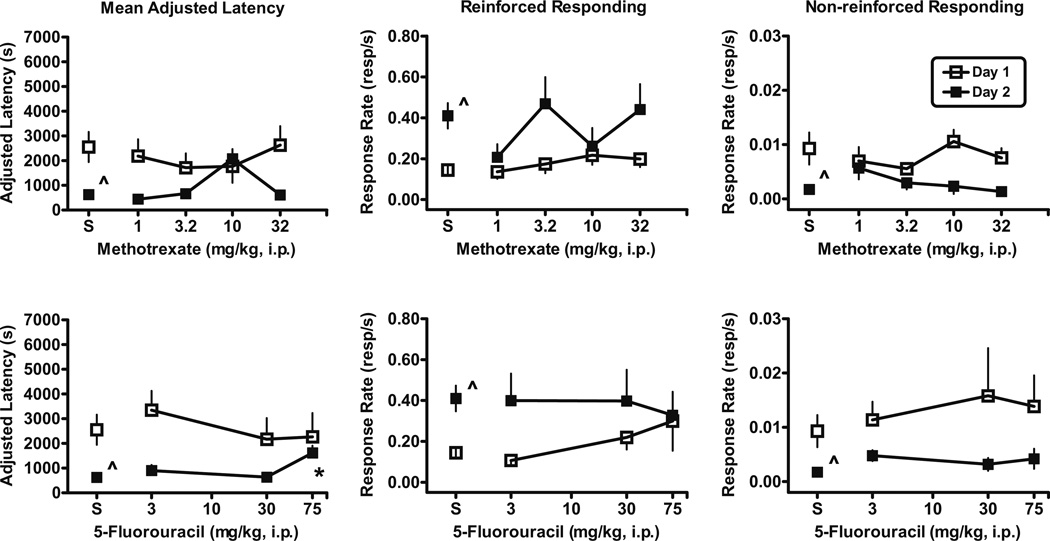
Single chemotherapeutic agents: methotrexate or 5-fluorouracil
Groups of mice were treated with one of the two chemotherapeutic agents: methotrexate or 5-fluorouracil. Mice treated with methotrexate did not differ significantly from saline controls in day-1-adjusted latency (Fig. 2, upper left panel), dipper response rates (Fig. 2, upper middle panel), or non-reinforced, nose-poke response rates (Fig. 2, upper right panel). Similarly, prior administration of methotrexate did not significantly alter day-2-adjusted latency (Fig. 2, upper left panel), dipper response rate (Fig. 2, upper middle panel), or non-reinforced, nose-poke response rate (Fig. 2, upper right panel). Although prior administration of 10 mg/kg methotrexate appeared to produce longer mean-adjusted latency and a corresponding decrease in dipper response rate and non-reinforced, nose-poke response rate relative to the next lower and next higher doses tested, neither measure was statistically significant.
Fig. 2.
Effects of the chemotherapeutic agents methotrexate (upper panels) and 5-fluorouracil (lower panels) on day 1 measures (open squares) and day 2 measures (filled squares) in male, Swiss–Webster mice. Adjusted latency on day 2 was significantly longer (F(3, 28)=4.8; p<0.01) after treatment with 5-fluorouracil than after saline. Total number of mice tested on day 1/number of mice completing ten reinforcers on day 1 and therefore included on day 2 per dose: methotrexate [1 mg/kg (9/7); 3.2 mg/kg (6/6); 10 mg/kg (9/8); 32 mg/kg (11/9)] and 5-fluorouracil [3 mg/kg (12/8); 30 mg/kg (6/5); 75 mg/kg (6/5)]. Abscissa Dose in milligram per kilogram administered i.p. Asterisk Significantly different than saline control determined by Dunnett’s multiple comparison tests, p<0.05. Caret Significantly different than day 1 as determined by paired t tests: mean-adjusted latency, p<0.01; dipper response rates, p<0.0001; non-reinforced nose-poke rate, p<0.02. Other details as in Fig. 1
Mice treated with 5-fluorouracil did not differ significantly from day 1 saline controls in adjusted latency (Fig. 2, lower left panel), dipper response rates (Fig. 2, lower middle panel), or non-reinforced, nose-poke response rates (Fig. 2, lower right panel). On day 2, prior administration of 5-fluorouracil on day 1 did not alter dipper response rate (Fig. 2, lower middle panel) or non-reinforced, nose-poke response rate (Fig. 2, lower right panel). However, injection of the highest dose of 75 mg/kg 5-fluorouracil on day 1 produced a significant increase (p<0.01) in day-2-adjusted latency (Fig. 2, lower left panel) relative to saline controls on day 2. When adjusted latency and dipper response rates on day 2 were compared to the values on day 1, paired t tests indicated that adjusted latency values and the dipper response rates were not significantly different on day 2 from day 1 after the injection of 75 mg/kg 5-fluorouracil, similar to the effects described above for scopolamine.
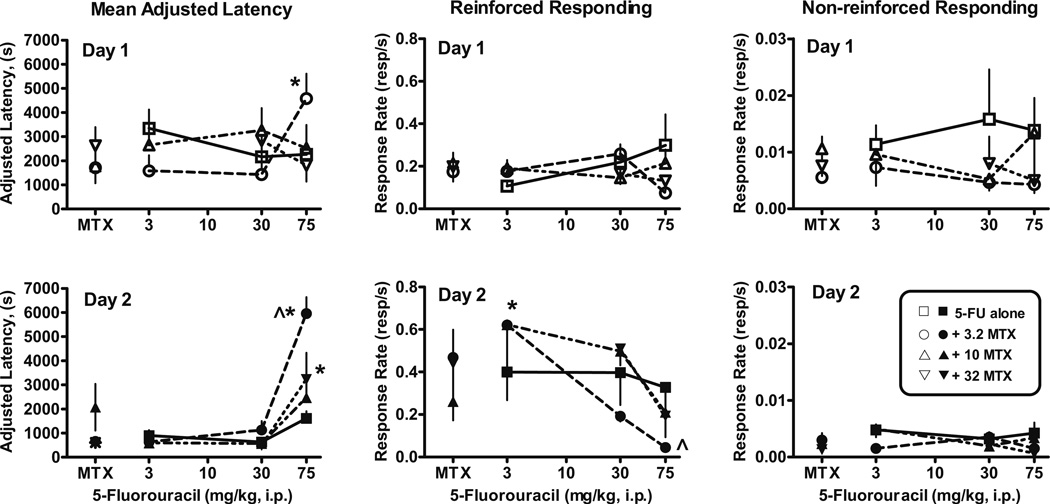
Combined administration of methotrexate and 5-fluorouracil
Mice were injected with methotrexate (3.2, 10, or 32 mg/kg) and one of three doses of 5-fluorouracil (3, 30, or 75 mg/kg) prior to the day 1 acquisition session. Combined administration of 3.2 mg/kg methotrexate with 75 mg/kg 5-fluorouracil prior to the day 1 acquisition session significantly increased adjusted latencies (p<0.05) compared to 3.2 mg/kg methotrexate alone (Fig. 3, upper left panel). An overall decrease in day 1 dipper response rates (Fig. 3, upper middle panel) relative to 3.2 mg/kg methotrexate alone was also observed, although no single 5-fluorouracil dose in combination with 3.2 mg/kg methotrexate achieved statistical significance. These acquisition effects occurred only when 3.2 mg/kg methotrexate was administered in combination with 75 mg/kg 5-fluorouracil, not when they were administered alone (Fig. 2, left panels) or in combination with other doses of methotrexate or 5-fluorouracil (Fig. 3, upper panels). Day 1 non-reinforced, nose-poke response rates were unaffected by combined administration of these agents (Fig. 3, upper right panel).
Fig. 3.
Effects of 5-fluorouracil (5-FU) alone (open squares and closed squares) and in combination with methotrexate (MTX) (open and closed circles) on autoshaped responses on day 1 (upper panels) and day 2 (lower panels). Combined administration of 3.2 mg/kg methotrexate and with 5-fluorouracil altered day-1-adjusted latency (F(3, 23)=4.4, p<0.02) relative to the effects of 3.2 mg/kg methotrexate alone and produced a decrease in day 1 dipper response rates (F(3, 23)=3.3, p<0.04). On day 2, adjusted latency was increased by combinations of 3.2 mg/kg (F(3, 21)=50, p<0.0001) and 32 mg/kg methotrexate (F(2, 18)=8.2, p<0.004) with 5-fluorouracil relative to methotrexate alone. In addition, 3.2 mg/kg methotrexate and 75 mg/kg 5-fluorouracil produced longer adjusted latencies than 75 mg/kg 5-fluorouracil alone (p<0.01). Day 2 dipper response rates were significantly different than methotrexate alone for 3.2 mg/kg (F(3, 23)=3.3, p<0.04) and 10 mg/kg (F(3, 26)=5.2, p<0.007) methotrexate in combination with 5-fluorouracil. Total number of mice tested on day 1/number of mice completing ten reinforcers on day 1 and therefore included in day 2 per dose: [3.2 mg/kg methotrexate+3 mg/kg 5-fluorouracil (6/6), +30 mg/kg 5-fluorouracil (6/6), +75 mg/kg 5-fluorouracil (6/4); 10 mg/kg methotrexate+3 mg/kg 5-fluorouracil (6/6), +30 mg/kg 5-fluorouracil (6/5), +75 mg/kg 5-fluorouracil (6/6); 32 mg/kg methotrexate+30 mg/kg 5-fluorouracil (6/4), +75 mg/kg 5-fluorouracil (6/5)]. Points above MTX are the effects of 3.2, 10, or 32 mg/kg methotrexate alone tested 3–6 months apart in two separate groups of mice per dose (Fig. 2). Asterisk Significantly different than methotrexate alone, p<0.05. Caret Significantly different than 75 mg/kg 5-fluorouracil alone, p<0.05 (lower left panel), p<0.0004 (lower middle panel). Other details as in Fig. 1
Combined administration of 3.2 (p<0.01) or 32 mg/kg methotrexate (p<0.01) and 75 mg/kg 5-fluorouracil significantly altered day 2 mean-adjusted latency compared to these doses of methotrexate alone (Fig. 3, lower left panel). Overall day 2 dipper response rates were decreased by combined administration of 3.2 mg/kg methotrexate with 5-fluorouracil (p<0.04), although no single 5-fluorouracil dose in combination with 3.2 mg/kg methotrexate achieved statistical significance (Fig. 3, lower middle panel). Day 2 non-reinforced, nose-poke response rates were unaffected by any combination of these agents relative to methotrexate alone (Fig. 3, lower right panel). Unpaired t tests indicated that the adjusted latency values were significantly increased (p<0.0004), and the dipper response rates were significantly decreased (p<0.04) after administration of 75 mg/kg 5-fluorouracil in combination with 3.2 mg/kg methotrexate compared to the effects of 75 mg/kg 5-fluorouracil alone.
Effects of scopolamine, methotrexate, and 5-fluorouracil on progressive ratio responding
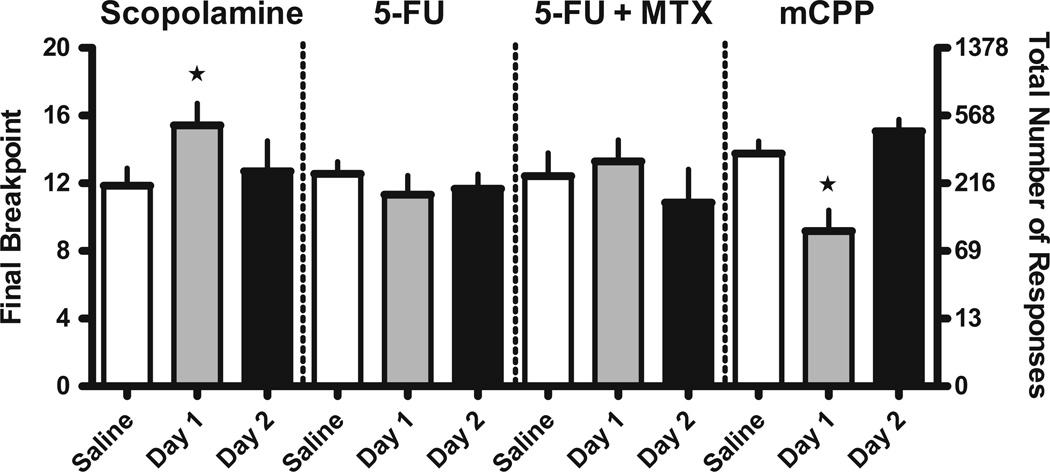
To eliminate the possibility that the significant behavioral changes described above for the chemotherapeutic agents were the result of an aversive response to the Ensure solution or changes in motor activity, we assessed 10 mg/kg scopolamine, 75 mg/kg 5-fluorouracil alone, 75 mg/kg 5-fluorouracil combined with 3.2 mg/kg methotrexate, and 3.2 mg/kg mCPP in the progressive ratio assay in mice. A dose of 10 mg/kg scopolamine, which produced retrieval deficits in the autoshaping-operant procedure (Fig. 1, upper panels), significantly increased breakpoint (p<0.05) but not response rates (0.40±0.12 vs. 0.38±0.10 resp/s) on day 1 compared to the saline baseline values obtained the day prior to scopolamine injection, as indicated by repeated measures one-way ANOVA (Fig. 4). This increased responding for the Ensure solution dissipated by day 2. Single administration of 75 mg/kg 5-fluorouracil and combined administration of 75 mg/kg 5-fluorouracil and 3.2 mg/kg methotrexate failed to change breakpoint or rates of responding (0.40±0.12 vs. 0.31±0.11–0.48±0.15 resp/s, respectively) for the Ensure solution relative to saline baseline values. Finally, 3.2 mg/kg mCPP did not alter adjusted latency, dipper response rates, or activity rates on day 1 or day 2 of testing in the autoshaping-operant procedure (data not shown) but significantly decreased breakpoint (p<0.01) and produced a small but insignificant decrease in response rates relative to the saline baseline values (0.43±0.12 vs. 0.21±0.10 resp/s; Fig. 4).
Fig. 4.
Effects of drugs on the motivation to respond for Ensure solution in mice. Doses of 10 mg/kg scopolamine (N=7), 75 mg/kg 5-fluorouracil alone (N=9) and in combination with 3.2 mg/kg methotrexate (N=7), or 3.2 mg/kg mCPP (N=12) administered i.p. prior to the session (day 1) or 24 h prior to the session (day 2). Scopolamine significantly increased breakpoint (F(2, 20)=3.9; p<0.05), whereas mCPP decreased breakpoint (F(2, 35)=17.6; p<0.0001). Ordinate Final number of ratios completed (left axis) and total number of responses emitted (right axis) on the log PR schedule. Abscissa Saline injection on the day prior to testing (baseline responding), drug injection 30 min prior to testing day 1, saline injection 30 min prior to testing on day 2 and 24 h after drug injection on day 1. Star Significantly different (p<0.05, p<0.01, respectively) than baseline responding. Vertical bars represent SEM
Discussion
In the current study, mice were conditioned to respond for a liquid reinforcer in the presence of an audible tone on day 1 as a measure of acquisition and were then required to perform the same response on day 2 as a measure of retrieval and retention. Consistent with the findings of previous studies (Liy-Salmeron and Meneses 2007; Mundy and Iwamoto 1987; Vanover and Barrett 1998), scopolamine significantly increased day-2-adjusted latency responding, decreased dipper rates of responding, and increased activity in the non-reinforced nose-poke holes when administered prior to the day 1 experimental session. These results demonstrate the sensitivity of the autoshaping-operant assay to detect subtle influences of drugs on behavior. For instance, the increased adjusted latency on day 2 suggests a reduced capacity to retain and/or retrieve the previously learned response. This conclusion is strengthened by the simultaneous decrease in overall dipper-well nose-poke rates and increase in non-reinforced nose-poke rate, suggesting a decrease in the ability to associate the dipper-well with the liquid reinforcer or to discriminate between reinforced and non-reinforced responding. When administered prior to the day 1 experimental session, the peripherally acting muscarinic acetylcholine receptor antagonist methylscopolamine did not duplicate the changes produced by scopolamine, suggesting that in order to alter retention and/or retrieval in the autoshaping-operant procedure, the agent must enter the CNS.
When the chemotherapeutic agent methotrexate was administered prior to the day 1 session, it did not alter mean latency acquisition, retention, or reinforced response rates on either day 1 or day 2. In previous studies, these doses of methotrexate alone disrupted conditioned taste aversion, conditioned emotional responses, spatial memory, and novel object recognition (Madhyastha et al. 2002; Seigers et al. 2008; Yanovski et al. 1989) but failed to alter responding in a different conditioned taste aversion assay and a Pavlovian appetitive conditioning assay (Stock et al. 1995). The doses of 3.2–32 mg/kg methotrexate used in mice and rats fall within the predicted 8–42-mg/kg-scaled mouse doses as determined from the range of clinical doses used to treat breast cancer in humans (40 mg/m2) according to pharmacokinetic studies (Lobo and Balthasar 2003; Ohdo et al. 1997; Peters et al. 1993). When 75 mg/kg 5-fluorouracil was administered prior to the day 1 session, it failed to alter response rates or acquisition latency on day 1. However, this dose of 5-fluorouracil significantly increased day 2 latency, a result similar to that obtained with known amnesic scopolamine in the autoshaping-operant procedure. In a single study examining 5-fluorouracil alone, four repeated injections of 150 mg/kg 5-fluorouracil failed to alter maze performance in young female rats (Lee et al. 2006), a result in contrast to the present findings. The doses of 75 mg/kg (present study) and 150 mg/kg (Lee et al. 2006) 5-fluorouracil in mice and rats, respectively, fall slightly below and slightly above the predicted 100-mg/kg-scaled mouse dose as determined from the typical clinical dose used to treat breast cancer in humans (500 mg/m2) (Gusella et al. 2006; Jin et al. 2005; Kralovanszky et al. 1999; Ooi et al. 2001; Peters et al. 1993). The findings in the present study underscore the importance of examining a range of relevant preclinical and clinical doses in more than one type of assay to capture the relationship of chemotherapeutic agents to changes in different types of learning and memory behaviors.
Patients rarely receive only one chemotherapeutic agent to treat breast cancer, so it is important to examine adverse effects of agents in combination. Indeed, there is preclinical evidence that certain combinations of chemotherapeutic drugs can cause behavioral effects in some assays (Mullenix et al. 1994; Winocur et al. 2006). For example, after three weekly injections of a combination of 37.5 mg/kg methotrexate and 75 mg/kg 5-fluorouracil, mice tested in variations of the Morris water maze exhibited deficits in spatial memory, non-matching-to-sample learning, and delayed-non-matching-to-sample learning, while there were no changes noted in cued memory or discrimination learning (Winocur et al. 2006). Similarly, rats treated with three weekly treatments of a combination of cyclophosphamide and doxorubicin showed impairments in contextual but not cue-specific fear responses (Macleod et al. 2007). In the present study, three doses of methotrexate were combined with three doses of 5-fluorouracil for a total of nine different acute dose combinations. The largest effect on learning and memory was produced by the combination of 3.2 mg/kg methotrexate with 75 mg/kg 5-fluorouracil. Of note, although 3.2 mg/kg methotrexate alone failed to alter day 2 mean-adjusted latency relative to controls (Fig. 2), the combination of this ineffective methotrexate dose with a moderately effective dose of 5-fluorouracil produced a much greater increase in mean-adjusted latency (Fig. 3). Perhaps even more striking, however, was that neither agent alone had a significant impact on day 1 measures, but the combination produced a significant increase in day 1 mean-adjusted latency. There appears to be a specific interaction at this dose ratio because combining higher doses of methotrexate with 5-fluorouracil produced less of an effect. Consistent with this idea, the interaction between combinations of methotrexate and prednisolone are antagonistic, neutral, or synergistic, further demonstrating the need to study multiple dose combinations (Mullenix et al. 1994). Indeed, in the treatment of cancer, certain combination ratios of maximally tolerated doses can be synergistic while others are antagonistic, such that the most efficacious combinations may not occur at the highest doses (Mayer and Janoff 2007). Taken together, these data suggest that certain dose-ratio combinations of methotrexate and 5-fluorouracil can yield therapeutic or adverse effects that exceed those of the drugs given alone.
Both methotrexate and 5-fluorouracil can produce anorexia, nausea, and emesis in patients (Chabner and Ratain 2007), raising the concern that the deficits in retention and retrieval observed in the autoshaping-operant procedure in mice may be a result of toxicity or altered motivation for the liquid reinforcer. To assess these possibilities, we examined the doses of 5-fluorouracil alone and in combination with methotrexate that increased adjusted latency values on day 2 in the autoshaping-operant procedure in another behavioral task, the progressive ratio procedure. The progressive ratio procedure is used to assess the motivational value of a reinforcer: If a drug increases the reinforcing value of the Ensure solution, the mice respond at higher breakpoint values; if a drug decreases the reinforcing value of the Ensure solution, the mice respond at lower breakpoint values. For example, the 5-HT1B/2C receptor agonist mCPP, which produces hypophagia in many assays (e.g., Kennett and Curzon 1988; Kennett et al. 1996), significantly decreased the breakpoint value for Ensure solution (Fig. 4) without altering response rates in the progressive ratio assay, as previously reported (Ward et al. 2008). The same dose of mCPP did not alter consolidation or retrieval in the autoshaping-operant procedure (data not shown). 5-Fluorouracil alone or in combination with methotrexate did not change either breakpoint (Fig. 4) or rates of responding for Ensure solution on day 1 or 2, suggesting that these doses are not acutely toxic and that the Ensure solution retains its palatability for at least 2 days after injections of methotrexate and 5-fluorouracil. Furthermore, the observation that 5-fluorouracil alone and in combination with methotrexate did not alter the rate at which the mice respond for the reinforcer in either the autoshaping-operant or progressive ratio procedures supports the notion that these doses are not behaviorally suppressive. A dose of 10 mg/kg scopolamine significantly increased breakpoint values but not response rates on day 1 in the progressive ratio experiment. Therefore, the increased mean-adjusted latencies and non-reinforced nose-poke response rates and decreased dipper response rates on day 2 (Fig. 1) in the autoshaping-operant procedure are most likely not due to an altered motivation for the Ensure solution.
In summary, these experiments demonstrate retrieval/retention deficits produced by 5-fluorouracil administration alone and acquisition and retrieval/retention deficits produced by a combination of methotrexate and 5-fluorouracil at doses that did not alone alter responding in an autoshaping-operant procedure in mice. In control procedures, these effects were shown to be unlikely the result of toxicity, taste aversion, or altered motivation to respond for the liquid reinforcer. Instead, the effects appear to be due to a more selective disruption in learning and memory processes and support a growing preclinical and clinical literature on chemotherapy-induced cognitive deficits.
Acknowledgments
The authors would like to acknowledge Timothy Lefever for his technical assistance and Drs. Swati Nagar, Ronald J. Tallarida, and Rachel Clark-Vetri for their discussions and assistance with dose scaling.
Footnotes
Conflict of interest statement The authors have no conflicts of interest to disclose.
Contributor Information
John J. Foley, Department of Pharmaceutical Sciences, Temple University School of Pharmacy, 3307 North Broad Street, Philadelphia, PA 19140, USA
Robert B. Raffa, Department of Pharmaceutical Sciences, Temple University School of Pharmacy, 3307 North Broad Street, Philadelphia, PA 19140, USA
Ellen A. Walker, Department of Pharmaceutical Sciences, Temple University School of Pharmacy, 3307 North Broad Street, Philadelphia, PA 19140, USA, ellen.walker@temple.edu
References
- Ahles TA, Saykin AJ. Breast cancer chemotherapy-related cognitive dysfunction. Clin Breast Cancer. 2002;3 Suppl 3:S84–S90. doi: 10.3816/cbc.2002.s.018. [DOI] [PubMed] [Google Scholar]
- Ahles TA, Saykin AJ. Candidate mechanisms for chemotherapy- induced cognitive changes. Nat Rev Cancer. 2007;7:192–201. doi: 10.1038/nrc2073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albritton RL, Coen DM, Golan DE. Principles of combination chemotherapy. In: Golan DE, Tashjian AH, Armstrong EJ, et al., editors. Principles of pharmacology, the pathophysiologic basis of drug therapy. Philadelphia PA: Lippincott Williams & Wilkins; 2005. pp. 603–613. [Google Scholar]
- Barrett JE, Vanover KE. Chapter 8 Assessment of learning and memory using the autoshaping of operant responding in mice. Current Protocols in Neuroscience. 2003 doi: 10.1002/0471142301.ns0805fs25. 8.5F.1-8.5F. [DOI] [PubMed] [Google Scholar]
- Brezden CB, Phillips KA, Abdolell M, Bunston T, Tannock IF. Cognitive function in breast cancer patients receiving adjuvant chemotherapy. J Clin Oncol. 2000;18:2695–2701. doi: 10.1200/JCO.2000.18.14.2695. [DOI] [PubMed] [Google Scholar]
- Chabner BA, Ratain MJ. Clarification regarding “phase II trials published in 2002: a cross-specialty comparison showing significant design differences between oncology trials and other medical specialties” and the accompanying commentary,“phase II cancer trials: out of control?”. Clin Cancer Res. 2007;13:6540. doi: 10.1158/1078-0432.CCR-07-1108. [DOI] [PubMed] [Google Scholar]
- Coveney JR, Sparber SB. Phencyclidine retards autoshaping at a dose which does not suppress the required response. Pharmacol Biochem Behav. 1982;16:937–942. doi: 10.1016/0091-3057(82)90049-1. [DOI] [PubMed] [Google Scholar]
- Davenport JW. Combined autoshaping-operant (AO) training: CS-UCS interval in rats. Bulletin of the Psychomonic Society. 1974;3:383–385. [Google Scholar]
- Davis L, Lindley C. Neoplastic disorders and their treatment: general principles. In: Koda-Kimble MA, Young LY, Kradjan WA, Guglielmo BJ, editors. Applied therapeutics: the clinical use of drugs. Philadelphia: Lippincott Williams & Wilkins; 2005. pp. 88–124. [Google Scholar]
- Dietrich J, Han R, Yang Y, Mayer-Proschel M, Noble M. CNS progenitor cells and oligodendrocytes are targets of chemotherapeutic agents in vitro and in vivo. J Biol. 2006;5:22.1–22.23. doi: 10.1186/jbiol50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donovan KA, Small BJ, Andrykowski MA, Schmitt FA, Munster P, Jacobsen PB. Cognitive functioning after adjuvant chemotherapy and/or radiotherapy for early-stage breast carcinoma. Cancer. 2005;104:2499–2507. doi: 10.1002/cncr.21482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gusella M, Crepaldi G, Barile C, Bononi A, Menon D, Toso S, Scapoli D, Stievano L, Ferrazzi E, Grigoletto F, Ferrari M, Padrini R. Pharmacokinetic and demographic markers of 5-fluorouracil toxicity in 181 patients on adjuvant therapy for colorectal cancer. Ann Oncol. 2006;17:1656–1660. doi: 10.1093/annonc/mdl284. [DOI] [PubMed] [Google Scholar]
- Hermelink K, Untch M, Lux MP, Kreienberg R, Beck T, Bauerfeind I, Munzel K. Cognitive function during neoadjuvant chemotherapy for breast cancer: results of a prospective, multicenter, longitudinal study. Cancer. 2007;109:1905–1913. doi: 10.1002/cncr.22610. [DOI] [PubMed] [Google Scholar]
- Hodos W. Progressive ratio as a measure of reward strength. Science. 1961;134:943–944. doi: 10.1126/science.134.3483.943. [DOI] [PubMed] [Google Scholar]
- Hodos W, Kalman G. Effects of increment size and reinforcer volume on progressive ratio performance. J Exp Anal Behav. 1963;6:387–392. doi: 10.1901/jeab.1963.6-387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inagaki M, Yoshikawa E, Matsuoka Y, Sugawara Y, Nakano T, Akechi T, Wada N, Imoto S, Murakami K, Uchitomi Y. Smaller regional volumes of brain gray and white matter demonstrated in breast cancer survivors exposed to adjuvant chemotherapy. Cancer. 2007;109:146–156. doi: 10.1002/cncr.22368. [DOI] [PubMed] [Google Scholar]
- Jenkins VA, Bloomfield DJ, Shilling VM, Edginton TL. Does neoadjuvant hormone therapy for early prostate cancer affect cognition? Results from a pilot study. BJU Int. 2005;96:48–53. doi: 10.1111/j.1464-410X.2005.05565.x. [DOI] [PubMed] [Google Scholar]
- Jin Y, Li J, Rong LF, Lu XW, Huang Y, Xu SY. Pharmacokinetics and tissue distribution of 5-fluorouracil encapsulated by galactosylceramide liposomes in mice. Acta Pharmacol Sin. 2005;26:250–256. doi: 10.1111/j.1745-7254.2005.00530.x. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Curzon G. Evidence that hypophagia induced by mCPP and TFMPP requires 5-HT1C and 5-HT1B receptors; hypophagia induced by RU 24969 only requires 5-HT1B receptors. Psychopharmacology (Berl) 1988;96:93–100. doi: 10.1007/BF02431539. [DOI] [PubMed] [Google Scholar]
- Kennett GA, Wood MD, Bright F, Cilia J, Piper DC, Gager T, Thomas D, Baxter GS, Forbes IT, Ham P, Blackburn TP. In vitro and in vivo profile of SB 206553, a potent 5-HT2C/5-HT2B receptor antagonist with anxiolytic-like properties. Br J Pharmacol. 1996;117:427–434. doi: 10.1111/j.1476-5381.1996.tb15208.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingma A, van Dommelen RI, Mooyaart EL, Wilmink JT, Deelman BG, Kamps WA. Slight cognitive impairment and magnetic resonance imaging abnormalities but normal school levels in children treated for acute lymphoblastic leukemia with chemotherapy only. J Pediatr. 2001;139:413–420. doi: 10.1067/mpd.2001.117066. [DOI] [PubMed] [Google Scholar]
- Kralovanszky J, Katona C, Jeney A, Pandi E, Noordhuis P, Erdelyi-Toth V, Otvos L, Kovacs P, Van der Wilt CL, Peters GJ. 5-Ethyl- 2′-deoxyuridine, a modulator of both antitumour action and pharmacokinetics of 5-fluorouracil. J Cancer Res Clin Oncol. 1999;125:675–684. doi: 10.1007/s004320050333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee GD, Longo DL, Wang Y, Rifkind JM, Abdul-Raman L, Mamczarz JA, Duffy KB, Spangler EL, Taub DD, Mattson MP, Ingram DK. Transient improvement in cognitive function and synaptic plasticity in rats following cancer chemotherapy. Clin Cancer Res. 2006;12:198–205. doi: 10.1158/1078-0432.CCR-05-1286. [DOI] [PubMed] [Google Scholar]
- Liy-Salmeron G, Meneses A. Role of 5-HT1-7 receptors in short- and long-term memory for an autoshaping task: intrahippocampal manipulations. Brain Res. 2007;1147:140–147. doi: 10.1016/j.brainres.2007.02.007. [DOI] [PubMed] [Google Scholar]
- Lobo ED, Balthasar JP. Pharmacokinetic-pharmacodynamic modeling of methotrexate-induced toxicity in mice. J Pharm Sci. 2003;92:1654–1664. doi: 10.1002/jps.10431. [DOI] [PubMed] [Google Scholar]
- Macleod JE, DeLeo JA, Hickey WF, Ahles TA, Saykin AJ, Bucci DJ. Cancer chemotherapy impairs contextual but not cuespecific fear memory. Behav Brain Res. 2007;181:168–172. doi: 10.1016/j.bbr.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Madhyastha S, Somayaji SN, Rao MS, Nalini K, Bairy KL. Hippocampal brain amines in methotrexate-induced learning and memory deficit. Can J Physiol Pharmacol. 2002;80:1076–1084. doi: 10.1139/y02-135. [DOI] [PubMed] [Google Scholar]
- Mayer LD, Janoff AS. Optimizing combination chemotherapy by controlling drug ratios. Mol Interv. 2007;7:216–223. doi: 10.1124/mi.7.4.8. [DOI] [PubMed] [Google Scholar]
- Mignone RG, Weber ET. Potent inhibition of cell proliferation in the hippocampal dentate gyrus of mice by the chemotherapeutic drug thioTEPA. Brain Res. 2006;1111:26–29. doi: 10.1016/j.brainres.2006.06.093. [DOI] [PubMed] [Google Scholar]
- Mullenix PJ, Kernan WJ, Schunior A, Howes A, Waber DP, Sallan SE, Tarbell NJ. Interactions of steroid, methotrexate, and radiation determine neurotoxicity in an animal model to study therapy for childhood leukemia. Pediatr Res. 1994;35:171–178. doi: 10.1203/00006450-199402000-00009. [DOI] [PubMed] [Google Scholar]
- Mundy WR, Iwamoto ET. Studies on desglycinamide arginine vasopressin and scopolamine in a modified/lever-touch autoshaping model of learning/memory in rats. Pharmacol Biochem Behav. 1987;27:307–315. doi: 10.1016/0091-3057(87)90574-0. [DOI] [PubMed] [Google Scholar]
- Ohdo S, Inoue K, Yukawa E, Higuchi S, Nakano S, Ogawa N. Chronotoxicity of methotrexate in mice and its relation to circadian rhythm of DNA synthesis and pharmacokinetics. Jpn J Pharmacol. 1997;75:283–290. doi: 10.1254/jjp.75.283. [DOI] [PubMed] [Google Scholar]
- Ooi A, Ohkubo T, Higashigawa M, Kawasaki H, Kakito H, Kagawa Y, Kojima M, Sakurai M. Plasma, intestine and tumor levels of 5-fluorouracil in mice bearing L1210 ascites tumor following oral administration of 5-fluorouracil, UFT (mixed compound of tegafur and uracil), carmofur and 5′-deoxy-5- fluorouridine. Biol Pharm Bull. 2001;24:1329–1331. doi: 10.1248/bpb.24.1329. [DOI] [PubMed] [Google Scholar]
- O’Shaughnessy JA. Effects of epoetin alfa on cognitive function, mood, asthenia, and quality of life in women with breast cancer undergoing adjuvant chemotherapy. Clin Breast Cancer. 2002;3 Suppl 3:S116–S120. doi: 10.3816/cbc.2002.s.022. [DOI] [PubMed] [Google Scholar]
- Peters GJ, Schornagel JH, Milano GA. Clinical pharmacokinetics of anti-metabolites. Cancer Surv. 1993;17:123–156. [PubMed] [Google Scholar]
- Reiriz AB, Reolon GK, Preissler T, Rosado JO, Henriques JA, Roesler R, Schwartsmann G. Cancer chemotherapy and cognitive function in rodent models: memory impairment induced by cyclophosphamide in mice. Clin Cancer Res. 2006;12:5000. doi: 10.1158/1078-0432.CCR-06-0138. author reply 5000-1. [DOI] [PubMed] [Google Scholar]
- Richardson NR, Roberts DC. Progressive ratio schedules in drug self-administration studies in rats: a method to evaluate reinforcing efficacy. J Neurosci Methods. 1996;66:1–11. doi: 10.1016/0165-0270(95)00153-0. [DOI] [PubMed] [Google Scholar]
- Richmond MA, Nichols BP, Deacon RM, Rawlins JN. Effects of scopolamine and hippocampal lesions on negative patterning discrimination performance in rats. Behav Neurosci. 1997;111:1217–1227. doi: 10.1037//0735-7044.111.6.1217. [DOI] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA. Mild cognitive impairment: conceptual issues and structural and functional brain correlates. Semin Clin Neuropsychiatry. 2003;8:12–30. doi: 10.1053/scnp.2003.50002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saykin AJ, Wishart HA, Rabin LA, Santulli RB, Flashman LA, West JD, McHugh TL, Mamourian AC. Older adults with cognitive complaints show brain atrophy similar to that of amnestic MCI. Neurology. 2006;67:834–842. doi: 10.1212/01.wnl.0000234032.77541.a2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schagen SB, van Dam FS, Muller MJ, Boogerd W, Lindeboom J, Bruning PF. Cognitive deficits after postoperative adjuvant chemotherapy for breast carcinoma. Cancer. 1999;85:640–650. doi: 10.1002/(sici)1097-0142(19990201)85:3<640::aid-cncr14>3.0.co;2-g. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Hamburger HL, Muller MJ, Boogerd W, van Dam FS. Neurophysiological evaluation of late effects of adjuvant high-dose chemotherapy on cognitive function. J Neurooncol. 2001;51:159–165. doi: 10.1023/a:1010635229762. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Rosenbrand RM, van Rhijn D, Rodenhuis S, van Dam FS. Late effects of adjuvant chemotherapy on cognitive function: a follow-up study in breast cancer patients. Ann Oncol. 2002a;13:1387–1397. doi: 10.1093/annonc/mdf241. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Van Dam FS. Cognitive dysfunction and chemotherapy: neuropsychological findings in perspective. Clin Breast Cancer. 2002b;3 Suppl 3:S100–S108. doi: 10.3816/cbc.2002.s.020. [DOI] [PubMed] [Google Scholar]
- Schagen SB, Muller MJ, Boogerd W, Mellenbergh GJ, van Dam FS. Change in cognitive function after chemotherapy: a prospective longitudinal study in breast cancer patients. J Natl Cancer Inst. 2006;98:1742–1745. doi: 10.1093/jnci/djj470. [DOI] [PubMed] [Google Scholar]
- Seigers R, Schagen SB, Beerling W, Boogerd W, van Tellingen O, van Dam FS, Koolhaas JM, Buwalda B. Long-lasting suppression of hippocampal cell proliferation and impaired cognitive performance by methotrexate in the rat. Behav Brain Res. 2008;186:168–175. doi: 10.1016/j.bbr.2007.08.004. [DOI] [PubMed] [Google Scholar]
- Shannon HE, Eberle EL. Effects of biasing the location of stimulus presentation, and the muscarinic cholinergic receptor antagonist scopolamine, on performance of a 5-choice serial reaction time attention task in rats. Behav Pharmacol. 2006;17:71–85. doi: 10.1097/01.fbp.0000189813.54178.e3. [DOI] [PubMed] [Google Scholar]
- Silberfarb PM, Philibert D, Levine PM. Psychosocial aspects of neoplastic disease: II. Affective and cognitive effects of chemotherapy in cancer patients. Am J Psychiatry. 1980;137:597–601. doi: 10.1176/ajp.137.5.597. [DOI] [PubMed] [Google Scholar]
- Silverman DH, Dy CJ, Castellon SA, Lai J, Pio BS, Abraham L, Waddell K, Petersen L, Phelps ME, Ganz PA. Altered frontocortical, cerebellar, and basal ganglia activity in adjuvanttreated breast cancer survivors 5–10 years after chemotherapy. Breast Cancer Res Treat. 2007;103:303–311. doi: 10.1007/s10549-006-9380-z. [DOI] [PubMed] [Google Scholar]
- Stafford D, LeSage MG, Glowa JR. Progressive-ratio schedules of drug delivery in the analysis of drug self-administration: a review. Psychopharmacology (Berl) 1998;139:169–184. doi: 10.1007/s002130050702. [DOI] [PubMed] [Google Scholar]
- Stemmer SM, Stears JC, Burton BS, Jones RB, Simon JH. White matter changes in patients with breast cancer treated with high-dose chemotherapy and autologous bone marrow support. AJNR Am J Neuroradiol. 1994;15:1267–1273. [PMC free article] [PubMed] [Google Scholar]
- Stock HS, Rosellini RA, Abrahamsen GC, McCaffrey RJ, Ruckdeschel JC. Methotrexate does not interfere with an appetitive Pavlovian conditioning task in Sprague�Dawley rats. Physiol Behav. 1995;58:969–973. doi: 10.1016/0031-9384(95)00147-b. [DOI] [PubMed] [Google Scholar]
- Tchen N, Juffs HG, Downie FP, Yi QL, Hu H, Chemerynsky I, Clemons M, Crump M, Goss PE, Warr D, Tweedale ME, Tannock IF. Cognitive function, fatigue, and menopausal symptoms in women receiving adjuvant chemotherapy for breast cancer. J Clin Oncol. 2003;21:4175–4183. doi: 10.1200/JCO.2003.01.119. [DOI] [PubMed] [Google Scholar]
- van Dam FS, Schagen SB, Muller MJ, Boogerd W, vd Wall E, Droogleever Fortuyn ME, Rodenhuis S. Impairment of cognitive function in women receiving adjuvant treatment for high-risk breast cancer: high-dose versus standard-dose chemotherapy. J Natl Cancer Inst. 1998;90:210–218. doi: 10.1093/jnci/90.3.210. [DOI] [PubMed] [Google Scholar]
- Vanover KE, Barrett JE. An automated learning and memory model in mice: pharmacological and behavioral evaluation of an autoshaped response. Behav Pharmacol. 1998;9:273–283. [PubMed] [Google Scholar]
- Ward SJ, Lefever TW, Jackson C, Tallarida RJ, Walker EA. Effects of a CB1 receptor antagonist and 5-HT2C receptor agonist alone and in combination on motivation for palatable food: a dose-addition analysis study in mice. J Pharmacol Exp Ther. 2008;325:567–576. doi: 10.1124/jpet.107.131771. [DOI] [PubMed] [Google Scholar]
- Wieneke MH, Dienst ER. Neuropsychological assessment of cognitive functioning following chemotherapy for breast cancer. Psychooncology. 1995;4:61–66. [Google Scholar]
- Winocur G, Vardy J, Binns MA, Kerr L, Tannock I. The effects of the anti-cancer drugs, methotrexate and 5-fluorouracil, on cognitive function in mice. Pharmacol Biochem Behav. 2006;85:66–75. doi: 10.1016/j.pbb.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Yanovski JA, Packer RJ, Levine JD, Davidson TL, Micalizzi M, D’Angio G. An animal model to detect the neuropsychological toxicity of anticancer agents. Med Pediatr Oncol. 1989;17:216–221. doi: 10.1002/mpo.2950170309. [DOI] [PubMed] [Google Scholar]