Abstract
Increasing evidence indicates that the noradrenergic system plays a key role in biasing the nervous system towards producing behaviors that help animals adapt to constantly changing environments. Most of the studies investigating noradrenergic function are performed in animals that have a limited repertoire of tractable natural behaviors. Songbirds, in contrast, with their rich set of precisely quantifiable vocal behaviors, provide a unique model system to study the noradrenergic system. An additional advantage of this system is the existence of a well-defined neural circuit, known as the song system, that is necessary for the production, learning and perception of song and can be studied at many different levels. These include the ability to investigate the effect of norepinephrine on synaptic function using brain slices, identifying its influence on singing-related gene expression and monitoring its impact on the activity of single neurons recorded in awake behaving birds. In this review article, we describe the similarities and differences, both anatomical and functional, between the avian and mammalian noradrenergic system and its role in sensory processing, learning, attention and synaptic modulation. We also describe how the noradrenergic system influences motor production, an under-explored aspect of norepinephrine function in mammalian studies. We argue that the richness of behaviors observed in songbirds provides a unique opportunity to study the noradrenergic system in a highly integrative manner that will ultimately provide important insights in the role of this system in normal behavior and disease.
Keywords: Locus coeruleus, Norepinephrine, Zebra finch, Song system, Attention, Perception, Learning
Introduction
As animals navigate through their environment, they face a constant barrage of sensory stimuli. They need to learn to extract relevant information in order to perform appropriate responses and remember these actions for future encounters. The noradrenergic system plays a pivotal role in shaping, regulating and maintaining several of the behaviors and neural processes involved in these adaptive responses (Kety, 1970; Berridge and Waterhouse, 2003; Aston-Jones and Cohen, 2005; Berridge, 2008). Based on the classification of the catecholaminergic system in rats, the noradrenergic system consists of several distinct cell groups in the brainstem. These include cell groups A4 through A7 with the most prominent being the locus coeruleus (the A6 group) (Dahlstrom and Fuxe, 1964).
The overall development, projection pattern and receptor distribution of the noradrenergic system appears to be conserved across vertebrates (Smeets and Gonzalez, 2000). The locus coeruleus has widespread projections throughout the vertebrate brain and a single neuron can often project to different areas and even hemispheres (Gibbs and Summers, 2002). As a result of this anatomical characteristic, the noradrenergic system is well placed to exert a coordinated activation of multiple brain areas. This led Seymour Kety to propose the idea that this system is responsible for the arousal of an animal, which is a behavioral state that facilitates the onset and execution of adaptive responses to the environment. Kety’s ideas focused particularly on the role of the locus coeruleus-noradrenergic system in causing the formation of long-term memories (Kety, 1970). Indeed, there is a large body of literature implicating the noradrenergic system in mediating learning and memory (Coull, 1994; Sirvio and Macdonald, 1999; Gibbs and Summers, 2002).
Since then, several general principles have emerged to suggest that norepinephrine also plays an important role in other processes such as the regulation of sensory responsiveness across behavioral states. Neurons in the locus coeruleus, for example, show elevated tonic firing rates during vigilant, aroused or attentive states and decreased rates during low states of vigilance. The presentation of a novel stimulus also increases firing in these neurons, and they rapidly habituate to repeated presentations of the same stimulus. Activity levels in the locus coeruleus are reset following presentation of a new and relevant stimulus (Jouvet, 1969; Berridge and Waterhouse, 2003; Bouret and Sara, 2005). These changes in locus coeruleus activity can have a strong impact on sensory responsiveness. Elevated levels of norepinephrine, for example, can increase the signal-to-noise ratio of sensory responses by increasing evoked sensory responses while decreasing background spontaneous activity. In many cases, norepinephrine can also increase sensitivity by allowing subthreshold stimuli to elicit responses (Hasselmo et al, 1997; Waterhouse et al, 1988).
Besides regulating sensory responsiveness when an animal is awake, recent evidence suggests that the noradrenergic system also plays an important role in mediating behavioral state transitions as well. For instance, the locus coeruleus is most active when an animal is awake and is at its most quiescent during rapid eye movement (REM) sleep. The expression of a wide range of genes is regulated by the activity of the locus coeruleus and this is related to the regulation of sleep, depression and other behavioral state dependent disorders (Cirelli et al, 1996; Cirelli and Tononi, 2000; 2004; Payne et al, 2002). There remain many aspects of noradrenergic function that are poorly understood. These include its role in shaping the acquisition of behavioral responses, its exact influence on sensory processing particularly within the context of naturally occurring behaviors and its potential impact on motor processes despite evidence that several disorders linked to the noradrenergic system have strong motor components (Berridge and Waterhouse, 2003). Also unknown is how the noradrenergic system interacts with other neurotransmitters, neuromodulators and hormones.
Songbirds offer a rich repository of natural behaviors whose neural control is becoming increasingly well understood (Catchpole and Slater, 1995; Zeigler and Marler, 2008). Given that the well-defined neural structures responsible for many of these behaviors, known collectively as the song system, are strongly innervated by the noradrenergic system, songbirds provide a potentially powerful model system for investigating the noradrenergic system. R1.2: Most of our current understanding of noradrenergic action comes from experiments with animals that are trained to perform in a task that bears a sparing resemblance to natural situations (for examples see Berridge and Waterhouse, 2003; Aston-Jones and Cohen, 2005). In songbirds, we can study the role of norepinephrine within the context of a well-defined suite of natural behaviors involved in song production which many other model systems of noradrenergic function lack.
Our knowledge of noradrenergic action in oscines (songbirds) is restricted to studies done on temperate zone R2.3:or captive bred species like zebra finches, starlings, canaries and sparrows, with zebra finches being the most widely studied songbird species in this regard. In zebra finches, singing is highly sexually dimorphic with only the males engaging in this behavior. In this article, we provide a comprehensive review of the major studies investigating how noradrenergic function influences brain function and behavior in both male and female songbirds. We also draw parallels between songbirds and mammals and discuss how songbird behaviors can be exploited to compose a richer picture of noradrenergic action. To begin with, we present a brief description of the anatomy of the noradrenergic system in songbirds.
1. Anatomy of the noradrenergic system in songbirds
a. The location and neurochemistry of the locus coeruleus in songbirds
There is considerable neuroanatomical evidence regarding the role of norepinephrine in the song system. In songbirds, studies of noradrenergic anatomy focus on projections originating from the locus coeruleus and the sub coeruleus in the brainstem (also called the A6 complex in vertebrates; Smeets and Gonzalez, 2000; Dahlstrom and Fuxe, 1964). According to the most recent convention in songbird nomenclature, this is the group of about 700 cells that lies at the lateral edge of the fourth ventricle on either side of the fiber tract called the fasciculus longitudinalis medialis. The locus coeruleus extends ventrolaterally into the sub coeruleus ventralis that contains about 600 cells. The locus coeruleus comprises mainly noradrenergic cells, but it overlaps with both serotonergic (more dorsally) and cholinergic (more ventrally) cell groups (Reiner et al, 2004b; Waterman and Harding, 2008; Puelles et al, 2007). In zebra finches the locus coeruleus has fewer cells than in non-oscines like quail (about 1300) or mammals like rats (about 3000), monkeys (about 7000) and humans (about 15000) (Barclay and Harding, 1988; Waterman and Harding, 2008; Berridge and Waterhouse, 2003).
The neurochemical signature of locus coeruleus and subcoeruleus cells in songbirds is currently unknown. In rats and primates, locus coeruleus neurons are predominantly noradrenergic while cats have more non-noradrenergic cell types (Grzanna and Molliver, 1980; Swanson, 1976; Leger and Hernandez-Nicaise, 1980). In songbirds, the locus coeruleus and sub coeruleus appear to have a mixture of noradrenergic and non-noradrenergic cells. This is based on the finding that there is incomplete overlap between cells labeled with tyrosine hydroxylase (TH; the rate limiting enzyme for catecholamine synthesis) and those labeled with tracers from different song control nuclei. The remaining cells still lie within the boundaries defined for the locus coeruleus and sub coeruleus but the proportion and the neurochemical signature of these non-noradrenergic cells is not known (Appeltants et al, 2001, 2002a; Castelino et al, 2007a).
R2.4: The synthesis of norepinephrine from dopamine is catalyzed by the enzyme dopamine beta hydroxylase (DBH) which is present in all noradrenergic neurons including those of the locus coeruleus. Additionally, in mammals locus coeruleus neurons are immunoreactive for several peptides like galanin, vasopressin, somatostatin, neuropeptide Y, enkephalin, neurotensin, and corticotrophin releasing hormone. Locus coeruleus activation can significantly change peptide levels in the brain and infusing these peptides into the locus coeruleus causes changes in locus coeruleus action. These interactions are involved in a wide array of behaviors related to stress, reproduction and aggression (reviewed in Berridge and Waterhouse, 2003; R2.12/13: Olpe and Steinmann, 1991). So far, we know that the locus coeruleus in songbirds has several vasotocin immunoreactive cells (Panzica et al, 1999; 2001) and in quail, the locus coeruleus has a strong galanin label (Azumaya and Tsutsui, 1996). We do not know if the peptide positive cells in the locus coeruleus are noradrenergic and we do not have information on the other peptides that may be present in this nucleus. It is intriguing however that many song control nuclei innervated by the locus coeruleus show immunoreactivity for all the peptides listed above ( Bottjer et al, 1997; Bottjer and Alexander, 1995; Ball et al, 1995a; 1995b; Ryan et al, 1981; Reiner et al, 2004b; Fiore et al, 1999 and reviewed in Blahser, 1984). It will be of interest to investigate the proportion of peptidergic fibers in the song control circuit that are also noradrenergic in nature or near noradrenergic neurons especially given the profound seasonal and sex differences that are observed in the distribution of both noradrenergic and peptidergic fibers (Bottjer et al, 1997; Ball et al, 1995a; 1995b; Blahser, 1984).
b. The locus coeruleus projects to several song nuclei
The song system consists of a series of interconnected groups of cells or nuclei that control the acquisition, production and perception of song. These nuclei are commonly segregated based on their main functions into a descending motor pathway, which is involved in song production and a specialized basal ganglia containing pathway, known as the anterior forebrain pathway, which is involved in sensorimotor learning. The motor pathway comprises projections from the nucleus HVC (acronym used as the proper name; Fortune and Margoliash, 1995; Reiner et al, 2004a) to RA (robust nucleus of the arcopallium). RA in turn projects to nuclei in the brainstem that either control respiration (Ram; nucleus retroambigualus and PAm; nucleus paraambigualus) or the muscles of the vocal organ, known as the syrinx in birds. The anterior forebrain pathway is made up of projections from HVC to area X of the medial striatum which projects to the midbrain thalamic nucleus DLM (dorsolateral division of the medial thalamus). This thalamic nucleus in turn projects back to the forebrain nucleus LMAN (lateral nucleus magnocellularis of the anterior neostriatum) which sends a projection to RA in the motor pathway (Ziegler and Marler, 2008; also chapter in this issue).
The locus coeruleus sends diffuse projections to several parts of the avian song control circuit (Figure 2A). We have evidence for noradrenergic projections to HVC (Appeltants et al, 2001), RA (Appeltants et al, 2002a) and area X (Castelino et al, 2007a) that are predominantly ipsilateral and non-reciprocal in nature. HVC, RA and area X receive both non-noradrenergic and noradrenergic projections from the locus coeruleus and the subcoeruleus (Appeltants et al, 2001, 2002a; Castelino et al, 2007a). There is considerable functional (e.g. based on action of norepinephrine in vitro and in vivo, Sizemore and Perkel, 2008; Cardin and Schmidt 2004b) and anatomical evidence (based on measurable levels of norepinephrine, Barclay et al, 1992; 1996 and receptor distribution, Casto et al, 1992; Bernard and Ball, 1995; Ball, 1994; Riters et al, 2002) for noradrenergic projections to other nuclei in the song system, including LMAN, NIf (nucleus interfacialis) and Uva (nucleus uvaeformis). These projections, however, have not yet been confirmed using tract tracing techniques.
Figure 2. Summary of known noradrenergic presence in the song system.
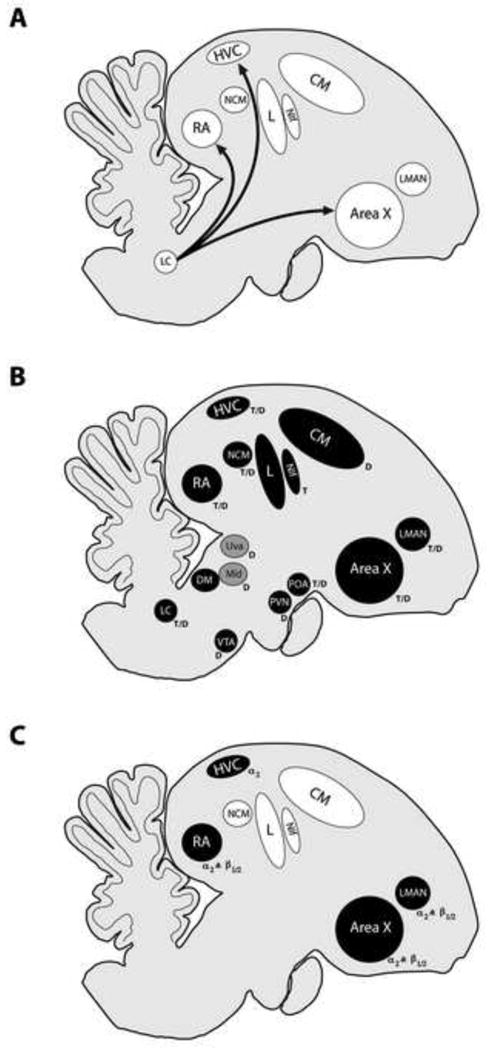
(A) Locus coeruleus projections to the song system. Although many more targets are likely, projections have only been characterized for a few nuclei in the songbird brain. From these studies, at least three nuclei of the song system receive projections from the locus coeruleus. These include HVC and RA in the forebrain and to area X of the medial striatum. (B) Norepinephrine levels in the song system. Many nuclei that form part of the song system or are associated with vocal behavior contain high levels of norepinephrine (nuclei shaded in black). These include HVC, RA, LMAN, area X, NIf and DM in the song system; areas NCM, Field L (L) and CM in the auditory forebrain; the auditory thalamic nucleus, Mld, two midbrain areas associated with motivation, POA and PVN, the norepinephrine synthesizing nucleus LC and the dopaminergic nuclei, VTA and SN. In addition to containing norepinephrine, all of these nuclei also stain for either TH (T) or DBH (D). The symbols T (TH) or D (DBH) are indicated in the figure beside the nuclei where staining has been shown. (C) Distribution of noradrenergic receptors in song nuclei. Although many structures have not been tested for the presence of the different subtypes of noradrenergic receptors, at least four major song system nuclei show strong staining for the α2 receptor subtype as well as both the β1 and β2 (β1/2) receptor subtypes. Abbreviations: CM (caudomedial mesopallium); DM (dorsomedial nucleus of the intercollicular complex); HVC (acronym used as proper name); LC (locus coeruleus); L (Field L complex); LMAN (lateral magnocellular nucleus of the anterior nidopallium); Mld (mesencephalicus lateralis, dorsalis); NCM (caudomedial nidopallium ); NIf (nucleus interfacialis); POA (preoptic area); PVN (paraventricular nucleus of the hypothalamus); RA (robust nucleus of the arcopallium); SN (substantia nigra); Uva (nucleus uvaeformis); VTA (ventral tegmental area).
The pattern of noradrenergic projections in the songbird brain reveals a difference between avian and mammalian noradrenergic systems. In mammals, the striatum and globus pallidus regions of the basal ganglia receive little to no noradrenergic input as compared to the rest of the brain. One exception to this, is the shell region of the nucleus accumbens which receives some fibers (albeit predominantly non-noradrenergic) from the locus coeruleus (Smeets and Gonzalez, 2002; Berridge and Waterhouse, 2003). This contrasts with birds, where the medial striatum receives projections from both the locus coeruleus and sub coeruleus (e.g. Chicken (Gallus gallus) Szekely et al, 1994; Japanese quail (Coturnix japonica) Bons and Oliver, 1986; pigeon (Columba livia) Kitt and Brauth, 1986; and zebra finch (Taeniopygia guttata) Castelino et al, 2007a). Future analyses describing the nature of these projections, such as whether they are noradrenergic and whether their terminals terminate differentially across subdivisions of the avian striatum (in particular the song nucleus area X), will reveal the degree to which avian and mammalian systems diverge. In the songbird, we still have little information regarding the exact projection patterns from the locus coeruleus to song nuclei. So far, we only know that many more locus coeruleus cells project to RA than to the surrounding arcopallium (Appeltants et al, 2002a). A systematic analysis of noradrenergic innervation to the different song nuclei may uncover specializations similar to those observed in the dopaminergic system where it appears that neurons in dopaminergic areas like the ventral tegmental area (VTA) and the substantia nigra pars compacta (SNc) can be divided into discrete populations depending on whether they project to area X or the surrounding striatum (Person et al, 2008).
c. Song nuclei contain high levels of norepinephrine
Consistent with the pattern of noradrenergic projections to song control nuclei, several studies have confirmed high levels of norepinephrine in these areas (Figure 2B). One of the key differences between the noradrenergic system in mammals and songbirds is that the latter contains up to 10 times the norepinephrine level observed in homologous brain areas in mammals, resulting in a significantly higher ratio of norepinephrine to dopamine in birds (Barclay and Harding, 1988; Waterman and Harding, 2008). Norepinephrine levels have been measured in key brain areas associated with song. In zebra finches, these include the song nuclei area X, LMAN, HVC, RA, NIf and DM (dorsomedial nucleus of the intercollicular complex) as well as the auditory forebrain areas Field L, NCM (caudomedial nidopallium) and CMM (caudomedial mesopallium) (Barclay et al, 1992, 1996; Sockman and Salvante, 2008). Additionally, the POA (preoptic area) and PVN (paraventricular nucleus), hypothalamic areas involved in song motivation (Riters and Ball, 1999; Riters et al, 2000; Heimovics and Riters, 2005; Alger and Riters, 2006; Alger et al, 2009), also contain high levels of norepinephrine (Barclay et al, 1992; Barclay et al, 1996). In birds the medial preoptic area (POM) sends a projection to the locus coeruleus (Riters and Alger, 2004) and POM lesions cause a decrease in the frequency of sexually related behaviors such as singing to a female (Riters and Ball, 1999, 2000). Norepinephrine levels in song nuclei are sensitive to several factors like changes in social cues in the environment, amount of song production and levels of circulating hormones. We discuss these interactions in later sections of this review.
d. Noradrenergic enzymes and receptors delineate nuclei in the song system
The distribution patterns of two enzymes are of particular interest with respect to the noradrenergic system (Figure 2B). One is TH, which is the rate limiting enzyme for catecholamine synthesis and is therefore present in all catecholaminergic neurons R2.5: and within the cell bodies of neurons in the VTA and SN (Bottjer, 1993; Appeltants et al, 2001). The other is DBH, which is the enzyme required for the conversion of norepinephrine to dopamine and therefore is a marker for noradrenergic neurons. The locus coeruleus contains several DBH labeled cells as well as TH fibers (Mello et al, 1998; Bottjer, 1993; Appeltants et al, 2001; Waterman and Harding, 2008). Immunohistochemistry for DBH positive fibers delineates several song nuclei like HVC, RA, LMAN and Uva (Mello et al, 1998; Waterman and Harding, 2008). Area X has very sparse albeit quantifiable DBH labeling that is sensitive to processing and fixation differences (Mello et al, 1998; Castelino et al, 2007a; Waterman and Harding, 2008). In addition to nuclei of the song system, areas that are important for song production or perception but that lie outside the song control circuit also contain high levels of DBH-immunoreactivity (DBH-ir). These include the auditory areas NCM and Mld (mesencephalicus lateralis, pars dorsalis) (Mello et al, 1998; Waterman and Harding, 2008), VTA and SN as well as the POA and the PVN, two structures implicated in motivated behaviors.
The labeling for TH is denser and more widespread than DBH-ir and its distribution pattern is known in several songbird species. In male canaries and zebra finches there TH-ir fibers are present in several song nuclei namely HVC, RA, LMAN, NIf and area X. Similar to DBH staining, there is also dense staining in the POA and NCM. Comparing TH staining levels in song nuclei between male and female birds of the same species reveal that staining is much stronger in males (Bottjer, 1993; Appeltants et al, 2001) suggesting that behavioral differences between the sexes extends to the distribution of key catecholaminergic enzymes. These differences further highlight the potential importance of catecholaminergic modulation of song production and learning.
The influence of noradrenergic projections to song nuclei are likely maximized by the density of noradrenergic receptors, which are much higher in locus coeruleus targets than in the surrounding tissue (Figure 2C). R2.14: The two main noradrenergic receptor subtypes are α and β and these are divided into α1, α2 and β1, β2, β3 subtypes. Activation of these receptor subtypes has different consequences on the firing rate and excitability of neurons and this allows for differences in the impact of noradrenergic release at target sites. (Feldman et al, 1997; Berridge and Waterhouse, 2003). We do not know whether the functional consequences of activating the different noradrenergic receptor subtypes in songbirds are the same as those in mammals. Like other anatomical markers, noradrenergic receptor distribution in the song system is also specialized. α-2 adrenergic receptors and β–adrenergic receptor distributions delineate the boundaries of several song nuclei in both zebra finches and starlings (Casto et al, 1992; Bernard and Ball, 1995; Ball, 1994; Riters et al, 2002). There is some evidence that suggests that most of the α-2 adrenergic receptors are located presynaptically (Solis and Perkel, 2006; Sizemore and Perkel, 2008). Noradrenergic receptors show profound seasonal and sex differences in their distribution, suggesting a close association between norepinephrine and sex steroid hormones (we discuss this in greater detail in section 6a). Song nuclei in male birds have much higher levels of α–2 adrenergic receptors than females. These differences are restricted to song nuclei and are not seen in non song nuclei such as the medial preoptic area (POM; Riters and Ball, 2002).α-2 adrenergic receptor distribution in song nuclei also shows seasonal fluctuations that are related to circulating hormone levels (Riters et al, 2002). We do not know the extent of α–1 receptor distribution in the song circuit.
Take together, increasing evidence suggests that noradrenergic receptors and enzymes show distinct patterns of expression that are specifically associated with nuclei in the song system as well as nuclei outside this system that are closely involved in song behavior (such as the auditory forebrain and hypothalamic motivational areas). In most cases, these patterns are strongly influenced by factors like age, sex, season and even social interactions. The dynamic nature underlying the noradrenergic projection patterns and receptor-expression levels suggest that this system is strongly implicated in the control of song behavior. We explore this link in more detail in the following sections.
2. A role for norepinephrine in song learning and song memory
Song learning can be divided into two general phases: an initial “sensory” phase when birds have to memorize a song template, followed by a “sensorimotor” phase when birds must match their vocal output to the memorized song template. In zebra finches, acquisition of the song template typically occurs between 20 days post hatch (dph) and 60 dph while sensorimotor learning occurs from approximately 35 dph to 90 dph. Song typically reaches a final “crystallized state around 90 dph (reviewed in Brainard and Doupe, 2002). In this section we discuss preliminary evidence that points to a role for norepinephrine in modulating changes in the song system that relate to learning. We also describe how norepinephrine might regulate the storage of the tutor song memory in the auditory forebrain.
a. Development of the noradrenergic system closely parallels song learning
The first suggestion that norepinephrine might be involved in song learning refers to the development of the noradrenergic system and its relation to the trajectory of song development in juvenile birds. In zebra finches, norepinephrine levels and turnover in the vocal control nuclei NIf, RA, LMAN and area X and in the auditory nucleus Mld peak at about 25 to 30 days after hatching and then decline steadily until approximately 60 dph. This developmentally regulated peak in norepinephrine appears to be specific for these song nuclei and not a general feature of the noradrenergic system because these changes are not observed in areas such as HVC, POA or Field L (Sakaguchi and Saito, 1989; Harding et al, 1998). TH-ir and catecholamine histofluorescence, which are markers for both the noradrenergic and dopaminergic systems, corroborate the increases in norepinephrine described above. The developmental time course and nuclei showing these changes are different, possibly because this methodology reflects increases in both dopamine as well as norepinephrine levels. R1.5: Specifically, increases in immunoreactivity occur at 35 dph and peak around 60 dph and are seen in more song nuclei such as HVC (Soha et al, 1996).
What then, is the functional significance of this norepinephrine surge during development? The period between 30–50 dph is when young birds are at the height of the vocal imitation process, which includes memorizing song and using this song memory to guide the sensorimotor process that underlies vocal learning. Key to this learning is the anterior forebrain pathway and its interaction with the descending motor pathway. A recent study performed on brain slices from 30–50 day old birds that contain HVC, RA and LMAN suggests that norepinephrine plays a key role in the interactions between these different nuclei. Specifically, by inhibiting synaptic inputs onto RA from LMAN without affecting those from HVC, norepinephrine is well situated to shift the balance toward RA toward being driven primarily by HVC. Norepinephrine also has developmentally specific effects on other synapses in RA, notably the suppression of inputs from inhibitory interneurons as well as excitatory inputs from axon collaterals of other projection neurons in RA (Figure 3; Sizemore and Perkel, 2008). The exact role norepinephrine plays in juvenile birds is unclear but the robustness of this effect coupled with what we know of its developmental regulation suggest that norepinephrine plays an important part in modifying RA circuitry in ways that are critical for vocal learning.
Figure 3. Effect of norepinephrine on synaptic inputs to RA.
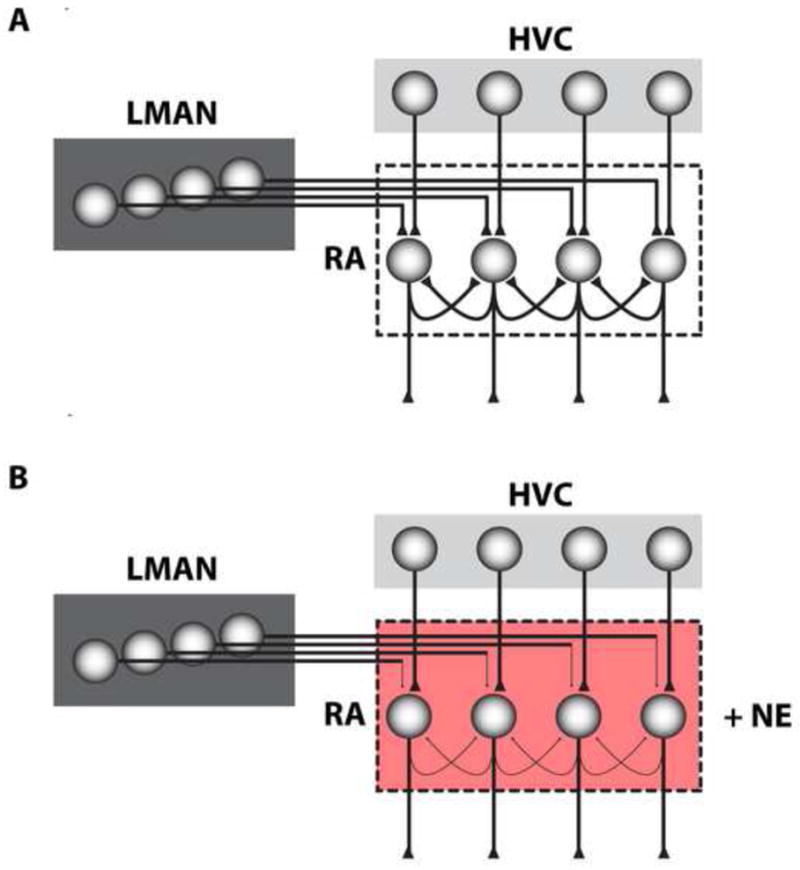
(A) RA receives glutamatergic inputs from both HVC and LMAN, with HVC inputs depending primarily on AMPA-receptors and those from LMAN on NMDA-receptors. In singing birds, HVC inputs represent the direct motor drive while LMAN inputs represent a more indirect motor input and are hypothesized to contribute to motor variability. Projection neurons in RA that receive inputs from HVC and LMAN send out a significant number of collateral glutamatergic projections to other projection neurons. RA also contains a large population of interneurons but these are not shown for simplicity. (B) In both adult and juvenile birds, addition of norepinephrine (shown in red) to RA causes a significant reduction in the synaptic strength of LMAN inputs while having no effect on inputs from HVC. In juveniles, norepinephrine also has the additional effect of suppressing collateral synaptic connectivity between RA projection neurons. This effect is not observed in adult birds. (Based on data from Sizemore and Perkel, 2008).
R1.4: In juvenile zebra finches norepinephrine plays an important role in song learning and its effect is most influential during the critical phase between 30 to 50 days post hatch. In the white-crowned sparrow, sensory and sensorimotor phases do not overlap and song learning takes place over a longer period of time. In canaries these phases most likely overlap however in this species even adult birds can learn new songs and song learning never stops (Brainard and Doupe, 2002). If a norepinephrine surge is important during a certain phase of song learning, then in other species this surge would occur at different times than in zebra finches. The study of noradrenergic action in songbirds with different learning trajectories will reveal the functional significance of peaks and decreases in norepinephrine during song learning and perhaps also help formulate general principles of norepinephrine action during learned behaviors.
b. How norepinephrine might regulate the formation of tutor song memory
While the neural substrate for sensorimotor learning is located within nuclei in the song system, the memory for song is most likely stored in NCM and CMM (Bolhuis and Gahr, 2006), areas homologous to the mammalian auditory cortex that are involved in the perception, representation and the memory of song (Ribeiro and Mello, 2000; Mello et al, 2004; Gobes and Bolhuis, 2007). NCM and CMM show increased responsiveness (measured either electrophysiologically or by the expression of several immediate-early genes (IEGs) including ZENK, Fos and Arc) to songs that birds have been exposed to previously (Mello et al, 1992; Mello and Clayton, 1994; Velho et al, 2005). Repeated song playback results in the habituation of auditory responses in NCM that rebound upon presentation of a new song R1.7: or a familiar song in a novel context (Mello et al, 1995; Kruse et al, 2000; 2004; Dong and Clayton, 2008) suggesting that this structure plays a role in discriminating familiar songs from novel ones. The discrimination between familiar and novel songs in NCM appears to extend to the tutor song. NCM responses habituate more rapidly to the tutor song than to novel songs and the rate of habituation is directly proportional to how well the bird copies the song template (Phan et al. 2006). Additional evidence comes from experiments showing that IEG expression levels (a molecular proxy for neural activation) in NCM are directly proportional to the strength of song learning (Bolhuis et al. 2000). R2.6:The most convincing evidence to date for a role for NCM in song learning comes from a study where the signaling cascade involved in plastic changes in NCM was blocked using an inhibitor of mitogen activated protein kinase (MEK) called U1026 which prevents the activation of the extracellular signal-regulated kinase (ERK) which is required for memory formation. This pharmacological manipulation was used in conjunction with a paradigm where tutor song presentation could be dissociated in time from vocal rehearsal. Blockade in NCM selectively during presentation of the tutor song prevented birds from storing the song template and caused them to develop highly abnormal songs that resembled “isolate” song (London and Clayton 2008).
Direct evidence for a role of norepinephrine in regulating the formation of the tutor song memory in NCM in juvenile birds does not exist, but several findings implicate norepinephrine in such a role. The first line of evidence is the ability of norepinephrine to modulate both the genomic as well as the electrophysiological responses in NCM in adult zebra finches. Specifically, application of the α–adrenergic antagonist phentolamine directly into NCM disrupts conspecific song induced IEG expression in this structure (Velho et al, 2005). Furthermore, phentolamine injections into NCM prevent the well-described neuronal habituation to repeated presentations of familiar songs (Lu et al, 2008). These data implicate norepinephrine in modulating gene expression and electrophysiological responses related to song playbacks. The second line of evidence comes from the literature related to norepinephrine and memory formation in young chicks. In domestic chickens, imprinting is accompanied by a surge in norepinephrine levels in forebrain areas that is related to visual experience (Davies et al, 1983) and the subsequent activation of adrenergic receptors is required for the formation of both short term memories and long term consolidation (Gibbs and Summers, 2002; Gibbs, 2008). These findings showing a role for norepinephrine in both imprinting in chicks as well as neural responsiveness in NCM of adult songbirds suggest a possible strong link between norepinephrine and song memory formation in NCM.
3. Norepinephrine and song production
Song is elicited and influenced by cues in the birds’ environment. In zebra finches, social context plays an important role in determining the type of song males produce. Norepinephrine regulates many of the effects of social context on male zebra finch song production (Barclay et al, 1992; Barclay et al, 1996; Castelino and Ball, 2005). Below we describe the evidence for these noradrenergic effects both on behavior and on the underlying neural circuitry that drives these behaviors.
a. Social context-dependent changes in singing behavior in male zebra finches
In zebra finches, the presence or absence of a conspecific bird has a profound impact on singing behavior (Sossinka and Bohner, 1980). While adult zebra finches only produce a single song that is quite stereotyped, they will produce slight variants of these songs depending on their audience. In the presence of female birds (and in some cases male birds as well) they will sing directly at the bird and produce a high tempo song that is referred to as “directed” song. In contrast, songs produced either in the absence of conspecific birds or when they are facing away from neighboring birds are referred to as “undirected” song (Zann, 1996; Sossinka and Bohner, 1980).
In the field and in the lab, directed singing is accompanied by an elaborate suite of postural displays. During directed song, a male bird engages in high levels of beak wiping, fluffs out the feathers at his throat and cheeks, sleeks back the feathers on his head, moves his head from side to side and hops down the perch turning his body at 180 degree angles with each hop (see Barclay et al 1992, 1996 and Williams, 2001 for examples and details). This display is always conducted with the male facing the female (or another male) bird, who invariably hops and calls (or sings in the case of another male) intermittently. Undirected song, in contrast, is produced without any of the accompanying hops and generally involves greater levels of neck stretches than directed song (Sossinka and Bohner, 1980). In the field, undirected song is produced in order to attract a mate, during nest guarding and mate separation (Dunn and Zann 1996a, 1996b, 1997). Song characteristics differ consistently between directed and undirected song. Directed song bouts are longer and delivered with a faster tempo. They also contain many more introductory notes and contain very little syllable variability from rendition to rendition. Undirected songs, on the other hand, are much shorter, are produced with a slower tempo and contain fewer introductory notes. Song syllables also show a significant degree of acoustic and temporal variation across song renditions (Sossinka and Bohner, 1980; Zann, 1996; Kao et al, 2005; Kao and Brainard 2006; Kao et al, 2008). R1.8: Female zebra finches are able to discriminate between and show a preference for directed songs over undirected songs (Woolley and Doupe, 2008).
b. Norepinephrine regulation of social context-dependent changes in singing behavior
A powerful method for investigating the role of norepinephrine in vocal behavior is the use of the noradrenergic specific neurotoxin DSP-4 (N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine) which is widely used in birds and mammals. DSP-4 depletes noradrenergic neurons by entering the cell through the norepinephrine reuptake pathway and destroying the axons. Since there is crosstalk between monoaminergic reuptake systems, DSP-4 is administered along with a serotonin reuptake inhibitor (zimelidine hydrochloride) so that the serotonergic system is unaffected and the neurotoxic effects are specific to the noradrenergic system (Dudley et al, 1990; Fritschy and Grzanna, 1991; Barclay et al, 1992; 1996).
Systemic and central (intracerebroventricular; icv) depletion of norepinephrine with DSP-4 consistently increases the latency with which males commence female directed song behavior. There is also a decrease in the frequency of courtship displays but these treatments have no effect on the intensity of courtship displays. Neither systemic nor central administration of DSP-4 cause any obvious changes in song as judged by length of song bouts or motifs per bout. The only subtle effect is a decrease in the number of song bouts with icv administration of DSP-4 (Barclay et al, 1992; Barclay et al, 1996). These findings suggest that increases in the latencies for female-directed singing might be caused by deficiencies in attentional mechanisms following DSP-4 treatment.
Taking advantage of the changes in song observed in different social contexts, a more recent study (Castelino and Ball, 2006) observed significant effects of DSP-4 treatment on song. Specifically, DSP-4 treatment abolished differences in the number of introductory notes, speed of song delivery and motif length across social contexts. In essence, depleting norepinephrine made directed song more like undirected song (Castelino and Ball, 2006). Some of the differences between these two studies with respect to the effect of DSP-4 treatment on song is that the Castelino and Ball (2006) study, rather than giving a single DSP-4 injection, gave two injections that were 10 days apart, which has recently been shown to be much more effective at depleting norepinephrine (Waterman and Harding, 2008). Nevertheless, both of these studies suggest that in male birds, norepinephrine influences attentional processes as well as the regulation of song production across different social contexts.
c. Norepinephrine regulation of social context-dependent changes in immediate-early gene expression
The act of singing drives the expression of many IEGs in the song control circuit of several songbird species (Jarvis and Nottebohm 1997; Mello and Ribeiro, 1998; Mello 2002). The expression levels of the IEGs Fos (Kimpo and Doupe, 1997), ZENK (Jarvis et al, 1998; avian homologue of egr-1) and the speech related gene FoxP2 (Teramitsu and White, 2006) are differentially regulated based on social context. ZENK and Fos expression, for example, is significantly lower during directed song than undirected song in a number of song control nuclei (Jarvis et al, 1998; Kimpo and Doupe, 1997) and FoxP2 mRNA expression levels are higher during directed song than undirected song (Teramitsu and White, 2006) in area X of the medial striatum (the avian homologue of the basal ganglia; Carillo and Doupe, 2004; Reiner et al, 2004b).
Area X shows the highest difference in ZENK and Fos expression across social contexts and this difference is strongly affected by norepinephrine (Figure 4). Specifically, systemic administration of DSP-4 to groups of male birds singing directed and undirected song abolishes the difference in ZENK expression in this area, with DSP-4 depleted males showing higher levels of ZENK expression during directed song as compared to control males. These results suggest that under normal conditions, norepinephrine plays an important role in down-regulating ZENK expression in area X during directed singing (Castelino and Ball, 2005).
Figure 4. Noradrenergic regulation of social-context mediated ZENK expression in Area X.
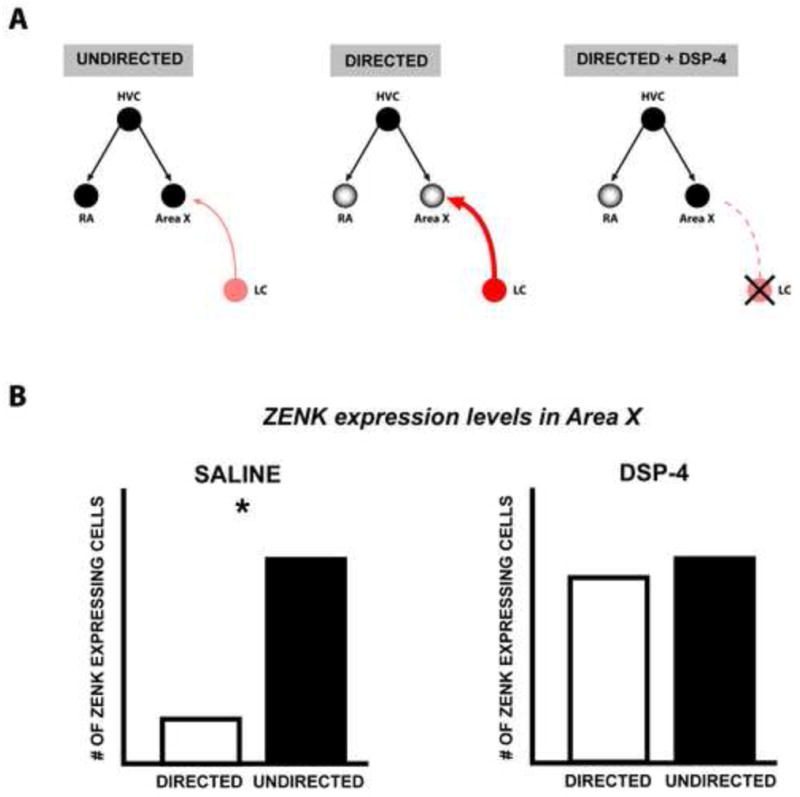
(A) During undirected song, the immediate-early gene, ZENK, shows increased expression in HVC, RA and Area X. In contrast, during directed singing, ZENK expression is significantly reduced in RA and Area X when compared to undirected song. Depleting norepinephrine using DSP-4 abolishes the context-dependent difference in ZENK expression in area X but not RA. This suggests that noradrenergic inputs from the locus coeruleus down-regulate ZENK expression in area X during directed singing. Input strength from LC is shown as low (pale red; thin arrow) during undirected song and strong (red; thick arrow) during directed song. (B) Graphs depicting ZENK expression levels in Area X across social contexts in saline treated control birds and in DSP-4 treated norepinephrine depleted birds. (Based on data from Castelino and Ball, 2005).
d. A possible role for norepinephrine in regulating neural activity related to singing in different social contexts
Social context has a considerable impact on singing-related neural activity in the anterior forebrain pathway, which is implicated in song learning and subsequent song maintenance (reviewed in Brainard and Doupe, 2002). Both Area X and LMAN are active during directed and undirected singing but activity during undirected song is more variable from rendition to rendition (Hessler and Doupe, 1999). In LMAN, neurons also transition from a single-spike mode to a burst firing mode during undirected song and bursts show significant trial-by-trial variability in their timing and structure (Kao et al, 2008). This increase in neural variability in the anterior forebrain pathway during undirected singing is correlated with the increase in song variability observed under those same conditions and implies that LMAN output onto RA plays a critical role in driving this motor variability. Consistent with this notion, lesions of LMAN remove many of acoustic and temporal differences between directed and undirected singing. Other aspects of song behavior such as the number of introductory elements, number of motifs, song stereotypy and courtship display are unaffected by LMAN lesions (Kao et al, 2005; Kao and Brainard, 2006).
Currently there is no direct evidence for norepinephrine in regulating neural activity across social contexts in any song nucleus. However, in vitro studies have shown that application of norepinephrine onto brain slices significantly reduces synaptic inputs from LMAN to RA without much effect on those from HVC (Figure 3; Sizemore and Perkel, 2008). Given the hypothesis that LMAN inputs onto RA drive some of the motor variability observed during undirected song, the selective influence of norepinephrine on LMAN inputs is likely to attenuate these inputs and turn undirected song into a song that resembles directed song. This prediction is consistent with the finding that DSP-4 treatment makes gene expression (Castelino and Ball, 2005) and singing behavior (Castelino and Ball, 2006) more undirected like since such treatment is expected to decrease norepinephrine levels and therefore increase the influence of LMAN inputs onto RA.
4. A role for norepinephrine in auditory gating and song perception in male birds
Neural activity patterns in thalamic and cortical brain areas vary significantly between wakefulness, sleep and anesthesia. These transitions between behavioral states often lead to changes in sensory processing and gating mechanisms at the level of the thalamus have been shown to modulate the flow of sensory information to the cortex (Weyand et al, 2001; Castro-Alamancos, 2001, 2002; Steriade et al, 1969) in ways that facilitate information transfer during wakefulness and attenuate it during sleep (Guecer, 1979; Livingstone and Hubel, 1981; however see Issa and Wang, 2008). Various neuromodulatory systems, including the noradrenergic system, play a major role in mediating the observed changes in thalamocortical transmission that occur between these different behavioral states (reviewed in McCormick, 1992).
The noradrenergic system, and the locus coeruleus in particular, shows a profound change in activation across behavioral states with activity increasing during wakefulness and decreasing during states of quiet resting or sleep. These changes in locus coeruleus activity are directly correlated with the release of norepinephrine in target areas (Devilbiss et al, 2006) and cause the increased expression of several plasticity-related genes (Tononi, et al, 1994, 2001; Cirelli et al, 1996; Cirelli and Tononi, 2000, 2004). In addition to its role in sleep-wake transition, the noradrenergic system is also implicated in attentional modulation during waking. In several mammalian species, including humans, attention-demanding tasks result in an increase in locus coeruleus firing (Foote et al, 1980; Aston-Jones and Bloom, 1981; Rasmussen et al, 1986; Aston-Jones et al, 1991, 1994; Coull et al, 1999; Minzenberg et al, 2008) and the resulting increase in norepinephrine levels is thought to strongly influences the response and representation of sensory stimuli in a number of different cortical areas (Devilbiss et al, 2006; reviewed in Berridge and Waterhouse, 2003). In this section we focus on the role of the noradrenergic system in regulating auditory processing in the song system. We begin with a brief description of behavioral state modulation of auditory responses.
a. Behavioral-state modulation of auditory responses in the song system
A hallmark characteristic of neurons in the song system is their robust response to, and preference for, the bird’s own song (BOS). HVC neurons respond strongly and specifically to BOS and not as much to manipulated versions of BOS (e.g. spectrally reversed versions of the BOS or the BOS with syllables placed in the reverse order) or playbacks of conspecific songs (Margoliash, 1986). Furthermore, BOS-specific responses in song control nuclei are strongly linked to the behavioral state of the bird. In sleeping or sedated birds recordings at the multiunit level reveal highly robust BOS responses in HVC (Schmidt and Konishi, 1998; Nick and Konishi, 2001; Dave et al, 1998; Cardin and Schmidt, 2003; Cardin and Schmidt, 2004a), whereas recordings from awake birds reveal a much more variable auditory response to BOS. Responsiveness in HVC of awake birds is generally much weaker than in the sedated or sleeping bird and in some cases is even completely absent. Interestingly, when responsive during wakefulness, neurons lose their selectivity to BOS and respond instead to a wide range of auditory stimuli that include conspecific songs, female calls and reverse BOS (Schmidt and Konishi, 1998; Dave et al, 1998; Cardin and Schmidt, 2003).
The strong and consistent BOS responses during sleep and the mostly weak and variable responses when awake are also observed in NIf, a sensorimotor area that forms part of the song system and is also one of the major auditory inputs to HVC. R1.9: Auditory areas outside the song circuit, such as the field L complex and other higher-order auditory areas, by contrast, do not show any behavioral-state dependent modulation of auditory responsiveness across the sleep-wake transition (Schmidt and Konishi, 1998; Cardin and Schmidt, 2003). Auditory responses in NIf and HVC show strong covariation of auditory response magnitude during all behavioral states. Furthermore, infusions of the GABAa receptor agonist muscimol into NIf abolish both spontaneous activity and BOS responses in HVC, while the GABAa receptor antagonist bicuculline enhances HVC responses. This evidence points to a role for NIf in shaping the activity and auditory responses in HVC (Cardin and Schmidt 2004a; but see below for CM and Uva influences on HVC activity).
b. Norepinephrine as a mediator of auditory gating in the song system
Norepinephrine is a key regulator of behavioral state dependent transitions in neural activity in mammals (Foote et al, 1980; Aston-Jones and Bloom, 1981; Coull et al, 1999; Devilbiss et al, 2006). The anatomical evidence supporting a role for norepinephrine in mediating behavioral state dependent changes in auditory responsiveness in the song system is very strong given the significant amounts of norepinephrine, TH, DBH and noradrenergic receptors that are present in both HVC and NIf (see anatomy section 3c & 3d for details and references).
To study the dependency of auditory responses on behavioral state in a controlled manner, Cardin and Schmidt (2003, 2004a,b) developed an experimental paradigm where they could briefly arouse birds from a sedated state using short air puffs applied to the bird. In this paradigm, arousing stimuli were delivered on randomly interleaved presentations of BOS to test the effect of arousal on auditory responsiveness (Figure 5). Presentation of a brief air puff a few seconds prior to the auditory stimulus reliably cause a near complete suppression of auditory responses in both HVC and NIf but not in field L (Cardin and Schmidt, 2003; 2004a). Such brief arousing stimuli are known to elicit robust responses in the locus coeruleus of mammals (Foote et al, 1980; Chen and Sara, 2007) and songbirds (Castelino et al, 2007b), making it possible to test the effect of norepinephrine on auditory suppression with this paradigm. Direct application of norepinephrine into NIf mimics the effect of the air puff by causing a complete suppression of the auditory response. Interestingly, similar to what has been observed in various sensory cortical areas in mammals (reviewed in Aston-Jones and Cohen, 2005), the effect of norepinephrine on auditory responsiveness shows an inverted-U shaped response. Low doses of norepinephrine increase auditory responses while higher doses suppress it. The increased responsiveness in the presence of low doses of norepinephrine is caused by both an increase in the evoked auditory response as well as a decrease in spontaneous activity. The addition of norepinephrine agonists targeted to α1 and α2 adrenergic receptors confirmed the specificity of this effect. Infusions of these drugs into NIf are able to mimic the dose responsiveness (α1) and the suppressive (α1 and α2) effects of norepinephrine in HVC. Furthermore, infusions of α1 and α2 adrenergic antagonists directly into NIf are able to prevent BOS suppression in HVC during air puff trials. This is a direct test of the role of norepinephrine in mediating the arousal suppression of BOS responses and suggests that norepinephrine plays an important role in the modulation of auditory responses in NIf and HVC.
Figure 5. Noradrenergic modulation of auditory responses in the song system.
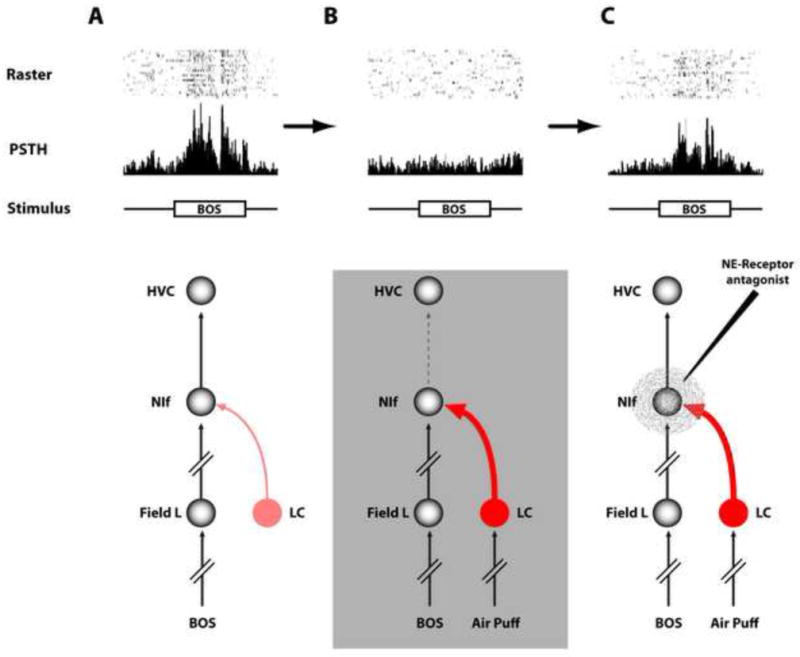
(A) Presentation to a sedated bird of the bird’s own song (BOS) through a speaker causes a robust auditory response in HVC. (B) Application of an air puff to the sedated bird briefly arouses it and causes a near complete suppression of auditory responses in NIf and HVC. Such arousing stimuli are known to activate the locus coeruleus. (C) Because of the discrete nature of the connectivity pattern in the song system and the restricted size of the different nuclei, it is possible to directly test the hypothesis that norepinephrine released onto NIf following the air puff causes the suppression of the auditory response in HVC. A cocktail of norepinephrine receptor antagonists targeted to NIf while auditory responses are recorded in HVC is able to block the action of noradrenergic inputs from the locus coeruleus and reverse the suppression of BOS responses causes by the air puff. (Based on data from Cardin and Schmidt, 2004b).
In addition to its role in gating auditory responses in NIf, norepinephrine is likely to play an important role in shaping auditory properties in the song system by its inputs to brain areas that project directly onto NIf and HVC. One prime candidate is the thalamic nucleus uvaeformis (Uva). Uva sends robust projections directly to NIf and HVC, and is likely innervated by the locus coeruleus given the dense staining of DBH-ir (Mello et al, 1998). Firing patterns in Uva have recently been shown to shape spontaneous activity as well as auditory responses in HVC. Specifically, when Uva is firing in a tonic single spike mode, it tends to suppress activity (both spontaneous and auditory-evoked) in HVC, while bursts in Uva tend to enhance spontaneous activity and auditory-evoked responses in HVC (Hahnloser et al, 2008). Because the transition from single-spike to burst firing mode in thalamic structures is typical of neuromodulator influences (McCormick, 1992), it seems likely that norepinephrine also plays an important role in modulating activity patterns in Uva and as a consequence in the rest of the song system.
Additional candidate areas that might shape auditory responses in a noradrenergic-dependent manner include other auditory forebrain areas that project directly to HVC. Recent experiments show that CM, a higher-order auditory forebrain area (that comprises both CMM and the lateral subdivision CLM; caudolateral mesopallium), sends direct projections to both NIf and HVC and is required for auditory responses in both of these structures (Bauer et al, 2008). CM shows strong DBH-ir (Mello et al, 1998) and contains high levels of norepinephrine in female starlings (Sockman and Salvante, 2008), making it a likely target of noradrenergic modulation. Given that CM is implicated in monitoring auditory feedback and detecting errors in song performance (Bauer et al, 2008), it is possible that noradrenergic inputs to CM might be involved in modulating auditory feedback.
c. A role for NE in song perception and attention
The influence of norepinephrine in the latency to song production (Barclay et al 1992, 1996) as well as in the state-dependence of auditory processing (Cardin and Schmidt 2003, 2004a) suggests that the noradrenergic system plays an important role in attentional processing in songbirds. Given that low levels of norepinephrine in NIf enhance auditory responses and that low levels of norepinephrine correlate with selective attention in several mammalian species, it appears reasonable that these increased responses might be linked to attentional processes. Additional support for an attentional role comes from song perception experiments in DSP-4 treated male canaries. In these experiments, DSP-4 treated birds perform more poorly and show increased response latencies in a two-alternative choice procedure where they must discriminate between masked auditory stimuli (Appeltants et al, 2002b). Studies showing that systemic noradrenergic changes are due to a lapse in the attentional abilities of the male birds still need to be performed to confirm a role for norepinephrine in shaping perception and auditory responses through attentional processes.
5. Norepinephrine regulates perceptual processes involved in female mate choice and mate choice plasticity
R1.10: Birdsong and the accompanying behaviors are an integral part of male courtship behavior, the goal of which is to attract a female mate. Female birds are often the primary targets of male song and many studies have assessed and shown behavioral preferences in females for aspects of male songs (Andersson, 1994). Female European starlings have a preference for long songs and higher song variation (Eens, 1991; Gentner and Hulse, 2000; Gentner et al, 2001; Sockman et al, 2002). Female zebra finches prefer abnormally long male songs over songs of normal length in a two choice test (Neubauer, 1999). Female canaries indicate their song preference to songs that contain “sexy syllables” (which is a syllable composed of two notes) by producing more copulation solicitation displays (CSDs), a behavioral response that indicates a female’s receptivity to mate with a partner. In many cases, this behavioral preference correlates with higher ZENK mRNA induction in NCM by songs containing sexy syllables as compared to ones that do not (Vallet et al, 1998; Leitner et al, 2005). The male song traits that females prefer could be a reflection of genetic qualities that are indicators of greater reproductive fitness (Duffy and Ball, 2002; Ball et al, 2006). Thus, female choice behaviors R2.8: may be a combination of female perceptual machinery that is ‘tuned’ to song traits that indicate ‘fit’ males and males exploiting the sensory predispositions of female conspecifics.
a. Norepinephrine mediates male song discrimination in female birds
Female birds choose between prospective mates based on songs in a noisy environment. This includes the songs of other singing male birds and environmental sounds. Recent work suggests that this task requires an intact noradrenergic system (Appeltants et al, 2002b). Systemic norepinephrine depletion (via DSP-4) in reproductively active female canaries affects their responses to playbacks of ‘sexy’ male canary songs that are masked with ‘non-sexy’ songs or white noise, with DSP-4 treated females performing significantly fewer and shorter CSDs. These birds also lose the ability to accurately discriminate and respond to ‘sexy’ songs when compared to intact females (Appeltants et al, 2002b). Activation of the neural substrates involved in this mate choice discrimination is also regulated by norepinephrine. In female canaries, playback of conspecific male songs causes a significantly higher level of ZENK protein expression in secondary auditory areas NCM and CMM than heterospecific songs (Mello et al, 1992). Treating females systemically with DSP-4 abolishes this difference by decreasing the amount of ZENK expression as a result of conspecific song (Lynch and Ball, 2008). Norepinephrine is also involved in activating other areas of the female brain in response to behaviorally relevant stimuli. Riters and Pawlisch (2007), for example, showed that intact female European starlings listening to conspecific songs had significantly higher levels of ZENK expression in area X (involved in song learning in males, and possibly song discrimination in females) and the ventromedial hypothalamus (an area involved in the control of female sexual behavior) than DSP-4 treated females. Therefore, norepinephrine is involved in modulating changes (via IEG induction) in the auditory cortex, motivational areas and the song circuit of females in the presence of behaviorally relevant stimuli (like “sexy” male song) and in the female bird’s ability to perceive this song (Appeltants et al, 2002b; Lynch and Ball, 2008).
The studies above tested a role for norepinephrine in mediating the discriminatory abilities of females choosing between different male songs or between songs in the presence of competing noisy stimuli. However, we also know that the perceptual machinery of female birds is tuned to the extremely subtle differences between a single male singing in different social contexts. Female zebra finches, for example, are able to reliably discriminate between directed and undirected song in a two-choice behavioral approach paradigm and they exhibit a strong preference for directed song over undirected song. Measurement of IEG expression in this paradigm reveals that ZENK induction in NCM is higher in response to unfamiliar directed songs and that ZENK induction in CMM is higher in response to directed song than it is to undirected song. Therefore, auditory processing of song novelty and social context appears to be compartmentalized in these different auditory forebrain areas (Woolley and Doupe, 2008). Given norepinephrine’s role in regulating both the neural and behavioral aspects of female song perception, it will be of interest to test whether it is also involved in female preference between directed and undirected song and in the differential modulation of ZENK expression patterns in these auditory areas.
b. Norepinephrine is involved in the perceptual processes underlying mate choice plasticity in female birds
Often the quality of available mates is governed by the prevailing environment and females need to demonstrate flexibility in their mate choice decisions in the face of such fluctuations. For this, they need to have an accurate depiction of the most prevalent male characteristics in the environment and change their decisions based on changes in the environment (Jennions and Petrie, 1997; Kokko and Rankin, 2006; Chaine and Lyon, 2008). The perceptual processes that are involved in directing this plasticity in female mate choice are presumably localized in higher-order auditory areas such as NCM and CMM. As a result, gene expression patterns in NCM and CMM reflect changes in song characteristics in the environment.
When female European starlings hear playbacks of long bout songs for a week, they show significantly higher ZENK expression levels in NCM and CMM in response to the presentation of long-bout songs when compared to short-bout songs. However, if females listen to short-bout songs for a week, there is no difference in ZENK expression in these same auditory areas between the presentation of long-bout songs and short-bout songs (Sockman et al, 2002). Interestingly, one week of long-bout song playback results in a significant increase in norepinephrine and dopamine metabolites and in the amount of DBH-ir in NCM when compared to similar playback but with short-bout songs (Sockman and Salvante, 2008). R2.15: These data therefore suggest that plasticity in female mate choice due to tracking the song environment is related to changes in catecholaminergic levels in the neural substrates related to the perception and memory of song (i.e. NCM and CMM).
The study of the neural substrates underlying mate-choice cues and norepinephrine influences on female mate choice and mate choice plasticity in songbirds are in their infancy (Sockman, 2007). So far, these studies reveal two key aspects about noradrenergic action that should be explored further. First, norepinephrine causes changes in nuclei (NCM, CMM, area X and VMN) that form part of separate neural circuits (auditory, song and motivational) and a key unanswered question is how these changes relate to each other? Second, future studies should investigate the development of song type preferences in female birds and examine whether this is also influenced by the noradrenergic system.
6. Interactions between norepinephrine and other systems
Norepinephrine action in the song circuit occurs in concert with many other factors and in this section we review some of the research relating to these interactions. The two main areas that we will focus on are the interactions between norepinephrine and hormones and between norepinephrine and dopamine.
a. Norepinephrine and sex steroid hormones
There is considerable evidence linking the noradrenergic system and sex steroid hormones to the control of singing behavior (Ball et al, 2003). Most of this evidence is related to anatomical coexistence of these two systems. For instance, TH-ir cells in the locus coeruleus of male canaries also contain mRNA for both androgen and estrogen receptors (Maney et al, 2001). Conversely, there are dense catecholaminergic inputs to aromatase (the synthesizing enzyme for estrogen) containing cells in the auditory forebrain in male canaries (Appeltants et al, 2004). The density of α2-adrenergic receptors is also related to levels of circulating hormones. In male European starlings the volumes of HVC, RA and area X are largest when testosterone levels are at their highest and the density of α2-adrenergic receptors in HVC and RA is inversely proportional to the level of testosterone. Interestingly, this effect is specific for HVC and RA because receptor density does not change with circulating hormone levels in area X and LMAN (Riters et al, 2002). Supportive evidence for a link between both systems comes from experiments where sex steroid hormone treatment restores norepinephrine and norepinephrine turnover levels in the song system back to normal baseline levels in castrated males (Barclay and Harding, 1988; Barclay and Harding, 1990).
Further support for a functional relationship between sex steroids and the noradrenergic system comes from experimental manipulations in female birds. Female canaries do not normally sing but if they are treated with exogenous testosterone, they will start to sing and this modified behavioral phenotype is accompanied by an enlargement of HVC and RA. Associated with this androgen-mediated increase in volume of song system nuclei is an increase in the area occupied by TH-ir fibers in nuclei of the song system, the auditory forebrain, the ventral tegmental area and the locus coeruleus (Appeltants et al, 2003; Leblanc et al, 2007). In female zebra finches, there is evidence that sex steroid hormones act via the noradrenergic system to cause changes in female responses to male songs. Ovariectomized female birds treated with estrogen, for example, respond significantly more to complex male songs but only when their noradrenergic system is intact (Vyas et al, 2008).
In male birds, sex steroid hormones both at the periphery and in the brain fluctuate with song production and social context. Directed song production requires higher levels of circulating plasma testosterone than undirected song production. The conversion of testosterone to 17β-estradiol is essential for directed song, but undirected song can occur even when this conversion is blocked by the administration of an aromatase inhibitor (Prove, 1978; Harding et al, 1983; Walters et al, 1991).
Like hormones, norepinephrine is implicated in regulating the switch between singing behavior in different social contexts (Barclay et al, 1992; 1996; Castelino and Ball, 2005; Castelino and Ball, 2006). Recent studies showing that steroid hormones such as estradiol can be regulated locally (i.e. within a single brain area such as NCM) and at surprisingly rapid rates (within minutes) (Remage-Healey et al, 2008) suggest that their time course of action is well matched with that of neuromodulators. The similar roles for these two systems in singing behavior and the anatomical evidence of their proximity suggest that they may be working in concert to cause changes in singing behavior. Future studies should attempt to consolidate what we know of the roles of hormones and norepinephrine in the neural control of social context-dependent changes in singing.
b. Norepinephrine and dopamine
Dopamine is found in more structures than norepinephrine, and two of the major dopamine containing areas in the midbrain, VTA and SN, show projection patterns that overlap significantly with those delineated by the noradrenergic system (Kandel et al, 2000; Smeets and Gonzalez, 2000; Bjorklund and Dunnett, 2007). There are also strong reciprocal connections between the locus coeruleus and the VTA (Swanson, 1982; Deutch, 1986; Meijas-Aponte et al, 2009) suggesting that these two systems might be tightly coupled. Locus coeruleus stimulation, for example, is able to modulate dopamine neuron activity in a noradrenergic receptor dependent manner (Arencibia-Albita et al, 2007; Grenhoff et al, 1995; Grenhoff and Svenson, 1989, 1993; Guiard et al, 2008; Paladini and Williams, 2004) and stimulation of dopaminergic areas causes norepinephrine to increase in the locus coeruleus (Deutch, 1986; Lin et al, 2008). The interactions between norepinephrine and dopamine also extend to reuptake mechanisms where the noradrenergic transporter can act as a reuptake mechanism for both norepinephrine and dopamine (Carboni et al, 1990, 2001; Moron et al, 2002; Torres et al, 2003). Finally, there is direct crosstalk between both systems at the receptor level, with dopamine and norepinephrine both being able to activate each other’s receptors (Newman-Tancredi, 1997; Lanau et al, 1997; Nyronen et al, 2001). Several studies have shown such crosstalk to occur in songbirds (Cornil et al, 2002; Cornil et al, 2008; Cornil and Ball, 2008). In the avian song system, dopamine is able to bind to α-2 adrenergic receptors in HVC, LMAN, area X and the VTA in both male and female zebra finches (Cornil et al, 2008; Cornil and Ball, 2008). Additionally, in area X, dopamine autoinhibition (a mechanism that regulates neurotransmitter release), which generally takes place by binding of dopamine to presynaptic D2 receptors, also occurs via binding to α-2 adrenergic receptors (Gale and Perkel, 2005). Widespread crosstalk at the receptor and transporter level therefore implies that these two systems likely overlap quite significantly in their effects on the song system.
Consistent with the notion of functional overlap between both systems, dopamine appears to be involved in many functions that have been associated with the noradrenergic system. Firing in neurons within VTA increases more during directed song than undirected song (Yanagihara and Hessler, 2006) and online in vivo microdialysis reveals significantly higher levels of dopamine in area X of males singing directed song than undirected song (Sasaki et al, 2006). Both neuromodulators might also complement each other’s actions within the context of social context and singing. Lesions of the VTA, for example, abolish the social context differences in gene expression in LMAN and RA but not in area X (Hara et al, 2007) whereas R1.11: depletion of norepinephrine (via systemic administration of a noradrenergic neurotoxin) only affects gene expression levels in area X (Castelino and Ball, 2005). At the song level, unilateral lesions to the VTA R2.9: alone do not have any noticeable effect on song parameters except for decreasing the rate of directed song. In contrast, lesioning both the locus coeruleus and the VTA causes a marked decrease in song rate as well as degradation of syllable, sequence and motif structure (Hara et al, 2007). Finally, there is evidence that dopamine levels are affected by circulating steroid sex hormones in similar ways as norepinephrine (Barclay and Harding, 1988; 1990).
Taken together, most studies tend to examine the role dopamine and norepinephrine in isolation. As evidence accumulates however that systems act in concert to regulate behavior, it will be critical for future studies to unravel the relative contributions of these various neuromodulators in shaping behaviors.
7. Summary and Conclusion
Many of the studies investigating the neural mechanisms by which norepinephrine influences sensory processing and behavior have been performed either in anesthetized animals or in animals performing relatively simple behaviors. Nevertheless, a framework has emerged to suggest that a major role of the noradrenergic system might be in biasing the nervous system towards producing behaviors that help animals adapt to constantly changing environments. Specifically, by modifying firing properties in the locus coeruleus from a tonic mode to a more phasic mode, the noradrenergic system is proposed to shift behavioral strategies from those that more exploratory in nature (high tonic levels) to those that are more exploitative in nature (high phasic levels) (Aston-Jones and Cohen, 2005). The potential complexity of this role for the noradrenergic system requires that it be studied within animal model systems that exhibit behaviors that are consistent with these functions.
The strength of using songbirds as a model to study the noradrenergic system lies in the confluence of sensorimotor processing that results in a large repository of behaviors that have naturally occurring transitions. The most important of these is song production, which is a learned complex motor program that we know, is regulated by norepinephrine. This is a potentially crucial area given that little is known regarding the motor aspects of noradrenergic function despite anatomical evidence for noradrenergic projections to motor areas that include the motor cortex, cerebellum and spinal cord (Berridge and Waterhouse, 2003). Songbird studies have also revealed the differences and similarities in noradrenergic function across sexes. In particular, we have the opportunity to examine the effects of norepinephrine and associated peptides on mediating both sex and seasonal differences in brain and behavior; thus exploring their putative interactions with sex steroid hormone systems as well. Finally, the link between the noradrenergic system, sensory processing and vocal learning in songbirds provides a potential powerful model for studying how the noradrenergic system might influence the storage of auditory memories and their retrieval during sensorimotor learning. One of the great advantages of the song system is that the influence of norepinephrine on neural circuitry and behavior can be studied at multiple levels (from brain slices to gene expression to single unit physiology and behavior) within the context of a learned behavior that is both complex and well quantifiable. Future studies will undoubtedly reveal the nature of noradrenergic control in this circuit and give us valuable insight into general principles governing noradrenergic action.
Norepinephrine is implicated in several disorders including attention deficit hyperactivity disorder, Alzheimer’s, Parkinson’s, depression, posttraumatic stress disorder and stroke-induced aphasias (Berridge and Waterhouse, 2003). These disorders involve impairment in both sensory and motor function. Studying the noradrenergic system in songbirds allows us to elucidate the intricacies of norepinephrine control of sensorimotor integration across several areas of the brain. This will help fill in many of the gaps in our understanding of the role that norepinephrine plays in the etiology of a gamut of neurological disorders.
Figure 1. Schematic depiction of the A6 complex in the brainstem.
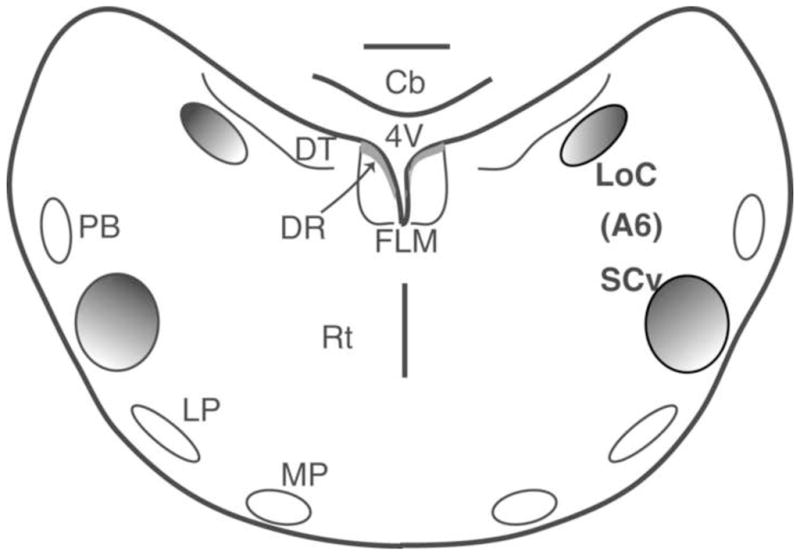
Coronal view of a slice of the songbird brainstem showing the locus coeruleus and sub coeruleus and a few key surrounding nuclei and fiber tracts. Abbreviations: Cb (cerebellum); DR (dorsal raphe); DT (dorsal tegmental nucleus); FLM (medial longitudinal fasciculus); LoC (locus coeruleus); LP (lateral pontine nucleus); MP (medial pontine nucleus); PB(parabrachial nucleus); Rt (reticular formation); SCv (sub coeruleus); 4V (fourth ventricle). (This figure was adapted from Mello et al, 1998; Reiner et al, 2004b and Puelles et al, 2007).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alger SJ, Maasch SN, Riters LV. Lesions to the medial preoptic nucleus affect immediate early gene immunolabeling in brain regions involved in song control and social behavior in male European starlings. Eur J Neurosci. 2009;29:970–982. doi: 10.1111/j.1460-9568.2009.06637.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Alger SJ, Riters LV. Lesions to the medial preoptic nucleus differentially affect singing and nest box-directed behaviors within and outside of the breeding season in European starlings (Sturnus vulgaris) Behav Neurosci. 2006;120:1326–1336. doi: 10.1037/0735-7044.120.6.1326. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Andersson M. Sexual Selection. Princeton University Press; Princeton, NJ: 1994. [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. The distribution of tyrosine hydroxylase in the canary brain: demonstration of a specific and sexually dimorphic catecholaminergic innervation of the telencephalic song control nulcei. Cell Tiss Res. 2001;304:237–259. doi: 10.1007/s004410100360. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. The origin of catecholaminergic inputs to the song control nucleus RA in canaries. Neuroreport. 2002a;13:649–653. doi: 10.1097/00001756-200204160-00023. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. Song activation by testosterone is associated with an increased catecholaminergic innervation of the song control system in female canaries. Neurosci. 2003;121:801–814. doi: 10.1016/s0306-4522(03)00496-2. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Ball GF, Balthazart J. Catecholaminergic inputs to aromatase cells in the canary auditory forebrain. Neuroreport. 2004;15:1727–1730. doi: 10.1097/01.wnr.0000135920.75925.1e. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Gentner TQ, Hulse SH, Balthazart J, Ball GF. The effect of auditory distractors on song discrimination in male canaries (Serinus canaria) Behav Process. 2005;69:331–341. doi: 10.1016/j.beproc.2005.01.010. [DOI] [PubMed] [Google Scholar]
- Appeltants D, Negro CD, Balthazart J. Noradrenergic control of auditory information processing in female canaries. Behav Brain Res. 2002b;00:1–15. doi: 10.1016/s0166-4328(02)00005-0. [DOI] [PubMed] [Google Scholar]
- Arencibia-Albite F, Paladini C, Williams JT, Jimenez-Rivera CA. Noradrenergic modulation of the hyperpolarization-activated cation current (Ih) in dopamine neurons of the ventral tegmental area. Neurosci. 2007;149:303–314. doi: 10.1016/j.neuroscience.2007.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Bloom FE. Norepinephrine containing locus coeruleus neurons in behaving rats exhibit pronounced responses to non noxious environmental stimuli. J Neurosci. 1981;1:887–900. doi: 10.1523/JNEUROSCI.01-08-00887.1981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aston-Jones G, Chiang C, Alexinsky T. Discharge of noradrenergic locus coeruleus neurons in behaving rats and monkeys suggests a role in vigilance. Prog Brain Res. 1991;88:501–520. doi: 10.1016/s0079-6123(08)63830-3. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Cohen JD. An integrative theory of locus coeruleus-norepinephrine function: Adaptive gain and optimal performance. Ann Rev Neurosci. 2005;28:403–450. doi: 10.1146/annurev.neuro.28.061604.135709. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Rajkowski J, Kubiak P, Alexinsky T. Locus coeruleus neurons in monkey are selectively activated by attended cues in a vigilance task. J Neurosci. 1994;14:4467–4480. doi: 10.1523/JNEUROSCI.14-07-04467.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Azumaya Y, Tsutsui K. Localization of galanin and its binding sites in the quail brain. Brain Res. 1996;727:187–195. doi: 10.1016/0006-8993(96)00379-4. [DOI] [PubMed] [Google Scholar]
- Ball GF. Neurochemical Specializations Associated with Vocal Learning and Production in Songbirds and Budgerigars. Brain Beh Evol. 1994;44:234–246. doi: 10.1159/000113579. [DOI] [PubMed] [Google Scholar]
- Ball GF, Absil P, Balthazart J. Assessment of volumetric sex differences in the song control nuclei HVC and RA in zebra finches by immunocytochemistry for methionine enkephalin and vasoactive intestinal polypeptide. Brain Res. 1995a;699:83–96. doi: 10.1016/0006-8993(95)00875-q. [DOI] [PubMed] [Google Scholar]
- Ball GF, Absil P, Balthazart J. Peptidergic delineations of nucleus interface reveal a sex difference in volume. Neuroreport. 1995b;6:957–960. doi: 10.1097/00001756-199505090-00002. [DOI] [PubMed] [Google Scholar]
- Ball GF, Auger CJ, Bernard DJ, Charlier TD, Sartor JJ, Riters LV, Balthazart J. Seasonal plasticity in the song control system: multiple brain sites of steroid hormone action and the importance of variation in song behavior. Ann N Y Acad Sci. 2004;1016:586–610. doi: 10.1196/annals.1298.043. [DOI] [PubMed] [Google Scholar]
- Ball GF, Castelino CB, Maney DL, Appeltants D, Balthazart J. The activation of birdsong by testosterone: multiple sites of action and role of ascending catecholamine projections. Ann N Y Acad Sci. 2003;1007:211–231. doi: 10.1196/annals.1286.021. [DOI] [PubMed] [Google Scholar]
- Ball GF, Sockman KW, Duffy DL, Gentner TQ. A neuroethological approach to song behavior and perception in European starlings: Interrelationships among testosterone, neuroanatomy, immediate early gene expression and immune function. Adv Stud Behav. 2006;36:59–121. [Google Scholar]
- Barclay SR, Harding CF. Androstenedione modulation of monoamine levels and turnover in hypothalamic and vocal control nuclei in the male zebra finch: steroid effects on brain monoamines. Brain Res. 1988;459:333–343. doi: 10.1016/0006-8993(88)90649-x. [DOI] [PubMed] [Google Scholar]
- Barclay SR, Harding CF. Differential modulation of monoamine levels and turnover rates by estrogen and/or androgen in hypothalamic and vocal control nuclei of male zebra finches. Brain Res. 1990;523:251–262. doi: 10.1016/0006-8993(90)91494-2. [DOI] [PubMed] [Google Scholar]
- Barclay SR, Harding CF, Waterman SA. Correlations between catecholamine levels and sexual behavior in male zebra finches. Pharmacol Biochem Behav. 1992;41:195–201. doi: 10.1016/0091-3057(92)90082-q. [DOI] [PubMed] [Google Scholar]
- Barclay SR, Harding CF, Waterman SA. Central DSP-4 treatment decreases norepinephrine levels and courtship behavior in male zebra finches. Pharmacol Biochem and Behav. 1996;53:213–220. doi: 10.1016/0091-3057(95)00183-2. [DOI] [PubMed] [Google Scholar]
- Bauer EE, Coleman MJ, Roberts TF, Roy A, Prather JF, Mooney R. A synaptic basis for auditory-vocal integration in the songbird. J Neurosci. 2008;28:1509–1522. doi: 10.1523/JNEUROSCI.3838-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bernard DJ, Ball GF. Two histological markers reveal a similar photoperiodic difference in the volume of the high vocal center in male European starlings. J Comp Neurol. 1995;360:726–734. doi: 10.1002/cne.903600415. [DOI] [PubMed] [Google Scholar]
- Berridge CW. Noradrenergic modulation of arousal. Brain Res Rev. 2008;58:1–17. doi: 10.1016/j.brainresrev.2007.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berridge CW, Waterhouse BD. The locus coeruleus-noradrenergic system: modulation of behavioral state and state-dependent cognitive processes. Brain Res Brain Res Rev. 2003;42:33–84. doi: 10.1016/s0165-0173(03)00143-7. [DOI] [PubMed] [Google Scholar]
- Bjorklund A, Dunnett SB. Dopamine neuron systems in the brain: an update. Trends Neurosci. 2007;30:194–202. doi: 10.1016/j.tins.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Blahser S. Peptidergic pathways in the avian brain. J Exp Zool. 1984;232:397–403. doi: 10.1002/jez.1402320304. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Gahr M. Neural mechanisms of birdsong memory. Nat Rev Neurosci. 2006;7:347–357. doi: 10.1038/nrn1904. [DOI] [PubMed] [Google Scholar]
- Bolhuis JJ, Zijlstra GGO, Boer-Visser AMd, Zee EAVd. Localized neuronal activation in the zebra finch brain is related to the strength of song learning. Proc Nat Acad Sci. 2000;97:2282–2285. doi: 10.1073/pnas.030539097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bons N, Oliver J. Origin of the afferent connections to the parolfactory lobe in quail shown by retrograde labelling with a fluorescent neuron tracer. Exp Brain Res. 1986;63:125–134. doi: 10.1007/BF00235654. [DOI] [PubMed] [Google Scholar]
- Bottjer SW. The Distribution of Tyrosine-Hydroxylase Immunoreactivity in the Brains of Male and Female Zebra Finches. J Neurobiol. 1993;24:51–69. doi: 10.1002/neu.480240105. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Alexander G. Localization of met-enkephalin and vasoactive intestinal polypeptide in the brains of male zebra finches. Brain Behav Evol. 1995;45:153–177. doi: 10.1159/000113547. [DOI] [PubMed] [Google Scholar]
- Bottjer SW, Roselinsky H, Tran NB. Sex differences in neuropeptide staining of song-control nuclei in zebra finch brains. Brain Behav Evol. 1997;50:284–303. doi: 10.1159/000113342. [DOI] [PubMed] [Google Scholar]
- Bouret S, Sara SJ. Network reset: a simplified overarching theory of locus coeruleus noradrenaline function. Trends Neurosci. 2005;28:574–582. doi: 10.1016/j.tins.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Brainard MS, Doupe AJ. What songbirds teach us about learning. Nature. 2002;417:351–358. doi: 10.1038/417351a. [DOI] [PubMed] [Google Scholar]
- Carboni E, Tanda GL, Frau R, Di Chiara G. Blockade of the noradrenaline carrier increases extracellular dopamine concentrations in the prefrontal cortex: evidence that dopamine is taken up in vivo by noradrenergic terminals. J Neurochem. 1990;55:1067–1070. doi: 10.1111/j.1471-4159.1990.tb04599.x. [DOI] [PubMed] [Google Scholar]
- Carboni E, Spielewoy C, Vacca C, Nosten-Bertrand M, Giros B, Di Chiara G. Cocaine and amphetamine increase extracellular dopamine in the nucleus accumbens of mice lacking the dopamine transporter gene. J Neurosci. 2001;21(RC141):1–4. doi: 10.1523/JNEUROSCI.21-09-j0001.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cardin J, Schmidt MF. Song system auditory responses are stable and highly tuned during sedation, rapidly modulated and unselective during wakefulness and suppressed by arousal. J Neurophysiol. 2003;90:2884–2899. doi: 10.1152/jn.00391.2003. [DOI] [PubMed] [Google Scholar]
- Cardin J, Schmidt MF. Auditory responses in multiple sensorimotor song system nuclei are co-modulated by behavioral state. J Neurophysiol. 2004a;91:2148–2163. doi: 10.1152/jn.00918.2003. [DOI] [PubMed] [Google Scholar]
- Cardin JA, Schmidt MF. Noradrenergic inputs mediate state dependence of auditory responses in the avian song system. J Neurosci. 2004b;24:7745–7753. doi: 10.1523/JNEUROSCI.1951-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carillo G, Doupe AJ. Is the songbird area X striatal, pallidal, or both? An anatomical study. J Comp Neurol. 2004;473:415–437. doi: 10.1002/cne.20099. [DOI] [PubMed] [Google Scholar]
- Castelino CB, Ball GF. A role for norepinephrine in the regulation of context-dependent ZENK expression in male zebra finches (Taeniopygia guttata) Eur J Neurosci. 2005;21:1962–1972. doi: 10.1111/j.1460-9568.2005.04028.x. [DOI] [PubMed] [Google Scholar]
- Castelino CB, Ball GF. 2006 Abstract Viewer/Itinerary Planner. Atlanta, GA: Society for Neuroscience; 2006. Differences in singing behavior by zebra finches across social contexts are abolished by systemic depletion of norepinephrine in the song control circuit. Online. [Google Scholar]
- Castelino CB, Diekamp B, Ball GF. Noradrenergic projections to the song control nucleus area X of the medial striatum in male zebra finches (Taeniopygia guttata) J Comp Neurol. 2007;502:544–562. doi: 10.1002/cne.21337. [DOI] [PubMed] [Google Scholar]
- Castelino CB, Yumul RE, Schmidt MF. 2007 Abstract Viewer/Itinerary Planner. San Diego, CA: Society for Neuroscience; 2007. Relating locus coeruleus activity to arousal- mediated suppression of song responses in HVC of male zebra finches. Online. [Google Scholar]
- Casto JM, Absil P, Balthazart J, Ball GF. Autoradiographic localization of beta-adrenergic receptors in the songbird vocal control system. Society for Neuroscience; 1992. [Google Scholar]
- Castro-Alamancos MA. Role of thalamocortical sensory suppression during arousal: focusing sensory inputs in neocortex. J Neurosci. 2002;22:9651–9655. doi: 10.1523/JNEUROSCI.22-22-09651.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castro-Alamancos MA, Calcagnotto ME. High-pass filtering of corticothalamic activity by neuromodulators released in the thalamus during arousal: in vitro and in vivo. J Neurophysiol. 2001;85:1489–1497. doi: 10.1152/jn.2001.85.4.1489. [DOI] [PubMed] [Google Scholar]
- Catchpole CK, Slater PJB. Bird Song- Biological themes and variations. Cambridge University Press; 1995. [Google Scholar]
- Chaine AS, Lyon BE. Adaptive plasticity in female mate choice dampens sexual selection on male ornaments in the lark bunting. Science. 2008;319:459–462. doi: 10.1126/science.1149167. [DOI] [PubMed] [Google Scholar]
- Chen FJ, Sara SJ. Locus coeruleus activation by foot shock or electrical stimulation inhibits amygdala neurons. Neurosci. 2007;144:472–481. doi: 10.1016/j.neuroscience.2006.09.037. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Pompeiano M, Tononi G. Neuronal gene expression in the waking state: a role for the locus coeruleus. Science. 1996;274:1211–1215. doi: 10.1126/science.274.5290.1211. [DOI] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Differential expression of plasticity-related genes in waking and sleep and their regulation by the noradrenergic system. J Neurosci. 2000;20:9187–9194. doi: 10.1523/JNEUROSCI.20-24-09187.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cirelli C, Tononi G. Locus ceruleus control of state-dependent gene expression. J Neurosci. 2004;24:5410–5419. doi: 10.1523/JNEUROSCI.0949-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Ball GF. Interplay among catecholamine systems: dopamine binds to alpha2-adrenergic receptors in birds and mammals. J Comp Neurol. 2008;511:610–627. doi: 10.1002/cne.21861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Balthazart J, Motte P, Massotte L, Seutin V. Dopamine activates noradrenergic receptors in the preoptic area. J Neurosci. 2002;22:9320–9330. doi: 10.1523/JNEUROSCI.22-21-09320.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cornil CA, Castelino CB, Ball GF. Dopamine binds to alpha(2)-adrenergic receptors in the song control system of zebra finches (Taeniopygia guttata) J Chem Neuroanat. 2008;35:202–215. doi: 10.1016/j.jchemneu.2007.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Coull JT. Pharmacological manipulations of the alpha-2 noradrenergic system. Effects on cognition. Drugs Aging. 1994;5:116–126. doi: 10.2165/00002512-199405020-00005. [DOI] [PubMed] [Google Scholar]
- Coull JT, Buchel C, Friston KJ, Frith CD. Noradrenergically mediated plasticity in a human attentional neuronal network. Neuroimage. 1999;10:705–715. doi: 10.1006/nimg.1999.0513. [DOI] [PubMed] [Google Scholar]
- Dahlstrom A, Fuxe K. Localization of monoamines in the lower brain stem. Experientia. 1964;20:398–399. doi: 10.1007/BF02147990. [DOI] [PubMed] [Google Scholar]
- Dave AS, Yu AC, Margoliash D. Behavioral state modulation of auditory activity in a vocal motor system. Science. 1998;282:2250–2254. doi: 10.1126/science.282.5397.2250. [DOI] [PubMed] [Google Scholar]
- Davies DC, Horn G, McCabe BJ. Changes in telencephalic catecholamine levels in the domestic chick. Effects of age and visual experience. Brain Res. 1983;312:251–255. doi: 10.1016/0165-3806(83)90141-4. [DOI] [PubMed] [Google Scholar]
- Deutch AY, Goldstein M, Roth RH. Activation of the locus coeruleus induced by selective stimulation of the ventral tegmental area. Brain Res. 1986;363:307–314. doi: 10.1016/0006-8993(86)91016-4. [DOI] [PubMed] [Google Scholar]
- Devilbiss DM, Page ME, Waterhouse BD. Locus ceruleus regulates sensory encoding by neurons and networks in waking animals. J Neurosci. 2006;26:9860–9872. doi: 10.1523/JNEUROSCI.1776-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong S, Clayton DF. Habituation in songbirds. Neurobiol Learn Mem. 2008 doi: 10.1016/j.nlm.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudley MW, Howard BD, Cho AK. The interaction of the beta-haloethyl benzylamines, xylamine, and DSP-4 with catecholaminergic neurons. Ann Rev Pharmacol Toxicol. 1990;30:387–403. doi: 10.1146/annurev.pa.30.040190.002131. [DOI] [PubMed] [Google Scholar]
- Duffy DL, Ball GF. Song predicts immunocompetence in male European starlings (Sturnus vulgaris) Proc Biol Sci. 2002;269:847–852. doi: 10.1098/rspb.2002.1956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn A, Zann RA. Undirected song encourages the breeding female zebra finch to remain in the nest. Ethology. 1996a;102:540–548. [Google Scholar]
- Dunn A, Zann RA. Undirected song in wild zebra finch flocks: contexts and effects of mate removal. Ethology. 1996b;102:529–539. [Google Scholar]
- Dunn A, Zann RA. Effects of pair bond and presence of conspecifics on singing in captive zebra finches. Behaviour. 1997;134:127–142. [Google Scholar]
- Eens M, Pinxten R, Verheyen RF. Male Song as a Cue for Mate Choice in the European Starling. Behaviour. 1991;116:210–238. [Google Scholar]
- Feldman RS, Meyer JS, Quenzer LF. Principles of Neuropsychopharmacology. Sinauer Associates; MA, USA: 1997. [Google Scholar]
- Fiore M, Clayton NS, Pistillo L, Angelucci F, Alleva E, Aloe L. Song behavior, NGF level and NPY distribution in the brain of adult male zebra finches. Behav Brain Res. 1999;101:85–92. doi: 10.1016/s0166-4328(98)00143-0. [DOI] [PubMed] [Google Scholar]
- Foote SL, Aston-Jones G, Bloom FE. Impulse activity of locus coeruleus neurons in awake rats and monkeys is a function of sensory stimulation and arousal. Proc Natl Acad Sci U S A. 1980;77:3033–3037. doi: 10.1073/pnas.77.5.3033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fortune ES, Margoliash D. Parallel Pathways and Convergence onto Hvc and Adjacent Neostriatum of Adult Zebra Finches (Taeniopygia-Guttata) J Comp Neurol. 1995;360:413–441. doi: 10.1002/cne.903600305. [DOI] [PubMed] [Google Scholar]
- Fritschy JM, Grzanna R. Selective effects of DSP4 on locus coeruleus axons - are there pharmacologically different types of noradrenergic axons in the CNS? Prog Brain Res. 1991;88:257–268. doi: 10.1016/s0079-6123(08)63815-7. [DOI] [PubMed] [Google Scholar]
- Gale SD, Perkel DJ. Properties of dopamine release and uptake in the songbird basal ganglia. J Neurophysiol. 2005;93:1871–1879. doi: 10.1152/jn.01053.2004. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH. Female European starling preference and choice for variation in conspecific male song. Anim Beh. 2000;59:443–458. doi: 10.1006/anbe.1999.1313. [DOI] [PubMed] [Google Scholar]
- Gentner TQ, Hulse SH, Duffy D, Ball GF. Response biases in auditory forebrain regions of female songbirds following exposure to sexually relevant variation in male song. J Neurobiol. 2001;46:48–58. doi: 10.1002/1097-4695(200101)46:1<48::aid-neu5>3.0.co;2-3. [DOI] [PubMed] [Google Scholar]
- Gibbs ME. Memory systems in the chick: regional and temporal control by noradrenaline. Brain Res Bull. 2008;76:170–182. doi: 10.1016/j.brainresbull.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Gibbs ME, Summers RJ. Role of adrenoceptor subtypes in memory consolidation. Prog Neurobiol. 2002;67:345–391. doi: 10.1016/s0301-0082(02)00023-0. [DOI] [PubMed] [Google Scholar]
- Gobes SM, Bolhuis JJ. Birdsong memory: a neural dissociation between song recognition and production. Curr Biol. 2007;17:789–793. doi: 10.1016/j.cub.2007.03.059. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, North RA, Johnson SW. Alpha 1-adrenergic effects on dopamine neurons recorded intracellularly in the rat midbrain slice. Eur J Neurosci. 1995;7:1707–1713. doi: 10.1111/j.1460-9568.1995.tb00692.x. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Clonidine modulates dopamine cell firing in rat ventral tegmental area. Eur J Pharmacol. 1989;165:11–18. doi: 10.1016/0014-2999(89)90765-6. [DOI] [PubMed] [Google Scholar]
- Grenhoff J, Svensson TH. Prazosin modulates the firing pattern of dopamine neurons in rat ventral tegmental area. Eur J Pharmacol. 1993;233:79–84. doi: 10.1016/0014-2999(93)90351-h. [DOI] [PubMed] [Google Scholar]
- Grzanna R, Molliver ME. The locus coeruleus in the rat: an immunohistochemical delineation. Neuroscience. 1980;5:21–40. doi: 10.1016/0306-4522(80)90068-8. [DOI] [PubMed] [Google Scholar]
- Gucer G. The effect of sleep upon the transmission of afferent activity in the somatic afferent system. Exp Brain Res. 1979;34:287–298. doi: 10.1007/BF00235674. [DOI] [PubMed] [Google Scholar]
- Guiard BP, El Mansari M, Blier P. Cross-talk between dopaminergic and noradrenergic systems in the rat ventral tegmental area, locus ceruleus, and dorsal hippocampus. Mol Pharmacol. 2008;74:1463–1475. doi: 10.1124/mol.108.048033. [DOI] [PubMed] [Google Scholar]
- Hahnloser RH, Wang CZ, Nager A, Naie K. Spikes and bursts in two types of thalamic projection neurons differentially shape sleep patterns and auditory responses in a songbird. J Neurosci. 2008;28:5040–5052. doi: 10.1523/JNEUROSCI.5059-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hara E, Kubikova L, Hessler NA, Jarvis ED. Role of the midbrain dopaminergic system in modulation of vocal brain activation by social context. Eur J Neurosci. 2007;25:3406–3416. doi: 10.1111/j.1460-9568.2007.05600.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harding CF, Barclay SR, Waterman SA. Changes in catecholamine levels and turnover rates in hypothalamic, vocal control, and auditory nuclei in male zebra finches during development. J Neurobiol. 1998;34:329–346. [PubMed] [Google Scholar]
- Harding CF, Sheridan K, Walters MJ. Hormonal Specificity and Activation of Sexual-Behavior in Male Zebra Finches. Horm Behav. 1983;17:111–133. doi: 10.1016/0018-506x(83)90021-1. [DOI] [PubMed] [Google Scholar]
- Hasselmo ME, Linster C, Patil M, Ma D, Cekic M. Noradrenergic suppression of synaptic transmission may influence cortical signal-to-noise ratio. J Neurophysiol. 1997;77:3326–3339. doi: 10.1152/jn.1997.77.6.3326. [DOI] [PubMed] [Google Scholar]
- Heimovics SA, Riters LV. Immediate early gene activity in song control nuclei and brain areas regulating motivation relates positively to singing behavior during, but not outside of, a breeding context. J Neurobiol. 2005;65:207–224. doi: 10.1002/neu.20181. [DOI] [PubMed] [Google Scholar]
- Hessler NA, Doupe AJ. Social context modulates singing related neural activity in the songbird forebrain. Nat Neurosci. 1999;2:209–211. doi: 10.1038/6306. [DOI] [PubMed] [Google Scholar]
- Issa EB, Wang X. Sensory responses during sleep in primate primary and secondary auditory cortex. J Neurosci. 2008;28:14467–14480. doi: 10.1523/JNEUROSCI.3086-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Nottebohm F. Motor-driven gene expression. Proc Nat Acad Sci USA. 1997;94:4097–4102. doi: 10.1073/pnas.94.8.4097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jarvis ED, Scharff C, Grossman MR, Ramos JA, Nottebohm F. For whom the bird sings: Context-dependent gene expression. Neuron. 1998;21:775–788. doi: 10.1016/s0896-6273(00)80594-2. [DOI] [PubMed] [Google Scholar]
- Jennions MD, Petrie M. Variation in mate choice and mating preferences: a review of causes and consequences. Biol Rev Camb Philos Soc. 1997;72:283–327. doi: 10.1017/s0006323196005014. [DOI] [PubMed] [Google Scholar]
- Jouvet M. Biogenic amines and the states of sleep. Science. 1969;163:32–41. doi: 10.1126/science.163.3862.32. [DOI] [PubMed] [Google Scholar]
- Kandel ER, Schwartz JH, Jessell TM. Principles of Neural Science. McGraw-Hill; 2000. [Google Scholar]
- Kao MH, Brainard MS. Lesions of an avian basal ganglia circuit prevent context-dependent changes to song variability. J Neurophysiol. 2006;96:1441–1455. doi: 10.1152/jn.01138.2005. [DOI] [PubMed] [Google Scholar]
- Kao MH, Doupe AJ, Brainard MS. Contributions of an avian basal ganglia-forebrain circuit to real-time modulation of song. Nature. 2005;433:638–643. doi: 10.1038/nature03127. [DOI] [PubMed] [Google Scholar]
- Kao MH, Wright BD, Doupe AJ. Neurons in a forebrain nucleus required for vocal plasticity rapidly switch between precise firing and variable bursting depending on social context. J Neurosci. 2008;28:13232–13247. doi: 10.1523/JNEUROSCI.2250-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kety SS. The biogenic amines in the central nervous system: their possible roles in arousal, emotion and learning. In: Schmitt FO, editor. The Neurosciences: Second Study Program. Rockefeller University Press; NY: 1970. pp. 324–336. [Google Scholar]
- Kimpo RR, Doupe AJ. FOS Is Induced by Singing in Distinct Neuronal Populations in a Motor Network. Neuron. 1997;18:315–325. doi: 10.1016/s0896-6273(00)80271-8. [DOI] [PubMed] [Google Scholar]
- Kitt CA, Brauth SE. Telencephalic projections from midbrain and isthmal cell groups in the pigeon. I. Locus coeruleus and subcoeruleus. J Comp Neurol. 1986;247:69–91. doi: 10.1002/cne.902470105. [DOI] [PubMed] [Google Scholar]
- Kokko H, Rankin DJ. Lonely hearts or sex in the city? Density-dependent effects in mating systems. Philos Trans R Soc Lond B Biol Sci. 2006;361:319–334. doi: 10.1098/rstb.2005.1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kruse AA, Stripling R, Clayton D. Minimum experience required for immediate-early gene induction in zebra finch neostriatum. Neurobiol Learn Mem. 2000;74:179–184. doi: 10.1006/nlme.2000.3968. [DOI] [PubMed] [Google Scholar]
- Kruse AA, Stripling R, Clayton DF. Context-specific habituation of the zenk gene response to song in adult zebra finches. Neurobiol Learn Mem. 2004;82:99–108. doi: 10.1016/j.nlm.2004.05.001. [DOI] [PubMed] [Google Scholar]
- Lanau F, Zenner MT, Civelli O, Hartman DS. Epinephrine and norepinephrine act as potent agonists at the recombinant human dopamine D4 receptor. J Neurochem. 1997;68:804–812. doi: 10.1046/j.1471-4159.1997.68020804.x. [DOI] [PubMed] [Google Scholar]
- LeBlanc MM, Goode CT, MacDougall-Shackleton EA, Maney DL. Estradiol modulates brainstem catecholaminergic cell groups and projections to the auditory forebrain in a female songbird. Brain Res. 2007;1171:93–103. doi: 10.1016/j.brainres.2007.06.086. [DOI] [PubMed] [Google Scholar]
- Leger L, Hernandez-Nicaise ML. The cat locus coeruleus. Light and electron microscopic study of the neuronal somata. Anat Embryol (Berl) 1980;159:181–198. doi: 10.1007/BF00304977. [DOI] [PubMed] [Google Scholar]
- Leitner S, Voigt C, Metzdorf R, Catchpole CK. Immediate early gene (ZENK, Arc) expression in the auditory forebrain of female canaries varies in response to male song quality. J Neurobiol. 2005;64:275–284. doi: 10.1002/neu.20135. [DOI] [PubMed] [Google Scholar]
- Lin Y, Quartermain D, Dunn AJ, Weinshenker D, Stone EA. Possible dopaminergic stimulation of locus coeruleus alpha1-adrenoceptors involved in behavioral activation. Synapse. 2008;62:516–523. doi: 10.1002/syn.20517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Livingstone MS, Hubel DH. Effects of sleep and arousal on the processing of visual information in the cat. Nature. 1981;291:554–561. doi: 10.1038/291554a0. [DOI] [PubMed] [Google Scholar]
- London SE, Clayton DF. Functional identification of sensory mechanisms required for developmental song learning. Nat Neurosci. 2008;11:579–586. doi: 10.1038/nn.2103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu K, Vicario DS, Velho TAF, Mello CV. Program No. 99.5. Neuroscience Meeting Planner. Washington DC: Society for Neuroscience; 2008. Noradrenergic modulation may contribute to long-term memory in songbird auditory telencephalon. Online. [Google Scholar]
- Lynch KS, Ball GF. Noradrenergic deficits alter processing of communication signals in female songbirds. Brain Behav Evol. 2008;72:207–214. doi: 10.1159/000157357. [DOI] [PubMed] [Google Scholar]
- Maney DL, Bernard DJ, Ball GF. Gonadal steroid receptor mRNA in catecholaminergic nuclei of the canary brainstem. Neurosci Lett. 2001;311:189–192. doi: 10.1016/s0304-3940(01)02157-7. [DOI] [PubMed] [Google Scholar]
- Margoliash D. Preference for autogenous song by auditory neurons in a song system nucleus of the white crowned sparrow. J Neurosci. 1986;6:1643–1661. doi: 10.1523/JNEUROSCI.06-06-01643.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormick DA. Neurotransmitter actions in the thalamus and cerebral cortex. J Clin Neurophysiol. 1992;9:212–223. doi: 10.1097/00004691-199204010-00004. [DOI] [PubMed] [Google Scholar]
- Mejias-Aponte CA, Drouin C, Aston-Jones G. Adrenergic and noradrenergic innervation of the midbrain ventral tegmental area and retrorubral field: prominent inputs from medullary homeostatic centers. J Neurosci. 2009;29:3613–3626. doi: 10.1523/JNEUROSCI.4632-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello C, Nottebohm F, Clayton D. Repeated Exposure to One Song Leads to a Rapid and Persistent Decline in an Immediate-Early Genes Response to That Song in Zebra Finch Telencephalon. J Neurosci. 1995;15:6919–6925. doi: 10.1523/JNEUROSCI.15-10-06919.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV. Mapping vocal communication pathways in birds with inducible gene expression. J Comp Physiol A. 2002;188:943–959. doi: 10.1007/s00359-002-0347-1. [DOI] [PubMed] [Google Scholar]
- Mello CV, Clayton DF. Song-induced ZENK gene expression in auditory pathways of songbird brain and its relation to the song control system. J Neurosci. 1994;14:6652–6666. doi: 10.1523/JNEUROSCI.14-11-06652.1994. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mello CV, Pinaud R, Ribeiro S. Noradrenergic system of the zebra finch brain: Immunocytochemical study of dopamine-beta-hydroxylase. J Comp Neurol. 1998;400:207–228. [PubMed] [Google Scholar]
- Mello CV, Ribeiro S. ZENK protein regulation by song in the brain of songbirds. J Comp Neurol. 1998;393:426–438. doi: 10.1002/(sici)1096-9861(19980420)393:4<426::aid-cne3>3.0.co;2-2. [DOI] [PubMed] [Google Scholar]
- Mello CV, Velho TA, Pinaud R. Song-induced gene expression: a window on song auditory processing and perception. Ann N Y Acad Sci. 2004;1016:263–281. doi: 10.1196/annals.1298.021. [DOI] [PubMed] [Google Scholar]
- Mello CV, Vicario DS, Clayton DF. Song presentation induces gene expression in the songbird forebrain. Proc Nat Acad Sci USA. 1992;89:6818–6822. doi: 10.1073/pnas.89.15.6818. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minzenberg MJ, Watrous AJ, Yoon JH, Ursu S, Carter CS. Modafinil shifts human locus coeruleus to low-tonic, high-phasic activity during functional MRI. Science. 2008;322:1700–1702. doi: 10.1126/science.1164908. [DOI] [PubMed] [Google Scholar]
- Moron JA, Brockington A, Wise RA, Rocha BA, Hope BT. Dopamine uptake through the norepinephrine transporter in brain regions with low levels of the dopamine transporter: evidence from knock-out mouse lines. J Neurosci. 2002;22:389–395. doi: 10.1523/JNEUROSCI.22-02-00389.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neubauer RL. Super-normal length song preferences of female zebra finches (Taeniopygia guttata) and a theory of the evolution of bird song. Evol Ecol. 1999;13:365–380. [Google Scholar]
- Newman-Tancredi A, Audinot-Bouchez V, Gobert A, Millan MJ. Noradrenaline and adrenaline are high affinity agonists at dopamine D4 receptors. Eur J Pharmacol. 1997;319:379–383. doi: 10.1016/s0014-2999(96)00985-5. [DOI] [PubMed] [Google Scholar]
- Nick TA, Konishi M. Dynamic control of auditory activity during sleep: Correlation between song response and EEG. Proc Nat Acad Sci USA. 2001;98:14012–14016. doi: 10.1073/pnas.251525298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nyronen T, Pihlavisto M, Peltonen JM, Hoffren AM, Varis M, Salminen T, Wurster S, Marjamaki A, Kanerva L, Katainen E, Laaksonen L, Savola JM, Scheinin M, Johnson MS. Molecular mechanism for agonist-promoted alpha(2A)-adrenoceptor activation by norepinephrine and epinephrine. Mol Pharmacol. 2001;59:1343–1354. doi: 10.1124/mol.59.5.1343. [DOI] [PubMed] [Google Scholar]
- Olpe HR, Steinmann M. Responses of locus coeruleus neurons to neuropeptides. Prog Brain Res. 1991;88:241–248. doi: 10.1016/s0079-6123(08)63813-3. [DOI] [PubMed] [Google Scholar]
- Paladini CA, Williams JT. Noradrenergic inhibition of midbrain dopamine neurons. J Neurosci. 2004;24:4568–4575. doi: 10.1523/JNEUROSCI.5735-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Panzica GC, Aste N, Castagna C, Viglietti-Panzica C, Balthazart J. Steroid-induced plasticity in the sexually dimorphic vasotocinergic innervation of the avian brain: behavioral implications. Brain Res Brain Res Rev. 2001;37:178–200. doi: 10.1016/s0165-0173(01)00118-7. [DOI] [PubMed] [Google Scholar]
- Panzica GC, Plumari L, Garcia-Ojeda E, Deviche P. Central vasotocin-immunoreactive system in a male passerine bird (Junco hyemalis) J Comp Neurol. 1999;409:105–117. [PubMed] [Google Scholar]
- Payne JL, Quiroz JA, Zarate CA, Jr, Manji HK. Timing is everything: does the robust upregulation of noradrenergically regulated plasticity genes underlie the rapid antidepressant effects of sleep deprivation? Biol Psychiatry. 2002;52:921–926. doi: 10.1016/s0006-3223(02)01676-1. [DOI] [PubMed] [Google Scholar]
- Person AL, Gale SD, Farries MA, Perkel DJ. Organization of the songbird basal ganglia, including area X. J Comp Neurol. 2008;508:840–866. doi: 10.1002/cne.21699. [DOI] [PubMed] [Google Scholar]
- Phan ML, Pytte CL, Vicario DS. Early auditory experience generates long-lasting memories that may subserve vocal learning in songbirds. Proc Natl Acad Sci U S A. 2006;103:1088–1093. doi: 10.1073/pnas.0510136103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prove E. Courtship and Testosterone in Male Zebra Finches (Taeniopygia-Guttata-Castanotis Gould) J Comp Ethol. 1978;48:47–67. [Google Scholar]
- Puelles L, Martinez-de-la-Torre M, Paxinos G, Watson C, Martinez S. The chick brain in stereotaxic coordinates: An atlas featuring neuromeric subdivisions and mammalian homologies. Academic Press; 2007. [Google Scholar]
- Rasmussen K, Morilak DA, Jacobs BL. Single unit activity of locus coeruleus neurons in the freely moving cat. I. During naturalistic behaviors and in response to simple and complex stimuli. Brain Res. 1986;371:324–334. doi: 10.1016/0006-8993(86)90370-7. [DOI] [PubMed] [Google Scholar]
- Reiner A, Laverghetta A, Meade C, Cuthbertson S, Bottjer SW. An immunohistochemical and pathway tracing study of the striatopallidal organization of area x in the male zebra finch. J Comp Neurol. 2004a;469:239–261. doi: 10.1002/cne.11012. [DOI] [PubMed] [Google Scholar]
- Reiner A, Perkel DJ, Bruce LL, Butler AB, Csillag A, Kuenzel W, Medina L, Paxinos G, Shimizu T, Striedter G, Wild M, Ball GF, Durand S, Gunturkun O, Lee DW, Mello CV, Powers A, White SA, Hough G, Kubikova L, Smulders TV, Wada K, Dugas-Ford J, Husband S, Yamamoto K, Yu J, Siang C, Jarvis ED, Guturkun O. Revised nomenclature for avian telencephalon and some related brainstem nuclei. J Comp Neurol. 2004b;473:377–414. doi: 10.1002/cne.20118. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Remage-Healey L, Maidment NT, Schlinger BA. Forebrain steroid levels fluctuate rapidly during social interactions. Nat Neurosci. 2008;11:1327–1334. doi: 10.1038/nn.2200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ribeiro S, Mello CV. Gene expression and synaptic plasticity in the auditory forebrain of songbirds. Learn Mem. 2000;7:235–243. doi: 10.1101/lm.34400. [DOI] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Lesions to the medial preoptic area affect singing in the male European starling (Sturnus vulgaris) Horm Behav. 1999;36:276–286. doi: 10.1006/hbeh.1999.1549. [DOI] [PubMed] [Google Scholar]
- Riters LV, Ball GF. Sex differences in the densities of alpha 2-adrenergic receptors in the song control system, but not the medial preoptic nucleus in zebra finches. J Chem Neuroanat. 2002;23:269–277. doi: 10.1016/s0891-0618(02)00005-4. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Ball GF. Seasonal changes in the densities of alpha(2) noradrenergic receptors are inversely related to changes in testosterone and the volumes of song control nuclei in male European starlings. J Comp Neurol. 2002;444:63–74. doi: 10.1002/cne.10131. [DOI] [PubMed] [Google Scholar]
- Riters LV, Eens M, Pinxten R, Duffy DL, Balthazart J, Ball GF. Seasonal changes in courtship song and the medial preoptic area in male European starlings (Sturnus vulgaris) Horm Behav. 2000;38:250–261. doi: 10.1006/hbeh.2000.1623. [DOI] [PubMed] [Google Scholar]
- Riters LV, Alger SJ. Neuroanatomical evidence for indirect connections between the medial preoptic nucleus and the song control system: possible neural substrates for sexually motivated song. Cell Tiss Res. 2004;316:35–44. doi: 10.1007/s00441-003-0838-6. [DOI] [PubMed] [Google Scholar]
- Riters LV, Pawlisch BA. Evidence that norepinephrine influences responses to male courtship song and activity within song control regions and the ventromedial nucleus of the hypothalamus in female European starlings. Brain Res. 2007;1149:127–140. doi: 10.1016/j.brainres.2007.02.059. [DOI] [PubMed] [Google Scholar]
- Ryan SM, Arnold AP, Elde RP. Enkephalin-like immunoreactivity in vocal control regions of the zebra finch brain. Brain Res. 1981;229:236–240. doi: 10.1016/0006-8993(81)90763-0. [DOI] [PubMed] [Google Scholar]
- Sakaguchi H, Saito N. The Acetylcholine and Catecholamine Contents in Song Control Nuclei of Zebra Finch During Song Ontogeny. Dev Brain Res. 1989;47:313–317. doi: 10.1016/0165-3806(89)90189-2. [DOI] [PubMed] [Google Scholar]
- Sasaki A, Sotnikova TD, Gainetdinov RR, Jarvis ED. Social context-dependent singing-regulated dopamine. J Neurosci. 2006;26:9010–9014. doi: 10.1523/JNEUROSCI.1335-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmidt MF, Konishi M. Gating of auditory responses in the vocal control system of awake songbirds. Nature. 1998;1:513–518. doi: 10.1038/2232. [DOI] [PubMed] [Google Scholar]
- Sirvio J, Macdonald E. Central alpha 1-adrenoceptors: their role in the modulation of attention and memory formation. Pharmacol Ther. 1999;83:49–65. doi: 10.1016/s0163-7258(99)00017-0. [DOI] [PubMed] [Google Scholar]
- Sizemore M, Perkel DJ. Noradrenergic and GABA B receptor activation differentially modulate inputs to the premotor nucleus RA in zebra finches. J Neurophysiol. 2008;100:8–18. doi: 10.1152/jn.01212.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smeets WJAJ, Gonzalez A. Catecholamine systems in teh brain of vertebrates: a new perspective through a comparative approach. Brain Res Rev. 2000;33:308–379. doi: 10.1016/s0165-0173(00)00034-5. [DOI] [PubMed] [Google Scholar]
- Sockman KW. Neural orchestration of mate-choice plasticity in songbirds. J Ornithol. 2007;148:S225–S230. [Google Scholar]
- Sockman KW, Gentner TQ, Ball GF. Recent experience modulates forebrain gene-expression in response to mate-choice cues in European starlings. Proc R Soc Lond B. 2002;269:2479–2485. doi: 10.1098/rspb.2002.2180. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sockman KW, Salvante KG. The integration of song environment by catecholaminergic systems innervating the auditory telencephalon of adult female European starlings. Dev Neurobiol. 2008;68:656–668. doi: 10.1002/dneu.20611. [DOI] [PubMed] [Google Scholar]
- Soha JA, Shimizu T, Doupe AJ. Development of the catecholaminergic innervation of the song system of the male zebra finch. J Neurobiol. 1996;29:473–489. doi: 10.1002/(SICI)1097-4695(199604)29:4<473::AID-NEU5>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- Solis MM, Perkel DJ. Noradrenergic modulation of activity in a vocal control nucleus in vitro. J Neurophysiol. 2006;95:2265–2276. doi: 10.1152/jn.00836.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sossinka R, Bohner J. Song types in the zebra finch (Poephila guttata castanotis) J Comp Ethol. 1980;53:123–132. [Google Scholar]
- Steriade M, Iosif G, Apostol V. Responsiveness of thalamic and cortical motor relays during arousal and various stages of sleep. J Neurophysiol. 1969;32:251–265. doi: 10.1152/jn.1969.32.2.251. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The locus coeruleus: a cytoarchitectonic, Golgi and immunohistochemical study in the albino rat. Brain Res. 1976;110:39–56. doi: 10.1016/0006-8993(76)90207-9. [DOI] [PubMed] [Google Scholar]
- Swanson LW. The projections of the ventral tegmental area and adjacent regions: a combined fluorescent retrograde tracer and immunofluorescence study in the rat. Brain Res Bull. 1982;9:321–353. doi: 10.1016/0361-9230(82)90145-9. [DOI] [PubMed] [Google Scholar]
- Szekely AD, Boxer MI, Stewart MG, Csillag A. Connectivity of the lobus parolfactorius of the domestic chicken (Gallus domesticus): an anterograde and retrograde pathway tracing study. J Comp Neurol. 1994;348:374–393. doi: 10.1002/cne.903480305. [DOI] [PubMed] [Google Scholar]
- Teramitsu I, White SA. FoxP2 Regulation during Undirected Singing in Adult Songbirds. J Neurosci. 2006;26:7390–7394. doi: 10.1523/JNEUROSCI.1662-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terleph TA, Lu K, Vicario DS. Response properties of the auditory telencephalon in songbirds change with recent experience and season. PLoS ONE. 2008;3:e2854. doi: 10.1371/journal.pone.0002854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tononi G, Cirelli C. Modulation of brain gene expression during sleep and wakefulness: a review of recent findings. Neuropsychopharmacology. 2001;25:S28–S35. doi: 10.1016/S0893-133X(01)00322-0. [DOI] [PubMed] [Google Scholar]
- Tononi G, Pompeiano M, Cirelli C. The locus coeruleus and immediate-early genes in spontaneous and forced wakefulness. Brain Res Bull. 1994;35:589–596. doi: 10.1016/0361-9230(94)90173-2. [DOI] [PubMed] [Google Scholar]
- Torres GE, Gainetdinov RR, Caron MG. Plasma membrane monamine transporters: structure, regulation and function. Nat Rev Neurosci. 2003;4:13–25. doi: 10.1038/nrn1008. [DOI] [PubMed] [Google Scholar]
- Vallet E, Beme I, Kreutzer M. Two-note syllables in canary songs elicit high levels of sexual display. Anim Behav. 1998;55:291–297. doi: 10.1006/anbe.1997.0631. [DOI] [PubMed] [Google Scholar]
- Velho TAF, Pinaud R, Rodrigues PV, Mello CV. Co-induction of activity-dependent genes in songbirds. Eur J Neurosci. 2005;22:1667–1678. doi: 10.1111/j.1460-9568.2005.04369.x. [DOI] [PubMed] [Google Scholar]
- Vyas A, Harding C, McGowan J, Snare R, Bogdan D. Noradrenergic neurotoxin, N-(2-chloroethyl)-N-ethyl-2-bromobenzylamine hydrochloride (DSP-4), treatment eliminates estrogenic effects on song responsiveness in female zebra finches (Taeniopygia guttata) Behav Neurosci. 2008;122:1148–1157. doi: 10.1037/0735-7044.122.5.1148. [DOI] [PubMed] [Google Scholar]
- Walters MJ, Collado D, Harding CF. Estrogenic Modulation of Singing in Male Zebra Finches - Differential-Effects on Directed and Undirected Songs. Anim Behav. 1991;42:445–452. [Google Scholar]
- Waterhouse BD, Sessler FM, Cheng JT, Woodward DJ, Azizi SA, Moises HC. New evidence for a gating action of norepinephrine in central neuronal circuits of mammalian brain. Brain Res Bull. 1988;21:425–432. doi: 10.1016/0361-9230(88)90154-2. [DOI] [PubMed] [Google Scholar]
- Waterman SA, Harding CF. Neurotoxic effects of DSP-4 on the central noradrenergic system in male zebra finches. Behav Brain Res. 2008;188:271–280. doi: 10.1016/j.bbr.2007.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weyand TG, Boudreaux M, Guido W. Burst and tonic response modes in thalamic neurons during sleep and wakefulness. J Neurophysiol. 2001;85:1107–1118. doi: 10.1152/jn.2001.85.3.1107. [DOI] [PubMed] [Google Scholar]
- Williams H. Choreography of song, dance and beak movements in the zebra finch (Taeniopygia guttata) J ExpBiol. 2001;204:3497–3506. doi: 10.1242/jeb.204.20.3497. [DOI] [PubMed] [Google Scholar]
- Woolley SC, Doupe AJ. Social context-induced song variation affects female behavior and gene expression. PLoS Biol. 2008;6:e62. doi: 10.1371/journal.pbio.0060062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yanagihara S, Hessler NA. Modulation of singing-related activity in the songbird ventral tegmental area by social context. Eur J Neurosci. 2006;24:3619–3627. doi: 10.1111/j.1460-9568.2006.05228.x. [DOI] [PubMed] [Google Scholar]
- Zann RA. Zebra Finch: A Synthesis of Lab and Field Studies. Oxford University Press Inc; 1996. [Google Scholar]
- Zeigler MH, Marler P. Neuroscience of Birdsong. Cambridge University Press; 2008. [Google Scholar]


