Abstract
OBJECTIVE
To assess whether functional maternal or fetal genotypes along well-characterized metabolic pathways (ie, CYP1A1, GSTT1, and CYP2A6) may account for varying associations with adverse outcomes among pregnant women who smoke.
METHODS
DNA samples from 502 smokers and their conceptuses, alongside women in a control group, were genotyped for known functional allelic variants of CYP1A1 (Ile462Val AA>AG/GG), GSTT1(del), and CYP2A6 (Lys160His T>A). Modification of the association between smoking and outcome by genotype was evaluated. Outcomes included birth weight, pregnancy loss, preterm birth, small for gestational age, and a composite outcome composed of the latter four components plus abruption.
RESULTS
No interaction between maternal or fetal genotype of any of the polymorphisms and smoking could be demonstrated. In contrast, the association of smoking with gestational age–adjusted birth weight (birth weight ratio) was modified by fetal GSTT1 genotype (P for interaction=.02). Fetuses with GSTT1(del) had a mean birth weight reduction among smokers of 262 g (P=.01), whereas in fetuses without the GSTT1(del) the effect of tobacco exposure was nonsignificant (mean reduction 87 g, P=.16). After adjusting for confounding, results were similar.
CONCLUSION
Fetal GSTT1 deletion significantly and specifically modifies the effect of smoking on gestational age–corrected birth weight.
LEVEL OF EVIDENCE
Maternal tobacco use has been identified in multiple population-based studies as the strongest modifiable risk factor for intrauterine growth restriction (IUGR) and multiple other adverse pregnancy outcomes.1–8 However, while many fetuses are exposed to tobacco smoke, not all experience adverse outcomes.1–4 This discrepancy cannot be accounted for by dose effect alone,5–8 and smoking-related weight reduction has historically been considered to be largely independent of other maternal and fetal risk factors that influence birth weight.1,7–13 Thus, current efforts aimed at understanding the potential genetic and metabolic basis of this variable susceptibility to tobacco smoke exposure are of importance in perinatal medicine.
Mechanisms leading to growth restriction following in utero tobacco exposure are poorly understood, but have generally often been attributed to chronic fetal hypoxia. Nicotine, a principal alkaloid of tobacco smoke, has been shown to mediate constriction of the intrauterine vessels and result in increased apoptosis of placental syncytiotrophoblasts.9,14–16 Nicotine, cotinine, and potentially harmful DNA adducts (metabolic products of polycyclic aromatic hydrocarbons) are known to cross or collect in the placenta of smokers.14–21 Thus while chronic hypoxia may be a mediator of growth restriction after in utero tobacco exposure, it is plausible that the discrepant variation in fetal susceptibility to smoking-related growth restriction results from fetal and/or maternal metabolic gene polymorphisms along the polycyclic aromatic hydrocarbons pathway.
Of the more than 4000 substances in tobacco smoke, polycyclic aromatic hydrocarbons and nitrosamine compounds comprise carcinogenic species in tobacco smoke.22–26 The majority of chemical carcinogens are metabolized in a sequential series of two-phase enzymatic metabolic reactions. Phase I enzymes, such as cytochrome P450s (ie, CYP1A1), metabolically activate polycyclic aromatic hydrocarbon compounds into oxidized derivatives, resulting in reactive oxygen intermediates capable of covalently binding DNA to form adducts.22–28 Of interest to the study reported herein, maternal smoking induces placental expression of CYP1A1.28 In turn, these reactive electrophilic intermediates can be detoxified by phase II enzymes, such as the glutathione S-transferase family, via conjugation with endogenous species to form hydrophilic glutathione conjugates, which are then readily excretable.23,25–27 Thus, the coordinated expression of these enzymes and their relative balance may determine the extent of cellular DNA damage and related development of adverse outcomes.
For these reasons, we hypothesized that maternal and/or fetal metabolic gene polymorphisms would alter the tobacco-related risk of adverse pregnancy outcomes. Utilizing prospectively acquired biologic samples from a multiinstitutional study, we assessed whether the functional polymorphisms associated with increased formation of carcinogenic adducts (phase I CYP1A1 Ile462Val polymorphism), inability to excrete reactive intermediates along the phase II metabolic pathways (GSTT1(del)), or altered metabolism of nicotine (CYP2A6 Lys160His)24,29–32 might account for the varying susceptibility to tobacco-mediated adverse pregnancy outcomes.
MATERIALS AND METHODS
The index study was a prospective, observational multicenter study conducted within the National Institute of Child Health and Human Development (NICHD) Maternal–Fetal Medicine Units (MFMU) Network and has been previously well described.33 The primary aim was to define the risk of venous thromboembolism and adverse pregnancy outcome in association with the factor V Leiden mutation.33 At each of the 13 enrollment sites, institutional review board approval and consent attainment was performed by trained research nurses for both the index study as well as for desired future disposition of samples in a uniform fashion as previously described.33,34 Of the 5,188 women who were enrolled between April 2000 and August 2001, 3,626 granted unrestricted permission for future use of whom 502 were self-admitted tobacco users (see below). A group of 502 nonsmokers was randomly selected from those who had granted unrestricted permission, matched by center and fetal/neonatal sample availability. Before initiating the analysis reported herein, we ascertained that this would be the first identified population-based report to investigate fetal genotype and adverse outcome susceptibility in response to in utero tobacco use (PubMed and Ovid with all-language search terms “fetal,” “genotype,” “pregnancy,” “growth restriction,” “abruption,” “miscarriage,” “stillbirth,” “preterm birth,” “smoking,” “tobacco;” 1986 to present).
Each woman had a venous blood sample drawn and submitted to a central reference laboratory (DNA Diagnostic Laboratory, University of Utah, Salt Lake City, UT), where analysis for the presence of the factor V Leiden mutation was performed as previously described.33 A neonatal cord blood sample was obtained after delivery. If cord blood was not available, a neonatal buccal swab, a 2-cm portion of umbilical cord, or a 2×2×2-cm portion of placenta was collected. DNA extractions were performed using a commercial DNA isolation kit, Puregene (Gentra Systems, Minneapolis, MN) as previously described.33 In the case of miscarriage or fetal death, abortus material was obtained whenever possible for DNA extraction.
At enrollment women were asked the questions “Have you smoked cigarettes at any time during this pregnancy?” and “Average number of cigarettes/day in the last week?” For this report, self-admitted tobacco use was not validated; however, a previous MFMU Network study (Ramsey P, et al. Do maternal serum cotinine levels correlate with self-reported smoking behavior during pregnancy? Presented at the 51st Annual Meeting of the American College of Obstetricians and Gynecologists, New Orleans, LA, April 2003) did validate self-reported maternal smoking behavior with 96% of “nonsmokers” having undetectable serum cotinine levels at less than 10 ng/mL, in accordance with other investigators.35
Gestational age at delivery was determined from best obstetric estimate at the time of enrollment and the interval between enrollment and delivery. Outcome data were abstracted from the patients’ medical records, and the diagnoses of placental abruption, pregnancy loss, small for gestational age (SGA)36–38 and preterm birth were the same as used in the primary analysis.33 Pregnancy loss was defined as either a spontaneous abortion at <20 completed gestational weeks or an antepartum or intrapartum fetal death. SGA was defined both as birth weight less than the 10th or 5th percentile derived from gender- and race-specific growth curves for gestational age for live births at 20 weeks or later with non-missing birth weight.36–38 Birth weight ratio was calculated in each case as birth weight divided by mean birth weight for gestation from the same reference data as for SGA and is a validated measure of adjusting for gestational age in a study population.38 For the definition of the primary composite outcome if any one of the outcome measures of “pregnancy loss,” “SGA,” “placental abruption,” and/or “preterm birth” occurred, then the composite adverse outcome was considered “true.” Baseline, demographic, laboratory, and outcome data were forwarded to the data coordinating center.
Samples were amplified using a set of primers specific for detection of the GSTT1(del) polymorphism as previously described25 and detailed in Appendix 2, available online at http://links.lww.com/AOG/A159.
An additional set of control primers were co-amplified with the insertion/deletion primers to insure detection of amplification of the sample in those individuals with deletions. The reverse primer for each primer pair contained a 5′ end fluorescent tag (56-FAMM) that allowed detection of the polymerase chain reaction (PCR) product during capillary electrophoresis (Appendix 2, available online at http://links.lww.com/AOG/A159). In this manner, we are testing a true functional deletion of GSTT1 and therefore assume it to be homozygous. Polymerase chain reactions were performed in a final volume of 15 microliters with 30 ng of genomic DNA, 0.5 micromolar GSTT1 specific primer, 0.2 micromolar amplification control primer, 2.0 mM MgCl2, 200 micromolar dNTPs, and 0.05 units/microliter of TaqGold Polymerase (Applied Biosystems Incorporated [ABI], Foster City, CA). Polymerase chain reactions in an ABI 9700 thermocycler were under the following conditions: 96°C 11 minutes, 5 cycles 95°C for 10 seconds, 60°C 10 seconds, 72°C 20 seconds; 30 cycles 94°C 10 seconds, 55°C 10 seconds, and 72°C 20 seconds. Of the PCR reaction, 0.1 microliter was added to 10 microliters of deionized formamide containing an internal sizing standard and the GSTT1(del) polymorphism was detected using the ABI 3730 XL fluorescent capillary electrophoresis instrument as the presence or absence of a 480-bp peak. The amplification control band was detected as a 312-bp peak.
Genotyping was accomplished using a Taqman assay (ABI). Primers and probes were selected using ABI selection software and as outlined in Appendix 2 (available online at http://links.lww.com/AOG/A159) in general accordance with previously published methods.26,29 Assays for the CYP1A1–01 (rs1048943) and CYP2A6–01 (rs1801272) corresponding to CYP1A1 (Ile462Val AA>AG/GG) and CYP2A6 (Lys160His T>A) allelic variants were validated and optimized as described in the SNP500 Cancer website (http://snp500cancer.nci.nih.gov). Polymerase chain reaction was performed in a 384-well plate in a final volume of 5 microliters with 30 ng of genomic DNA with 2.5 microliters of Taqman Universal Mastermix (ABI), 3.1 micromoles of each primer and 0.7 micromoles of each probe. The PCR reactions were cycled under the following conditions in an ABI 9700 thermocycler: 95°C 10 minutes, followed by 45 cycles of 92°C 15 seconds and 60°C 60 seconds. Genotypes were determined by an end-point reading on an ABI 7900HT real-time PCR instrument with four replicates of a DNA control sample and 4 nontemplated (no DNA added) controls to insure quality control.
Univariable analyses of genotype compared with smoking status and of smoking status and outcome were performed using χ2 or Fisher exact test for discrete variables and the Wilcoxon test for the continuous variables. To examine the modification of the effect of smoking on each outcome, logistic regression analysis was used for dichotomous outcomes, and analysis of covariance for birth weight and birth weight ratio, with genotype, smoking status, and an interaction term between smoking and genotype. For SGA, birth weight and birth weight ratio, multivariable analyses were also conducted adjusting for race, maternal body mass index (BMI), and for multiparity versus primiparity, history of a SGA neonate, and for birth weight only, gestational age at delivery. For the remaining outcomes, logistic regression was conducted adjusting for race, maternal BMI, and multiparity. Analyses combining maternal/fetal genotypes were also examined. SAS (SAS Institute, Inc., Cary NC) was used for analysis. A nominal P<.05 was considered significant. No adjustment was made for multiple comparisons. Outcomes included birth weight, pregnancy loss, preterm birth, small for gestational age, and a composite outcome composed of the latter four components plus abruption.
RESULTS
Appendix 3, available online at http://links.lww.com/AOG/A160, shows the characteristics of the study population by smoking status. The association of smoking with different outcomes in the full cohort of 1,004 women is reported in Appendix 4, available online at http://links.lww.com/AOG/A161. To summarize these data, overall there were 99 patients with SGA less than the 10th percentile and 46 less than the 5th percentile, 150 patients who delivered preterm, 63 who had a pregnancy loss, 150 with preterm delivery, and 285 with at least one of the events making up the composite adverse outcome. Since there were only eight cases of abruption, further analysis of this as a separate outcome was not undertaken. Smoking was significantly associated with an increased risk of SGA less than the 10th percentile (14.4% SGA among smokers and 8.2% among nonsmokers, odds ratio [OR] 1.9, 95% confidence interval [(CI)] 1.2–2.9; P=.004). Smoking was not significantly associated with an increase in SGA less than the 5th percentile (OR 1.7, 95% CI 0.9–3.2, P=.07), although the point estimate of the OR was very similar; this was likely due to the smaller numbers. Birth weight (mean for smokers 3,115±657 g compared 3,243±631 g, P=.009) and birth weight ratio (mean for smokers 0.97±0.14 compared with 1.01±0.15 for nonsmokers, P were significantly lower among the smokers. Smoking was marginally associated with an increased risk of the composite adverse outcome (OR 1.3, 95% CI 1.0 1.7, P=.05). However, smoking was not associated with pregnancy loss, preterm birth, or gestational age at delivery.
Based on the outcomes in our cohort, we sought to characterize the genetic basis for the differential susceptibility in fetal growth among smokers. To first orient the reader to the genotypes examined, a few comments regarding their gene products are in order. CYP1A1 is a phase I metabolic enzyme which encodes the aryl hydrocarbon hydroxylase enzymes responsible for the activation of the polycyclic aromatic hydrocarbons compounds to their potentially harmful reactive intermediates. Following high-affinity binding of polycyclic aromatic hydrocarbons compounds to their intracellular aryl hydrocarbon ligands, the complex is translocated to the nucleus where it dissociates then heterodimerizes to form a DNA binding complex to modulate chromatin disruption and regulate induction of CYP1A1 expression.22,25–27 The CYP1A1 Ile462Val (AA>AG/GG) allele carriers exhibit higher levels of CYP1A1 enzymatic activity and inducibility, and smokers who carry this variant have increased cellular polycyclic aromatic hydrocarbons-DNA adducts.22,25–27 Polymorphisms leading to enzymatic inactivity in the phase II metabolic enzyme glutathione S-transferase theta 1 (GSTT1) (eg, GSTT1[del]) are prevalent and have been extensively studied in the context of individual susceptibility to tobacco-mediated carcinogenesis, albeit with variable attributable risk associations.23,25–27 Finally, variable expression of alternate cytochrome P450 enzymes (eg, CYP2A6) have been shown to modify daily cigarette consumption.24,29,30 CYP2A6 is a highly polymorphic allele and functions as the rate-limiting enzyme in the metabolism of nicotine to cotinine.24,29,30 Individuals with diminished activity of CYP2A6 activity at the CYP2A6*2 allele (CYP2A6 Lys160His T>A) inherit the slowest metabolism of nicotine and have been associated with lower cigarette consumption, shorter duration of smoking, and increased ability to quit smoking.24,29–32 Theoretically, any combinatorial association of increase phase I activity (eg, increased expression of CYP1A1 via functional polymorphisms) in combination with decreased phase II activity (eg, decreased GSTT1 expression) may yield increased susceptibility to tobacco-related adverse outcomes.
The availability of DNA and the distribution of each of these genotypes are given in Appendix 5, available online at http://links.lww.com/AOG/A162. Of the 1,004 maternal samples, 213 (21%) were discarded for an inability to determine the GSTT1 allelic deletion variant or for poor-quality DNA. For CYP1A1 and CYP2A6, only 45 (4.5%) and 55 (5.5%) samples could not be genotyped respectively. Of the corresponding 1,004 conceptuses, DNA samples were available for 772 (76.9%). Of these, GSTT1 deletion could not be determined in 197 samples (25.5%), and CYP1A1 and CYP2A6, in 157 (20.3%) and 205 (26.6%) samples, respectively. The distribution of maternal and fetal genotypes was consistent with that reported for the general population and did not demonstrate bias with respect to maternal tobacco use (Appendix 4, available online at http://links.lww.com/AOG/A161).22–32 Since the detected prevalence of the CYP1A1 and CYP2A6 recessive alleles was uncommon in the study population and prior studies have not supported a recessive model, the AG/GG (CYP1A1) and AT/AA (CYP2A6) genotypes were combined in the data analyses.22
Univariable analyses of the relationship between smoking and the dichotomous adverse outcomes by maternal and fetal genotype are given in Tables 1 and 2. Within each genotype, the ORs and 95% CIs for the association of smoking with the adverse outcome is given. The P values showed that, despite different magnitudes and even different directions of ORs between the two genotypes of each polymorphism, there was no statistically significant interaction between any maternal or fetal genotype and smoking (Tables 1 and 2). This indicates that maternal and fetal genotype did not significantly modify any association between smoking and adverse outcome. Odds ratios for each less-common genotype with the adverse outcome were then calculated, adjusting for smoking status in addition to confounders (Tables 1 and 2).
Table 1.
Association of Smoking, Maternal Genotype, and Adverse Pregnancy Outcome
| Maternal Genotype | Smoker [N (%)]* |
Nonsmoker [N (%)]* |
OR (95% CI)† | P‡ | Adjusted OR§ (95% CI) for Genotype |
P‖ |
|---|---|---|---|---|---|---|
| Pregnancy loss | ||||||
| GSTT1(del) | 7 (7.1) | 3 (3.2) | 2.28 (0.57–9.10) | .26 | 0.92 (0.44–1.92) | .82 |
| GSTT1 | 17 (6.1) | 18 (6.5) | 0.94 (0.48–1.87) | |||
| CYP1A1 AG/GG (Ile426Val) | 3 (7.9) | 3 (6.3) | 1.29 (0.24–6.76) | .72 | 1.00 (0.38–2.64) | 1.00 |
| CYP1A1 AA | 26 (6.2) | 27 (6.6) | 0.94 (0.54–1.63) | |||
| CYP2A6 AT/AA (Lys160His) | 1 (4.8) | 0 (0) | — | .98 | 0.44 (0.06–3.31) | .42 |
| CYP2A6 TT | 28 (6.5) | 30 (6.9) | 0.95 (0.55–1.61) | |||
| Preterm delivery | ||||||
| GSTT1(del) | 16 (17.8) | 10 (11.5) | 1.67 (0.71–3.90) | .18 | 0.67 (0.40–1.10) | .11 |
| GSTT1 | 43 (16.3) | 49 (18.6) | 0.86 (0.55–1.35) | |||
| CYP1A1 AG/GG (Ile426Val) | 5 (6.1) | 6 (4.7) | 1.14 (0.32–4.08) | .90 | 1.04 (0.50–2.16) | .91 |
| CYP1A1 AA | 68 (8.8) | 63 (8.2) | 1.05 (0.72–1.53) | |||
| CYP2A6 AT/AA (Lys160His) | 2 (10.0) | 2 (16.7) | 0.56 (0.07–4.57) | .56 | 0.60 (0.18–2.04) | .42 |
| CYP2A6 TT | 70 (17.3) | 68 (16.7) | 1.05 (0.72–1.51) | |||
| SGA less than 10th percentile | ||||||
| GSTT1(del) | 13 (14.4) | 7 (8.1) | 1.91 (0.72–5.03) | .91 | 0.93 (0.53–1.64) | .81 |
| GSTT1 | 40 (15.4) | 21 (8.2) | 2.03 (1.16–3.56) | |||
| CYP1A1 AG/GG (Ile426Val) | 7 (20.0) | 3 (6.8) | 3.42 (0.81–14.35) | .41 | 1.42 (0.64–3.15) | .39 |
| CYP1A1 AA | 55 (14.2) | 31 (8.4) | 1.81 (1.14–2.89) | |||
| CYP2A6 AT/AA (Lys160His) | 3 (15.0) | 3 (25.0) | 0.53 (0.09–3.18) | .15 | 1.78 (0.68–4.63) | .24 |
| CYP2A6 TT | 58 (14.5) | 30 (7.6) | 2.08 (1.30–3.30) | |||
| SGA less than 5th percentile | ||||||
| GSTT1(del) | 5 (5.6) | 2 (2.3) | 2.47 (0.47–13.1) | .80 | 0.77 (0.33–1.80) | .54 |
| GSTT1 | 19 (7.3) | 10 (3.9) | 1.94 (0.88–4.26) | |||
| CYP1A1 AG/GG (Ile426Val) | 3 (8.6) | 1 (2.3) | 4.03 (0.4–40.55) | .50 | 1.27 (0.41–3.96) | .68 |
| CYP1A1 AA | 25 (6.5) | 14 (3.8) | 1.76 (0.90–3.43) | |||
| CYP2A6 AT/AA (Lys160His) | 1 (5.0) | 1 (8.3) | 0.58 (0.03–10.21) | .43 | 1.05 (0.24–4.70) | .95 |
| CYP2A6 TT | 26 (6.5) | 14 (3.5) | 1.90 (0.98–3.70) | |||
| Composite adverse outcome | ||||||
| GSTT1(del) | 34 (35.1) | 17 (19.1) | 2.29 (1.17–4.48) | .12 | 0.77 (0.53–1.12) | .18 |
| GSTT1 | 92 (33.2) | 79 (28.6) | 1.24 (0.86–1.78) | |||
| CYP1A1 AG/GG (Ile426Val) | 14 (36.8) | 10 (20.8) | 2.22 (0.85–5.78) | .31 | 1.16 (0.66–2.02) | .61 |
| CYP1A1 AA | 137 (33.1) | 109 (27.4) | 1.31 (0.97–1.77) | |||
| CYP2A6 AT/AA (Lys160His) | 6 (28.6) | 5 (41.7) | 0.56 (0.13–2.48) | .24 | 1.11 (0.51–2.41) | .79 |
| CYP2A6 TT | 144 (33.6) | 115 (26.9) | 1.38 (1.03–1.85) |
OR, odds ratio; CI, confidence interval; SGA, small for gestational age.
N (%) with outcome.
Odds ratios and 95% CIs for smoking and adverse outcome within each genotype.
The P value for interaction between genotype and smoking.
Odds ratio and 95% CI for the first listed genotype, adjusted for smoking status, race/ethnicity, maternal body mass index, and nulliparity compared with multiparity.
The P value for adjusted OR for genotype.
Table 2.
Association of Smoking, Maternal Genotype, and Adverse Pregnancy Outcome
| Fetal Genotype | Smoker [N (%)]* |
Nonsmoker [N (%)]* |
OR (95% CI)† | P‡ | Adjusted OR§ (95% CI) for Genotype |
P‖ |
|---|---|---|---|---|---|---|
| Pregnancy loss | ||||||
| GSTT1(del) | 1 (1.1) | 1 (1.3) | 0.90 (0.06–14.59) | .96 | 3.12 (0.40–24.66) | .28 |
| GSTT1 | 0 (0) | 2 (1.0) | —¶ | |||
| CYP1A1 AG/GG (Ile426Val) | 0 (0) | 1 (2.7) | —¶ | .98 | 0.69 (0.06–8.14) | .77 |
| CYP1A1 AA | 1 (0.4) | 2 (0.7) | 0.51 (0.05–5.65) | |||
| CYP2A6 AT/AA (Lys160His) | 0 (0) | 0 (0) | —¶ | .98 | —¶ | |
| CYP2A6 TT | 0 (0) | 3 (1.1) | —¶ | |||
| Preterm birth | ||||||
| GSTT1(del) | 12 (13.6) | 10 (12.5) | 1.11 (0.45–2.72) | .68 | 0.77 (0.45–1.30) | .32 |
| GSTT1 | 30 (14.8) | 33 (16.3) | 0.89 (0.52–1.52) | |||
| CYP1A1 AG/GG (Ile426Val) | 6 (14.3) | 5 (13.5) | 1.07 (0.30–3.83) | .92 | 1.34 (0.65–2.76) | .43 |
| CYP1A1 AA | 38 (14.4) | 39 (14.4) | 1.00 (0.61–1.61) | |||
| CYP2A6 AT/AA (Lys160His) | 2 (14.3) | 0 (0) | — | .98 | 0.57 (0.13–2.48) | .45 |
| CYP2A6 TT | 36 (13.8) | 41 (14.6) | 0.94 (0.58–1.53) | |||
| SGA less than 10th percentile | ||||||
| GSTT1(del) | 15 (17.0) | 4 (5.1) | 3.85 (1.22–12.16) | .46 | 1.21 (0.67–2.19) | .54 |
| GSTT1 | 28 (13.8) | 13 (6.5) | 2.31 (1.16–4.61) | |||
| CYP1A1 AG/GG (Ile426Val) | 5 (11.9) | 0 (0)- | —¶ | .96 | 0.56 (0.21–1.51) | .25 |
| CYP1A1 AA | 39 (14.8) | 21 (7.8) | 2.05 (1.17–3.59) | |||
| CYP2A6 AT/AA (Lys160His) | 1 (7.1) | 2 (18.2) | 0.35 (0.03–4.42) | .13 | 0.92 (0.26–3.31) | .90 |
| CYP2A6 TT | 42 (16.2) | 19 (6.8) | 2.64 (1.49–4.67) | |||
| SGA less than 5th percentile | ||||||
| GSTT1(del) | 7 (8.0) | 1 (1.3) | 6.74 (0.81–56.05) | .32 | —¶ | |
| GSTT1 | 12 (5.9) | 6 (3.0) | 2.04 (0.75–5.55) | |||
| CYP1A1 AG/GG (Ile426Val) | 2 (4.8) | 0 (0) | —¶ | .97 | 0.60 (0.13–2.72) | .50 |
| CYP1A1 AA | 14 (5.3) | 9 (3.3) | 1.62 (0.69–3.81) | |||
| CYP2A6 AT/AA (Lys160His) | 0 (0) | 0 (0) | —¶ | 1.00 | —¶ | |
| CYP2A6 TT | 18 (6.9) | 9 (3.2) | 2.23 (0.69–3.81) | |||
| Composite adverse outcome | ||||||
| GSTT1(del) | 26 (29.2) | 14 (17.5) | 1.95 (0.93–4.06) | .31 | 0.96 (0.62–1.47) | .85 |
| GSTT1 | 53 (26.1) | 45 (22.2) | 1.24 (0.79–1.96) | |||
| CYP1A1 AG/GG (Ile426Val) | 10 (23.8) | 5 (13.5) | 1.34 (0.90–1.99) | .53 | 0.90 (0.48–1.69) | .74 |
| CYP1A1 AA | 72 (27.2) | 59 (21.8) | 2.00 (0.62–6.51) | |||
| CYP2A6 AT/AA (Lys160His) | 3 (21.4) | 2 (18.2) | 1.23 (0.17–9.02) | .89 | 0.79 (0.29–2.19) | .66 |
| CYP2A6 TT | 71 (27.3) | 59 (20.9) | 1.42 (0.96–2.11) |
OR, odds ratio; CI, confidence interval; SGA, small for gestational age.
N (%) with outcome.
Odds ratios and 95% CIs for smoking and adverse outcome within each genotype.
The P value for interaction between genotype and smoking.
Odds ratio and 95% CI for the first listed genotype, adjusted for smoking status, race/ethnicity, maternal body mass index, and nulliparity compared with multiparity.
The P value for adjusted OR for genotype.
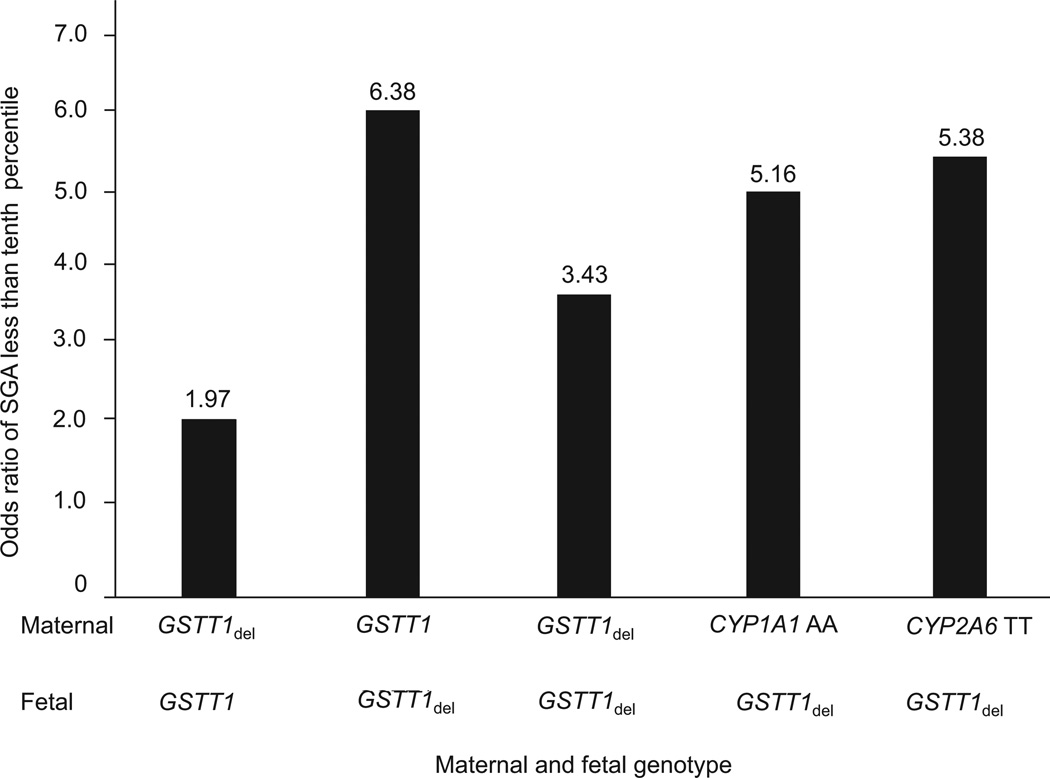
When the effect of the simultaneous presence of maternal and fetal gene polymorphisms at each of the three alleles was examined for association with SGA 10th percentile, fetal GSTT(del) was unique in its observed association with growth restriction in combinatorial allelic models (Fig. 1). For example, maternal GSTT1(del) with a fetal GSTT1 nondeletion did not show an increased risk of smoking-associated growth restriction (OR 1.97, 95% CI 0.40 –9.71), while fetal GSTT1(del) with maternal GSTT1 nondeletion (OR 6.38, 95% CI 1.30 –31.41), fetal GSTT1(del) with CYP1A1 AA (OR 5.16, 95% CI 1.42–18.7), and fetal GSTT1(del) with CYP2A6 TT (OR 5.38, 95% CI 1.49 –19.46) fetal–maternal associations bore a significant increased risk (Fig. 1). When all potential combinatorial associations were applied (maternal and/or fetal GSTT1 nondeletion compared with [del], with maternal and/or fetal CYP1A1 AA compared with CYP1A1 AG/GG, with maternal and/or fetal CYP2A6 TT compared with CYP2A6 AT/AA) for each of the adverse pregnancy outcome(s) tested, homozygous deletion of fetal GSTT1 persisted as the only allelic variant with an observed significant association between maternal smoking and adverse pregnancy outcome (data not shown).
Fig. 1.
Odds ratio of smoking for small for gestational age (SGA) for fetal GSTT1(del) allelic combinations. Calculation of SGA was defined as birth weight less than the 10th percentile, derived from sex- and race-specific growth curves for gestational age for live births after 20 weeks of gestation with nonmissing birth weight.36–39 GSTT1, normal GSTT1 allele; GSTT1del, GSTT1 deletion; CYP1A1 AA, CYP1A1 AA genotype; CYP2A6 TT, CYP2A6 TT genotype.36–39
Analyses of birth weight and birth weight ratio by smoking status and maternal/fetal GSTT1 genotype are shown in Table 3. There was no interaction between genotype and smoking except for fetal GSTT1 (P=.02). The mean difference (Δ=0.03) in the birth weight ratio between smokers and nonsmokers among GSTT1 nondeletion fetuses was nonsignificant (Table 3), whereas the mean birth weight ratio difference (Δ=0.09) between smokers and nonsmokers among GSTT1(del) fetuses was significant (P<.0001).
Table 3.
Association of In Utero Tobacco Exposure and GSTT1 Genotype, Birth Weight, and Birth Weight Ratio
| Genotype | ||||||||
|---|---|---|---|---|---|---|---|---|
| Maternal | Fetal | |||||||
| GSTT1 | GSTT1(del) | GSTT1 | GSTT1(del) | |||||
| Smoker | Nonsmoker | Smoker | Nonsmoker | Smoker | Nonsmoker | Smoker | Nonsmoker | |
| Birth weight (g) | ||||||||
| Mean | 3122 | 3266 | 3086 | 3276 | 3151 | 3238 | 3084 | 3346 |
| Standard deviation | 664 | 605 | 561 | 607 | 683 | 572 | 624 | 684 |
| P* | .01 | .03 | .16 | .01 | ||||
| P Interaction | .54 | .14 | ||||||
| Birth weight ratios | ||||||||
| Mean | 0.97 | 1.01 | 0.98 | 1.01 | 0.97 | 1.00 (Δ=0.03) | 0.97† | 1.06 (Δ=0.09)† |
| Standard deviation | 0.14 | 0.16 | 0.14 | 0.15 | 0.15 | 0.14 | 0.13† | 0.17† |
| P* | .001 | .10 | .05 | <.001† | ||||
| P Interaction | .82 | .02† | ||||||
Birth weight and birth weight ratio among singleton live births were analyzed as a continuous variable in an analysis of covariance with maternal smoking status, genotype, and the interaction term (maternal smoking status × genotype).
P value derived from univariate analysis (t test) between smokers and nonsmokers.
Statistical significance at a P<.05, indicating that the genotype significantly altered the association between smoking and mean birth weight ratio.
Although in univariable analysis there was no significant interaction term between GSTT1 fetal genotype and smoking (P=.14), after adjusting for race, maternal BMI, and multiparity compared with primiparity, the interaction term was significant (P=.002). Thus, we observed a significant decrease in birth weight in tobacco-exposed compared with non–tobacco-exposed fetuses with the GSTT1(del) (mean reduction of 262 g, P=.01). For fetuses without a deletion of GSTT1 (ie, the nonvariant GSTT1 allele) the decrease in birth weight in tobacco-exposed compared with non-exposed fetuses was not significant (mean 87 g, P=.16; Table 3). This corresponded to an additional 256-g reduction between -xposed and non-exposed fetuses for the GSTT1 deletion group. After adjusting for race, maternal BMI, and multiparity compared with primiparity, fetal GSTT1(del) persisted as an effect modifier for the relationship between smoking and birth weight ratio (corresponding to an additional reduction [Δ=0.07] between tobacco-exposed and non-exposed fetuses for the GSTT1 deletion group reduction, P=.006; Table 3).
DISCUSSION
While one other study examined an association of maternal genotype with fetal growth,13 our findings suggest that fetal homozygous deletion of a phase II gene (GSTT1) integral to excretion of reactive intermediates is significantly and specifically associated with modified fetal growth patterns in response to maternal smoking. These observations persisted in multiple allelic models to suggest an interaction between the fetal metabolic gene GSTT1, maternal smoking, and modification of birth weight (Fig. 1, Tables 1 and 2).
While our findings were significant for the association with fetal GSTT1(del) on birth weight ratio, there was no observed association among tested polymorphisms on the other adverse pregnancy outcomes. Thus, there are a number of limitations that ought to be considered when broadly interpreting our findings. First, our samples were obtained from a prospective, observational study designed to assess the rate of venous thromboembolism among factor V Leiden carriers.33 The index study was not powered to detect the adverse pregnancy outcomes among rare allelic variants (eg, CYP1A1 [Ile462Val AA>AG/GG] and CYP2A6 [Lys160His T>A]) nor was it designed to assess the effect of smoking among a relatively “low risk” population. Second, tobacco smoke contains multiple compounds, and while we have tested functional polymorphisms along well-established metabolic pathways related to varying cancer susceptibility, the relative role of these genes in perinatal outcomes is unknown. Third, we cannot ascertain a dose-dependent effect, although good correlations between the presence or absence of detectable serum cotinine and self-reported smoking status in pregnancy have been reported.7,35 Fourth, the fact that there were a significant number of missing fetal samples may have provided a bias underestimating the relative fetal contribution specifically with respect to risk of fetal loss or miscarriage, since we matched the nonsmokers on the availability of fetal/neonatal samples. Finally, we did not formally exclude the possibility that unrecognized linkage disequilibrium between the examined allelic variant(s) and another (unexamined) actual susceptibility gene has occurred raising the possibility that we have not specifically evaluated the “true” susceptibility locus.
Despite these limitations, the strengths of our study are several. First, the index study enrolled more than 5,100 pregnant women who had considerable geographic and sociodemographic diversity.33 Second, consent for subsequent use of DNA was given before enrollees and their providers would have any potential knowledge of mutation status and without consideration of maternal smoking status, therefore eliminating the possibility of bias based on discovery of a genetic condition, subsequent adverse pregnancy outcome, or tobacco use per se. Third, our secondary genotype analyses explored the association between maternal smoking and adverse pregnancy outcomes as stratified by maternal and fetal genotype according to both known maternal factors, as well as ad hoc factors unknown at enrollment. Fourth, we used a priori defined strict clinical criteria for the establishment of adverse outcome diagnoses.
Given our study design, we were able to overcome limitations in prior limited analyses. Wang et al13 initially reported an association between maternal cigarette smoking and infant birth weight and gestational age by maternal polymorphisms in CYP1A1 and GSTT1; however, their findings did not reach significance when a test of interaction was applied. Several studies have examined the relationship between maternal metabolic gene polymorphisms and polycyclic aromatic hydrocarbons–DNA adduct formation but without relation to adverse pregnancy outcome.13,39–40 Finally, Nukui et al41 examined genotypes of a panel of phase I and II metabolic enzymes from maternal peripheral and umbilical cord blood samples among 955 maternal/newborn pairs to evaluate the association with premature birth (defined as less than 2,500 g and less than 37 weeks of week gestation). In their analysis, the combined presence of a double at-risk genotype together with maternal third-trimester smoking resulted in an approximate doubling of either genotypic or exposure risks for preterm birth.41 While we did not observe a significant association with preterm birth, we benefitted from a well-defined study population enrolled at less than 14 weeks of gestation, which allowed us to differentiate preterm neonates from small for gestational age neonates utilizing obstetric dating criteria alongside sex- and race-specific growth curves. It remains a formal possibility that by virtue of their definition of premature births as less than 2,500 g and less than 37 weeks of gestation Nukui et al41 may be reporting a true association of SGA rather than pre-maturity. In summary, our data ultimately reflect previous findings, with the notable and significant additional observation that it is specifically a fetal, rather than maternal, genotype (GSTT1[del]) that associates with modifications in fetal growth following in utero tobacco exposure.
Our observations bear biologic plausibility and merit. First, while mechanisms leading to IUGR after in utero tobacco exposure have generally often been attributed to chronic fetal hypoxia, it is known that nicotine, cotinine, and DNA adducts cross or collect in the placenta of smokers.14–21 As discussed, phase I gene products, such as CYP1A1, are integral in metabolic activation of polycyclic aromatic hydrocarbon compounds into oxidized derivatives, resulting in reactive oxygen intermediates capable of covalently binding DNA to form adducts; as a balance to such intermediary forming reactions, conjugation with endogenous species to form hydrophilic glutathione conjugates, which are then readily excretable, occurs. Our data support the notion that the variation in fetal susceptibility to smoking-related growth restriction results from the diminished ability of the fetus to excrete these reactive intermediates (fetal phase II GSTT1[del]).
There is an increasing recognition of the role genetic and epigenetic mechanisms play in modifying individual susceptibility to perinatal health and disease. Our secondary analysis recognizes such mechanisms. The implications of our findings are twofold. First, our data illustrate that a fetal metabolic gene (GSTT1), which is integral in the excretion of reactive intermediates of aromatic hydrocarbons, modifies fetal growth specifically in response to in utero tobacco exposure. These findings imply that tobacco metabolites may reach the fetus and thus modify fetal growth if not excreted. Second, future studies aimed at illuminating the complex interplay of genomic–epigenomic–environmental interactions may help dissect multifactorial etiologies and identify at-risk populations for the common adverse pregnancy outcomes.
Supplementary Material
Acknowledgments
Supported by the Eunice Kennedy Shriver National Institute of Child Health and Human Development through the Maternal-Fetal Medicine Units Network (U10-HD27869, U10-HD21414, U01-HD36801, U10-HD34208, U10-HD27860, U10-HD34116, U10-HD34136, U10-HD27861, U10-HD34122, U10-HD21410, U10-HD27915, U10-HD34210, U10-HD27905, and U10-HD27917) and the National Institutes of Health’s Office of Research on Women’s Health.
The authors thank Dr. Donna Dizon-Townson for her initial conception and execution of the index study with the Maternal-Fetal Medicine Units Network and Yuan Zhao, MS, for biostatistical analysis. In addition, the authors thank Julia Gold, RN, for protocol development and coordination between clinical research centers, Valerija Momirova, MS, for protocol and data management, and Michael Varner, MD, for protocol development and oversight and manuscript development.
Footnotes
For a list of other members of the NICHD MFMU who participated in this study, see Appendix 1 online at http://links.lww.com/AOG/A158. There are also supplementary tables for this article, also available online at http://links.lww.com/AOG/A159, http://links.lww.com/AOG/A160, http://links.lww.com/AOG/A161, and http://links.lww.com/AOG/A162.
Financial Disclosure
The authors did not report any potential conflicts of interest.
REFERENCES
- 1.Hammoud AO, Bujold E, Sorokin Y, Schild C, Krapp M, Baumann P. Smoking in pregnancy revisited: Findings from a large population-based study. Am J Obstet Gynecol. 2005;192:1856–1863. doi: 10.1016/j.ajog.2004.12.057. [DOI] [PubMed] [Google Scholar]
- 2.Kyrklund-Blomberg N, Cnattingius S. Preterm birth and maternal smoking; risks related to gestational age and onset of delivery. Am J Obstet Gynecol. 1998;179:1051–1055. doi: 10.1016/s0002-9378(98)70214-5. [DOI] [PubMed] [Google Scholar]
- 3.Cnattingius S, Granath F, Petersson G, Harlow BL. The influence of gestational age and smoking habits on the risk of subsequent preterm deliveries. N Engl J Med. 1999;341:943–948. doi: 10.1056/NEJM199909233411303. [DOI] [PubMed] [Google Scholar]
- 4.Peacock JL, Bland JM, Anderson R. Preterm delivery: effects of socioeconomic factors, psychological stress, smoking, alcohol, and caffeine. BMJ. 1995;311:531–535. doi: 10.1136/bmj.311.7004.531. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Peacock JL, Cook DG, Carey IM, et al. Maternal cotinine level during pregnancy and birth weight for gestational age. Int J Epidemiol. 1998;27:647–656. doi: 10.1093/ije/27.4.647. [DOI] [PubMed] [Google Scholar]
- 6.Everson R, Randerath E, Santella RM, et al. Quantitative associations between DNA damage in human placenta and maternal smoking and birth weight. J Natl Cancer Inst. 1998;80:567–576. doi: 10.1093/jnci/80.8.567. [DOI] [PubMed] [Google Scholar]
- 7.Cnattingius S. The epidemiology of smoking during pregnancy: Smoking prevalence, maternal characteristics, and pregnancy outcomes. Nicotine Tob Res. 2004;6:S125–S140. doi: 10.1080/14622200410001669187. [DOI] [PubMed] [Google Scholar]
- 8.Secker-Walker RH, Vacek PM. Relationship between cigarette smoking during pregnancy, gestational age, maternal weight gain, and infant birth weight. Addict Behav. 2003;28:55–66. doi: 10.1016/s0306-4603(01)00216-7. [DOI] [PubMed] [Google Scholar]
- 9.Vogt-Isaksen C, Austgulen R, Chedwick L, et al. Maternal smoking, intrauterine growth restriction, and placental apoptosis. Ped Develop Pathol. 2004;7:433–442. doi: 10.1007/s10024-004-0105-1. [DOI] [PubMed] [Google Scholar]
- 10.Wen SW, Goldenberg RL, Cutter GR, et al. Smoking, maternal age, fetal growth, and gestational age at delivery. Am J Obstet Gynecol. 1990;162:53–58. doi: 10.1016/0002-9378(90)90819-s. [DOI] [PubMed] [Google Scholar]
- 11.Cnattingius S, Haglund B. Decreasing smoking prevalence during pregnancy in Sweden: the effect on small-for-gestational-age births. Am J Public Health. 1997;87:410–413. doi: 10.2105/ajph.87.3.410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Aagaard-Tillery KM, Porter TF, Lane RH, Varner MW, LaCoursiere DY. In utero tobacco exposure modifies the affect of maternal factors on fetal growth. Am J Obstet Gynecol. 2008;198:66.e1–66.e6. doi: 10.1016/j.ajog.2007.06.078. [DOI] [PubMed] [Google Scholar]
- 13.Wang X, Zuckerman B, Pearson C, et al. Maternal cigarette smoking, metabolic gene polymorphism, and infant birth weight. JAMA. 2002;287:195–202. doi: 10.1001/jama.287.2.195. [DOI] [PubMed] [Google Scholar]
- 14.Pastrakuljic A, Derewlany LO, Koren G. Maternal cocaine use and cigarette smoking in pregnancy in relation to amino acid transport and fetal growth. Placenta. 1999;20:499–512. doi: 10.1053/plac.1999.0418. [DOI] [PubMed] [Google Scholar]
- 15.Castro LC, Allen R, Ogunyerni D, Roll K, Platt LD. Cigarette smoking during pregnancy: acute effects on uterine flow velocity waveforms. Obstet Gynecol. 1993;81:551–555. [PubMed] [Google Scholar]
- 16.Lambers DS, Clark K. Maternal and fetal physiologic effects of nicotine. Semin Perinatol. 1996;20:115–126. doi: 10.1016/s0146-0005(96)80079-6. [DOI] [PubMed] [Google Scholar]
- 17.Marana HRC, Andrade JM, Martins GA, et al. A morphometric study of maternal smoking on apoptosis in the syncytiotrophoblast. Int J Gynaecol Obstet. 1998;61:21–27. doi: 10.1016/s0020-7292(98)00014-9. [DOI] [PubMed] [Google Scholar]
- 18.Perera F, Jedrychowski W, Rauh V, Whyatt RM. Molecular epidemiologic research on the effects of environmental pollutants on the fetus. Environ Health Perspect. 1999;107 Supp 3:451–460. doi: 10.1289/ehp.99107s3451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Huel G, Godin J, Moreau T, et al. Aryl hydrocarbon hydroxylase activity in human placenta of passive smokers. Environ Res. 1989;50:173–183. doi: 10.1016/s0013-9351(89)80056-8. [DOI] [PubMed] [Google Scholar]
- 20.Gladen BC, Zadorozhnaja TD, Chislovska N, et al. Polycyclic aromatic hydrocarbons in placenta. Hum Experiment Toxicol. 2000;19:597–603. doi: 10.1191/096032700671433928. [DOI] [PubMed] [Google Scholar]
- 21.Daube H, Scherer G, Riedel K, et al. DNA adducts in human placenta in relation to tobacco smoke exposure and plasma antioxidant status. J Canc Res Clin Oncol. 1997;123:141–151. doi: 10.1007/BF01214666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Anttila S, Hakkola J, Tuominen P, et al. Methylation of Cytochrome P4501A1 promoter in the lung is associated with tobacco smoking. Cancer Res. 2003;63:8623–8628. [PubMed] [Google Scholar]
- 23.Smits KM, Benhamou S, Garte S, et al. Association of metabolic gene polymorphisms with tobacco consumption in healthy controls. Int J Cancer. 2004;110:266–270. doi: 10.1002/ijc.20114. [DOI] [PubMed] [Google Scholar]
- 24.Raunio H, Rautio A, Gullsten H, Pelkonen O. Polymorphisms of CYP2A6 and its practical consequences. Br J Clin Pharmacol. 2001;52:357–363. doi: 10.1046/j.0306-5251.2001.01500.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Larsen JE, Colosimo ML, Yang IA, et al. CYP1A1 Ile462Val and MPO G-463A interact to increase risk of adenocarcinoma but not squamous cell carcinoma of the lung. Carcinogenesis. 2006;27:525–532. doi: 10.1093/carcin/bgi227. [DOI] [PubMed] [Google Scholar]
- 26.Hou L, Chatterjee N, Huang W-Y, et al. CYP1A1Val462 and NQO1Ser187 polymorphisms, cigarette use, risk of colorectal carcinoma. Carcinogenesis. 2005;26:1122–1128. doi: 10.1093/carcin/bgi054. [DOI] [PubMed] [Google Scholar]
- 27.Mooney LA, Bell DA, Santella RM, et al. Contribution of genetic and nutritional factors to DNA damage in heavy smokers. Carcinogenesis. 1997;18:503–509. doi: 10.1093/carcin/18.3.503. [DOI] [PubMed] [Google Scholar]
- 28.Czekaj P, Wiaderkiewicz A, Florek E, Wiaderkiewicz R. Tobacco smoke-dependent changes in cytochrome P450 1A1, 1A2, and 2E1 protein expressions in fetuses, newborns, pregnancy rats, and human placenta. Arch Toxicol. 2005;79:13–24. doi: 10.1007/s00204-004-0607-7. [DOI] [PubMed] [Google Scholar]
- 29.Schoedel KA, Hoffmannn EB, Rao Y, Sellers EM, Tyndale RF. Ethnic variation in CYP2A6 and association of genetically slow nicotine metabolism and smoking in adult Caucasions. Pharmacogenetics. 2004;14:615–626. doi: 10.1097/00008571-200409000-00006. [DOI] [PubMed] [Google Scholar]
- 30.Pianezza ML, Sellers EM, Tyndale R. Nicotine metabolism defect reduces smoking. Nature. 1998;393:750. doi: 10.1038/31623. [DOI] [PubMed] [Google Scholar]
- 31.Oscarson M, McLellan RAN, Gullsten H, et al. Identification and characterization of novel polymorphisms in the CYP2A locus: implications for nicotine metabolism. FEBS Lett. 1999;460L:321–327. doi: 10.1016/s0014-5793(99)01364-2. [DOI] [PubMed] [Google Scholar]
- 32.Hong Y-C, Lee K-H, Son B-K, et al. Effects of the GSTM1 and GSTT1 polymorphisms on the relationship between maternal exposure to environmental tobacco smoke and neonatal birth weight. J Occup Environ Med. 2003;45:492–498. doi: 10.1097/01.jom.0000063627.37065.a1. [DOI] [PubMed] [Google Scholar]
- 33.Dizon-Townson DS, Miller C, Sibai B, et al. The relationship of the factor V Leiden mutation and pregnancy outcomes for mother and fetus. Obstet Gynecol. 2005;106:517–524. doi: 10.1097/01.AOG.0000173986.32528.ca. [DOI] [PubMed] [Google Scholar]
- 34.Aagaard-Tillery KM, Sibai B, Spong CY, et al. Sample bias among women with retained DNA samples for future genetic studies. Obstet Gynecol. 2006;108:1115–1120. doi: 10.1097/01.AOG.0000241536.19539.14. [DOI] [PubMed] [Google Scholar]
- 35.McDonald SD, Perkins SL, Walker MC. Correlation between self-reported smoking status and serum cotinine during pregnancy. Addict Behav. 2005;30:853–857. doi: 10.1016/j.addbeh.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 36.Alexander GR, Tompkins ME, Allen MC, Hulsey TC. Trends and racial differences in birth weight and related survival. Matern Child Health J. 1999;3:71–79. doi: 10.1023/a:1021849209722. [DOI] [PubMed] [Google Scholar]
- 37.Alexander GR, Kogan MD, Himes JH. 1994–1996 U.S. singleton birth weight percentiles for gestational age by race, Hispanic origin, and gender. Matern Child Health J. 1999;3:225–231. doi: 10.1023/a:1022381506823. [DOI] [PubMed] [Google Scholar]
- 38.Wilcox MA, Johnson IR, Maynard PV, Smith SJ, Chilvers CE. The individualized birthweight ratio: a more logical outcome measure of pregnancy than birthweight alone. Br J Obstet Gynaecol. 1993;100:342–347. doi: 10.1111/j.1471-0528.1993.tb12977.x. [DOI] [PubMed] [Google Scholar]
- 39.Whyatt RM, Jedrychowski W, Hemminki K, Santella RM, Tsai WY, Yang K, Perera FP. Biomarkers of polycyclic aromatic hydrocarbon-DNA damage and cigarette smoke exposures in paired maternal and newborn blood samples as a measure of differential susceptibility. Cancer Epidemiol. 2001;10:581–588. [PubMed] [Google Scholar]
- 40.Whyatt RM, Perera FP, Jedrychowski W, Santella RM, Garte S, Bell DA. Association between polycyclic aromatic hydrocarbon-DNA adduct levels in maternal and newborn white blood cells and glutathione S-transferase P1 and CYP1A1 polymorphisms. Cancer Epidemiol. 2000;9:207–212. [PubMed] [Google Scholar]
- 41.Nukui T, Day RD, Sims CS, Ness RB, Romkes M. Maternal/newborn GSTT1 null genotype contributes to risk of preterm, low birthweight infants. Pharmacogenetics. 2004;14:569–576. doi: 10.1097/00008571-200409000-00001. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.