Abstract
The Hem/Kette/Nap1 protein is involved in many biological processes. We have recently reported that Hem is required for the normal migration of neurons in the Drosophila embryo. In this paper, we report that Hem regulates the asymmetric division of neural precursor cells. We find that a well-studied Hem/Kette mutant allele produces at least two main, but possibly more, phenotypic classes of mutant embryos, and these phenotypes correlate with variable levels of maternal wild type Hem protein in the developing embryo. While the weaker class exhibits weak axon guidance defect and the mis-migration of neurons, the stronger class causes severe axon guidance defects, mis-migration of neurons and symmetric division of ganglion mother cells (GMC) of the RP2/sib lineage. We also show that the basis for the loss of asymmetric division is due to non-localization of Inscuteable and Numb in GMC-1. A non-asymmetric Numb segregates to both daughter cells of GMC-1, which then prevents Notch signaling from specifying a sib fate. This causes both cells to adopt an RP2 fate. Furthermore, loss of function for Abelson tyrosine kinase also causes loss of asymmetric localization of Inscuteable and Numb and symmetric division of GMC-1, the loss of function for WAVE has a very weakly penetrant loss of asymmetry defect. These results define another role for Hem/Kette/Nap1 in a neural precursor cell during neurogenesis.
Keywords: Drosophila, Hem protein, WAVE, asymmetric division
1. Introduction
In Drosophila embryos, the ventral nerve cord (VNC) consists of segmentally repeated units, each of which contains ~320 neurons and ~ 30 glia. The neurons are generated by about 30 neuroblast (NB) stem cells (Bossing et al., 1996; Schmidt et al., 1997; Doe, 1992). A NB, upon delamination from the neuroectoderm, divides via a series of self-renewing asymmetric divisions to produce a chain ganglion mother cells (GMCs). Although a GMC is bipotential, it does not normally self-renew (Bhat and Apsel, 2004). Instead, it divides asymmetrically to generate two different post-mitotic neurons. The phenomenon of asymmetric division in the Drosophila nerve cord has been extensively studied using a typical lineage, the NB4-2→GMC-1→RP2/sib lineage(Bhat, 1999; Bhat et al., 2011; Gaziova and Bhat, 2007). NB4-2 is formed in row 4, column 2 around 4.5 hours of development. It generates its first GMC, GMC-1 (also known as GMC4-2a, see Doe, 1992) around 6 hours of development. This GMC-1 then divides asymmetrically into a motor neuron called RP2 and its sibling cell named sib, around 7.5 hours of development.
Several players have been identified as required for asymmetric division of these precursor cells (reviewed in Gaziova and Bhat, 2007). Of most importance are Inscuteable (Insc), a cytoplasmic adaptor protein, Numb (Nb), and Notch (N). How do these proteins regulate asymmetric cell identity specification? Previous studies have shown that the N-signaling plays a crucial role not only in selecting a neural versus ectodermal fates during early neurogenesis, but also in the later asymmetric fate specification of daughter cells of GMCs (Bhat et al., 2011; Buescher et al., 1998; Gaziova and Bhat, 2007; Lu et al., 1999; Mehta and Bhat, 2001; Wai et al., 1999; Yedvobnick et al., 2004). This later function of N-signaling has an interesting, antagonistic relationship to the function of cytoplasmic protein Numb. Numb localizes to the basal end of a GMC and during division, it segregates into one of its two daughter cells, where it inhibits the cleavage of Nintra. This blocks the ability of Notch to specify a different fate (Buescher et al., 1998; Wai et al., 1999). In the GMC-1->RP2/sib lineage, for example, the loss of function for Notch causes both the daughter cells of the GMC-1 to adopt an RP2 fate (Buescher et al., 1998; Wai et al., 1999). This indicates that Notch specifies a sib fate. In the absence of Numb, both cells adopt the sib fate, thus, Numb is necessary to specify an RP2 fate. In the absence of both Notch and Numb, however, the two daughter cells adopt the RP2 fate indicating that Numb is necessary to specify an RP2 fate only when there is an intact N-signaling; Numb, thus, blocks Notch-signaling from specifying a sib fate to a cell.
The Hem protein (or Kette/dhem2/Hem-2/Nap1/Nap125) belongs to a highly conserved family, from invertebrates to mammals (Baumgartner et al., 1995). In humans, there two genes of the Hem family, Hem1 (NCKAP1L) and Hem2 (NCKAP1). Both Hem1 and Hem2 in humans generate two isoforms each. In Drosophila, however, there is only one Hem gene, which is homologous to the human Hem2. All the members of Hem family are predominantly expressed in the nervous system, whereas the Hem-1 gene in humans is predominantly expressed in the hematopoietic cells (Weiner et al., 2006). On the other hand, in C. elegans, the Hem-2 protein, known as GEX-3 (and Hem in Drosophila) is expressed everywhere, and has an essential function in the migration of epithelial cells in embryos (Soto et al., 2002). In Drosophila, Hem is maternally expressed during early embryogenesis but then becomes specifically expressed in the brain and the VNC. Hem has predicted six transmembrane domains (Baumgartner et al., 1995), however, in Drosophila S2 cells, most of Hem/Kette is in the cytosol and only very little is present in the membrane (Bogdan and Klambt, 2003). Therefore, it seems more likely that Hem/Kette is a cytosolic protein with some hydrophobic regions.
Hem is involved in many biological events such as apoptosis, myoblast fusion, formation and maturation of neuromuscular junction, axon pathfinding, neuronal differentiation and neuronal migration (Hummel et al., 2000; Nakao et al., 2008; Schenck et al., 2004; Schroter et al., 2004; Suzuki et al., 2000; Yokota et al., 2007; Zhu and Bhat, 2011). These studies suggest that Hem dynamically regulates the polymerization of actin. Hem binds to the first Src homology 3 (SH3) domain of Nck/Dock, which is an adaptor molecule containing one SH2 domain and three SH3 domains and links several receptor tyrosine kinases to the cytoskeleton (Li et al., 2001). This indicates that Hem may also link extracellular signals to the cytoskeleton. On the other hand, Hem is also part of the WAVE-complex and appears to regulate the activity of this complex to promote polymerization of actin. Our recent results indicate that in vivo Hem protects WAVE from degradation (Zhu and Bhat, 2011; see also Kunda et al., 2003; Schenck et al., 2004).
In this paper, we further characterize Hem mutant phenotypes and define at least two main phenotypic classes of mutant embryos from the same, well-studied antimorphic Hem mutant allele, HemJ4–48 (see Zhu and Bhat, 2011). We have classified them as weak (most embryos belong to this class) and strong classes. We correlate these classes to the variable levels of maternal wild type Hem protein in the developing embryo. We further show that in the weak class such defects as axon guidance is also weak, and it is strong in the strong class. While the migration defect can be observed in both weak and strong classes, we found that the strong class exhibited a loss of asymmetric division of GMC-1 of the RP2/sib lineage. We further show that this loss of asymmetric division is due to an effect on Inscuteable localization, a cytoplasmic adaptor protein, as well as localization of Numb, an antagonizer of the Notch signaling. A non-localized Numb in GMC-1, due to its segregation into both daughter cells, instead of one cell as in wild type, blocks Notch signaling from specifying a sib fate. Thus, both cells adopt an RP2 fate. We further show that the loss of function for Abl also causes loss of asymmetric division. This appears to be also due to loss of asymmetric localization of Insc and Numb. These results show that the Hemplays a crucial role in generating asymmetry via regulating localization of these key proteins in the embryonic CNS.
2. Results
2.1. The HemJ4–48 mutation generates two classes of mutant embryos
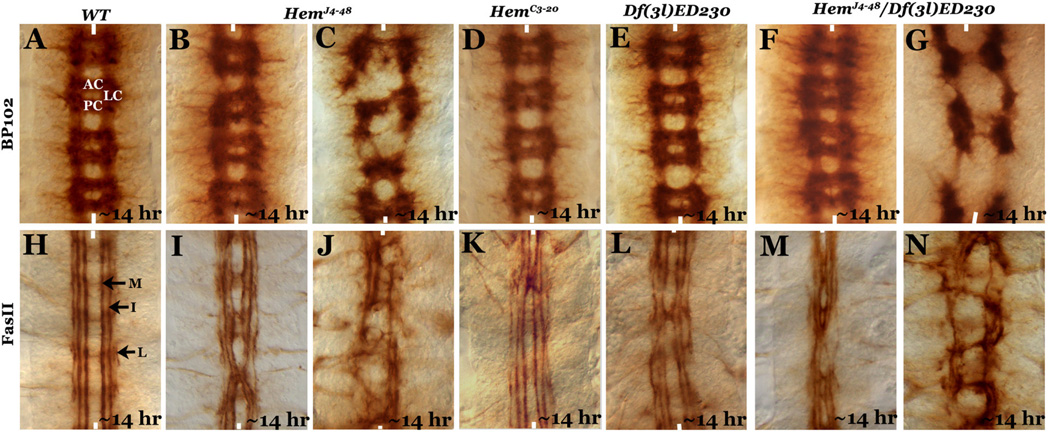
We have recently reported that loss of function for the Hem gene causes a mis-migration of RP2 neurons to the contralateral hemisegments resulting in a duplication of the RP2 neurons in one hemisegment and loss in the contralateral hemisegment (Zhu and Bhat, 2011). This defect was observed in all Hem alleles, including in deficiencies that remove the gene. The penetrance was the highest in the HemJ4–48 allele. We also showed that this allele behaves as an antimorphic mutation (Zhu and Bhat, 2011). During our analysis of the Hem mutant embryos, we stained embryos from the two mutant alleles, HemJ4–48 and HemC3–20, and a deficiency for Hem[Df (3L)ED230] with antibodies against BP102 and Fasciculin II (Fas II). BP102 is a monoclonal antibody that stains the ladder-like commissural structure in the embryonic nerve cord revealing the three major tracts: the anterior (AC) and the posterior (PC) commissures, and the longitudinal connectives (Fig. 1A). The Fas II antibody stains longitudinal connectives and reveals three major tracts, the medial (M), intermediate (I) and lateral (L), on either side of the midline (Fig. 1H). These stainings revealed that the HemJ4–48 allele (which expresses a truncated Hem protein, see Zhu and Bhat, 2011; also Fig. 3) produced mainly two categories of mutant embryos. In the first category, mutant embryos were morphologically nearly normal and the BP102 staining showed very little defects in the commissural architecture (Fig. 1B). However, Fas II staining revealed midline crossing of longitudinal connectives, although this phenotype was also relatively mild (Fig. 1I). In the second category, both the BP102 and the Fas II stainings revealed a much stronger phenotype with disrupted commissural tracts (Fig. 1C) and longitudinal connectives (Fig. 1J), as was also previously reported (Bogdan and Klambt, 2003; Schenck et al., 2004).
Figure 1. Axon guidance defect in Hem mutants.
Embryos (~14 hr old) in panels A–G are stained with BP102 and in H–N are stained with 22C10 antibodies. Anterior end is up and the midline is marked by vertical lines. Wild type (WT) and embryos from different Hem alleles are indicated at the top of the panels. AC, anterior commessure; PC, posterior commessure; LC, longitudinal connectives; M, medial tract; I, intermediate tract; L, lateral tract.
Figure 3. Duplication of RP2 neurons in Hem mutants.
Embryos are stained with anti-Eve. Anterior end is up, midline is marked by vertical lines. Panel A: Wild type embryo showing the ventral nerve cord with one RP2 neuron (arrow) per hemisegment. Panels B and C: HemJ4–48 strong mutant embryo and HemJ4–48/Hem-deficiency embryo, note the duplication of RP2 without accompanying loss from the contralateral hemisegment (double arrows) as well as migration of RP2 from one hemisegment to the contralateral hemisegment (arrowhead in panel B). Panel D: HemJ4–48–strong embryo showing the duplication of RP2 (as well as mismigration of RP2s). Panel E: HemJ4–48-weak embryo showing only the mis-migration of RP2 but not the duplication. Both these embryos are HemJ4–48 mutant embryos as confirmed by single-embryo PCR of the Hem gene from these embryos. Panel F: Line drawing showing the Hem gene and the Hem protein with molecular lesions in the gene in two of the Hem mutant alleles. Panel G: Hem-deficiency embryo with truncated Hem (which is expected to produce a truncated Hem protein similar to the one in HemJ4–48 mutant embryos) expressed from a UAS-•Hem transgene.
Staining of embryos from the HemC3–20 allele (which is closer to a null allele), as well as embryos from a Hem deficiency revealed that only the first, weaker phenotypic category of embryos, could be found in these mutant alleles, with the nerve cord showing minimal disruption of axon tracts (Fig. 1D and K; E and L). However, embryos that are transheterozygous for the HemJ4–48 allele and the Hem deficiency had both the categories of mutant embryos similar to the HemJ4–48 allele (Fig. 1F, G, M and N).
Given these results, we carefully determined the frequency of penetrance of these two types of phenotypes. We placed the HemJ4–48 mutant chromosome in trans to a balancer carrying the lacZ gene. We collected embryos from this line and double stained them with Fas II (or BP102; we also used Even-skippped as a third marker) and anti-LacZ antibodies. We examined the frequency of the two classes of mutants in both LacZ negative and LacZ positive embryos (to determine if this allele has any dominance/semi-dominance). The results are shown in Table 1, which indicates that among LacZ negative embryos (N=53; presumably all are homozygous mutant embryos), 57% had a weak phenotype, 24% had a strong phenotype and interestingly, 18% had no obvious defects. Moreover, the strong phenotypic class (24%) can be further subdivided with very strong defects, with a frequency of about 4% (see also Zhu and Bhat, 2011). The frequency of occurrence of weak phenotypic embryos is 2.3 times greater than the strong phenotypic embryos. We note that the ratio between LacZ positive and LacZ negative embryos is the expected 1:3, therefore, it is unlikely that some of these normal looking embryos are heterozygous or balancer only embryos that are not stained with anti-LacZ antibody.
Table 1. HemJ4–48 allele generates phenotypically variable mutant embryos.
Embryos from HemJ4–48 balanced using a lacZ carrying balancer and from HemJ4–48/+ were analyzed for the defects using BP102, Fas II and Eve staining. Note that the 24% strong phenotypic embryos can be again subdivided, with at least ~4% showing very strong defect (see also Zhu and Bhat, 2011). N is the number of embryos examined.
| Embryos from HemJ4–48/TM6B, LacZ X HemJ4–48/TM6B, LacZ | |||
| weak phenotype | strong phenotype | wild type | |
| LacZ negative embryos (HemJ4–48/HemJ4–48) N = 53 | 57 % | 24 % | 18 % |
| LacZ positive embryos (HemJ4–48/TM6B, LacZ or TM6B, LacZ/TM6B,LacZ) N = 74 | 5 % | 19 % | 76 % |
| Embryos from HemJ4–48/+ X HemJ4–48/+ | |||
| weak phenotype | strong phenotype | wild type | |
| Embryos N = 54 | 20 % | 4 % | 76 % |
Among the LacZ positive embryos (N=74; these are presumably the heterozygous embryos or homozygous for the balancer chromosome), interestingly, we found that 5%, 19% and 76% of the embryos showing the weak, strong and normal-looking embryos, respectively (Table 1). This suggests that there is a semidominance of the defects in this mutant allele. Either that some of the strong phenotypic embryos from these lacZ positive groups are embryos that are homozygous for the balancer, or that these are heterozygous embryos but the phenotype is caused by an interaction between the balancer and the mutant chromosome. It is unlikely that all are embryos that are homozygous for the balancer chromosome since we also observed the weak phenotypic class among these LacZ positive embryos.
Given these results, we took another approach to eliminate any balancer-induced effects. We out-crossed the mutant chromosome to wild type and collected males and virgins from this cross that are mutant over wild type chromosome (with no balancers) and then crossed these mutant heterozygous flies to each other (mutant/+ × mutant/+). Embryos from this cross were collected and stained with Fas II, or BP102 or Eve (all separately). From this cross, we found a 20%, 4% and 76% embryos with weak, strong and normal looking embryos (N=54; Table 1). This gives us a ratio close to the expected ratio (for a recessive mutation) of 1:3 of embryos (20+4 versus 76) that are mutant/mutant chromosome (and therefore with the mutant defect) versus mutant/balancer and balancer/balancer chromosomes. Note that these percentage calculations are for the total embryos as opposed to the previous calculations, which are for only the LacZ-negative embryos. These results therefore argue that there is a balancer-mediated enhancement of the penetrance of the HemJ4–48 defects in this mutant allele. This effect that we have previously named as balancer-induced maternal effect has been observed in other cases as well (Bhat et al., 2007; Gaziova and Bhat, 2009). Nonetheless, we do see the strong phenotypic class of embryos even when the balancer chromosome is eliminated from the parents. This suggests that these embryos are indeed Hem mediated mutant defects/embryos (see below).
We next sought to confirm these above results that HemJ4–48 allele indeed generates two classes of mutants, specifically the strong phenotypic class of embryos. This was particularly important since several of the previous studies have characterized only this strong phenotypic embryo as Hem mutant embryo (Hummel et al., 2000). We sought to sequence the Hem gene from single embryos belonging to both the categories. We collected single embryos from the balancer bearing HemJ4–48 parents and the sequencing of the Hem gene revealed that every single embryo with weak phenotype was homozygous for the Hem mutation, whereas only 25% of the strong phenotypic embryos were homozygous for the mutation, and the remaining 75% were all heterozygous for the mutation. These results suggest a dominant maternal effect of the Hem mutation enhanced by a balancer-mediated maternal effect (see also Bhat et al., 2007; Gaziova and Bhat, 2009).
2.2. Levels of maternal Hem is variable from embryo to embryo in the HemJ4–48 mutant allele
Next, we sought to determine the basis for these two phenotypes in the HemJ4–48 allele. As shown in Fig. 2A, in wild type, the maternally deposited Hem protein (~110 kDa) is still present in embryos that are 12–16 hours old, thus, this maternal Hem protein can last throughout embryogenesis. Interestingly, the maternal Hem protein is lower in HemJ4–48 mutant embryos compared to wild type or Hem-deficiency embryos (see also Zhu and Bhat, 2011). This lower levels of maternal Hem protein in HemJ4–48 allele may also explain why we observe two classes of mutant embryos in this allele, weaker and stronger, but only weaker embryos in the deficiency embryos (zygotically null). While the level of the Hem protein in Hem deficiency is much lower than in wild type (Fig. 2A, lane 3), the level in HemJ4–48 is even lower (Fig. 2A, lane 2, compare with lane 1). This suggests that somehow the HemJ4–48 mutant (truncated) protein down-regulates the levels of the maternal wild type Hem protein. However, this down regulation is variable, thus, producing two different classes of embryos.
Figure 2. Variability in the levels of maternal Hem protein in HemJ4–48 embryos.
Panel A: Western analysis of wild type, HemJ4–48, and Hem-deficiency embryos with Hem antibody; Anti-Tub was used to determine the loading amount. Panel B: Western analysis of single individual embryo extract per lane from wild type, and Hem-deficiency embryos with Hem antibody; Anti-Tub was used to determine the loading amount. Panel C: Western analysis of single individual embryo extract per lane of wild type and HemJ4–48 mutant embryos; star indicates a mutant embryo with a significantly lower level of Hem protein compared to other mutant embryos or wild type. Tubulin is used as loading control. The intensity of the signals for Hem, normalized against the loading control Tubulin, is given below each of the Westerns.
To test this possibility, we did Western analysis of extracts from single HemJ4–48 mutant embryos. As shown in Fig. 2C, we observed mutant embryos with different levels of the maternal Hem protein among HemJ4–48 mutant embryos. In some cases, the level of maternal Hem was quite low (lane 2) but in some other cases the level was higher. These results suggest that the stronger phenotype is likely due to these reduced levels of the maternal Hem protein. Consistent with this possibility, no such variability in the levels of the maternal Hem protein was observed among individual embryos from the deficiency (Fig. 2B).
2.3. The stronger Hem mutant embryos show duplication of RP2 neurons
We stained HemJ4–48 mutant embryos with an antibody against Even-skipped (Eve) to determine if Hem affects the GMC-1->RP2/sib lineage, one of the well-studied lineages in Drosophila (reviewed in Gaziova and Bhat, 2007). The GMC-1->RP2/sib lineage is generated by NB4-2, formed as an S2 NB around 4.5 hours of development. It generates its first GMC (GMC-1) by a self-renewing asymmetric division at around 6–6.5 hours of development. This GMC-1 divides to generate two asymmetrically sized daughters, the larger RP2 and the smaller sib, at around 7.45 hours of development. The RP2 begins to project its axon ipsilaterally towards the intersegmental nerve bundle (ISN) by about 9.5 hours of development and innervates muscle numbers 2, 9 and 11. There are several well-established ways to distinguish a GMC-1, an RP2, and a sib (Bhat and Schedl, 1994; Doe, 1992). Both nuclear division and cytokinesis of GMC-1 is asymmetric and thus, there is a size difference between a GMC-1 (7.5µm), an RP2 (~5µm), and a sib (~3µm). Similarly, the nuclear size of a GMC-1 is ~6.5µm, an RP2 is 4µm and a sib is 2.5µm. There is also a level difference in marker gene expression between an RP2 and a sib as well as a difference in the temporal dynamics of expression of these markers; the future RP2 cell has a stronger expression of markers like Even-skipped (Eve) compared to a future sib. The cell that assumes a sib identity undergoes a size reduction and further down-regulation of expression of RP2-specific marker genes. By ~14 hours of development, expression of all those markers is completely lost from the sib and only the RP2 is seen (Fig. 3A).
While the weak phenotypic HemJ4–48 mutant embryos showed a loss of RP2 neurons from one hemisegment with a corresponding gain of RP2 in the contralateral hemisegment (Fig. 3E), we showed that this is due to mis-migration of an RP2 neuron from one hemisegment to the contralateral hemisegment (Zhu and Bhat, 2011). However, in the strong phenotypic HemJ4–48 mutant embryos, in addition to this mis-migration phenotype (in a maximum of 28% of the hemisegments), we also observed a duplication of RP2s without any loss from the contralateral hemisegment (Fig.3B, and 3D) in a maximum of 33% of the hemisegments among strong phenotypic embryos (Table 2). This phenotype was also seen in embryos that are transheterozygous for HemJ4–48 and Hem deficiency, Df(3L)ED230 (Fig. 3C; a maximum of 31% of hemisegments in affected embryos, see Table 2). Note that about 4% of the strong phenotypic HemJ4–48 embryos have the GMC-1 of the RP2/sib lineage that are either not dividing and not migrating, or its progeny not migrating and residing in the location of a GMC-1 (see Zhu and Bhat, 2011). Moreover, the duplication without any loss was observed only in HemJ4–48 mutant embryos or embryos transheterozygous for HemJ4–48 and Hem-deficiency, but not in Hem-df or other mutant alleles of Hem (Table 2). This is consistent with the finding that strong phenotypic mutant embryos were observed only in the HemJ4–48 allele and this phenotype(s) is likely due to reduced levels of the maternal Hem.
Table 2. Frequency of penetrance of the symmetric division defects in various Hem mutant alleles.
N is the number of hemisegments examined. Eve staining was used to determine the symmetric division defect in mutant embryos.
| Genotype | Symmetric division of GMC-1 |
|
|---|---|---|
| HemJ4–48 | Weak phenotype embryo (N = 732) | 0 |
| Strong phenotype embryo (N = 348) | 33 % | |
| HemC3–20 (N = 386) | 0 | |
| Df(3L)ED230 (N = 412) | 0 | |
| HemJ4–48/Df(3L)ED230 | Weak phenotype embryo (N = 316) | 0 |
| Strong phenotype embryo (N = 322) | 31 % | |
| WAVEΔ37 (N = 296) | 0 | |
| WAVEmat,zyg (N = 302) | Very rare | |
| Abl2 (N = 516) | 9 % | |
| Ablmat,zyg (N = 234) | ~9 % | |
As has been previously reported, the molecular lesion in the gene in HemJ4–48 is a stop codon at amino acid 490 (Hummel et al., 2000; Zhu and Bhat, 2011; see Fig. 3F). Whereas the molecular lesion in the other Hem allele, HemC3–30 is a stop codon at amino acid 256 (Fig.3F). The molecular lesion in the HemJ4–48 allele is consistent with the possibility that it can produce a truncated protein that behaves as a dominant negative protein (the truncated Hem is not detectable in the Western blot since the antibody is raised against the portion beyond the truncation). Indeed, our previous results have shown that this allele behaves as an antimorphic mutation (Zhu and Bhat, 2011). Previously, we had constructed a synthetic mutation that mimicked the HemJ4–48 mutation by generating a UAS-HemJ4–48 transgenic line (Zhu and Bhat, 2011). This line, when induced with a GAL4 driver is expected to produce the same protein as in HemJ4–48 allele. Indeed, it showed, although weakly the same RP2 mis-migration phenotype as the HemJ4–48 allele (Zhu and Bhat, 2011). We examined if the induction of this antimorphic UAS-Hem transgene in embryos with a GAL4 driver (VP16-nos.UTR) in the background of Hem deficiency (to reduce the maternal load of wild type Hem protein) can also induce a symmetric division of GMC-1 into two RP2 neurons. As shown in Figure 3G, we observed duplication of RP2s without any loss in the contralateral hemisegment, or presence of the RP2-sib cells, indicating that this transgene is able to induce a symmetric division of GMC-1 (RP2 duplication is not observed in embryos homozygous for the deficiency). However, the frequency of penetrance of the defect is weak (0.5%, N=832 hemisegments), which is likely due to the presence of enough of the maternal wild type Hem protein for the asymmetric division of GMC-1 in most of the hemisegments. We could not generate germline mosaics for Hem mutations to confirm this possibility because of the location of the Hem gene on the chromosome, which is very close to the centromere. However, the expression of the truncated protein appears to down-regulate the levels of the wild type Hem protein (Zhu and Bhat, 2011).
We further examined the duplication phenotype to confirm that these duplicated cells are indeed RP2 neurons by staining mutant embryos with Eve and Zfh1. Zfh-1 is expressed at very low levels in a late GMC-1 just before its division, at high levels in an RP2 (Fig. 4A) and occasionally and transiently in a newly formed sib (Gaziova and Bhat, 2009). In HemJ4–48 mutant embryos we observed Eve and Zfh1 positive RP2 neurons with no sibs (Fig. 4B). This indicates that the RP2 duplication is indeed due to a symmetrical division of GMC-1 into two RP2s as opposed to scenarios such as symmetrical division of GMC-1 into two GMC-1s (which would generate two RP2s and two sibs). Additionally, the duplicated RP2 neurons in HemJ4–48 mutant embryos were expressing MAPIB/22C10 on the membrane and axon projections, with the axon projections properly fasciculating with the ISN (Fig. 4D) as in wild type (Fig. 4C). These results show that loss of function for Hem results in loss of asymmetric division of GMCs.
Figure 4. The duplication of RP2 neuron in Hem mutant embryos.
Anterior end is up, midline is marked by vertical lines. Panels A, B: Eve and Zfh1 double-stained embryos. RP2 neurons co-express both Eve and Zfh1 in wild type and in the mutant. Panels C, D: Embryos are double-stained with Eve and 22C10. 22C10 stains the membrane of an RP2 as well as its axon projection (small arrow); in the mutant embryo, the duplicated RP2s project their axons ipsilaterally as in wild type.
2.4. Loss of Hem function causes loss of asymmetric localization of Insc and Numb
In a dividing GMC-1, Insc is asymmetrically localized to the apical pole (Buescher et al., 1998). We sought to determine if Insc localization in GMC-1 is normal in HemJ4–48 mutant embryos. We double stained embryos with Insc and Eve antibodies, Eve being the marker for GMC-1. In wild type, Insc is on the apical side of a GMC-1 (Fig. 5A). In HemJ4–48 mutant embryos, we observed GMC-1s where the localization of Insc is non-asymmetric (Fig. 5B). This phenotype was observed in a maximum of ~15% of the hemisegments (N, the number of hemisegments counted, 55). This lower maximum penetrance of the Insc localization defect compared to the maximum penetrance of the terminal duplication defect is likely due to the fact that Insc defect can be observed only in a narrow window of time and that not all GMC-1s are formed at the same exact time, nor their division occurs at the same exact time, but with a window of almost 30 min. This gives a reduced penetrance count compared to the penetrance count for the terminal defect. We also point out that this non-asymmetric localization also makes it appear as if the level of Insc is lower in the mutant GMC-1. These results indicate that localization of Insc to the apical pole is dependent on Hem protein, although we do not know if the Hem protein itself is localized (the available Hem antibody does not work in whole mount immunohistochemistry).
Figure 5. Localization of Insc and Numb is non-asymmetric in GMC of the RP2/sib pairs in the HemJ4–48 mutant.
Embryos are double stained with anti-Eve (red) and anti-Insc (green; panels A and B), and anti-Eve (red) and anti-Numb (green; panels C and D). Images are taken from the side of the embryo, apical surface is up. Panel A: A GMC-1 in wild type embryo with nuclear Eve and apically localized Insc. Panel B: A GMC-1 in the mutant embryo with nuclear Eve and non-asymmetrc Insc. Panel C: A GMC-1 in wild type embryo with nuclear Eve and basally localized Numb. Panel D: A GMC-1 in the mutant embryo with nuclear Eve and non-asymmetric Numb.
Because Numb localization to the basal pole depends upon proper localization of Insc to the apical pole, we next determined if the localization of Numb is disrupted in HemJ4–48 mutants. If the Numb localization is affected in HemJ4–48 mutants, loss of asymmetric division of GMC-1 in HemJ4–48 mutants could be due to mis-localization of Numb. We double stained HemJ4–48 embryos with Numb and Eve and examined the localization of Numb in GMC-1 (Fig. 5C and D). Unlike in wild type where Numb is localized to the basal end (Fig. 5C), in HemJ4–48 mutants, Numb is not localized, but is uniformly distributed along the entire cortex of GMC-1 (Fig. 5D). The level of Numb appears to be lower in the GMC-1 of the mutant, but this is likely due to the fact that Numb is no longer concentrated in an area but uniformly distributed along the cortex of the entire cell (note that this is also the case with Insc, see Fig. 5B). We observed a maximum of ~17% of the hemisegments (N= 55) showing this defect. When such GMC-1s divide, both cells inherit Numb. Since Numb will block Notch-signaling from specifying a sib fate, the daughter cells of those GMCs in which the distribution of Numb is non-asymmetric will inherit Numb and are expected to adopt an RP2 fate. This would account for the duplicated RP2 neurons in HemJ4–48 mutants.
2.5. Role of WAVE/SCAR and Abelson tyrosine kinase in the Hem-mediated asymmetric division of GMC
Hem is part of the WAVE-complex. WAVE-complex, which activates the Arp2/3 complex to promote actin polymerization, has the following proteins: WAVE, Hem, Abi, Hspc300, Sra-1. In addition to the WAVE-complex, WASp is also part of the Hem-mediated process. WASp is located both in the membrane and in the cytoplasm and Hem is thought to activate WASp in the membrane (Bogdan and Klambt, 2003). While WASp appears to be not involved in the migration of neurons (Zhu and Bhat, 2011), WASp has been previously shown to be involved in the asymmetric division of GMC-1 of the RP2/sib lineage (Ben-Yaacov et al., 2001). However, it was suggested that the asymmetric division mediated by WASp is via Notch-signaling. Loss of function for Notch also causes symmetric division of GMC-1 into two RP2s (see for example, Wai et al., 1999) and this is due to the failure to specify a sib fate to one of the progeny of GMC-1. The WASp scenario appears to be different from that of our findings with Hem, in that Hem appears to mediate asymmetric division via regulating the asymmetric localization of Inscuteable/Numb proteins. Since the issue of WASp in asymmetric division was not thoroughly examined in the previous work, it is possible that WASp is indeed part of the same Hem-mediated process in generating asymmetry, instead of working through Notch.
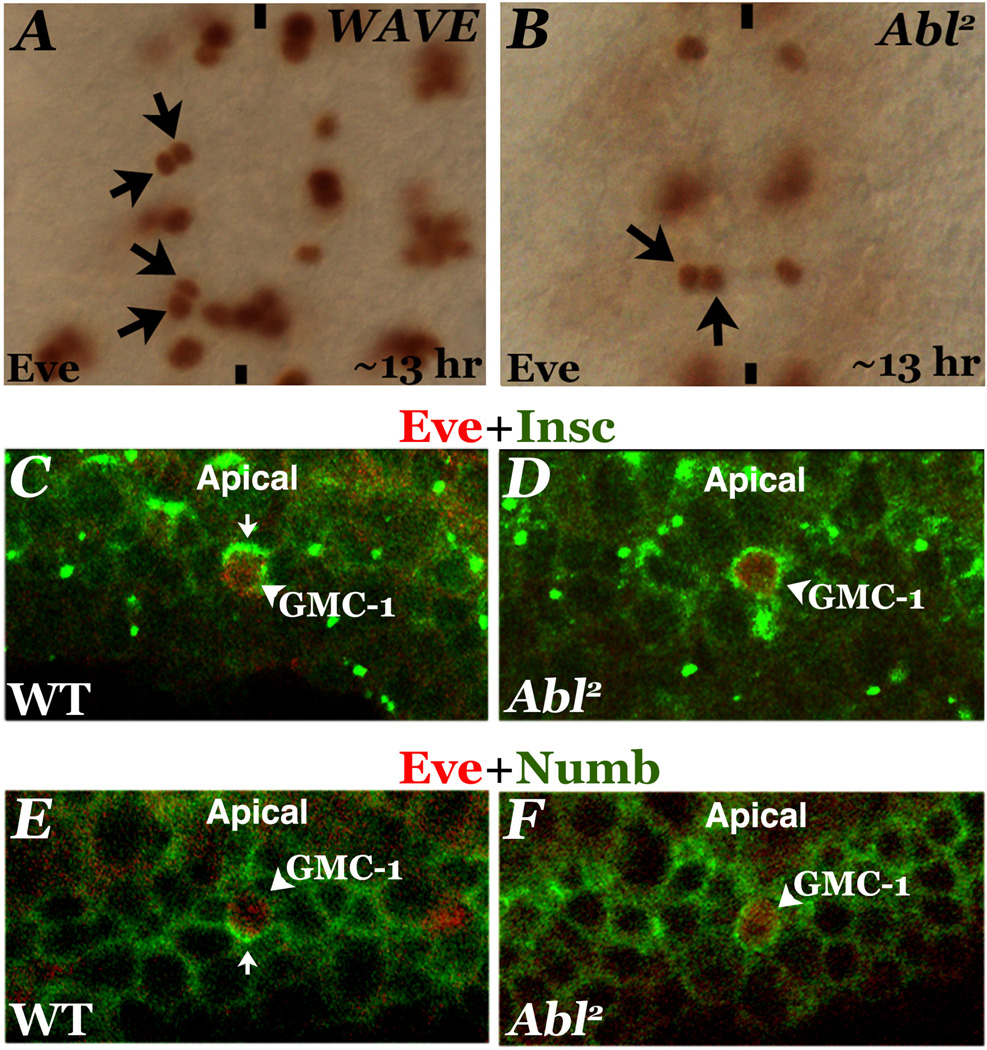
Therefore, first we examined the loss of function effects of WAVE on GMC-1 division. However, we did not observe any RP2 duplication due to symmetric division of GMC-1 in the zygotic mutants of WAVE, which could be due to the maternal deposition of WAVE (Zallen et al, 2002; Zhu and Bhat, 2011). Therefore, mosaic embryos for WAVE were generated and examined for the RP2 division defects. A weaker WAVE allele, SCARk13811, was used since stronger alleles fail to yield any mosaic embryos (Zallen et al., 2002). In these mosaic embryos we found the same phenotype of symmetric division of GMC-1 into two RP2 neurons (Fig. 6A). However, this frequency was very low (Table 2). This could be because there is still some WAVE gene product(s) left since we had to use a hypomorph due to practical difficulty in generating mosaic embryos. This is preseumably due to a germline requirement of WAVE. Alternatively, WAVE does not play any significant role in asymmetric division.
Figure 6. WAVE and Abl in asymmetric division of GMC-1 of the RP2/sib lineage.
Embryos in panels A (WAVEmat,zyg mutant) and B (Abl2 mutant) are stained with anti-Eve. Anterior end is up, midline is marked by vertical lines. Arrows indicate duplicated RP2 neurons. Panels C–F: Localization of Insc and Numb is non-asymmetric in GMC of RP2/sib pairs in the Abl mutant. Embryos are double stained with anti-Eve (red) and anti-Insc (green; panels A and B), and anti-Eve (red) and anti-Numb (green; panels C and D). Images are taken from the side of the embryo, apical surface is up. Panel C: A GMC-1 in wild type embryo with nuclear Eve and apically localized Insc. Panel D: A GMC-1 in the mutant embryo with nuclear Eve and non-asymmetrc Insc. Panel E: A GMC-1 in wild type embryo with nuclear Eve and basally localized Numb. Panel F: A GMC-1 in the mutant embryo with nuclear Eve and non-asymmetrc Numb.
Abi is part of the WAVE-complex (Eden et al., 2002) and in vitro cell culture studies suggests Abi recruits Abl to the WAVE-complex, where Abl mediates WAVE-activation (Leng et al., 2005). When we examined Abi mutant embryos, no RP2 division defect was observed. The absence of division defect in Abi could be due to its maternal deposition. On the other hand, we found that Abl mutants have the same RP2 division defect as Hem (Fig. 6B) with 9% of the hemisegments showing the defect (see Table 2). This partial penetrance is likely due to the perdurance of the maternal Abl gene products. Therefore, we generated Abl mosaic embryos with both maternal and zygotic Abl products lacking. In these embryos also, we found GMC-1 symmetrically dividing to give rise to two RP2 neurons (Table 2). However the frequency was the same as Abl2 zygotic mutant. Since we had to use a weaker allele in this case also for generating the mosaic, there will be still some functional Abl protein in developing embryos and we think this may be the reason for the low penetrance of the defect in these mosaic embryos as well.
We next examined if the localization of Insc and Numb is affected in Abl mutant embryos. Indeed, as shown in Fig. 6, the localization of both Insc and Numb were affected in Abl mutant embryos: localization of both Insc and Numb were non-asymmetric in these embryos (Fig. 6D and F) as in Hem muatnts.
3. Discussion
In this paper, we have shown that a well-studied mutation in the Hem gene, HemJ4-4, generates two main phenotypic classes of mutant embryos: weak and strong. While the strong phenotypic embryos have been charcterized in terms of axon guidance defects (Bogdan and Klambt, 2003; Hummel et al., 2000; Schenck et al., 2004), the weaker class of mutant embryos has not received much attention although this class forms the majority of embryos in one specific allele and the only type of embryos in Hem zygotic null embryos. That is, in zygotic null alleles of Hem, or in Hem deficiency embryos, only the weaker class of embryos are seen. Our work described in this paper shows that the two classes of mutant embryos in the HemJ4–48 allele are generated mainly due to individual variability in the amount of Hem protein present in embryos. In contrast, embryos from null alleles or deficiencies that eliminate Hem have a higher, non-variable levels of the Hem protein compared to the HemJ4–48 allele, which is consistent with the single, weaker class of phenotypic embryos from these alleles. We have recently shown that mutations in Hem (as well as in other players of the WAVE-complex: WAVE and Abl) causes an abnormal migration of a well-studied neuron, the RP2 neuron, from one hemisegment to the contralateral hemisegment (Zhu and Bhat, 2011). In this paper, we show that the GMC-1 of the RP2/sib lineage in the stronger class of mutant embryos divide symmetrically into two RP2 neurons.
3.1. Variability in the levels of maternal Hem is responsible for generating different phenotypic classes of mutant embryos in HemJ4–48 allele
Given the importance of the Hem activity to a diverse array of cellular processes, in this work we have attempted to characterize the Hem mutant alleles in terms of CNS defects. Indeed, there was this possibility that the severe axon guidance defects described for Hem using one of the alleles of Hem, HemJ4–48 may be a background effect unrelated to Hem. While this phenotype is a minority phenotype in this allele, our characterization of this allele in detail shows that these severe axon guidance defects (Fig. 1) are indeed due to loss of function for Hem. Our results show that the presence of different phenotypic classes of embryos in this allele is due to a variable, embryo-to-embryo, effect on the levels of maternally deposited wild type Hem gene product(s). The HemJ4–48 allele appears to be an antimorphic allele (see Zhu and Bhat, 2011) and given this property of the allele, it is not surprising that it produced embryos that are stronger than from alleles that are null. This allele also produces embryos that have morphologically variable defects. However, we have broadly catagorised these embryos into two classes: the weaker class of embryos and the stronger class. At the least, our work shows that the variability is likely due to the variability in the levels of maternal Hem protein (Fig. 2). Our previous results suggest that the truncated Hem protein in HemJ4–48 allele is somehow downregulating the levels of wild type maternal Hem (Zhu and Bhat 2011). The results shown in Fig. 2 is consistent with this possibility and that the variability inherent to this downrgulation/degradation process is resulting in embryos with variable levels of maternal Hem. This is also consistent with the semidominance of this allele.
These results are also important because, most of the previous work has dealt with the strong phenotypic class of mutant embryos using the HemJ4–48 allele, which are minority mutant embryos. But our detailed examination of this mutant allele reveals that these embryos are indeed Hem mutant embryos and the strong mutant defects such as axon guidance defects are not because of a background effect but the strongest loss of function effects of Hem. While the weaker class appears to be uniform in their phenotypic presentation, the stronger class embryos are variable, which is again likely due to the variable levels of maternal Hem in these embryos. One issue we have not been able to determine is the mode by which the truncated Hem protein in HemJ4–48 allele down-regulates maternal wild type Hem protein. Also, we do not know where this down regulation of Hem occurs, in the embryo or in the germline.
3.2. Regulation of asymmetric division by Hem
This work shows that the asymmetric division defect observed in Hem mutants is restricted only to the strong phenotypic class of embryos of HemJ4–48 allele, but not observed in the weak class of embryos. The weak class of embryos show only the mis-migration defect as we have recently reported (Zhu and Bhat, 2011). However, embryos that show the asymmetric division defect also show an enhanced mis-migration defect. That is, the maximum frequency of penetrance of the mis-migration defect in weak phenotypic class was 20%, whereas in strong phenotypic class, the maximum penetrance was 45% (see Zhu and Bhat, 2011). It is possible that the two processes, migration and asymmetric division, have different senesitivity to the levels of Hem. Furthermore, the Hem requirement for migration extends a much longer developmental period than the asymmetric division, which occurs within a narrow window of time.
Hem is necessary for axon pathfinding during development of the nervous system. Axon pathfinding defects were observed in Hem mutants (Hummel et al., 2000). It is also required for the formation and maturation of neuromuscular junction (NMJ) in that certain hypomorphic Hem allele combinations show smaller neuromuscular junctions (Bogdan et al., 2004; Schenck et al., 2004). Moreover, mutations in Hem led to enlarged foci that did not dissolve, similar to the observed block in myoblast fusion, which is crucial for the formation and repair of skeletal muscle (Richardson et al., 2007; Schäfer et al., 2007; Schroter et al., 2004). More recently, we showed that Hem regulates migration of GMC-1->RP2/sib cells (Zhu and Bhat, 2011; see also Bhat, 2007). All these processes in which Hem plays a role requires cytoskeletal reorganization, and therefore that Hem is required for the asymmetric division of neural precursor GMC-1 is perhaps not surprising. This asymmetric division of GMC-1 into RP2 and sib is a very well studied process and a number of players in this process have been identified (see Bhat and Schedl, 1994; Bhat et al., 1995; Buescher et al., 1998; Wai et al., 1999; Bhat et al., 2011; reviewed in Gaziova and Bhat, 2007). Briefly, an apically localized Insc causes a basal localization of Numb (see Fig. 7). This basal Numb gets segregated to one of the two daughter cells of GMC-1 during division. This Numb then blocks Notch signaling from specifying the sib identity, as a result, this cell adopts an RP2 fate (Fig. 7); whereas the other Numb-negative daughter is specified as sib by the Notch signaling (Fig. 7). The partial penetrance of the asymmetric division defect in Hem (or WAVE or Abl), however, is likely due to the maternal deposition of the protein and not because the pathway itself is partially redundant. An effective way to demonstrate this, however, is not possible since we cannot generate mosaic embryos that lack all of the Hem gene products given the germline requirement and technical difficulty.
Figure 7. Summary of the role of Hem in the GMC-1->RP2/sib lineage development.
Panel A: In a late GMC-1 of a wild type CNS, Insc apically localized, whereas Numb is basally localized. When this GMC-1 divides, Numb segregates into one of the two daughter cells where it prevents Notch signaling from specifying a sib fate to this cell. As a consequence, this cell adopts an RP2 identity. The other daughter cell that does not inherit Numb is specified as a sib by the Notch-signaling. Panels B and C: In bothHemJ4–48 and Abl mutant embryos, a GMC-1 has non-asymmetric Insc and a non-asymmetric Numb. Hence, both daughter cells inherit Numb and therefore the Notch signaling is blocked in both cells. These daughters then adopt an RP2 fate.
WASp is believed to be activated by Hem (Bogdan and Klaembt, 2003) and it appears that WASp is required for the asymmetric division of GMC-1. This is based on the finding that in the WASp mutant, both daughter cells become RP2 (as in Hem mutants). However, it has been thought that Wasp mediates asymmetric division via Notch (Ben-Yaacov et al., 2001) and in the WASp mutant, Notch signaling is prevented from specifying a sib fate to one of the two cells. On the other hand, this issue has not been fully examined and the connection between WASp and Notch is based on the finding that in both mutants the GMC-1 produces two RP2s (Ben-Yaacov et al., 2001). However, there is a caveat with this conclusion. When a GMC-1 divides into an RP2 and a sib, the cytokinesis of this division is also asymmetric. That is, one of the two cells is smaller than the other. The smaller cell eventually adopts a sib identity. In Notch mutants, the two RP2 neurons produced from a GMC-1 are often unequal (though not always) but the smaller cell still adopts an RP2 fate (see Wai et al., 1999). This means that while the asymmetric fate specification is altered in Notch mutants, the asymmetric cytokinesis is not always affected. The published data on the loss of asymmetric division in WASp mutants shows that the duplicated RP2s are all of the same size (Ben-Yaacov et al., 2001) as is the case with the Hem mutant, and this argues against WASp acting through Notch. Therefore, the function of Wasp is likely distinct from that of Notch. If WASp indeed works through Notch, then Hem and Abl appears to be required at an earlier stage, namely, for the asymmetric localization of Insc and Numb (see Fig. 7). We think that Hem, in collaboration with Abl, via regulating cytoskeletal organization, mediates proper asymmetric localization of Insc and Numb. In embryos mutant for these genes, a non-asymmetric localization of these proteins occurs and as a result GMC-1 division becomes symmetric.
What is the role of WAVE in this process? The answer is not very clear. Zygotic loss of function for WAVE does not show any symmetric division defects. When we examined WAVE mosaic embryos for this defect, rarely we observed the defect. The caveat here, however, is that these mosaic embryos are not null for WAVE: because of germline requirement for WAVE, we had to use a hypomorph to generate these mosaic embryos. Since we do see a low penetrance of the symmetric division defect in these embryos, it is more likely that WAVE is also required for the asymmetric localization of Insc and Numb, and asymmetric division of GMC-1.
4. Experimental Procedures
4.1. Fly strains, genetics
All the flies and crosses were kept/done at 22 °C unless otherwise indicated. The following strains were used: HemJ4–48, HemC3–20, Df(3L)ED230, insc22, nb796, Abl2, Abl1 FRT(w[hs])2A, UAS-Abl, Df(3L)st-j7, SCARΔ37, Df(2L)BSC32, UAS-WAVE, w;p[SCARK13811,W+] FRT40A/CyO, sca-Gal4, P[GAL4::VP16-nos.UTR]MVD2. Various mutant combinations are generated by standard genetics. To exclude possible maternal modifier effects of balancers (Bhat et al, 2007; Gaziova and Bhat, 2009), homozygous mutant embryos were also tested by out-crossing the balancer-bearing mutants to wild type and back-crossing the non-balancer bearing mutant adults. Staging of embryos was as described by Wieschaus and Nusslein-Volhard (Wieschaus and Nusslein-Volhard, 1986).
4.2. Generating mosaic animals
Germline clones for WAVE were generated as described by Zallen et al (2002), by heat shocking hsFLP; SCAR13811FRT40A/ovoD FRT40A early stage larvae at 37°C for 1-hour and another heat shock 24-hr later. Adult germline clones carrying females were crossed to SCARΔ37 males and embryos (WAVEmat, zyg) were collected for analysis. Germline clones for Abl were generate by heat shocking hsFLP; Abl1 FRT(w[hs])2A/ovoD FRT(w[hs])2A early stage larvae at 37°C for 1-hour and another heat shock 24-hr later. Adult germline clone females were crossed to Abl2 males and embryos (Ablmat,zyg) were collected for analysis.
4.3. Generating Transgenic animals
We have described this step recently (Zhu and Bhat, 2011). Briefly, To determine the antimorphic effects of the truncated Hem protein in HemJ4–48 allele (ΔHemJ4–48), the Hem truncated gene corresponding to the coding fragment of the first 489 amino acids of Hem was generated by PCR. The following primers that carried specific restriction sites and a stop codon were used: 5’- ATAAGAATGCGGCCGCTAAACTATTGCACGCCTCCCAATACG-3’ and 5’-GCTCTAGATTAGTCCAGGCGGAATGGTC-3’. The PCR product was digested with NotI and XbaI and subcloned into NotI/XbaI digested pUAST and the cloned truncated Hem gene was sequenced. Several independent transgenic lines were generated and used for analysis.
4.4. Immunohistochemistry
Standard immunostaining procedures were used with some modifications; modifications to the general fixation conditions and staining can be obtained by request. Embryos were fixed and stained with the following antibodies: Eve (rabbit, 1:2000 dilution), Eve (mouse, 1:5), Zfh1 (mouse, 1:400), 22C10 (mouse, 1:4), LacZ (rabbit, 1:3000 or mouse, 1:400), BP102 (mouse 1:10) FasII (mouse; 1:5). For confocal microscopy of embryos, cy5 and FITC-conjugated secondary antibodies were used. For light microscopy, alkaline phosphatase or DAB-conjugated secondary antibodies were used.
4.5. Sequencing of the mutant alleles and confirmation of the mutant homozygous identity by single embryo PCR
Mutant embryos were individually selected using the GFP-balancer as well as by using the visible mutant phenotype (which the Hem mutant embryos exhibit) under microscope. They were individually placed in 1.5 mL eppendorf tubes with 10 µL Lysis buffer (10 mM Tris pH=8.0, 1 mM EDTA, 25 mM NaCl, 200 µg/ml Proteinase K) and homogenized with autoclaved pestle. After incubation at 37 °C for 30 minutes, they were boiled for 5 minutes in a water bath (to deactivate Proteinase K). Lysates were used immediately for PCR.
4.6. Western-blotting experiments
For each genotype examined, twenty embryos were picked under GFP microscope and homogenized in 40µL of Lysis buffer (0.15 M NaCl, 0.02 M Tris pH 7.5, 0.001M EDTA, 0.001 M MgCl2, 1% Triton-X-100, PIC). After centrifugation, the supernatant (37.5µL) was collected and 12.5µL of 4X Laemmli sample buffer was added. Out of this 50µL, 10µL was subjected for SDS-PAGE and Western analysis. Primary antibodies used were against: WAVE (guinea pig 1:1500), Hem (rabbit 1:1000), and Tubulin (Abcam, rabbit, 1:2000). X-ray films from various Western blotting analyses were scanned and the densities of the signals were determined by using AlphaEaseFC (AlphaInnotech, V6.0). Anti-Tubulin was used as loading control and intensity of the band were normalized against Tubulin signal. Several independent experiments were done and the intensity values followed the same trends with narrow variations in all the experiments.
Research Highlights.
We show that the mutant alleles of Hem show significant phenotypic variability.
The variability correlates with the amount of maternal Hem
We show that the Hem pathway regulates asymmetric division of neural precursors.
Hem regulates asymmetric localization of determinants.
Acknowledegements
We thank Drs. Christian Klämbt, Manfred Frasch, Eyal Schejter, Jennifer Zallen, Zun Lai, Iowa Hybridoma Bank (antibodies against Eve, 22C10, BP102 and FasII), and the Bloomington stock center for various mutant stocks and antibodies. We also thank the members of the Bhat lab for their help and comments on the work. This work is supported by an R01 grant from NIH-NIGMS to KB (GM080538).
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Baumgartner S, Martin D, Chiquet-Ehrismann R, Sutton J, Desai A, Huang I, Kato K, Hromas R. The HEM proteins: a novel family of tissue-specific transmembrane proteins expressed from invertebrates through mammals with an essential function in oogenesis. J Mol Biol. 1995;251:41–49. doi: 10.1006/jmbi.1995.0414. [DOI] [PubMed] [Google Scholar]
- Ben-Yaacov S, Le Borgne R, Abramson I, Schweisguth F, Schejter ED. Wasp, the Drosophila Wiskott-Aldrich syndrome gene homologue, is required for cell fate decisions mediated by Notch signaling. J Cell Biol. 2001;152:1–13. doi: 10.1083/jcb.152.1.1-b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KM. Segment polarity genes in neuroblast formation and identity specification during Drosophila neurogenesis. Bioessays. 1999;21:472–485. doi: 10.1002/(SICI)1521-1878(199906)21:6<472::AID-BIES4>3.0.CO;2-W. [DOI] [PubMed] [Google Scholar]
- Bhat KM, Apsel N. Upregulation of Mitimere and Nubbin acts through Cyclin E to confer self-renewing asymmetric division potential to neural precursor cells. Development. 2004;131:1123–1134. doi: 10.1242/dev.01014. [DOI] [PubMed] [Google Scholar]
- Bhat KM, Gaziova I, Katipalla S. Neuralized mediates asymmetric division of neural precursors by two distinct and sequential events: promoting asymmetric localization of Numb and enhancing activation of Notch-signaling. Dev Biol. 2011;351:186–198. doi: 10.1016/j.ydbio.2010.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KM, Gaziova I, Krishnan S. Regulation of Axon Guidance by Slit and Netrin Signaling in the Drosophila Ventral Nerve Cord. Genetics. 2007;176:2235–2246. doi: 10.1534/genetics.107.075085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhat KM, Schedl P. The Drosophila miti-mere gene, a member of the POU family, is required for the specification of the RP2/sibling lineage during neurogenesis. Development. 1994;120:1483–1501. doi: 10.1242/dev.120.6.1483. [DOI] [PubMed] [Google Scholar]
- Bogdan S, Grewe O, Strunk M, Mertens A, Klambt C. Sra-1 interacts with Kette and Wasp and is required for neuronal and bristle development in Drosophila. Development. 2004;131:3981–3989. doi: 10.1242/dev.01274. [DOI] [PubMed] [Google Scholar]
- Bogdan S, Klambt C. Kette regulates actin dynamics and genetically interacts with Wave and Wasp. Development. 2003;130:4427–4437. doi: 10.1242/dev.00663. [DOI] [PubMed] [Google Scholar]
- Bossing T, Udolph G, Doe CQ, Technau GM. The embryonic central nervous system lineages of Drosophila melanogaster. I. Neuroblast lineages derived from the ventral half of the neuroectoderm. Dev Biol. 1996;179:41–64. doi: 10.1006/dbio.1996.0240. [DOI] [PubMed] [Google Scholar]
- Schmidt H, Rickert C, Bossing T, Vef O, Urban J, Technau G. The embryonic nervous system lineages of Drosophila melanogaster. II. Neuroblast lineages derived from the dorsal part of the neuroectoderm. Dev Biol. 1997;189:186–204. doi: 10.1006/dbio.1997.8660. [DOI] [PubMed] [Google Scholar]
- Buescher M, Yeo SL, Udolph G, Zavortink M, Yang X, Tear G, Chia W. Binary sibling neuronal cell fate decisions in the Drosophila embryonic central nervous system are nonstochastic and require inscuteable-mediated asymmetry of ganglion mother cells. Genes Dev. 1998;12:1858–1870. doi: 10.1101/gad.12.12.1858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doe CQ. Molecular markers for identified neuroblasts and ganglion mother cells in the Drosophila central nervous system. Development. 1992;116:855–863. doi: 10.1242/dev.116.4.855. [DOI] [PubMed] [Google Scholar]
- Eden S, Rohatgi R, Podtelejnikov AV, Mann M, Kirschner MW. Mechanism of regulation of WAVE1-induced actin nucleation by Rac1 and Nck. Nature. 2002;418:790–793. doi: 10.1038/nature00859. [DOI] [PubMed] [Google Scholar]
- Gaziova I, Bhat KM. Generating asymmetry: with and without self-renewal. Prog Mol Subcell Biol. 2007;45:143–178. doi: 10.1007/978-3-540-69161-7_7. [DOI] [PubMed] [Google Scholar]
- Gaziova I, Bhat KM. Ancestry-independent fate specification and plasticity in the developmental timing of a typical Drosophila neuronal lineage. Development. 2009;136:263–274. doi: 10.1242/dev.027854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hummel T, Leifker K, Klambt C. The Drosophila HEM-2/NAP1 homolog KETTE controls axonal pathfinding and cytoskeletal organization. Genes Dev. 2000;14:863–873. [PMC free article] [PubMed] [Google Scholar]
- Kunda P, Craig G, Dominguez V, Baum B. Abi, Sra1, and Kette control the stability and localization of SCAR/WAVE to regulate the formation of actin-based protrusions. Curr Biol. 2003;13:1867–1875. doi: 10.1016/j.cub.2003.10.005. [DOI] [PubMed] [Google Scholar]
- Leng Y, Zhang J, Badour K, Arpaia E, Freeman S, Cheung P, Siu M, Siminovitch K. Abelson-interactor-1 promotes WAVE2 membrane translocation and Abelson-mediated tyrosine phosphorylation required for WAVE2 activation. Proceedings of the National Academy of Sciences of the United States of America. 2005;102:1098–1103. doi: 10.1073/pnas.0409120102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li W, Fan J, Woodley DT. Nck/Dock: an adapter between cell surface receptors and the actin cytoskeleton. Oncogene. 2001;20:6403–6417. doi: 10.1038/sj.onc.1204782. [DOI] [PubMed] [Google Scholar]
- Lu B, Ackerman L, Jan LY, Jan YN. Modes of protein movement that lead to the asymmetric localization of partner of Numb during Drosophila neuroblast division. Mol Cell. 1999;4:883–891. doi: 10.1016/s1097-2765(00)80218-x. [DOI] [PubMed] [Google Scholar]
- Mehta B, Bhat KM. Slit signaling promotes the terminal asymmetric division of neural precursor cells in the Drosophila CNS. Development. 2001;128:3161–3168. doi: 10.1242/dev.128.16.3161. [DOI] [PubMed] [Google Scholar]
- Nakao S, Platek A, Hirano S, Takeichi M. Contact-dependent promotion of cell migration by the OL-protocadherin-Nap1 interaction. J Cell Biol. 2008;182:395–410. doi: 10.1083/jcb.200802069. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson BE, Beckett K, Nowak SJ, Baylies MK. SCAR/WAVE and Arp2/3 are crucial for cytoskeletal remodeling at the site of myoblast fusion. Development. 2007;134:4357–4367. doi: 10.1242/dev.010678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schäfer G, Weber S, Holz A, Bogdan S, Schumacher S, M¸ller A, Renkawitz-Pohl R, Önel SF. The Wiskott-Aldrich syndrome protein (WASP) is essential for myoblast fusion in Drosophila. Developmental Biology. 2007;304:664–674. doi: 10.1016/j.ydbio.2007.01.015. [DOI] [PubMed] [Google Scholar]
- Schenck A, Qurashi A, Carrera P, Bardoni B, Diebold C, Schejter E, Mandel JL, Giangrande A. WAVE/SCAR, a multifunctional complex coordinating different aspects of neuronal connectivity. 2004;274:260–270. doi: 10.1016/j.ydbio.2004.07.009. [DOI] [PubMed] [Google Scholar]
- Schroter RH, Lier S, Holz A, Bogdan S, Klambt C, Beck L, Renkawitz-Pohl R. kette and blown fuse interact genetically during the second fusion step of myogenesis in Drosophila. Development. 2004;131:4501–4509. doi: 10.1242/dev.01309. [DOI] [PubMed] [Google Scholar]
- Soto MC, Qadota H, Kasuya K, Inoue M, Tsuboi D, Mello CC, Kaibuchi K. The GEX-2 and GEX-3 proteins are required for tissue morphogenesis and cell migrations in C. elegans. Genes Dev. 2002;16:620–632. doi: 10.1101/gad.955702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suzuki T, Nishiyama K, Yamamoto A, Inazawa J, Iwaki T, Yamada T, Kanazawa I, Sakaki Y. Molecular Cloning of a Novel Apoptosis-Related Gene, Human Nap1 (NCKAP1), and Its Possible Relation to Alzheimer Disease. Genomics. 2000;63:246–254. doi: 10.1006/geno.1999.6053. [DOI] [PubMed] [Google Scholar]
- Wai P, Truong B, Bhat KM. Cell division genes promote asymmetric interaction between Numb and Notch in the Drosophila CNS. Development. 1999;126:2759–2770. doi: 10.1242/dev.126.12.2759. [DOI] [PubMed] [Google Scholar]
- Weiner OD, Rentel MC, Ott A, Brown GE, Jedrychowski M, Yaffe MB, Gygi SP, Cantley LC, Bourne HR, Kirschner MW. Hem-1 complexes are essential for Rac activation, actin polymerization, and myosin regulation during neutrophil chemotaxis. PLoS Biol. 2006;4:e38. doi: 10.1371/journal.pbio.0040038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wieschaus E, Nusslein-Volhard C. Drosophila, A practical Approach. Oxford U. Press; 1986. Looking at embryos. [Google Scholar]
- Yedvobnick B, Kumar A, Chaudhury P, Opraseuth J, Mortimer N, Bhat KM. Differential effects of Drosophila mastermind on asymmetric cell fate specification and neuroblast formation. Genetics. 2004;166:1281–1289. doi: 10.1534/genetics.166.3.1281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yokota Y, Ring C, Cheung R, Pevny L, Anton ES. Nap1-regulated neuronal cytoskeletal dynamics is essential for the final differentiation of neurons in cerebral cortex. Neuron. 2007;54:429–445. doi: 10.1016/j.neuron.2007.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zallen JA, Cohen Y, Hudson AM, Cooley L, Wieschaus E, Schejter ED. SCAR is a primary regulator of Arp2/3-dependent morphological events in Drosophila. J. Cell Biol. 2002;156:689–701. doi: 10.1083/jcb.200109057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Z, Bhat KM. The Hem protein mediates neuronal migration by inhibiting WAVE degradation and functions opposite of Abelson tyrosine kinase. Dev Biol. 2011 doi: 10.1016/j.ydbio.2011.06.025. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]