Abstract
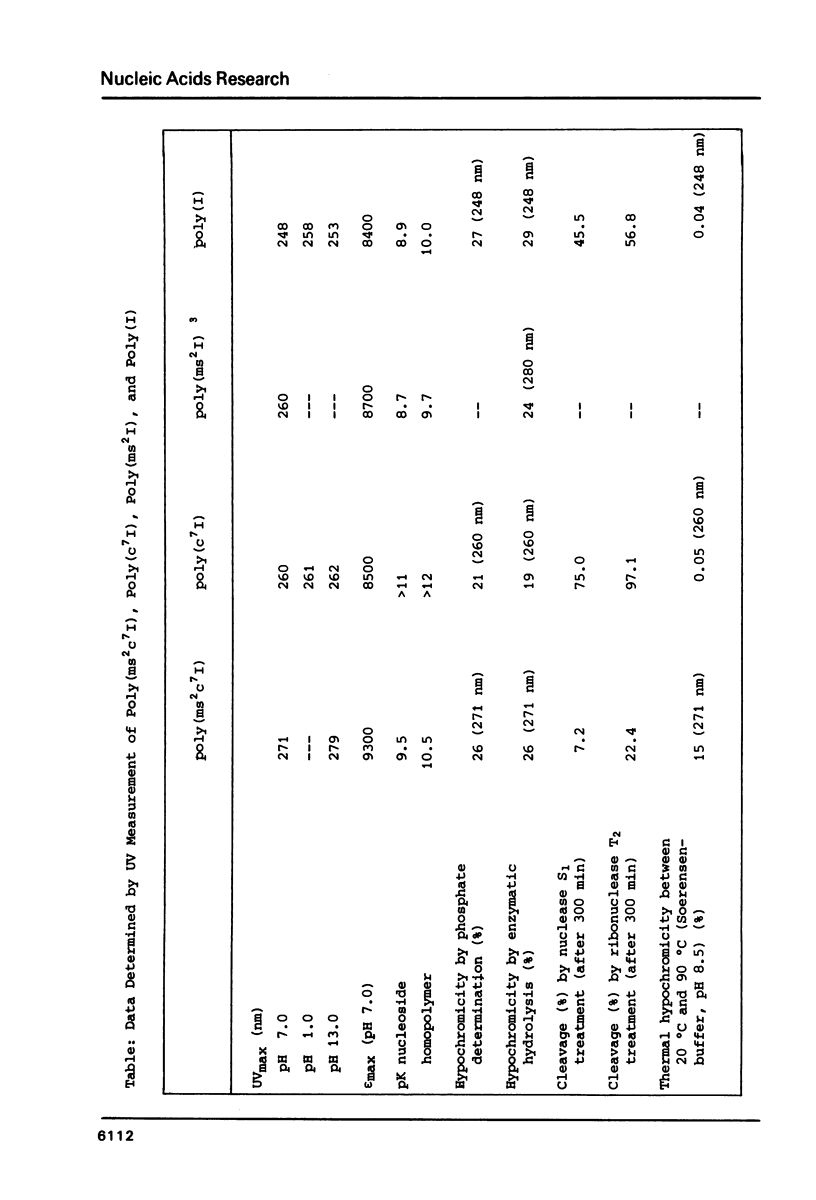
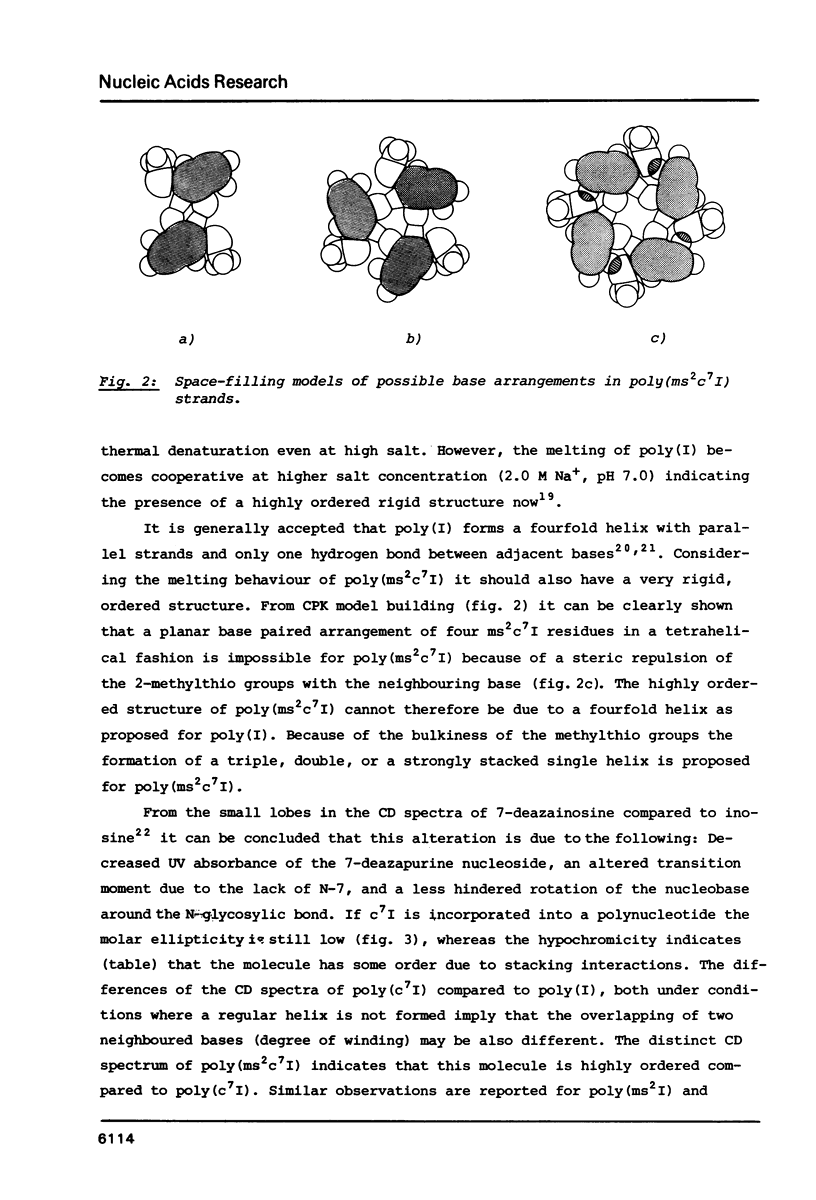
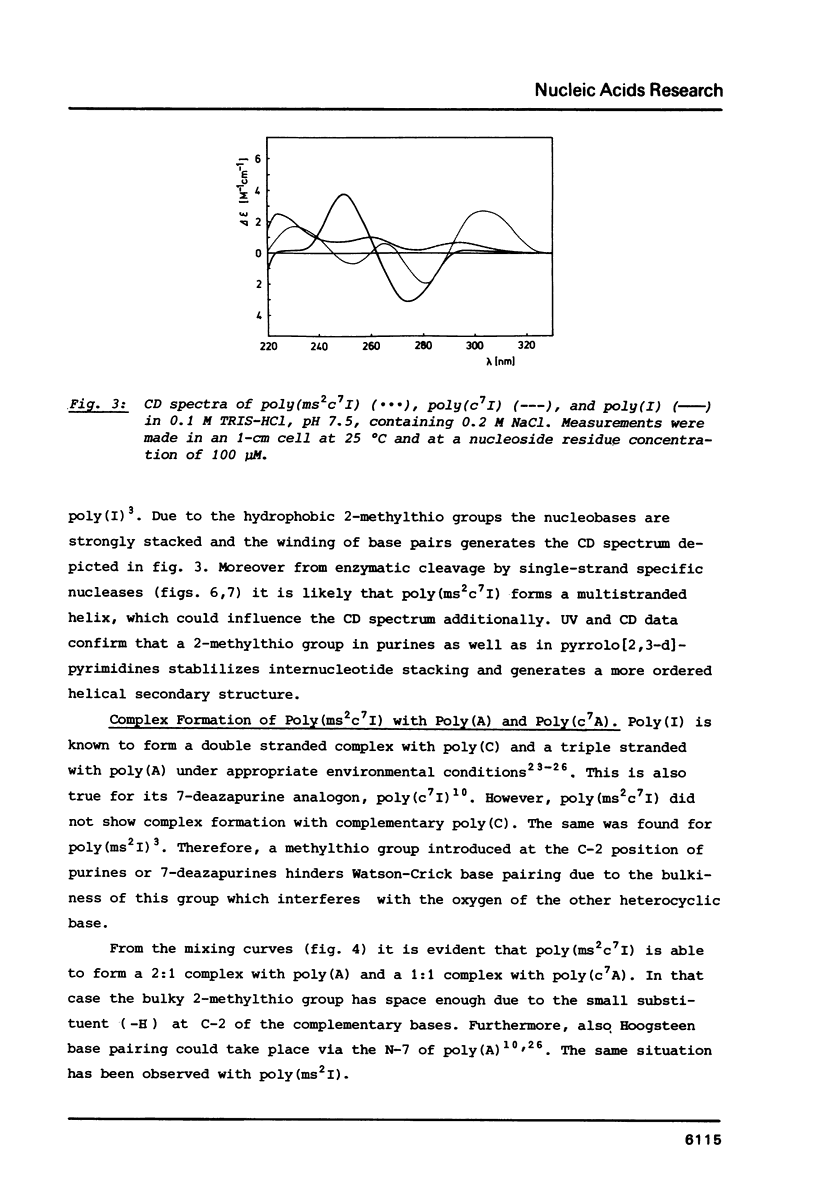
Poly(2-methylthio-7-deazainosinic acid) [poly(ms2c7I)] was enzymatically synthesized by polymerization of 2-methylthio-7-deazainosine 5'-diphosphate with polynucleotide phosphorylase from Micrococcus luteus in high yield. The homopolymer shows much higher thermal stability than its parent polynucleotides poly(7-deazainosinic acid) [poly(c7I)] and poly(I). Its sigmoidal melting curve and pronounced hypochromicity imply a rigid, ordered structure. Poly(ms2c7I), like poly(2-methylthio-inosinic acid) [poly(ms2I)], does not form a complex with poly(C) because of the bulky 2-methylthio substituent. On the other hand, two poly(ms2c7I) strands form very rigid triple strands with poly(A). Different from poly(I) and poly(c7I) the homopolymer poly(ms2c7I) is very stable against cleavage by nuclease S1 and ribonuclease T2 as expected from its rigid secondary structure.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnott S., Bond P. J. Triple-stranded polynucleotide helix containing only purine bases. Science. 1973 Jul 6;181(4094):68–69. doi: 10.1126/science.181.4094.68. [DOI] [PubMed] [Google Scholar]
- Carter W. A., Pitha P. M., Marshall L. W., Tazawa I., Tazawa S., Ts'o P. O. Structural requirements of the rI n -rC n complex for induction of human interferon. J Mol Biol. 1972 Oct 14;70(3):567–587. doi: 10.1016/0022-2836(72)90560-8. [DOI] [PubMed] [Google Scholar]
- Chou C. H., Thomas G. J., Jr, Arnott S., Smith P. J. Raman spectral studies of nucleic acids. XVII. Conformational structures of polyinosinic acid. Nucleic Acids Res. 1977 Jul;4(7):2407–2419. doi: 10.1093/nar/4.7.2407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fukui T., Ikehara M. Polynucleotides XLVII. Synthesis and properties of poly(2-methylthio- and 2-ethylthioadenylic acid). Formation of non-Watson-Crick type complexes. Biochim Biophys Acta. 1979 May 24;562(3):527–533. doi: 10.1016/0005-2787(79)90115-1. [DOI] [PubMed] [Google Scholar]
- HOARD D. E., OTT D. G. CONVERSION OF MONO- AND OLIGODEOXYRIBONUCLEOTIDES TO 5-TRIPHOSPHATES. J Am Chem Soc. 1965 Apr 20;87:1785–1788. doi: 10.1021/ja01086a031. [DOI] [PubMed] [Google Scholar]
- Harada F., Gross H. J., Kimura F., Chang S. H., Nishimura S., RajBhandary U. L. 2-Methylthio N6-(delta 2-isopentenyl) adenosine: a component of E. coli tyrosine transfer RNA. Biochem Biophys Res Commun. 1968 Oct 24;33(2):299–306. doi: 10.1016/0006-291x(68)90784-5. [DOI] [PubMed] [Google Scholar]
- Hashimoto J., Uchida T., Egami F. Action of ribonucleases T1, T2, and U2 on dinucleoside monophosphates containing 7-deazapurine base. Biochim Biophys Acta. 1970 Feb 18;199(2):535–536. doi: 10.1016/0005-2787(70)90099-7. [DOI] [PubMed] [Google Scholar]
- Howard F. B., Miles H. T. Interaction of poly(A) and poly(I). A reinvestigation. Biochemistry. 1977 Oct 18;16(21):4647–4650. doi: 10.1021/bi00640a018. [DOI] [PubMed] [Google Scholar]
- Howard F. B., Miles H. T. Poly(inosinic acid) helices: essential chelation of alkali metal ions in the axial channel. Biochemistry. 1982 Dec 21;21(26):6736–6745. doi: 10.1021/bi00269a019. [DOI] [PubMed] [Google Scholar]
- Ikehara M., Fukui T., Koide T., Inaba J. Polynucleotides. XXII. Synthesis and properties of poly 7-deazainosinic acid. Nucleic Acids Res. 1974 Jan;1(1):53–61. doi: 10.1093/nar/1.1.53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikehara M., Hattori M. Synthesis and properties of poly(2-methylthioinosinic acid). Biochim Biophys Acta. 1972 Apr 26;269(1):27–36. doi: 10.1016/0005-2787(72)90070-6. [DOI] [PubMed] [Google Scholar]
- Potter B. V., Romaniuk P. J., Eckstein F. Stereochemical course of DNA hydrolysis by nuclease S1. J Biol Chem. 1983 Feb 10;258(3):1758–1760. [PubMed] [Google Scholar]
- RICH A. Formation of two- and three-stranded helical molecules by polyinosinic acid and polyadenylic acid. Nature. 1958 Feb 22;181(4608):521–525. doi: 10.1038/181521a0. [DOI] [PubMed] [Google Scholar]
- Seela F., Ott J., Franzen D. Poly(adenylic acids) containing the antibiotic tubercidin -- base pairing and hydrolysis by nuclease S1. Nucleic Acids Res. 1982 Feb 25;10(4):1389–1397. doi: 10.1093/nar/10.4.1389. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seela F., Tran-Thi Q. H., Franzen D. Poly(7-deazaguanylic acid), the homopolynucleotide of the parent nucleoside of queuosine. Biochemistry. 1982 Aug 31;21(18):4338–4343. doi: 10.1021/bi00261a024. [DOI] [PubMed] [Google Scholar]
- Thiele D., Guschlbauer W. The structures of polyinosinic acid. Biophysik. 1973 May 30;9(3):261–277. doi: 10.1007/BF01184691. [DOI] [PubMed] [Google Scholar]
- Torrence P. F., De Clercq E., Waters J. A., Witkop B. A potent interferon inducer derived from poly (7-deazainosinic acid). Biochemistry. 1974 Oct 8;13(21):4400–4408. doi: 10.1021/bi00718a025. [DOI] [PubMed] [Google Scholar]
- Torrence P. F., Witkop B. Polynucleotide duplexes based on poly(7-deazaadenylic acid). Biochim Biophys Acta. 1975 Jun 2;395(1):56–66. doi: 10.1016/0005-2787(75)90233-6. [DOI] [PubMed] [Google Scholar]
- Uchida T., Egami F. The specificity of ribonuclease T2. J Biochem. 1967 Jan;61(1):44–53. doi: 10.1093/oxfordjournals.jbchem.a128519. [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Kato T., Takenishi T. A novel method for phosphorylation of nucleosides to 5'-nucleotides. Tetrahedron Lett. 1967 Dec;50:5065–5068. doi: 10.1016/s0040-4039(01)89915-9. [DOI] [PubMed] [Google Scholar]



