Abstract
The large meso-substituted porphine, meso-tetra(4-N-methylpyridyl)porphine has been identified as a DNA-interactive ligand with a capacity for intercalation (1,2). Subsequently, the 2-N-methyl, 3-N-methyl and N-trimethylanilinium analogues of this porphyrin intercalator have been obtained for physico-chemical analyses (absorption spectroscopy, viscometry, circular dichroism, unwinding of supercoiled DNA). In this paper we discuss the factors affecting the character of porphyrin binding (intercalative, as is the case for the 4-N-methyl and 3-N-methyl porphines, versus non-intercalative, as is the case for the 2-N-methyl and N-trimethylanilinium porphines) and the impact that porphyrins' binding has upon the structure of DNA. The molecular conformation of the porphyrin ligand varies slightly within this series so that the ability of a given porphyrin to intercalate may be correlated with the arrangement of charged groups, the planarity of the porphine ring and the effective width of the individual molecules. The results from these studies indicate that sequence selective binding occurs within a small aperture of solution conditions.
Full text
PDF















Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Baase W. A., Johnson W. C., Jr Circular dichroism and DNA secondary structure. Nucleic Acids Res. 1979 Feb;6(2):797–814. doi: 10.1093/nar/6.2.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer W., Vinograd J. The interaction of closed circular DNA with intercalative dyes. I. The superhelix density of SV40 DNA in the presence and absence of dye. J Mol Biol. 1968 Apr 14;33(1):141–171. doi: 10.1016/0022-2836(68)90286-6. [DOI] [PubMed] [Google Scholar]
- Carvlin M. J., Datta-Gupta N., Fiel R. J. Circular dichroism spectroscopy of a cationic porphyrin bound to DNA. Biochem Biophys Res Commun. 1982 Sep 16;108(1):66–73. doi: 10.1016/0006-291x(82)91832-0. [DOI] [PubMed] [Google Scholar]
- Chan A., Kilkuskie R., Hanlon S. Correlations between the duplex winding angle and the circular dichroism spectrum of calf thymus DNA. Biochemistry. 1979 Jan 9;18(1):84–91. doi: 10.1021/bi00568a013. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. A new model for DNA containing A.T and I.C base pairs. EMBO J. 1982;1(6):663–667. doi: 10.1002/j.1460-2075.1982.tb01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Early T. A., Kearns D. R., Hillen W., Wells R. D. A 300-MHz proton nuclear magnetic resonance investigation of deoxyribonucleic acid restriction fragments: dynamic properties. Biochemistry. 1981 Jun 23;20(13):3764–3769. doi: 10.1021/bi00516a015. [DOI] [PubMed] [Google Scholar]
- Englander S. W., Kallenbach N. R., Heeger A. J., Krumhansl J. A., Litwin S. Nature of the open state in long polynucleotide double helices: possibility of soliton excitations. Proc Natl Acad Sci U S A. 1980 Dec;77(12):7222–7226. doi: 10.1073/pnas.77.12.7222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Espejo R. T., Canelo E. S. Properties of bacteriophage PM2: a lipid-containing bacterial virus. Virology. 1968 Apr;34(4):738–747. doi: 10.1016/0042-6822(68)90094-9. [DOI] [PubMed] [Google Scholar]
- Fiel R. J., Beerman T. A., Mark E. H., Datta-Gupta N. DNA strand scission activity of metalloporphyrins. Biochem Biophys Res Commun. 1982 Aug;107(3):1067–1074. doi: 10.1016/0006-291x(82)90630-1. [DOI] [PubMed] [Google Scholar]
- Fiel R. J., Howard J. C., Mark E. H., Datta Gupta N. Interaction of DNA with a porphyrin ligand: evidence for intercalation. Nucleic Acids Res. 1979 Jul 11;6(9):3093–3118. doi: 10.1093/nar/6.9.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiel R. J., Munson B. R. Binding of meso-tetra (4-N-methylpyridyl) porphine to DNA. Nucleic Acids Res. 1980 Jun 25;8(12):2835–2842. doi: 10.1093/nar/8.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
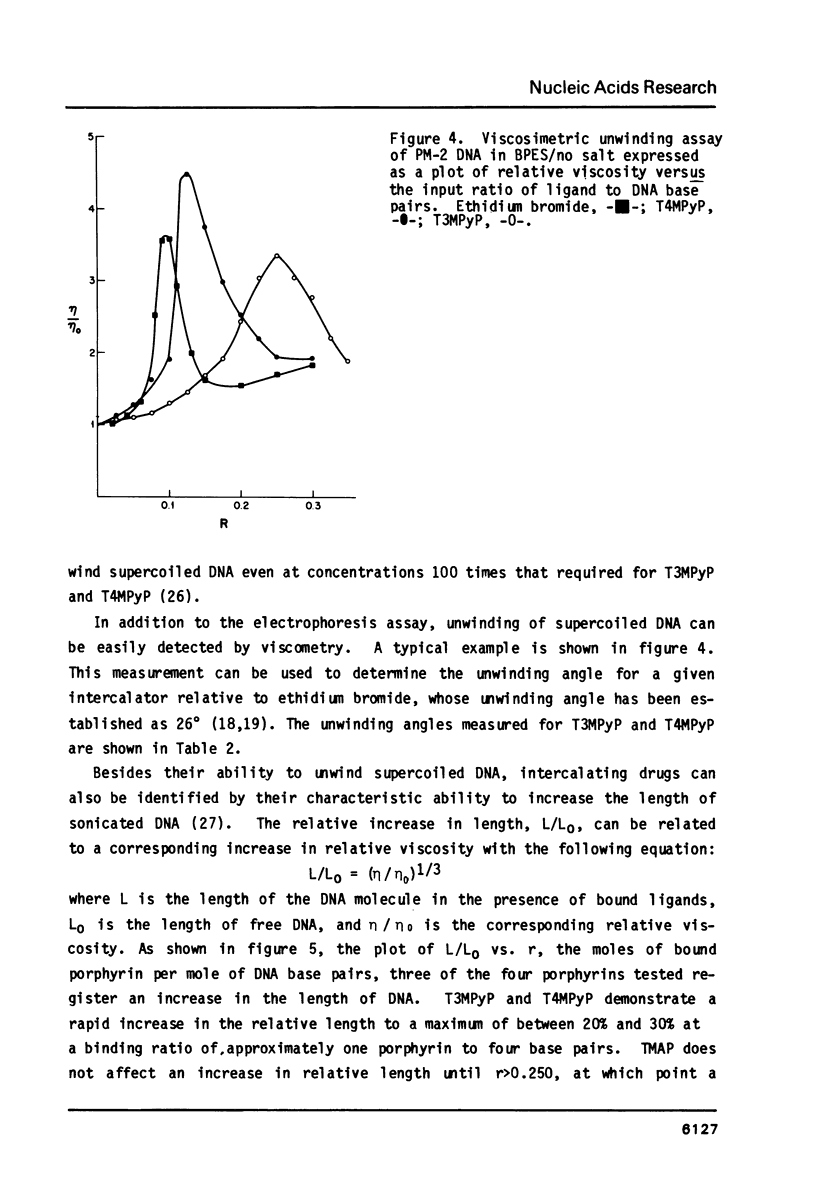
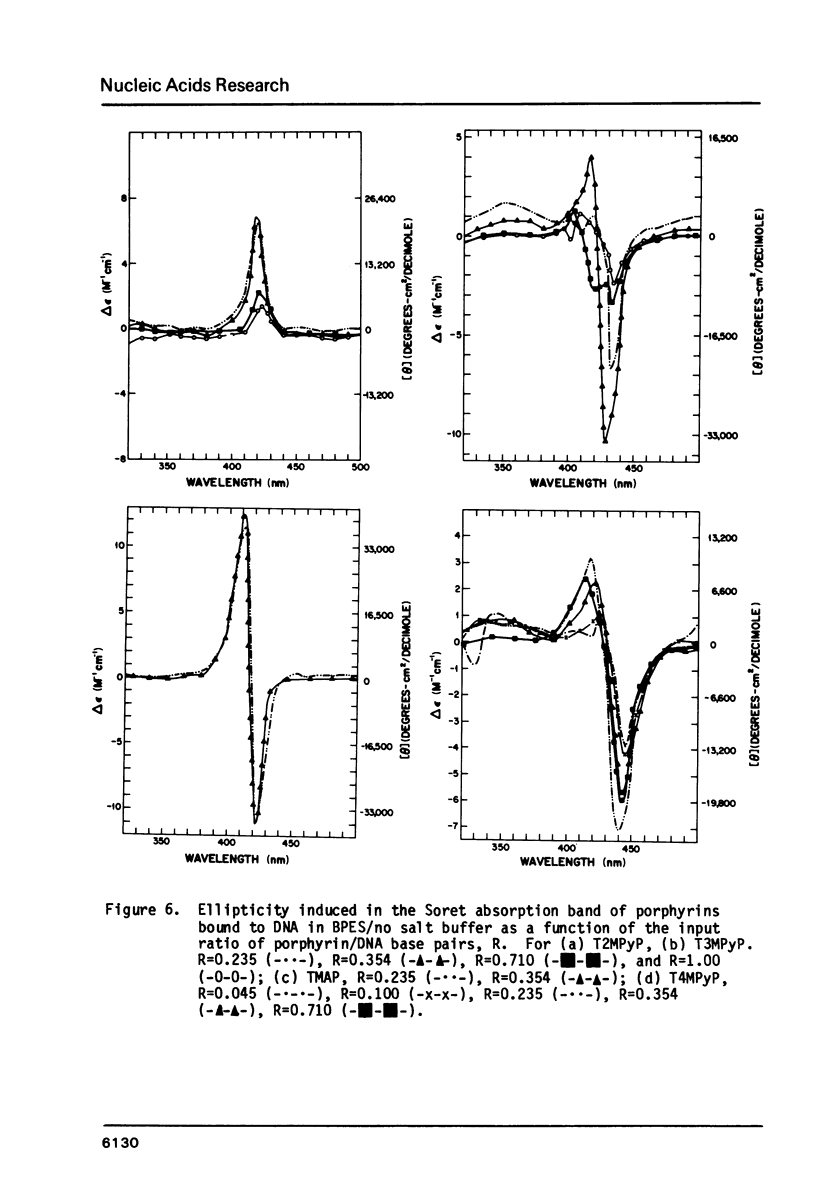
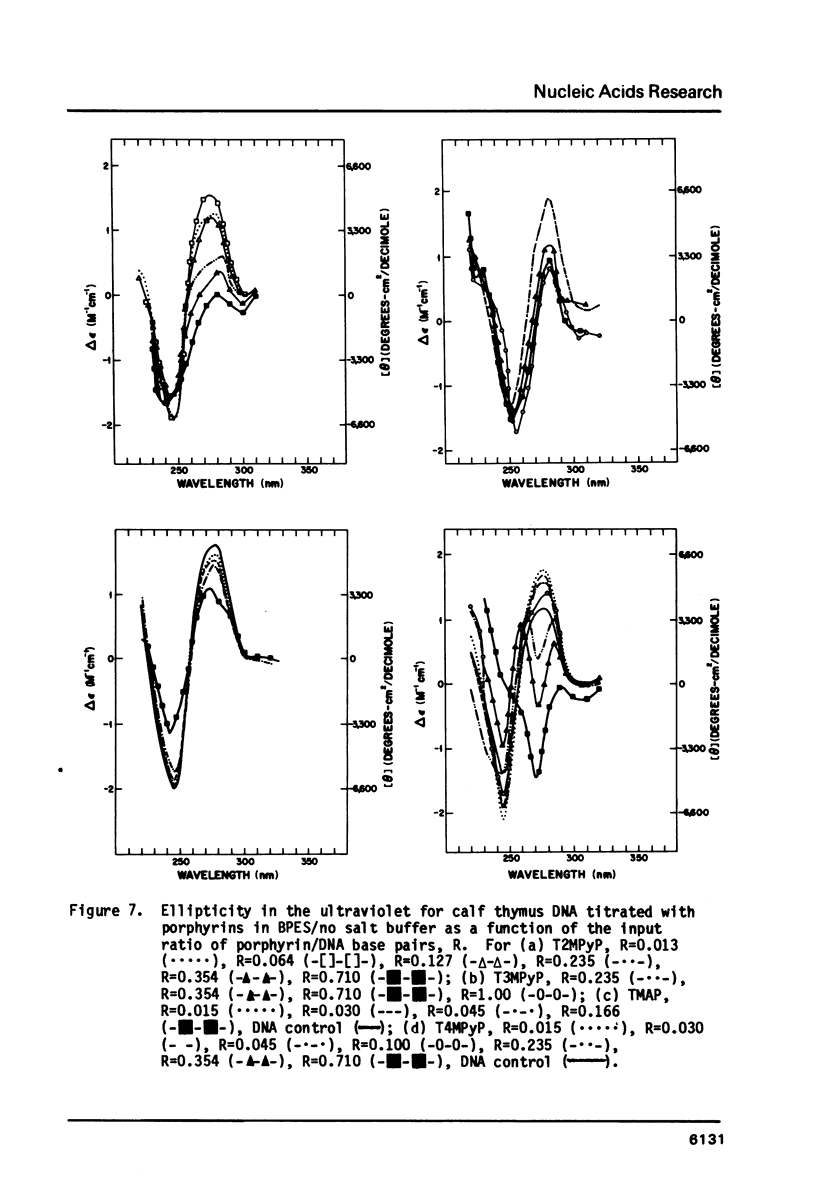
- Girod J. C., Johnson W. C., Jr, Huntington S. K., Maestre M. F. Conformation of deoxyribonucleic acid in alcohol solutions. Biochemistry. 1973 Dec 4;12(25):5092–5096. doi: 10.1021/bi00749a011. [DOI] [PubMed] [Google Scholar]
- Gralla J., Crothers D. M. Free energy of imperfect nucleic acid helices. 3. Small internal loops resulting from mismatches. J Mol Biol. 1973 Aug 5;78(2):301–319. doi: 10.1016/0022-2836(73)90118-6. [DOI] [PubMed] [Google Scholar]
- Hanlon S., Brudno S., Wu T. T., Wolf B. Structural transitions of deoxyribonucleic acid in aqueous electrolyte solutions. I. Reference spectra of conformational limits. Biochemistry. 1975 Apr 22;14(8):1648–1660. doi: 10.1021/bi00679a017. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minchenkova L. E., Schyolkina A. K., Poletayev A. I. Different conformations of double-stranded nucleic acid in solution as revealed by circular dichroism. Biopolymers. 1973;12(1):89–110. doi: 10.1002/bip.1973.360120109. [DOI] [PubMed] [Google Scholar]
- Li H. J., Crothers D. M. Relaxation studies of the proflavine-DNA complex: the kinetics of an intercalation reaction. J Mol Biol. 1969 Feb 14;39(3):461–477. doi: 10.1016/0022-2836(69)90138-7. [DOI] [PubMed] [Google Scholar]
- Mandal C., Kallenbach N. R., Englander S. W. Base-pair opening and closing reactions in the double helix. A stopped-flow hydrogen exchange study in poly(rA).poly(rU). J Mol Biol. 1979 Dec 5;135(2):391–411. doi: 10.1016/0022-2836(79)90443-1. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Formaldehyde as a probe of DNA structure. 3. Equilibrium denaturation of DNA and synthetic polynucleotides. Biochemistry. 1977 Jul 26;16(15):3267–3276. doi: 10.1021/bi00634a001. [DOI] [PubMed] [Google Scholar]
- McGhee J. D., von Hippel P. H. Formaldehyde as a probe of DNA structure. r. Mechanism of the initial reaction of Formaldehyde with DNA. Biochemistry. 1977 Jul 26;16(15):3276–3293. doi: 10.1021/bi00634a002. [DOI] [PubMed] [Google Scholar]
- Mong S., Huang C. H., Prestayko A. W., Crooke S. T. Interaction of cis-diamminedichloroplatinum(II) with PM-2 DNA. Cancer Res. 1980 Sep;40(9):3313–3317. [PubMed] [Google Scholar]
- Pilet J., Blicharski J., Brahms J. Conformations and structural transitions in polydeoxynucleotides. Biochemistry. 1975 May 6;14(9):1869–1876. doi: 10.1021/bi00680a011. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- Pohl F. M. Polymorphism of a synthetic DNA in solution. Nature. 1976 Mar 25;260(5549):365–366. doi: 10.1038/260365a0. [DOI] [PubMed] [Google Scholar]
- Pritchard N. J., Blake A., Peacocke A. R. Modified intercalation model for the interaction of amino acridines and DNA. Nature. 1966 Dec 17;212(5068):1360–1361. doi: 10.1038/2121360a0. [DOI] [PubMed] [Google Scholar]
- Révet B. M., Schmir M., Vinograd J. Direct determination of the superhelix density of closed circular DNA by viscometric titration. Nat New Biol. 1971 Jan 6;229(1):10–13. doi: 10.1038/newbio229010a0. [DOI] [PubMed] [Google Scholar]
- Shindo H., Simpson R. T., Cohen J. S. An alternating conformation characterizes the phosphodiester backbone of poly(dA-dT) in solution. J Biol Chem. 1979 Sep 10;254(17):8125–8128. [PubMed] [Google Scholar]
- Sobell H. M., Reddy B. S., Bhandary K. K., Jain S. C., Sakore T. D., Seshadri T. P. Conformational flexibility in DNA structure as revealed by structural studies of drug intercalation and its broader implications in understanding the organization of DNA in chromatin. Cold Spring Harb Symp Quant Biol. 1978;42(Pt 1):87–102. doi: 10.1101/sqb.1978.042.01.010. [DOI] [PubMed] [Google Scholar]
- Tinoco I., Jr, Bustamante C., Maestre M. F. The optical activity of nucleic acids and their aggregates. Annu Rev Biophys Bioeng. 1980;9:107–141. doi: 10.1146/annurev.bb.09.060180.000543. [DOI] [PubMed] [Google Scholar]
- Tunis-Schneider M. J., Maestre M. F. Circular dichroism spectra of oriented and unoriented deoxyribonucleic acid films--a preliminary study. J Mol Biol. 1970 Sep 28;52(3):521–541. doi: 10.1016/0022-2836(70)90417-1. [DOI] [PubMed] [Google Scholar]
- Vorlícková M., Sedlácek P., Kypr J., Sponar J. Conformational transitions of poly(dA-dT)poly(dA-dT) in ethanolic solutions. Nucleic Acids Res. 1982 Nov 11;10(21):6969–6979. doi: 10.1093/nar/10.21.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wang J. C. The degree of unwinding of the DNA helix by ethidium. I. Titration of twisted PM2 DNA molecules in alkaline cesium chloride density gradients. J Mol Biol. 1974 Nov 15;89(4):783–801. doi: 10.1016/0022-2836(74)90053-9. [DOI] [PubMed] [Google Scholar]
- Waring M. J., Henley S. M. Stereochemical aspects of the interaction between steroidal diamines and DNA. Nucleic Acids Res. 1975 Apr;2(4):567–586. doi: 10.1093/nar/2.4.567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waring M. Variation of the supercoils in closed circular DNA by binding of antibiotics and drugs: evidence for molecular models involving intercalation. J Mol Biol. 1970 Dec 14;54(2):247–279. doi: 10.1016/0022-2836(70)90429-8. [DOI] [PubMed] [Google Scholar]
- Wartell R. M., Benight A. S. Fluctuational base-pair opening in DNA at temperatures below the helix-coil transition region. Biopolymers. 1982 Oct;21(10):2069–2081. doi: 10.1002/bip.360211011. [DOI] [PubMed] [Google Scholar]
- Widom J., Baldwin R. L. Inhibition of cation-induced DNA condensation by intercalating dyes. Biopolymers. 1983 Jun;22(6):1621–1632. doi: 10.1002/bip.360220613. [DOI] [PubMed] [Google Scholar]
- Zacharias W., Martin J. C., Wells R. D. Condensed form of (dG-dC)n X (dG-dC)n as an intermediate between the B- and Z-type conformations induced by sodium acetate. Biochemistry. 1983 May 10;22(10):2398–2405. doi: 10.1021/bi00279a015. [DOI] [PubMed] [Google Scholar]