Abstract
BAFF inhibition is a new B cell-directed therapeutic strategy for autoimmune disease. Our purpose was to analyze the effect of BAFF/APRIL availability on the naive and Ag-activated B cell repertoires in systemic lupus erythematosus, using the autoreactive germline D42 H chain (glD42H) site-directed transgenic NZB/W mouse. In this article, we show that the naive Vκ repertoire in both young and diseased glD42H NZB/W mice is dominated by five L chains that confer no or low-affinity polyreactivity. In contrast, glD42H B cells expressing L chains that confer high-affinity autoreactivity are mostly deleted before the mature B cell stage, but are positively selected and expanded in the germinal centers (GCs) as the mice age. Of these, the most abundant is VκRF (Vκ16-104*01), which is expressed by almost all IgG anti-DNA hybridomas derived from the glD42H mouse. Competition with nonautoreactive B cells or BAFF/APRIL inhibition significantly inhibited selection of glD42H B cells at the late transitional stage, with only subtle effects on the glD42H-associated L chain repertoire. However, glD42H/VκRF-encoded B cells were still vastly overrepresented in the GC, and serum IgG anti-DNA Abs arose with only a slight delay. Thus, although BAFF/APRIL inhibition increases the stringency of negative selection of the naive autoreactive B cell repertoire in NZB/W mice, it does not correct the major breach in B cell tolerance that occurs at the GC checkpoint.
Systemic lupus erythematosus (SLE) is an autoimmune disorder in which pathogenic autoantibodies directed to nuclear material initiate inflammation in target tissues. SLE patients have defects in negative selection of autoreactive B cells at the immature and transitional checkpoints (1) and also fail to restrain pathogenic effector B cells that arise in the germinal center (GC) (2, 3). Understanding how these defects contribute to the production of pathogenic autoantibodies will allow therapy for SLE to be directed to the appropriate B cell developmental stage.
Signals transduced by interaction of the B cell homeostatic cytokine BAFF with its receptor, BAFF-R, regulate B cell selection at the late transitional stage and act as a rheostat for the size of the mature B cell compartment (4, 5). Autoreactive B cells that escape negative selection in the bone marrow often downregulate both cell surface IgM and BAFF-R and therefore do not compete well for BAFF in the periphery. Conversely, when BAFF levels are high, autoreactive B cells may be rescued (6, 7). The monoclonal anti-BAFF Ab belimumab has recently been approved for the treatment of SLE in humans (8), but it is unclear whether its therapeutic effect is actually due to alterations in B cell selection, nor is it known whether BAFF or APRIL inhibition alters the selection of effector autoreactive B cells.
The purpose of our experiments was to use germline D42 H chain (glD42H) NZB/W mice bearing a site-directed anti-dsDNA Ab–derived VH11 transgene to determine how BAFF availability and BAFF/APRIL blockade influence the selection of naive and Ag-activated autoreactive B cells during the evolution of SLE. Non-autoimmune glD42H mice have a low frequency of anti-dsDNA producing B cells as a result of clonal deletion, anergy. and receptor editing (9, 10). When glD42H is introduced into lupus-prone NZB/W mice, high-affinity IgG anti-DNA Abs appear in the serum by 6–7 mo of age, and nearly all spontaneous IgG anti-DNA Ab–producing hybridomas use the original D42 L chain VκRF (Vκ16-104*01)/Jκ5, which confers high-affinity anti-DNA reactivity (11, 12).
In this article, we show that B cells expressing a repertoire of L chains that confer no or low-affinity autoreactivity are positively selected into the naive B cell pool of glD42H NZB/W mice. In contrast, B cells with high-affinity autoreactivity are mostly deleted in the bone marrow and at the early transitional B cell stage, but are preferentially selected and expanded in the GCs of diseased mice. Competition with a diverse repertoire markedly inhibits selection of glD42H-expressing B cells into the naive B cell repertoire, and selection of these cells is further inhibited by BAFF/APRIL blockade with TACI-Ig. However, TACI-Ig does not prevent selection or expansion of high-affinity autoreactive B cells in the GCs. These findings show that BAFF/APRIL inhibition does not prevent the major breach in B cell tolerance that occurs during the GC response in NZB/W mice.
Materials and Methods
Mice
Female NZB/W glD42H mice were bred with NZW males (The Jackson Laboratory, Bar Harbor, ME). Transgenic (IgDb allotype-positive) female offspring were tested for proteinuria and anti-dsDNA Abs every 4 wk, as previously described (13). Groups of mice were sacrificed for analysis at 8 and 32 wk of age.
Bone marrow chimeras were generated by transfer of either 30:70 or 50:50% glD42H/wild-type (wt) bone marrow into totally irradiated 8- to 12-wk-old NZB/W F1 females. Groups of four or five chimeric mice were given 100 μg TACI-Ig (13) three times per week continuously, starting at day 3 after transplantation or no treatment. Mice were sacrificed 12–16 wk after transplant.
Flow cytometry
Spleen and bone marrow B cells from 8- and 32-wk-old glD42H mice and from chimeric mice harvested 12–16 wk after bone marrow transplant were gated using anti-CD19 (spleens) or anti-B220 (bone marrows), as previously described (13, 14) (Fig. 1). Transitional 1 (T1) cells were CD23lo/IgMhi/CD21−, transitional 2 (T2)-MZP cells were CD23hi/IgMhi/CD21+, marginal zone (MZ) cells were CD23lo/IgMhi/CD21hi, follicular (FO) cells were IgDhi/IgMint, GC cells were CD19+/IgM−/IgD−/PNA+/Fas+, and class-switched cells were IgD−/IgM−. Plasma cells (PCs) were B220int/IgD−/CD138hi (15). Bone marrow B cells were separated into B220lo/IgD−/IgMlo (pro- + pre-B cells), B220hi/IgD−/IgMlo (immature), and B220lo/int/IgD−/CD138hi (PC) subsets. For the bone marrow chimeras, anti-IgDb was used to sort the FO subset.
FIGURE 1.

Sorting strategy for spleen and bone marrow cells. A, Spleen: After exclusion of clumps and gating on lymphocytes, CD19+ cells were gated into CD23+ and CD23− (a), IgD+/IgM into (FO) (b) and IgM−/IgD− (class-switched) (c) fractions. d, T2-MZP cells are CD23+/IgMhi/CD21hi. e, T1 cells are CD23−/IgMlo/CD21lo. MZ cells are CD23−/IgMhi/CD21hi. f, GC cells are IgM−/IgD−/Fas+/PNAhi. g, Lymphocytes were gated into the B220lo/IgDlo fraction. h, PCs are B220lo/IgDlo/CD138hi. B, Bone marrow: B220+ B cells were gated into R1 (immature and recirculating) and R2 (pro- + pre-) B cell fractions. Upper panels show that the R1 population is CD43−. IgM+/IgD− immature B cells were sorted from R1 after gating for the B220lo population. The lower panels show that 60% of the R2 gate consists of pre-B cells (CD43−/IgM−/IgD−). Pro-B cells do not express Vκ L chains.
Single-cell sorting and PCR
Single cells were sorted from B cell subsets from three to five individual mice from the groups of mice described above, and cDNA was synthesized as previously described (16). cDNA was used as template for a 20-μL PCR reaction containing 2 μL cDNA, 10 μL FastStart PCR master (Roche), and 62.5 nM of each primer (Supplemental Table I). The PCR program was as follows: 4 min at 94°C; 50 cycles of 30 s at 94°C, 30 s at 60°C, and 55 s at 72°C, followed by 10 min at 72°C. A second round was then performed using primers specific for CDR1 and CDR3 of glD42H. Wells that yielded a product of the correct size contained transgenic B cells, and these were then subjected to PCR for Vκ, as previously described (17). PCR products were sequenced by Genewiz (South Plainfield, NJ), and sequences were identified using the International ImMunoGeneTics database.
Real-time PCR was performed as previously described (18). Primers are listed in Supplemental Table I. The average of the raw data for each sample (Ct value) was normalized to the internal control (housekeeping gene β-actin). Ratios of D42/CD19, VκRF/CD19, and VκRF/D42 were then calculated.
Expression studies
The glD42H H chain and selected germline L chain variable regions (Supplemental Table II; synthesized by Genscript, New Piscataway, NJ) were cloned into mouse IgG2a and κ expression vectors, respectively (InvivoGen, San Diego, CA), using the manufacturer’s instructions. H and L chains were cotransfected into 293T, cells using the LyoVec Kit (InvivoGen). Then 48-h supernatants were normalized to a concentration of 1 μg/ml and tested by ELISA for reactivity to cardiolipin, dsDNA, histones, phosphatidyl serine, phosphatidyl choline, insulin, chymotryp-sinogen A, cytochrome C, and keyhole limpet hemocyanin, all plated at 10 μg/ml. To increase the relative avidity of the Abs for dsDNA, we also preincubated the supernatant with the secondary Ab to generate preformed complexes.
Statistical analysis of repertoire data
The data on the L chain repertoire in the various B cell subsets come in the form of very large and sparse r × c contingency tables (Supplemental Tables II and III). The columns of the table represent the B cell subsets (e.g., pro- + pre-B, immature B, T1, T2, MZ cells, FO cells, GC cells, PCs) in each group of mice (8-wk-old glD42H, 32-wk-old glD42H, untreated and treated bone marrow chimeras), and the rows represent the many possible L chains.
The table entries were the frequency of L chains of a given Vκ genotype within B cell subsets. Column totals were considered “fixed” (i.e., column sampling), so that L chain “percentages” were computed as so-called column percentages. Thus, the column totals were denoted by n1, n2, …, nc, where c = the total number of B cell subsets being considered in the analysis. By convention, r, the number of rows, corresponded to the number of L chains that have representation in the table. In other words, an L chain must have had at least one nonzero frequency count to be considered in the table and the calculations cited below.
Owing to the typically small sample sizes of single cells, the usual Pearson χ2 statistic is not valid for determining whether differences exist in the distributions of L chains across B cell subsets. Instead, the Fisher exact test is applicable. However, the computing time required for running the exact test on large, sparse tables is prohibitive and, moreover, the power of the test is low. Accordingly, identifying L chains of interest without using formal inferential methods (i.e., without using p values or confidence intervals) is needed. This exploratory approach is frequently used in identifying interesting genes in gene expression microarray studies (19-21).
To this end, we used a novel method for “screening” the r × c table for cell frequencies that appear to be inconsistent, with the null hypothesis that the B cell subset and L chain distributions are independent. This approach was founded on a method advocated by Snedecor and Cochran (22), which is based on identifying table cells that contribute large values to the overall χ2 statistic (even though the χ2 is not being used for inference). The contribution of the ijth cell is χ2ij = (Oij − Eij)2/Eij, where Oij is the observed frequency in cell ij and Eij is the expected cell frequency, computed in the usual way. The values of χ2ij for i = 1, 2,…, r and j = 1, 2,…, c were computed. Then, Pij = 100*χ2ij/χ2, the percentage of overall χ2 contributed by the ijth cell, was calculated.
The values of Pij were ordered from largest to smallest, and the order statistics, P(1) ≥ P(2), ≥ …, ≥ P(rc) were enumerated. P(i) was plotted against i to form a scree plot, as is commonly done in principal components analysis (23). Typically, one would look for the “elbow,” or turning point, of the scree plot to determine a cut-off point of interest. In effect, this cut-off value would aid in identifying L chains whose contributions to χ2 are clearly deviant from the null hypothesis of independence (see Supplemental Fig. 1 for examples). Because visualizing the elbow is a subjective determination, a more objective analytical approach was used, whereby the cumulative order statistics were computed [i.e., P(1), P(1) + P(2), P(1) + P(2) + P(3), and so on]. When a cumulative sum of 50% was reached, then the L chains that contributed to that sum were targeted for more in-depth investigation. By this, we mean further investigation in the laboratory, using other methods, but not by conducting a usual Pearson χ2 test for proportions because performing the χ2 test at the usual significance levels (e.g., p < 0.05 or 0.01) is invalid when the decision to carry out the comparison is based on preselection of results that appeared to be interesting based on the scree plot methodology. With the arbitrary cumulative sum of 50% as a guide, 49 of the 84 identified Vκ genes were represented within the top 50% or contributed >5% to the χ2 in any of the comparisons performed (Table I, Supplemental Tables II, III). Of these 49, 40 genes contributed >2% of the total Vκ repertoire in any of the subsets.
Table I.
Comparison of Vκ repertoires between subsets
| Contribution to χ2a | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Mouse | Mouse Group Comparison | B Cell Subset Comparison | No. Vκ Genes Representedb | No. Genes Contributing >5% | No. Genes Contributing 50% | Top Gene Contributes (%) | Top 3 Genes Contribute (%) | Top 5 Genes Contribute (%) | Top 10 Genes Contribute (%) |
| Chimera | Pre-/Pro- versus Imm | 48 | 3 | 13 | 9.2 | 20.7 | 27.8 | 42.0 | |
| Chimera | Imm versus FO | 48 | 4 | 9 | 9.8 | 24.7 | 34.1 | 53.8 | |
| Chimera | FO versus GC | 43 | 3 | 6 | 24.7 | 40.0 | 48.6 | 70.1 | |
| Chimera | Control versus TACI-Ig | FO | 43 | 3 | 12 | 5.5 | 16.6 | 25.9 | 45.7 |
| Chimera | Control versus TACI-Ig | GC | 34 | 2 | 11 | 9.2 | 21.5 | 29.3 | 48.6 |
| Chimera | Control versus TACI-Ig | PC | 31 | 5 | 6 | 16.9 | 34.6 | 47.3 | 67.1 |
| Chimera | Versus young | FO | 39 | 10 | 7 | 12.0 | 27.8 | 39.8 | 69.4 |
| Chimera | Versus old | FO | 37 | 7 | 6 | 16.3 | 32.6 | 48.9 | 68.4 |
| Chimera | Versus old | GC | 27 | 4 | 8 | 12.4 | 28.6 | 40.0 | 58.9 |
| WT | Young versus old | T1 | 37 | 5 | 7 | 8.8 | 25.9 | 41.1 | 59.9 |
| WT | Young versus old | T2 | 26 | 10 | 8 | 10.2 | 26.3 | 37.6 | 63.0 |
| WT | Young versus old | MZ | 11 | 8 | 3 | 22.1 | 52.1 | 76.1 | 99.8 |
| WT | Young versus old | FO | 28 | 5 | 7 | 12.3 | 28.6 | 40.3 | 60.4 |
| WT | Young | T1 versus FO | 31 | 2 | 9 | 14.5 | 29.5 | 37.5 | 54.0 |
| WT | Old | T1 versus FO | 37 | 5 | 7 | 13.6 | 32.7 | 43.0 | 58.7 |
| WT | Old | FO versus GC | 22 | 2 | 1 | 49.7 | 61.3 | 69.2 | 81.2 |
In the two subsets being compared.
See Materials and Methods
Other statistical analyses
Comparisons in Figs. 2, 4, 5, and 8 were performed using the Mann–Whitney U test. The p values ≤ 0.05 were considered significant.
FIGURE 2.
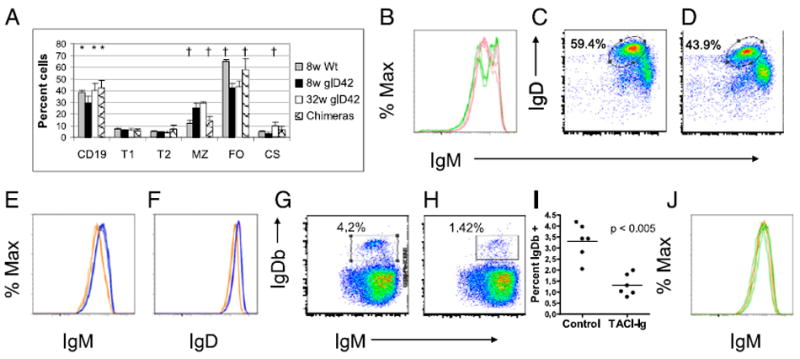
Phenotype of glD42H transgenic mice. A, glD42H mice have mild B cell lymphopenia, with an increase in MZ B cells and a decrease in FO B cells, compared with wt NZB/W. These defects are corrected in bone marrow chimeras (mean + 1 SD; *p < 0.05, †p < 0.01 compared with 8-wk-old glD42H). B, Increased IgM expression on B cells of glD42H (green) compared with wt NZB/W littermates (pink) reflects the enlarged IgMhi MZ subset in transgenic mice. C–F, IgM and IgD staining of CD19 gated cells from wt (C; representative blue histograms in E and F) and glD42H (D; representative orange histograms in E and F) mice shows modest IgM and IgD downregulation in the FO population (E and F gated on ovals in C and D) of age-matched transgenic compared with wt NZB/W mice. G and H, Representative FACS plots of transgenic IgDb B cells in the spleens of 50:50 wt/glD42H bone marrow chimeras reconstituted without (G) or with (H) TACI-Ig treatment. I, The percentage of IgDb B cells is significantly decreased in TACI-Ig–treated chimeras. Horizontal bar indicates the mean. J, IgM expression on transgenic IgDb B cells (representative green histograms) from glD42H chimeras is the same as that on endogenous IgDa B cells (representative orange histograms). Five or six mice were analyzed per group, and experiments were repeated once.
FIGURE 4.
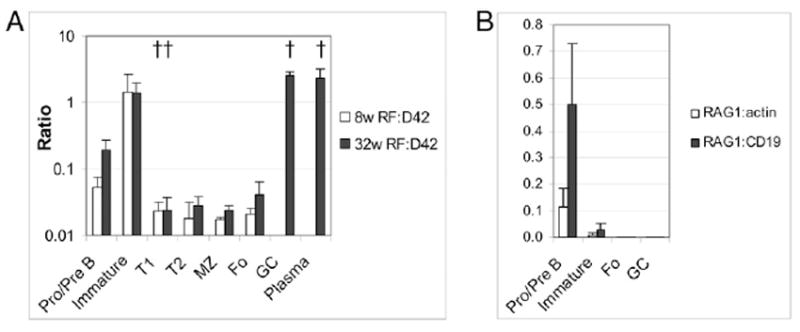
Real-time PCR of B cell subsets. A, Ratio of VκRF to glD42H in sorted subset pellets. Negative selection of VκRF occurs at the immature to T1 stage, and positive selection occurs at the GC stage (†p < 0.01 compared with previous B cell developmental stage). B, RAG-1 was detected in bone marrow B cells, but not in FO or GC B cells (similar results were obtained for RAG-2). Pellets from three individual mice were used per group, and experiments were repeated once.
FIGURE 5.
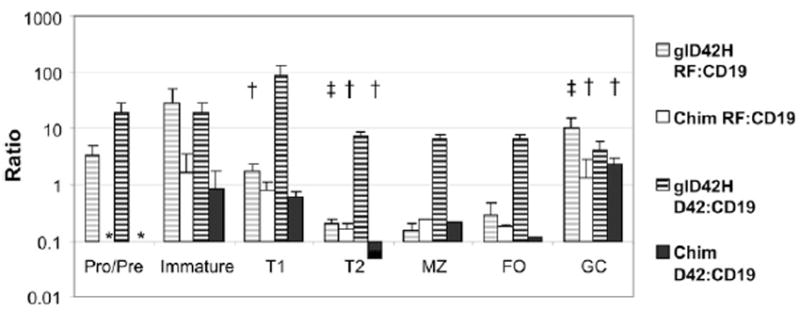
Quantitative analysis of H and L chains from each B cell subset from glD42H mice and 50:50 wt/glD42H chimeric mice. Ratio of VκRF and glD42H to CD19 expression in B cell subsets. Negative selection of glD42H/VκRF-expressing B cells occurs at the T1 stage, and positive selection occurs at the GC stage. Pellets from three individual mice were used per group, and experiments were repeated once. *Not done, †p < 0.01, ‡p < 0.05 compared with previous B cell developmental stage.
FIGURE 8.
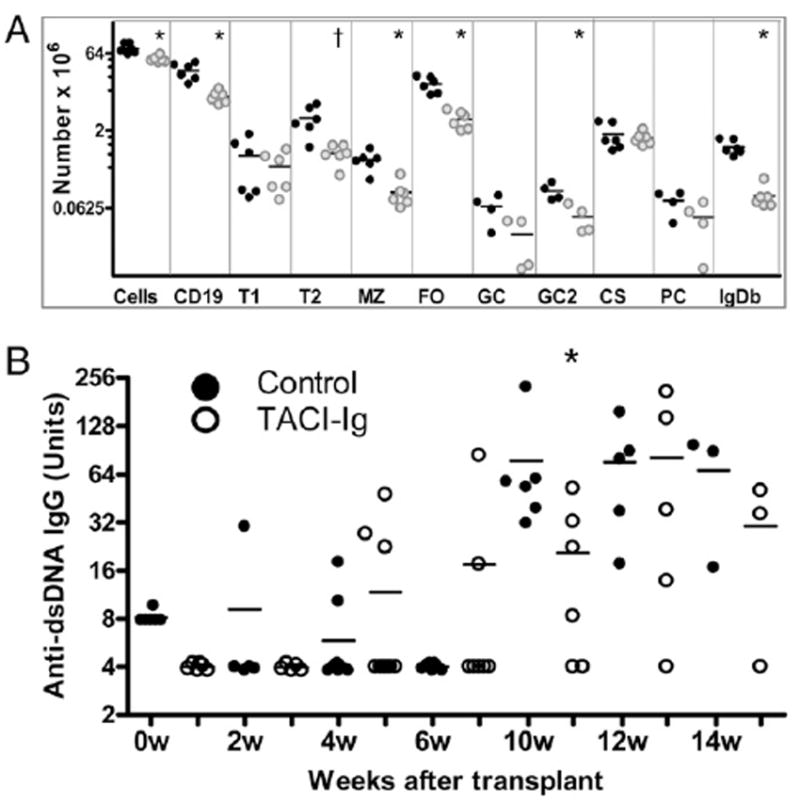
Splenic B cell counts and autoantibodies from control and TAI-Ig–treated 50:50 wt/glD42H bone marrow chimeras. A, B cell counts per spleens of the six individual mice in each group used for Experiment 2. Data for GC and PC subsets come from Experiment 1. Data for the GC2 subset comes from a third subset of chimeras (Experiment 3, four per group). Similar results for all other subsets were obtained for the mice used in Experiments 1 and 3. Each symbol represents the total number of cells per spleen from an individual mouse (control: black circles; TACI-Ig: gray circles). Bars represent the mean for each group; *p < 0.005, †p < 0.01. B, Serial serum IgG anti-dsDNA Abs in TACI-Ig–treated and untreated bone marrow chimeras. Titers were normalized to those of a high titer control and shown in arbitrary units. The only difference was observed at 10 wk; *p < 0.02, five to eight mice per group.
Results
B cell phenotype of glD42H mice
The 8-wk-old glD42H mice have mild B cell lymphopenia with an expanded MZ (Fig. 2A, 2B). The percentage of CD19-positive cells and of class-switched B cells increases as the mice age to 32 wk (Fig. 2A). We have previously reported that in nonautoimmune glD42H mice surface levels of IgM are decreased and a large proportion of B cells are anergic (9, 11). In contrast, in the autoimmune glD42H NZB/W mice we observed only a modest downregulation of surface IgM and IgD in the FO population compared with nontransgenic NZB/W littermates (Fig. 2 C–F). As previously reported (11), IgM anti-dsDNA Abs were first detected in the serum of glD42H NZB/W mice at 9–11 wk of age and IgG anti-dsDNA Abs at 16–18 wk (data not shown).
Single-cell PCR analysis of the B cell repertoire in glD42H mice
We performed single-cell PCR of splenic B cell subpopulations (Fig. 1A) from 8-wk-old and 32-wk-old glD42H mice (three to five per group) and analyzed the L chain repertoire associated with use of the glD42H H. A total of 84 Vκ genes from the International ImMunoGeneTics database were represented, indicating good coverage by our methodology. Of these, 40 contributed >5% of the χ2 value in any of the comparisons we performed (Table I, Supplemental Table II); these 40 genes are shown in Fig. 3.
FIGURE 3.
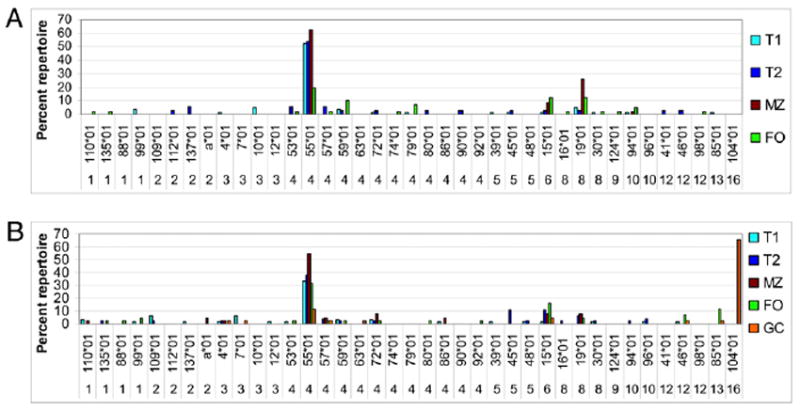
Repertoire analysis of glD42H-associated Vκ chains in 8-wk-old (A) and 32-wk-old (B) glD42H NZB/W mice. Genes that contribute >5% of the χ2 value in any of the comparisons are shown. A total of 40–85 sequences from three to five individual mice were analyzed per subset. The y-axis shows the percentage of the total repertoire for each subset. The complete dataset for the glD42H mice is shown in Supplemental Table II.
Both the MZ and FO B cell repertoires in young mice and old mice were dominated by three L chain genes: Vκ4–55*01, Vκ6–15*01, and Vκ8–19*01 (Fig. 3A). Jκ5 was the most frequent Jκ associated with Vκ4–55*01 and Vκ8–19*01 in both compartments; Jκ5 was associated with Vκ6–15*01 in the MZ compartment, whereas Jκ2 and Jκ4 were used in the FO compartment. In old mice, Vκ1 family members and Vκ12–46*01 and Vκ13–85*01 appeared in the FO repertoire; together, these genes constituted 32% of the FO repertoire in old mice compared with 5% in young mice (Supplemental Tables II, III). VκRF was not detected in the naive B cell repertoire of either 8-wk-old or 32-wk-old mice but accounted for 65% of the GC repertoire in 32-wk-old mice (Fig. 3B).
D42/VκRF ratios
The expression of D42 and VκRF in each B cell subset was measured by real-time PCR. We found a high ratio of VκRF/D42 in bone marrow pre-B and immature cells, with a decrease in the transitional (T1 and T2-MZP), MZ, and FO compartments and an expansion in the GC and in bone marrow PCs (Fig. 4A). Together, our single-cell PCR and real-time PCR data show that B cells expressing glD42/VκRF are generated in the bone marrow but are mostly deleted after the immature stage.
RAG expression
To determine whether peripheral receptor editing might account for the increased expression of VκRF/Jκ5 in the GCs, we measured RAG-1 and RAG-2 expression in sorted bone marrow and spleen B cells, using real-time PCR. RAG was expressed by the bone marrow pro- + pre-B cell fraction as expected, but not by FO or GC populations (Fig. 4B). Similarly, we did not detect an increase in RAG expression in the spleens by immunohistochemical methods (data not shown). Thus the high expression of VκRF/Jκ5 in GCs appears to be due to selection and expansion of rare cells.
Bone marrow chimeras
To examine the effect of BAFF/APRIL availability on selection of the B cell repertoire, we generated bone marrow chimeras, thus providing an environment in which immature autoreactive B cells were subject to normal competition for survival (24, 25). In some of these mice we depleted BAFF/APRIL by administering TACI-Ig, starting immediately after bone marrow transplant to ensure that all B cells matured in the low BAFF/APRIL environment.
The B cell phenotype in the control chimeric mice was restored to that of wt NZB/W mice, with normal numbers of B cells and normal distribution of the MZ and FO populations (Fig. 2A). However, reconstitution of transgenic B cells was poor even when the ratio of transgenic/wt cells was increased from 30:70 to 50:50 (Fig. 2G). Furthermore, IgM was no longer downregulated on the transgenic B cells (Fig. 2J), suggesting that surviving cells were not anergic. Thus, when competition from nonautoreactive cells is provided, glD42H confers a high enough affinity for autoantigen to result in deletion of the majority of transgenic B cells. To further explore this possibility, we analyzed sorted bone marrow B cells (Fig. 1B) of 50:50 wt/glD42H chimeras for expression of the glD42H transgene, using single-cell PCR. Although some mouse to mouse variability was present, we found that >90% of the B cells from the pro- + pre-B cell fraction and a variable percentage, up to 90%, from the immature bone marrow fraction expressed the glD42H, indicating a survival advantage of B cells with an already rearranged H chain. In the FO repertoire, however, only 4% of B cells expressed the glD42H (Fig. 2G, 2I), indicating that deletion of most glD42H-expressing B cells occurred after the immature B cell stage.
We confirmed these results by measuring the ratio of glD42H and VκRF/Jκ5 to CD19. The ratio of glD42H/CD19 decreased after the bone marrow immature stage and before the T2 stage. Similarly, although VκRF/Jκ5 was detected in the bone marrow and in the T1 subset, most B cells expressing glD42H/VκRF/Jκ5 were deleted by the T2 stage (Fig. 5). Importantly, the frequency of B cells bearing the major specificities Vκ4–55*01, Vκ6–15*01, Vκ8–19*01, Vκ12–46*01, and Vκ13–85*01 in the chimeric mice was similar to that in control glD42H mice (Fig. 6A), indicating that most naive glD42H B cells did not compete effectively for survival when a diverse B cell repertoire was present. Only subtle changes in the FO repertoire occurred in the chimeric mice (Fig. 6B). As in control glD42H mice, VκRF was vastly overrepresented in the GCs and PCs of the chimeric mice (Fig. 6C).
FIGURE 6.
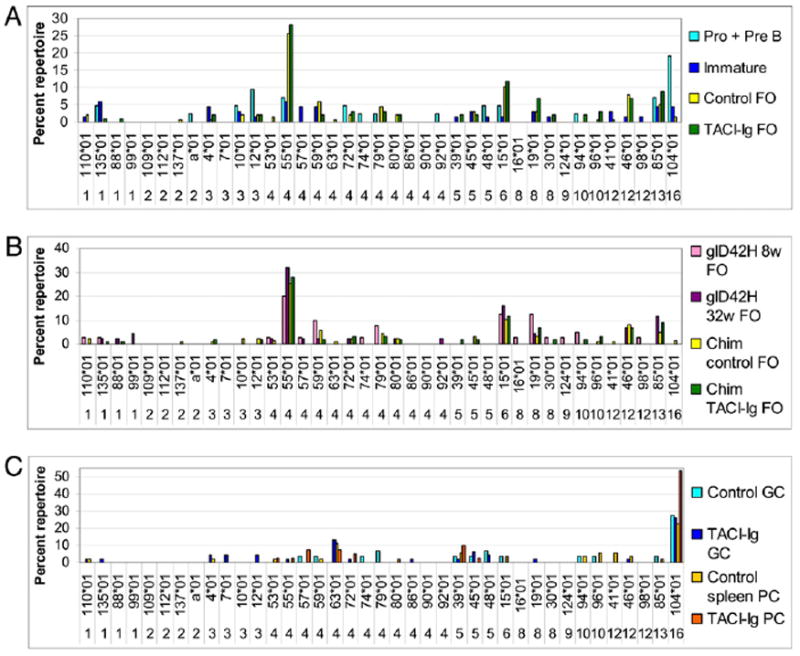
Effect of TACI-Ig on the L chain repertoire in 50:50 wt/glD42H chimeric mice. A–C, Repertoire analysis of glD42H-associated Vκ chains in control chimeric and TACI-Ig–treated mice harvested 12–16 wk after transplant. Genes that contribute >5% of the χ2 value in any of the comparisons are shown. The y-axis shows percentage of total repertoire. A, A total of 42–137 sequences from 10 mice per group sorted as individuals (4 mice per group, Experiment 1) or in pools of 3 (6 mice per group, Experiment 2) were analyzed per subset. B, A total of 40–137 sequences were analyzed per subset from 4 individual and 3 pools of chimeric mice and 4 individual wt mice in each group. C, A total of 29–53 sequences were analyzed per subset from 4 individual and 3 pools of control chimeric mice and 4 individual TACI-Ig–treated mice. The complete dataset is shown in Supplemental Table III.
To analyze the DNA-binding characteristics of the various glD42H-encoded Abs, we cotransfected glD42H/IgG2a with 13 individual L chains, including VκRF (bolded in Supplemental Table II), into 293T cells. For some L chains, more than one Jκ region was used (Supplemental Table II). Binding was observed only to dsDNA, cardiolipin, and histones, with no binding to any of the other Ags in the panel. Apart from VκRF, we identified seven other autoreactive L chains with variable binding to DNA, cardiolipin, or histones (Fig. 7). Importantly, of the five dominant L chains in the naive repertoire, only Vκ12–46*01 conferred weak reactivity with histones alone. Thus these L chains have no or low autoreactivity because they do not bind to autoantigens when they are expressed with glD42H as a dimeric IgG2a. The other six autoreactive L chains, Vκ3–12*01, Vκ4–57*01, Vκ4–63*01, Vκ5–45*01, Vκ5–48*01, and Vκ10–94*01, like VκRF, are expressed in the bone marrow, are infrequent in the FO repertoire, and appear in the GCs or among PCs.
FIGURE 7.
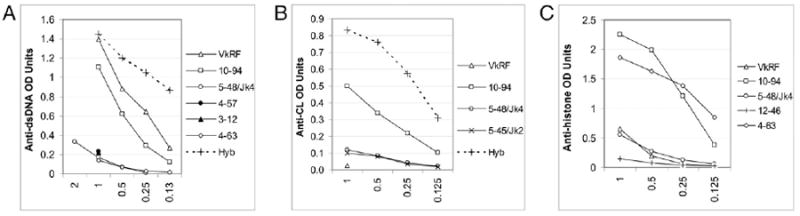
Binding of expressed glD42H Abs to autoantigens: Graphs show binding characteristics of transfected cell line supernatants that displayed positive binding to dsDNA (A), histones (B), or cardiolipin (C) compared with a positive hybridoma. Filled symbols in A represent dsDNA binding of preaggregated supernatants from cell lines that were negative in the initial ELISA.
The effect of BAFF/APRIL inhibition on the B cell repertoire
We next analyzed chimeric mice that had been treated with continuous TACI-Ig at a dose sufficient to deplete 50–60% of naive splenic B cells, an amount similar to that observed in primates treated with belimumab (26). TACI-Ig further depleted D42-positive B cells after the T1 stage (Fig. 8), with a 60% decrease in mature transgenic B cells (1.2 ± 0.7% of CD19+ B cells versus 3.3 ± 0.8% in untreated chimeras, p < 0.01; Figs. 2H, 2I, and 8A). To determine whether there was preferential deletion of B cells expressing particular L chains, we compared the FO repertoires of untreated and TACI-Ig–treated chimeric mice. The frequency of the five most common L chain genes, Vκ4–55*01, Vκ6–15*01, Vκ8–19*01, Vκ12–46*01, and Vκ13–85*01, was not substantially different in TACI-Ig–treated mice than in control glD42H or untreated chimeras. Thus, although TACI-Ig significantly impairs survival of naive glD42H B cells that escape from the bone marrow, it causes only subtle alterations in the accompanying L chain repertoire (Fig 6B, Supplemental Table III).
We next determined whether TACI-Ig altered selection of the GC repertoire in chimeric mice. The percentage of GC, class-switched cells, and PCs as a proportion of CD19+ cells was increased in the spleens of TACI-Ig–treated mice (data not shown). A modest decrease in the total number of GC B cells was noted in the spleens of TACI-Ig–treated chimeras, compared with untreated chimeras, reflecting the decrease in total numbers of CD19+ cells per spleen. However, the total number of class-switched cells and PCs in the spleens was not significantly decreased by TACI-Ig treatment, despite the modest decrease in spleen size and marked FO B cell depletion (Fig. 8A). In accord with these data, TACI-Ig–treated mice developed high-titer IgG anti-DNA Abs with only a 2-wk delay (Fig. 8B). As in the control glD42H transgenics, VκRF/Jκ5 was the dominant L chain specificity in the GC repertoire, and VκRF/Jκ5-expressing PCs were dominant in the spleens of both treated and untreated chimeras (Fig. 6C).
Discussion
B cell tolerance to self-antigen is maintained at a series of “checkpoints” that purge high-affinity self-reactive B cells from the naive B cell repertoire and prevent the emergence of autoreactivity during protective responses to foreign Ags (27, 28). Newly formed autoreactive B cells may be deleted, anergized, or subject to receptor editing in the bone marrow. Large numbers of autoreactive B cells are subsequently deleted or anergized in the periphery before reaching the mature stage (16). BCR signal strength is the major determinant of B cell fate in the bone marrow and in early transitional (T1) cells. In the late transitional stage, BCR signaling cooperates with BAFF signaling to promote B cell survival by upregulating expression of BAFF-R, by producing antiapoptotic molecules, and by generating p100, a substrate for the nonclassic NF-κB signaling pathway used by BAFF-R (29). The interaction of BAFF with BAFF-R is required for the survival of most B cells past the T1 stage, and complete absence of BAFF results in depletion of >90% of splenic B cells, even in auto-immune-prone mice. Conversely, excess BAFF increases the number of MZ and FO B cells in the spleen. In contrast, the homologous molecule APRIL, which does not bind to BAFF-R, is not required for mature B cell development or survival.
Studies in transgenic mice have shown that BAFF levels may influence the selection of the peripheral B cell repertoire. These studies in sum have shown that supraphysiologic BAFF excess does not alter the negative selection that occurs before or at the T1 stage, but rescues autoreactive cells that are anergized after the T1 stage and promotes their maturation into FO and or MZ cells (30). Because autoreactive B cells may have downregulated their BCR as a consequence of Ag stimulation at the early transitional stage, they signal less well through the classic NF-κB pathway and provide less of the p100 substrate needed for BAFF-R signaling. A lower BCR signal also results in lower cell surface expression of BAFF-R. Because BCR and BAFF-R signals synergize in promoting B cell survival, autoreactive B cells may therefore compete less well for survival as they progress to the T2 stage (6, 30, 31). When B cell numbers and BAFF levels are normal, deletion of many autoreactive B cells occurs; conversely, an increase in serum BAFF levels may result in relaxation of B cell selection (5, 7, 32). Increased serum levels of BAFF and overrepresentation of autoreactive specificities in the naive repertoire have been reported in human SLE patients (33-36).
Mature germline-encoded autoreactive B cells are usually excluded from the GC (27) or fail to progress past the early GC stage (37) and are therefore unlikely to undergo the class switching and somatic hypermutation that may yield pathogenic high-affinity self-reactivity. This checkpoint is defective in SLE patients (3, 38). Autoreactivity acquired by somatic mutation triggers a poorly defined deletion mechanism in GC B cells that purges the emerging memory B cell and PC repertoire of high-affinity autoreactivity (28). This checkpoint is defective in human SLE and in MRL/lpr and Nba2 mice (3, 39). In SLE, attenuation of B cell expression of the inhibitory receptor FcRIIB also contributes to the failure to delete autoreactive B cells in GCs (40, 41).
Although supraphysiologic excess of BAFF alters naive B cell selection in nonautoimmune strains of mice (5-7, 32), and BAFF inhibition depletes transitional and naive B cells in humans (42), it is not known whether limiting BAFF reverses the described abnormalities of naive B cell selection in SLE patients or whether either BAFF or APRIL inhibition has any impact on the selection of autoreactive B cells that arise after Ag activation. These questions need to be answered so that BAFF/APRIL-inhibiting drugs are used appropriately in SLE. Importantly, it has been shown in transgenic models that BAFF excess has much less effect on B cell selection if competition is provided by nonautoreactive B cells (reviewed in Ref. 30). The factors that contribute to the deletion of autoreactive B cells under conditions of normal competition are not fully understood, but, in addition to the availability of BAFF, could include intrinsic BCR signal strength, BCR cell surface levels, autoantigen availability and composition, ability of internalized autoantigens to stimulate TLRs or other innate receptors, other exogenous factors such as sex hormones, and availability of Ags that drive positive selection into the naive repertoire. It is therefore important, when using transgenic models, to perform studies of BAFF inhibition in model systems such as the one we describe in this article, in which competition is provided.
The studies we present have addressed the fate of B cells expressing either a germline-encoded low-affinity polyreactive autoantibody (glD42H/Vκ4-55) or a germline-encoded high-affinity anti-dsDNA autoantibody (glD42H/VκRF) under conditions of relative BAFF excess, of normal competition with nonautoreactive B cells, and of BAFF/APRIL inhibition in autoimmune NZB/W mice. The fates of these two types of B cells are quite different.
In normal humans as well as SLE patients, autoreactive specificities are very common in newly arising bone marrow B cells, but most of these are removed before the mature FO B cell stage (16); the defect in tolerance in SLE patients occurs both in the bone marrow and during peripheral deletion of autoreactive transitional cells (33, 34). In glD42H NZB/W mice, we found that B cells expressing the low-affinity polyreactive receptor are not deleted in the bone marrow and are positively selected into the T1 compartment of NZB/W mice, perhaps reflecting the abnormalities of immature B cell selection in SLE that occur in the bone marrow and transitional compartment and can be present even during periods of remission (34). In nonautoimmune glD42H mice, LPS-induced anti-dsDNA IgM hybridomas use a diverse L chain repertoire (11). In contrast, 67% of LPS-induced IgM hybridomas elicited from D42/Vκ1-Jκ1 NZB/W mice receptor edit to Vκ4–55*01 (12). IgM D42/Vκ4–55*01 binds DNA with only low affinity (12); we were not able to demonstrate dsDNA, cardiolipin, or histone binding of the germline-encoded but class-switched glD42H/Vκ4–55*01 Ab. Apart from Vκ4–55*01, we found only two other L chain genes, Vκ6–15*01 and Vκ8–19*01, in the MZs of young mice; these three L chains also constitute almost 50% of the FO repertoire. Selection of these L chains into both the FO and MZ repertoires may be due to differences in J region usage as well as variations in BCR expression.
Positive selection is a major contributor to the shape of the mature B cell repertoire (43, 44), and as shown by our single-cell PCR experiments, the highly skewed naive repertoire is positively selected before the BAFF-dependent T2 stage. Some diversification of the naive repertoire occurs as the mice age, with the recruitment of Vκ12-46*01, which confers low-affinity antihistone reactivity, into the FO repertoire, and of a more diverse MZ repertoire, suggesting a shift in the stringency for selection. Of importance, however, the dominant L chains in the naive repertoire have no or low autoreactivity because they are not autoreactive when expressed as dimeric IgG2a. Furthermore, the B cells most commonly selected into the naive repertoire are uncommon among the GC and PC repertoires, especially when the repertoire is diverse, suggesting that they may not be the appropriate target for therapeutic intervention.
In this article, we show that the GC entry checkpoint is defective in glD42H NZB/W mice and this defect is the major reason for loss of tolerance to dsDNA in these mice. In particular, the VκRF/Jκ5 L chain, which confers high-affinity anti-DNA reactivity and is used by nearly all IgG hybridomas from this mouse (12), is rare in the naive repertoire but constitutes >60% of the GC repertoire in old mice. This vast overrepresentation of VκRF in the GCs does not appear to be due to peripheral receptor editing, as we were unable to detect an increase in RAG expression in sorted GC B cells, compared with FO B cells, using quantitative PCR, nor were we able to detect RAG proteins in the spleen, using immunohistochemical methods. Our data suggest that rare VκRF-expressing B cells are preferentially selected into and expand in the GCs. Furthermore, the checkpoint that prevents clonal expansion and differentiation of autoreactive GC B cells into effector cells is also defective in glD42H mice, as PCs also express VκRF. Importantly, our repertoire data also show failure of exclusion of several other germline-encoded autoreactive B cells from the GCs. A similar defect in GC tolerance has been reported in human SLE patients (3, 38, 45, 46).
Within the GC microenvironment, B cells compete both for Ag, based on BCR affinity and availability of Ag presented by follicular dendritic cells, and for growth and costimulatory factors provided by T cells, resulting in selection of those high-affinity B cells that receive help, but in active death of B cells that receive low-affinity BCR signals or BCR signals without cognate T cell help (47). Downregulation of BCR expression on GC B cells, low levels of BAFF, and high expression of Fas all help ensure that only those high-affinity B cells that receive additional help from T cells are selected. Three alternative hypotheses could explain the preferential selection of VκRF-expressing B cells into the GCs of NZB/W mice. First, a loss of T cell tolerance may occur. Second, the DNA-binding activity of VκRF-expressing B cells may result in both a strong BCR signal and increased signaling through nucleic acid-recognizing TLRs; this could drive preferential selection and survival of these cells even if partial downregulation of BCR expression is present and cognate T cell help is absent (48). TACI-Ig might be expected to affect this mechanism because BAFF and APRIL amplify TLR signals (49). Alternatively, BCR downregulation resulting from the high affinity of glD42H/VκRF for DNA may result in insufficient BCR signals to induce tolerance. This mechanism for tolerance loss has been postulated in Sle1 mice (2).
Inhibition of BAFF is the first successful biologic therapy for human SLE (8). Because the naive and Ag-activated repertoires in NZB/W F1 glD42H mice are distinct, we were able to study the effects of BAFF/APRIL inhibition on B cell selection at two different B cell developmental checkpoints, using TACI-Ig. The effects of TACI-Ig on selection of the naive repertoire are due to BAFF inhibition, as APRIL plays no role in promoting naive B cell survival (50). Provision of B cells expressing a diverse B cell repertoire was sufficient to impair the survival of most glD42H B cells after the immature stage. The L chain repertoire of these surviving B cells was similar to that of control glD42H mice, indicating that most glD42H-bearing B cells compete inefficiently for survival under conditions of normal B cell competition. The disappearance of glD42H B cells that had lower levels of surface IgM and IgD in the chimeric mice indicates that these B cells are preferentially susceptible to deletion under conditions of normal competition.
Few studies have addressed the fate of autoreactive B cells within a diverse repertoire under conditions of either BAFF excess or BAFF inhibition. Studies in HEL/anti-HEL transgenic mice have shown that BAFF excess does not rescue anergic high-affinity autoreactive transitional B cells but alters the localization of intermediate-affinity cells from the FO region to the MZ. Similarly, in the IgK-reactive macroself Ag transgenic mouse, excess BAFF does not rescue anergic transitional B cells once competition is provided. In the 3H9 model, excess BAFF increases the maturity of transgenic B cells but does not rescue them into B cell follicles or induce ANA production. In contrast, in the R4A H chain transgenic mouse, excess BAFF induces the production of anti-DNA Abs. In this mouse, BAFF inhibition decreases autoreactivity in the reconstituting B cell repertoire after B cell depletion. A recent study using two H/L transgenic B cells encoded by highly related Ig genes that confer differential affinity for autoantigen showed that competition from nonautoreactive B cells significantly decreased selection of both types of transgenic autoreactive B cells, but neither a moderate excess of BAFF nor BAFF inhibition further altered their selection, despite BCR downregulation in one case and high affinity for autoantigen in the other (51). In our study, in which only the H chain was constrained by a transgene, we show clearly that BAFF/APRIL inhibition with TACI-Ig induces a significant decrease in the survival of glD42H-expressing B cells in the periphery in chimeric mice, but with only subtle alterations to the accompanying L chain repertoire. These findings in sum suggest that the effect of excess BAFF on naive B cell selection can be quite variable, and that not all autoreactive B cells are equally susceptible to BAFF inhibition at the transitional B cell checkpoint. It will be very important to further dissect what factors determine BAFF responsiveness of autoreactive B cells and to find a means of determining which individuals are responsive to BAFF inhibition.
The role of either BAFF or APRIL in regulating selection of the Ag experienced repertoire is not clearly defined. BAFF-deficient mice fail to form a mature GC follicular dendritic cell network, resulting in premature termination of the GC response (52, 53). Although somatic mutation and affinity maturation still occur in BAFF-deficient mice, the magnitude of the Ab response to T cell-dependent Ags is significantly attenuated (53). In contrast, APRIL appears to have a minimal role in GCs (52). Neither BAFF nor APRIL is required for the development of memory B cells but either is sufficient to support PCs, especially IgM PCs (14).
We have reported that when NZB/W mice are treated with TACI-Ig, they still produce IgG anti-DNA Abs that are capable of depositing in the glomeruli, but with less glomerular damage than in untreated controls (7, 10). We therefore wished to know whether differences in the class-switched repertoire exist between TACI-Ig treated mice and untreated chimeras. We found that despite the ability of TACI-Ig to modulate the naive B cell repertoire, it did not prevent the selection of VκRF/Jκ5 into the GC or the terminal differentiation of VκRF/Jκ5-expressing B cells to PCs or the secretion of IgG anti-DNA Abs. These data show that TACI-Ig treatment fails to completely eliminate D42/VκRF/Jκ5-expressing B cells that escape negative selection from the naive repertoire, confirming that not all germline-encoded autoreactive B cells are susceptible to BAFF inhibition. Our findings are also consistent with the observed failure of BAFF/APRIL inhibition (13, 14) or even complete BAFF (54) or BAFF-R (55) deficiency to prevent an anti-DNA response in autoimmune mice. Because the autoreactive GC repertoire in glD42H mice is highly restricted to B cells that are already autoreactive in their germline state, we were not able to address the effect of BAFF/APRIL inhibition on autoreactive effector B cells generated de novo in the GC by somatic mutation. This endeavor will require a different transgenic system. Nevertheless, our studies suggest that regulation of GC entry and expansion should be an important therapeutic goal to prevent autoantibody production in SLE and that such an approach may synergize with BAFF/APRIL inhibition.
Supplementary Material
Acknowledgments
We thank Haiou Tao for technical assistance and Herbert Borero for assistance with cell sorting.
This work was supported by National Institutes of Health, National Institute of Arthritis and Musculoskeletal and Skin Diseases Grant R01 AI083901 and National Institute of Allergy and Infectious Diseases Grant R01 AR 049938-01 (to A.D.) and Rheuminations (to R.B.).
Abbreviations used in this article
- FO
follicular
- GC
germinal center
- glD42H
germline D42 H chain
- MZ
marginal zone
- PC
plasma cell
- SLE
systemic lupus erythematosus
- T1
transitional 1
- T2
transitional 2
- wt
wild-type
Footnotes
The online version of this article contains supplemental material.
Disclosures The authors have no financial conflicts of interest.
References
- 1.Wardemann H, Nussenzweig MC. B-cell self-tolerance in humans. Adv Immunol. 2007;95:83–110. doi: 10.1016/S0065-2776(07)95003-8. [DOI] [PubMed] [Google Scholar]
- 2.Vuyyuru R, Mohan C, Manser T, Rahman ZS. The lupus susceptibility locus Sle1 breaches peripheral B cell tolerance at the antibody-forming cell and germinal center checkpoints. J Immunol. 2009;183:5716–5727. doi: 10.4049/jimmunol.0804215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pugh-Bernard AE, Silverman GJ, Cappione AJ, Villano ME, Ryan DH, Insel RA, Sanz I. Regulation of inherently autoreactive VH4-34 B cells in the maintenance of human B cell tolerance. J Clin Invest. 2001;108:1061–1070. doi: 10.1172/JCI12462. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Thompson SA, Jones JL, Cox AL, Compston DA, Coles AJ. B-cell reconstitution and BAFF after alemtuzumab (Campath-1H) treatment of multiple sclerosis. J Clin Immunol. 2010;30:99–105. doi: 10.1007/s10875-009-9327-3. [DOI] [PubMed] [Google Scholar]
- 5.Kawabata D, Venkatesh J, Ramanujam M, Davidson A, Grimaldi CM, Diamond B. Enhanced selection of high affinity DNA-reactive B cells following cyclophosphamide treatment in mice. PLoS ONE. 2010;5:e8418. doi: 10.1371/journal.pone.0008418. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lesley R, Xu Y, Kalled SL, Hess DM, Schwab SR, Shu HB, Cyster JG. Reduced competitiveness of autoantigen-engaged B cells due to increased dependence on BAFF. Immunity. 2004;20:441–453. doi: 10.1016/s1074-7613(04)00079-2. [DOI] [PubMed] [Google Scholar]
- 7.Thien M, Phan TG, Gardam S, Amesbury M, Basten A, Mackay F, Brink R. Excess BAFF rescues self-reactive B cells from peripheral deletion and allows them to enter forbidden follicular and marginal zone niches. Immunity. 2004;20:785–798. doi: 10.1016/j.immuni.2004.05.010. [DOI] [PubMed] [Google Scholar]
- 8.Navarra SV, Guzmán RM, Gallacher AE, Hall S, Levy RA, Jimenez RE, Li EK, Thomas M, Kim HY, León MG, et al. BLISS-52 Study Group. Efficacy and safety of belimumab in patients with active systemic lupus erythematosus: a randomised, placebo-controlled, phase 3 trial. Lancet. 2011;377:721–731. doi: 10.1016/S0140-6736(10)61354-2. [DOI] [PubMed] [Google Scholar]
- 9.Pewzner-Jung Y, Friedmann D, Sonoda E, Jung S, Rajewsky K, Eilat D. B cell deletion, anergy, and receptor editing in “knock in” mice targeted with a germline-encoded or somatically mutated anti-DNA heavy chain. J Immunol. 1998;161:4634–4645. [PubMed] [Google Scholar]
- 10.Yachimovich N, Mostoslavsky G, Yarkoni Y, Verbovetski I, Eilat D. The efficiency of B cell receptor (BCR) editing is dependent on BCR light chain rearrangement status. Eur J Immunol. 2002;32:1164–1174. doi: 10.1002/1521-4141(200204)32:4<1164::AID-IMMU1164>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 11.Friedmann D, Yachimovich N, Mostoslavsky G, Pewzner-Jung Y, Ben-Yehuda A, Rajewsky K, Eilat D. Production of high affinity autoanti-bodies in autoimmune New Zealand Black/New Zealand white F1 mice targeted with an anti-DNA heavy chain. J Immunol. 1999;162:4406–4416. [PubMed] [Google Scholar]
- 12.Yachimovich-Cohen N, Fischel R, Bachar N, Yarkoni Y, Eilat D. Autoimmune NZB/NZW F1 mice utilize B cell receptor editing for generating high-affinity anti-dsDNA autoantibodies from low-affinity precursors. Eur J Immunol. 2003;33:2469–2478. doi: 10.1002/eji.200324025. [DOI] [PubMed] [Google Scholar]
- 13.Ramanujam M, Wang X, Huang W, Schiffer L, Grimaldi C, Akkerman A, Diamond B, Madaio MP, Davidson A. Mechanism of action of transmembrane activator and calcium modulator ligand interactor-Ig in murine systemic lupus erythematosus. J Immunol. 2004;173:3524–3534. doi: 10.4049/jimmunol.173.5.3524. [DOI] [PubMed] [Google Scholar]
- 14.Ramanujam M, Wang X, Huang W, Liu Z, Schiffer L, Tao H, Frank D, Rice J, Diamond B, Yu KO, et al. Similarities and differences between selective and nonselective BAFF blockade in murine SLE. J Clin Invest. 2006;116:724–734. doi: 10.1172/JCI26385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Liu Z, Bethunaickan R, Huang W, Lodhi U, Solano I, Madaio MP, Davidson A. Interferon-α accelerates murine systemic lupus eryth-ematosus in a T cell-dependent manner. Arthritis Rheum. 2011;63:219–229. doi: 10.1002/art.30087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wardemann H, Yurasov S, Schaefer A, Young JW, Meffre E, Nussenzweig MC. Predominant autoantibody production by early human B cell precursors. Science. 2003;301:1374–1377. doi: 10.1126/science.1086907. [DOI] [PubMed] [Google Scholar]
- 17.Fukuyama H, Nimmerjahn F, Ravetch JV. The inhibitory Fcgamma receptor modulates autoimmunity by limiting the accumulation of immunoglobulin G+ anti-DNA plasma cells. Nat Immunol. 2005;6:99–106. doi: 10.1038/ni1151. [DOI] [PubMed] [Google Scholar]
- 18.Schiffer L, Bethunaickan R, Ramanujam M, Huang W, Schiffer M, Tao H, Madaio MP, Bottinger EP, Davidson A. Activated renal macrophages are markers of disease onset and disease remission in lupus nephritis. J Immunol. 2008;180:1938–1947. doi: 10.4049/jimmunol.180.3.1938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Arango DA, Wilson J, Shi Q, Corner GA, Arañes MJ, Nicholas C, Lesser M, Mariadason JM, Augenlicht LH. Molecular mechanisms of action and prediction of response to oxaliplatin in colorectal cancer cells. Br J Cancer. 2004;91:1931–1946. doi: 10.1038/sj.bjc.6602215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Gentleman R, Carey V, Huber W, Irizarry R, Dudoit S. Bio-informatics and Computational Biology Solutions Using R and Bioconductor. Springer Science+Business Media; New York: 2005. pp. 161–179. [Google Scholar]
- 21.Simon RM, Korn EL, McShane LM, Radmacher MD, Wright GW, Zhao Y. Design and Analysis of DNA Microarray Investigations. Springer Science+Business Media; New York: 2004. pp. 121–155. [Google Scholar]
- 22.Snedecor GW, Cochran WG. Statistical Methods. Iowa State University Press; Ames, IA: 1989. [Google Scholar]
- 23.Khattree R, Naik DN. Multivariate Data Reduction and Discrimination with SAS Software. SAS Institute; Cary, NC: 2000. [Google Scholar]
- 24.Martin F, Kearney JF. B-cell subsets and the mature preimmune repertoire. Marginal zone and B1 B cells as part of a “natural immune memory”. Immunol Rev. 2000;175:70–79. [PubMed] [Google Scholar]
- 25.Halverson R, Torres RM, Pelanda R. Receptor editing is the main mechanism of B cell tolerance toward membrane antigens. Nat Immunol. 2004;5:645–650. doi: 10.1038/ni1076. [DOI] [PubMed] [Google Scholar]
- 26.Baker KP, Edwards BM, Main SH, Choi GH, Wager RE, Halpern WG, Lappin PB, Riccobene T, Abramian D, Sekut L, et al. Generation and characterization of LymphoStat-B, a human monoclonal antibody that antagonizes the bioactivities of B lymphocyte stimulator. Arthritis Rheum. 2003;48:3253–3265. doi: 10.1002/art.11299. [DOI] [PubMed] [Google Scholar]
- 27.Goodnow CC, Vinuesa CG, Randall KL, Mackay F, Brink R. Control systems and decision making for antibody production. Nat Immunol. 2010;11:681–688. doi: 10.1038/ni.1900. [DOI] [PubMed] [Google Scholar]
- 28.Shlomchik MJ. Sites and stages of autoreactive B cell activation and regulation. Immunity. 2008;28:18–28. doi: 10.1016/j.immuni.2007.12.004. [DOI] [PubMed] [Google Scholar]
- 29.Stadanlick JE, Kaileh M, Karnell FG, Scholz JL, Miller JP, Quinn WJ, III, Brezski RJ, Treml LS, Jordan KA, Monroe JG, et al. Tonic B cell antigen receptor signals supply an NF-kappaB substrate for prosurvival BLyS signaling. Nat Immunol. 2008;9:1379–1387. doi: 10.1038/ni.1666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Liu Z, Davidson A. BAFF and selection of autoreactive B cells. Trends Immunol. 2011;32:388–394. doi: 10.1016/j.it.2011.06.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Stadanlick JE, Cancro MP. BAFF and the plasticity of peripheral B cell tolerance. Curr Opin Immunol. 2008;20:158–161. doi: 10.1016/j.coi.2008.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ota M, Duong BH, Torkamani A, Doyle CM, Gavin AL, Ota T, Nemazee D. Regulation of the B cell receptor repertoire and self-reactivity by BAFF. J Immunol. 2010;185:4128–4136. doi: 10.4049/jimmunol.1002176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yurasov S, Wardemann H, Hammersen J, Tsuiji M, Meffre E, Pascual V, Nussenzweig MC. Defective B cell tolerance checkpoints in systemic lupus erythematosus. J Exp Med. 2005;201:703–711. doi: 10.1084/jem.20042251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yurasov S, Tiller T, Tsuiji M, Velinzon K, Pascual V, Wardemann H, Nussenzweig MC. Persistent expression of autoantibodies in SLE patients in remission. J Exp Med. 2006;203:2255–2261. doi: 10.1084/jem.20061446. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Cheema GS, Roschke V, Hilbert DM, Stohl W. Elevated serum B lymphocyte stimulator levels in patients with systemic immune-based rheumatic diseases. Arthritis Rheum. 2001;44:1313–1319. doi: 10.1002/1529-0131(200106)44:6<1313::AID-ART223>3.0.CO;2-S. [DOI] [PubMed] [Google Scholar]
- 36.Petri M, Stohl W, Chatham W, McCune J, Butler T, Ryel J, Zhong J, Recta J, Freimuth W. BLyS plasma concentrations correlate with disease activity and levels of anti-dsDNA autoantibodies and immunoglobulins (IgG) in a SLE patient observational study. Arthritis Rheum. 2003;48:S655. abstract. [Google Scholar]
- 37.Paul E, Lutz J, Erikson J, Carroll MC. Germinal center checkpoints in B cell tolerance in 3H9 transgenic mice. Int Immunol. 2004;16:377–384. doi: 10.1093/intimm/dxh035. [DOI] [PubMed] [Google Scholar]
- 38.Jacobi AM, Zhang J, Mackay M, Aranow C, Diamond B. Phenotypic characterization of autoreactive B cells—checkpoints of B cell tolerance in patients with systemic lupus erythematosus. PLoS ONE. 2009;4:e5776. doi: 10.1371/journal.pone.0005776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Aït-Azzouzene D, Kono DH, Gonzalez-Quintial R, McHeyzer-Williams LJ, Lim M, Wickramarachchi D, Gerdes T, Gavin AL, Skog P, McHeyzer-Williams MG, et al. Deletion of IgG-switched autoreactive B cells and defects in Fas(lpr) lupus mice. J Immunol. 2010;185:1015–1027. doi: 10.4049/jimmunol.1000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Mackay M, Stanevsky A, Wang T, Aranow C, Li M, Koenig S, Ravetch JV, Diamond B. Selective dysregulation of the FcgammaIIB receptor on memory B cells in SLE. J Exp Med. 2006;203:2157–2164. doi: 10.1084/jem.20051503. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Tiller T, Kofer J, Kreschel C, Busse CE, Riebel S, Wickert S, Oden F, Mertes MM, Ehlers M, Wardemann H. Development of self-reactive germinal center B cells and plasma cells in autoimmune Fc gammaRIIB-deficient mice. J Exp Med. 2010;207:2767–2778. doi: 10.1084/jem.20100171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Jacobi AM, Huang W, Wang T, Freimuth W, Sanz I, Furie R, Mackay M, Aranow C, Diamond B, Davidson A. Effect of long-term belimumab treatment on B cells in systemic lupus erythematosus: extension of a phase II, double-blind, placebo-controlled, dose-ranging study. Arthritis Rheum. 2010;62:201–210. doi: 10.1002/art.27189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gu H, Tarlinton D, Müller W, Rajewsky K, Förster I. Most peripheral B cells in mice are ligand selected. J Exp Med. 1991;173:1357–1371. doi: 10.1084/jem.173.6.1357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Levine MH, Haberman AM, Sant’Angelo DB, Hannum LG, Cancro MP, Janeway CA, Jr, Shlomchik MJ. A B-cell receptor-specific selection step governs immature to mature B cell differentiation. Proc Natl Acad Sci USA. 2000;97:2743–2748. doi: 10.1073/pnas.050552997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zhang J, Jacobi AM, Wang T, Berlin R, Volpe BT, Diamond B. Polyreactive autoantibodies in systemic lupus erythematosus have pathogenic potential. J Autoimmun. 2009;33:270–274. doi: 10.1016/j.jaut.2009.03.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Mietzner B, Tsuiji M, Scheid J, Velinzon K, Tiller T, Abraham K, Gonzalez JB, Pascual V, Stichweh D, Wardemann H, Nussenzweig MC. Auto-reactive IgG memory antibodies in patients with systemic lupus erythematosus arise from nonreactive and polyreactive precursors. Proc Natl Acad Sci USA. 2008;105:9727–9732. doi: 10.1073/pnas.0803644105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Gatto D, Brink R. The germinal center reaction. J Allergy Clin Immunol. 2010;126:898–907. doi: 10.1016/j.jaci.2010.09.007. quiz 908–899. [DOI] [PubMed] [Google Scholar]
- 48.Herlands RA, Christensen SR, Sweet RA, Hershberg U, Shlomchik MJ. T cell-independent and toll-like receptor-dependent antigen-driven activation of autoreactive B cells. Immunity. 2008;29:249–260. doi: 10.1016/j.immuni.2008.06.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.He B, Santamaria R, Xu W, Cols M, Chen K, Puga I, Shan M, Xiong H, Bussel JB, Chiu A, et al. The transmembrane activator TACI triggers immunoglobulin class switching by activating B cells through the adaptor MyD88. Nat Immunol. 2010;11:836–845. doi: 10.1038/ni.1914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Dillon SR, Gross JA, Ansell SM, Novak AJ. An APRIL to remember: novel TNF ligands as therapeutic targets. Nat Rev Drug Discov. 2006;5:235–246. doi: 10.1038/nrd1982. [DOI] [PubMed] [Google Scholar]
- 51.Nikbakht N, Migone TS, Ward CP, Manser T. Cellular competition independent of BAFF/B lymphocyte stimulator results in low frequency of an autoreactive clonotype in mature polyclonal B cell compartments. J Immu-nol. 2011;187:37–46. doi: 10.4049/jimmunol.1003924. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kalled SL. Impact of the BAFF/BR3 axis on B cell survival, germinal center maintenance and antibody production. Semin Immunol. 2006;18:290–296. doi: 10.1016/j.smim.2006.06.002. [DOI] [PubMed] [Google Scholar]
- 53.Rahman ZS, Rao SP, Kalled SL, Manser T. Normal induction but attenuated progression of germinal center responses in BAFF and BAFF-R signaling-deficient mice. J Exp Med. 2003;198:1157–1169. doi: 10.1084/jem.20030495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Jacob CO, Pricop L, Putterman C, Koss MN, Liu Y, Kollaros M, Bixler SA, Ambrose CM, Scott ML, Stohl W. Paucity of clinical disease despite serological autoimmunity and kidney pathology in lupus-prone New Zealand mixed 2328 mice deficient in BAFF. J Immunol. 2006;177:2671–2680. doi: 10.4049/jimmunol.177.4.2671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ju ZL, Shi GY, Zuo JX, Zhang JW, Sun Jian. Unexpected development of autoimmunity in BAFF-R-mutant MRL-lpr mice. Immunology. 2007;120:281–289. doi: 10.1111/j.1365-2567.2006.02500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.


