Abstract
Animal models can certainly be useful to find out more about the biological bases of anxiety disorders and develop new, more efficient pharmacological and/or behavioral treatments. However, many of the current “models of anxiety” in animals do not deal with pathology itself, but only with extreme forms of anxiety which are still in the normal, adaptive range. These models have certainly provided a lot of information on brain and behavioral mechanisms which could be involved in the etiology and physiopathology of anxiety disorders, but are usually not satisfactory when confronted directly with clinical syndromes. Further progress in this field will probably depend on the finding of endophenotypes which can be studied in both humans and animals with common methodological approaches. The emphasis should be on individual differences in vulnerability, which have to be included in animal models. Finally, progress will also depend on refining theoretical constructs from an interdisciplinary perspective, including psychiatry, psychology, behavioral sciences, genetics, and other neurosciences.
Keywords: anxiety, animal model, threat, defense, coping strategy
Abstract
Ciertamente los modelos animales pueden resultar útiles para descubrir más acerca de las bases biológicas de los trastornos ansiosos y desarrollar nuevos y más eficientes tratamientos farmacológicos ylo conductuales. Sin embargo, muchos de los actuates “modelos de ansiedad” en animales no abordan lo patológico propiamente tal, sino que sólo formas extremas de ansiedad que están en un rango normal, adaptativo, Realmente estos modelos han provisto de bastante información sobre los mecanismos cerebrales y conductuales que podrían estar involucrados en la etiología y fisiopatología de los trastornos ansiosos, pero habitualmente no resultan satisfactorios cuando se confrontan directamente con los síndromes clínicos, Los futuros progresos en este campo dependerán probablemente de hallazgos de endofenotipos que puedan ser estudiados tanto en humanos como en animales con abordajes metodológicos comunes. El énfasis debe colocarse en las diferencias individuales de la vulnerabilidad, lo que tiene que estar incluido en los modelos animales. Finalmente el progreso también dependerá del refinamiento de los constructos teóricos desde una perspectiva interdisciplinaria, que incluya la psiquiatría, la psicología, las ciencias de la conducta, la genética y otras neurociencias.
Abstract
Les modèles animaux se sont montrés utiles pour étudier les bases biologiques des troubles anxieux et pour développer de nouveaux traitements, pharmacologiques ou mêmes comportementaux. Cependant, la plupart des modèles actuels ne concernent pas directement la pathologie, mais seulement des formes extrêmes d'anxiété, qui restent toutefois dans une norme permettant l'adaptation. Ces modèles ont certainement donné une quantité d'informations sur les mécanismes nerveux et comportementaux qui pourraient être impliqués dans l'étiologie et la physiopathologie des troubles anxieux, mais ne sont pas toujours satisfaisants lorsqu'ils sont confrontés à la réalité clinique. Le progrès dans ce domaine va dépendre de la découverte d'endophénotypes qui pourront être étudiés chez l'homme et chez l'animal avec des approches méthodologiques communes. Les différences interindividuelles dans la vulnérabilité doivent aussi être prises en compte dans les modèles animaux. Finalement, le progrès dépendra également d'une approche interdisciplinaire des bases théoriques de l'anxiété, approche qui devrait impliquer non seulement la psychiatrie et la psychologie, mais aussi les sciences du comportement et les neurosciences.
Introduction
This brief review will focus on rodent (rat and mouse) models of anxiety disorders. There are of course models of anxiety disorders in other species, including nonhuman primates,1 but rat and mouse models are byfar the most commonly used in the current preclinical and psychiatric (genetic) research. Our aim is not to present an exhaustive view of all existing models, but to discuss some conceptual issues related to these models.
A first and important issue is whether various animal species can really be used as “models” of human pathologies, given the highly subjective nature of anxiety. Do animals experience something like human anxiety, and how can we measure it, since we cannot “think like a rat”?2 We will argue that, as mentioned by many authors, the behavioral responses and brain mechanisms associated with an “anxious state” are so essential for survival that they must have evolved very early in the development of mammalian species and are probably highly conserved—the phylogenetic argument.3 In view of the relationship between anxiety and coping styles, or of the pivotal role of fear-conditioning processes (to be developed in the following sections), it is not unlikely that some form of anxiety exists in other vertebrate classes or lower organisms; even primitive ones have a capacity to detect danger and react to threat.4 This may offer new opportunities to design as-yet unexpected models.5-8 The probability of finding the various emotional responses and “logistic systems” involved in anxiety across phyla is discussed in a recent review.9
Some of the other issues that we would like to discuss here are the following: (i) How does anxiety relate to individual stress/fear coping strategies, and how are these determined? (ii) Do anxiety disorders arise from the failure to adapt successfully to life challenges (the maldaptation or dysadaptation hypothesis), and/or do they have more distant causes? (iii) What is the relative importance of genetic/epigenetic factors and life events in determining increased or decreased individual vulnerability to anxiety disorders? (iv) How accurately can animal models predict the efficacy of pharmacological or other kinds of treatments, and help in the development of new therapeutic approaches? We believe that some of the existing animal models of anxiety disorders have been or will be able to provide important information regarding these issues. However, in order to do so, these models will have to be permanently confronted with clinical practice, and also understood and discussed by clinicians. This is an absolute prerequisite for a successful translational approach.
Anxiety and its disorders
Anxiety is usually described as “a psychological, physiological, and behavioral state induced in animals and humans by a threat to well-being or survival, either actual or potential.”10 It is characterized by increased arousal, expectancy, autonomic and neuroendocrine activation, and specific behavior patterns, often with a behavioral transition from ongoing behaviors (eg, exploration, feeding) to an escape (eg, flight) or other defensive behaviors. The function of these changes is to facilitate coping with an adverse or unexpected situation. However, if the adaptive function of anxiety is not successful, anxiety can become a pathological state, which may later on interfere with the ability to cope with various challenges or stressful events in daily life, and even alter body condition. Pathological anxiety can also be a consequence of predisposing factors (or traits), which result from numerous gene-environment interactions during development (particularly during the perinatal period), and experience (life events). Conceptually, it is important to distinguish fear, which is a response to an immediate, real danger, from anxiety, which is a response to threat, ie, a potential danger.10
Threat and coping strategies
The term “coping” refers to physiological, psychological, and behavioral responses aimed at avoiding harm or distress, is conceptually more or less equivalent to “defense mechanisms,” and applies to both humans and animals.11 , 12
Coping mechanisms are clearly important for health and disease; a proper, successful coping strategy decreases the impact of stress and protects the organism from its longterm consequences. It is more and more evident that vulnerability to stress-induced diseases is highly individual and may in part depend on coping styles. A coping style can be defined as: “[...] a coherent set of behavioural and physiological stress responses which is consistent over time and which is characteristic to a certain group of individuals.” 11 Coping styles are more or less comparable to “temperament” or “personality” traits in humans, and form the basis of individual differences, which are essential to maintain the species' (or population's) adaptive capacity under changing environmental conditions.13 The genetic, epigenetic, and learned aspects of individual coping style are still a matter of debate. Individuals can be broadly classified into two categories: the active (or proactive) copers, and the passive (or reactive) copers; within each category of coping style, there is still ample space for large interindividual variability. As far as animal models are concerned, we have suggested using the concept of “psychobiological profile” to characterize individual sets of particular physiological and behavioral parameters, and to categorize individuals.14 Indeed, for a long time, researchers have tried to avoid or ignore the problem of interindividual differences in groups of experimental animals, especially when designing animal models. This apparent “homogeneity” was even an argument to use animals rather than human subjects! However, it is now clear that such differences do exist and are important, particularly as regards translational studies in psychiatry. For instance, the search for vulnerability (or predisposition) factors requires tools to describe these individual differences adequately. Guidelines for defining personality differences in rats have been recently proposed.15 Two theoretical models more directly related to individual differences in stress coping in rodents have also been published.16 , 17
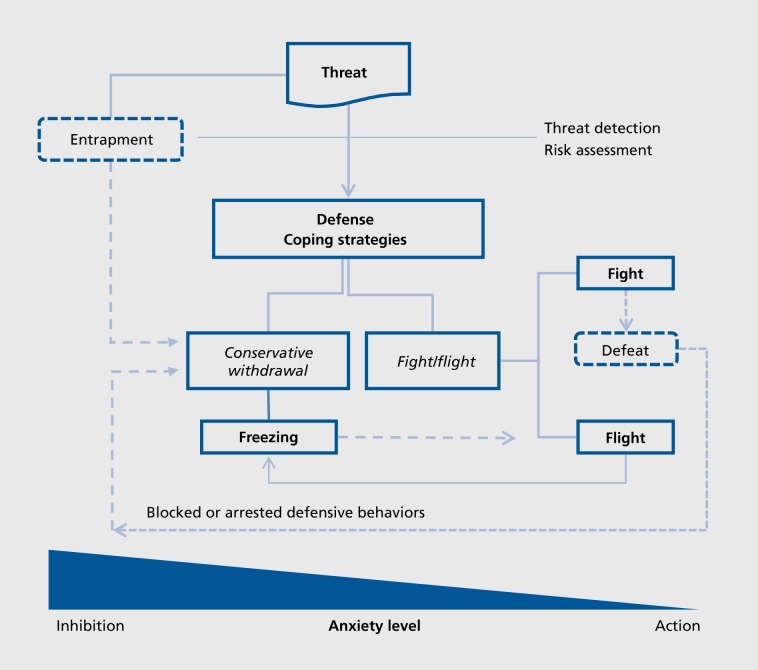
As shown in Figure 1., there are two main, alternative strategies to face environmental and/or psychosocial challenges, or threat.18 One is a passive (or reactive) strategy (conservation/withdrawal) aimed at protecting the organism from the possible consequences of threat, as originally described by Engel and Schmale.19 The other one is an active (or proactive) strategy aimed at eliminating the source of threat, either byescaping (“flight”) or facing it (“fight)”, originally described as the “fight-or-flight” response by Walter Cannon.20 The physiological responses associated with these alternative strategies are described in Henry's account of how individuals may adapt under threat situations:
Figure 1. Figure 1. Alternative defense (coping) strategies in response to threat. Depending on their psychobiobgical profile (temperament), previous experiences, appraisal of the situation, and/or environmental constraints, individuals can choose between an active fight-or-flight strategy, or a more passive, conservative-withdrawal response.If flight is not possible (“entrapment” situation, see ref 22) or aborted (“Arrested flight,” see ref 23), they have to rely on a passive coping strategy. The same occurs if, after fighting, they are defeated. Anxiety is markedly increased when the more passive coping strategies are used, or when action is inhibited, even momentarily (in choice and/or conflict situations). See text for details.
The sympathetic-adrenal medullary system (SAM) is activated when the organism is challenged but remains in control. With the increasing perception that loss of control and helplessness may occur, there is activation of the hypothalamic-pituitary-adrenal (IIPA) axis and the oxytocingonadotrophic species preservative system shuts down. There is a shift from active defense to a passive nonaggressive coping style. The emphasis is now on “self-preservation.21
Thus, there are many situations where individuals can use alternative coping strategies (Figure 1). The choice will depend on their constitutive psychobiological profile (individual coping style, or temperament, see above), previous experience, appraisal of the situation and/or environmental constraints. If escape (flight) is not possible, as in an “entrapment” situation,22 or aborted—the “arrested flight”—,23 individuals will have to rely on a passive coping strategy. The same occurs if, after fighting, they are defeated.22 As shown at the bottom of Figure I, anxiety is markedly increased when the more passive coping strategies are used, or when action is inhibited, even momentarily (in choice and/or conflict situations). However, if some kind of action can be undertaken, even under conditions of restricted choice, a blunting of the physiological, anxious response can be observed. If rats subjected to inescapable electric shocks can start fighting with a partner, if rats tightly restrained (immobilization stress) can bite a piece of wood,24 or if rats exposed to a novel situation perform self -grooming (a displacement activity), all these actions decrease the impact of stress and associated anxiety. Displaced aggression probably has a similar function.25 Anxiety disorders are characterized by the avoidance of situations that can lead to the arousal of fear and anxiety. Passive avoidance is part of some disorders; if it can be turned into an active coping strategy (at the cognitive and/or behavioral level), then things are much better off.26 Turning passive into more active coping strategies is indeed the basis of many cognitive-behavioral therapies, which are probably among the most effective interventions regarding a number of anxiety disorders.
The inhibition of ongoing behavior is one of the first behavioral symptoms of an anxious or fearful state. In the 1970s, Jeffrey Gray suggested that vulnerability to anxiety is associated with individual differences in the activity of a septo-hippocampal “behavioral inhibition system,” or BIS.27 Anxiety reflects a central state mediated by BIS activation, which is elicited by threats of punishment or failure, and by novelty or uncertainty. The results of BIS activation are an inhibition of the ongoing behavior, increased arousal and attention to environmental stimuli, especially novel stimuli.28 A number of human studies, in both community samples and clinical groups, have indicated that anxiety symptoms generally show positive associations with BIS sensitivity.29 Gray's Reinforcement Sensitivity Theory (RST), which has been revised and adapted many times,30 may thus not only be a good framework for research on personality-psychopathology associations,29 but also for translational studies, because of its relevance for animal behavior and natural defence mechanisms.
The central role of behavioral inhibition in generating an “anxious state” has also be pointed out by Laborit.31 For this author, anxiety is associated with the “alarm reaction,” as defined in Selye's original description of the stress response (or general adaptation syndrome).32 Anxiety appears when one realizes that a proper adaptive action is not possible, ie, that there is loss of control over the situation, and it involves an activation of the HPA axis.
According to Panksepp,33 the two opposite, spontaneous behavior patterns (ie, freezing, crouching, and passive avoidance, or flight, escape and active avoidance) seen in the response of animals to threat may have distinct and successive roles. Flight and other active coping behaviors are unconditional responses to proximate threat, whereas passive coping strategies such as freezing are conditioned responses to distal stimuli predictive of danger. These strategies are modulated by the (cognitive) apprehension of the environment and probability of success, eg, whether or not there is a route of escape. Thus, when an animal faces a predator, freezing is preferentially activated when the source of known danger is still far away. When danger gets closer, and the stimulus passes through some critical “psychometric” distance, it becomes a true unconditional stimulus and a flight pattern is activated.34
Coping strategies provide a new, interesting theoretical framework for models investigating the role of individual differences in vulnerability to anxiety disorders, and their genetic and epigenetic determinants.
Fear conditioning
Learning the relationships between aversive events and environmental stimuli which predict these events is essential for survival. The neurobiological bases of fear conditioning have been extensively investigated during the last decades.35 , 36 The major aspects of the relationship between conditioned fear and anxiety are the fact that a fearful response can be associated with specific cues (conditional stimuli, CS), and that this learned association can be “unlearned,” ie, not forgotten, but actively repressed.37 This fear conditioning (or learned fear) paradigm is highly relevant for some anxiety disorders, eg, phobias and post-traumatic stress disorder (PTSD) in particular, and is used in several animal models. The critical stage appears to be not the training (conditioning) phase, when the conditional (CS) and unconditional (US) stimuli are presented in a meaningful temporal relationship, but the extinction phase, when the CS is presented alone (without the reinforcement stimulus), during the time necessary for extinction to occur; some individuals fail to repress the memory of fear and show all the behavioral and physiological signs normally triggered in the presence of an actual threat.
Thus, fear conditioning provides another relevant theoretical framework for translational studies on anxiety disorders.
Conflict
Motivational conflict
This can be a major source of anxiety. Indeed, making a wrong decision in the face of danger can be fatal, and having to decide on the right way of doing constitutes a form of “psychological threat.” More frequently, the consequences of a wrong choice are not life-threatening, but can change an inidividual's life (losing one's partner, territory, or social status), or be only unpleasant (being momentarily deprived of food). In any case, making a decision when the consequences are unpredictable is a source of stress.
Frustration
Frustration can also be a source of anxiety, could be considered as a particular form of conflict. Frustration occurs when there is a discrepancy between the expected and actual outcome of an action. For instance, if you train animals to reach a goal by obeying certain rules, and then change the rules, they get very upset and enter a motivational conflict. This situation is very frequent in humans, and can lead not only to various anxiety disorders, but also to depression.
Conflict situations are present, directly or indirectly, in most animal models of anxiety. Thus, during exploration of a novel environment (a situation encountered in many of the tests), there is always a conflict between curiosity (knowing more about it) and fear (how risky is it?). In rats, this conflict may be displayed in the form of a displacement activity such as self-grooming.38
Memories and anticipation
The capacity to remember past events and situations (particularly frightful or traumatic ones), and to anticipate them, parallels the development of the corticolimbic system during evolution. Higher mammals, including humans, are thus better able to integrate past experiences and to “prepare for the worst.” This is an obvious adaptive advantage, but it also has severe drawbacks if the mechanisms involved are not constantly adjusted to “the real world.” It seems quite clear that some forms of anxiety disorders are a direct consequence of this (in)capacity to take into account past and future events. Generalized Anxiety Disorder (GAD) is probably linked to a bias in anticipating adverse circumstances (often without any obvious threat), whereas PTSD certainly results from a deficit in repressing traumatic memories. This is also the case, on a more elementary level, lor various kinds of phobic disorders, although some of these may be associated with more primitive, species-related fear memories.39,40
Individual differences in coping styles, and in the capacity to deal with learned fear, conflict, fear memories, and anticipation of adverse events are thus the most important factors determining vulnerability to anxiety disorders. Genetic and epigenetic predisposition factors do also play an important role, either per se or in combination with the above.41
In the following sections, we will see how and to what extent these concepts are applied in various animal models of anxiety disorders.
Trait vs state anxiety
Reference is sometime made to two sorts of anxiety: “state” anxiety is experienced at a particular moment and is enhanced by anxiogenic stimuli, whereas “trait” anxiety does not vary over time and is an innate characteristic of an “anxious” individual.42,43 These definitions are certainly open to criticism: it seems difficult to assess trait anxiety in the absence of anxiogenic stimuli, and these stimuli also increase anxiety in “naturally anxious” individuals... However, rat and mouse lines selected for high anxiety (see below) certainly present a form of “trait” anxiety. Trait anxiety is supposed to be a predisposing factor for anxiety disorders.
Anxiety disorders: a dysadaptation hypothesis
The mismatch hypothesis and the concept of adaptive phenotypic plasticity have been recently proposed in the context of animal models of depression in order to explain why some individuals may develop depression-like symptoms in adulthood when exposed to chronic stress in early life, while others do well or even better.44 A similar concept could be applied to models of anxiety disorders, where early life events have also been shown to influence the anxious phenotype. Thus, rat pups born from mothers having been stressed during pregnancy tend to be more anxious than their counterparts raised by non-stressed mothers.45-47 This phenomenon, called adaptive phenotypic plasticity, which has a limited range, or ”norm of reaction,“ 48 is the basis for Darwinian fitness49 and is mediated in part by epigenetic mechanisms: gene expression is modulated to fit the most probable environmental demands during the lifetime of the individual.50 Mismatch occurs when the expected conditions are not met in later life, eg, when early adverse conditions increase the sensitivity of the stress-response systems and when this hypersensitivity remains even when environmental pressure becomes lower in adulthood. Indeed, most individuals seem to adapt to this type of change in environmental conditions (a phenomenon known as ”resilience“), but a few fail to do so—a situation which is somehow reminiscent of what is observed in lear conditioning when extinction of learned fear does not occur, as described above. For this reason, it has been recently proposed that the basis for vulnerability to disease could involve genes (yet to be discovered) that would be responsible for different forms of brain and behavioral plasticity.51 Behavioral flexibility is a form of plasticity that may favor optimal coping17,48 and therefore decrease the risk of developing a pathology—or increase resilience, a phenomenon that certainly deserves more attention in future studies.
The term “dysadaptation” has been used previously in ophtalmology to describe “[...] the inability of the retina and iris to accommodate well to varying intensities of light.” 52 By analogy, this term could be applied to anxiety disorders, inasmuch as it would describe “the inability of defence/coping mechanisms to adapt to varying degrees of threat,” or the individual's inability to evaluate correctly the risks actually associated with signals of danger (perceived threat). The term “dysadaptation” seems to be better suited than “maladaptation,” in the sense that the psychophysiological and behavioral responses are still potentially adaptive, but inappropriate to the context, or the situation (the mismatch hypothesis).
Animal models and tests
What is a model?
In biomedical research, a model is usually described as an experimental setup or protocol (sometimes also called “a paradigm”) developed in a nonhuman species with the aim of replicating humans physiological, pathophysiological, or behavioral features. In other scientific disciplines (eg, mathematics or physics), the term “model” usually refers to a theoretical construct from which specific hypotheses can be deduced and tested experimentally. Animal models of psychiatric disorders can belong to both categories. The most simple models, notably those aimed at testing psychotropic drugs or other treatments—“empirical validity models”53—often have a limited, if any, theoretical background. This is also the case for those developed to simulate a specific sign or symptom (“Behavioral similarity models”). However, “theory-driven” and “mechanistic” models (according to McKinney's terminology), in particular those developed to study etiological aspects and/or the neurochemical and genetic mechanisms underlying anxiety disorders, often have an elaborate theoretical background.
How do we measure anxiety in animals?
The only variables that can be observed and measured in animals are the behavioral and physiological responses elicited when they are exposed to more or less naturalistic, potentially anxiogenic situations under controlled laboratory conditions. Setup and protocols used to record these experimental data are usually called “tests,” and constitute instruments (or tools) to measure anxiety-related parameters. It should be mentioned that, in the animal research literature, particularly as regards the so-called preclinical (pharmacological) studies, the term “model” is often used abusively to characterize a test, ie, a particular experimental setup (eg, “The elevated plus-maze as a model of anxiety in rodents”!). This usage should be avoided, because it is misleading: a model in the true sense has a more elaborate theoretical background and may include several tests. In the following section, we will mention a few examples of (mainly ethological) anxiety tests for rodents, which are by far the most common species used as animals models nowadays. There are over 30 different procedures (and many variations) described in the literature, with two main categories: unconditioned response tests (which require no training and usually have a high eco/ethological validity) and conditioned response tests (which often require extensive training and may show interference with mnemonic and motivational processes).54 A few examples are shown in Table I. More information regarding practical aspects of testing can be found in the literature55-58 and in the references in Table I.
Although measurements can be done using a single test, it is better to use a battery of these tests (for instance, the open field, the EPM, and a dark/light transition test) to assess each individual's behavioral phenotype, since these tests measure anxiety under different conditions.59
Data obtained from different tests can be combined to create ”derived“ variables which offer a more complete description of the individual behavioral profiles.14 Other elaborate forms of data analysis can (and should) be used: factorial analysis,60,61 structural analysis,62 or multivariate (principal component) analysis.63 Some of these tests are time-consuming, and therefore not always appropriate for large screening studies, but the throughput of behavioral assessment has been markedly improved in recent years by the use of automated monitoring, computer data processing, and the development of dedicated software for behavioral analysis.64
TABLE I. Table I. Models or tests of anxiety in rodents. For a definition of tests vs models, see text. See also refs 95, 96. Adapted from ref 54: Rodgers RJ. Animal models of 'anxiety': where next? Behav Pharmacol. 1997;8:477-496. Copyright© Lippincott Williams and Wilkins 1997.
| Unconditioned responses ("ethological" models) | Refs | Conditioned responses | Refs |
| Exploratory behavior | Conflict tests | ||
| Open fieid(s) | 97 | Geller-Seifter | 98 |
| Elevated Plus Maze (EPM) | 99; 100 | Vogel | 101, 102 |
| Elevated T-Maze | 103 | ||
| Zero-Maze | 104 | ||
| Holeboard(s) | 105 | ||
| Free exploration paradigm | 106 | ||
| Dark/light transition | Avoidance tests | ||
| Black/white box | 107, 108 | Passive avoidance | 109 |
| Dark/light open field | 16 | (Two-way) Active avoidance | 110 |
| Social/behavior | Inhibitory avoidance (Elevated T-Maze) | 111, 112 | |
| Separation-induced US vocalizations (pups) | 113 | ||
| Stress-induced vocalization (adults) | 114 | ||
| Social interaction | 115 | Fear-potentiated behavior in the EPM | 116 |
| Antipredator/defence | |||
| Anxiety test battery | 59 | Defensive (probe or prod) burying | 117, 118 |
| Mouse defence test battery | 119 | ||
| Predator odor avoidance | 120 | ||
| Others | |||
| Hyponeophagia | 121 | ||
| Open field drink test (OFDT) | 122 | Conditioned suppression | 56 |
| Startle response (baseline) | 123 | Fear-potentiated startle (FPS) | 56 |
| Freezing (unconditioned) (SNT) | 124 | Conditioned emotional response (CER) | 56 |
| Stress-induced hyperthermia (SIH) | 79 |
How can we assess the validity of models?
In the mid 1980s, Willner proposed three sets of criteria for assessing animal models of human mental disorders: predictive validity (performance in the test predicts performance in the condition being modeled), face validity (phenomenological similarity), and construct validity (theoretical rationale).65 , 66
To these “classical” criteria, we would like to add a new one, recently proposed by Mathias Schmidt in the context of animal models for depression: the “population validity“ criterion.44 This is a specific extension of the ”face validity“ criterion: the occurrence rate of a disease-like phenotype in an (epi)genetically heterogeneous population should match the human situation (same odds ratio for that risk or predisposition factor). Thus, risk factors such as adverse early life events should only affect a subpopulation of more vulnerable individuals. Application of this criterion poses a number of problems, notably regarding the number of animals which have to be used. However, the occurrence of anxiety disorders is quite frequent (lifetime prevalence 15% to 30%) in the general population,67,68 and similar values can be expected in a rat or mice population, as this has been shown for instance in animal models of PTSD.69
It would seem that application of the population validity criteria is probably essential if we want to develop models of anxiety disorders, and not only models of anxiety within the ”reaction norm“ (ie, in the normal adaptive range), although these models are still useful to delineate the biological and neural mechanisms underlying ”normal“ anxiety, or to evaluate the efficacy of (pharmacological) treatments.
Should models be based on clinical symptom classification?
In our views, the obvious answer to that question is: no, or at least not exclusively. First, the classifications of psychiatric diseases (either with the DSM-IV or ICD-10 systems) remain essentially syndromic and is constantly being revised.70,71 Second, currently recognized categories of psychiatric disorders include heterogeneous populations of patients, with subpopulations featuring a great diversity in underlying (epi)genetic and other predisposition factors, neurobiological mechanisms, life history, and comorbidities. And third, some important aspects of human pathology will probably never be accessible in animal models (eg, sadness or suicidal ideation in depression). However, some symptoms found in anxiety disorders can probably be modeled quite accurately in rats or mice.72
Recent advances suggest that translational research should preferably be based on “functional modules,” or particular sets (or patterns) of psychophysiological and behavioral responses related to coping with stress, fear, and anxiety, not on psychiatric symptoms as such. These functional modules correspond to specific neural circuits, hormonal systems, and behavioral/psychophysiological responses which are found both in humans and our existing animal models. These modules are conceptually equivalent or similar to what has been described as “endophenotypes” in neurogenetic research.73 , 74 The concepts of “endophenotype” (as opposed to “exophenotype”) was originally proposed by John and Lewis to describe features that were “...not the obvious and external but the microscopic and internal.”75 In Gottesman's own words:
Development of animal partial-models in psychiatry relies on identifying critical components of behavior (or other neurobiological traits) that are representative of more complex phenomena. Animals will never have guilty ruminations, suicidal thoughts, or rapid speech. Thus, animal models based on endophenotypes that represent, evolutionarily selected and quantifiable traits may better lend themselves to investigation of psychiatric phenomena than models based on face-valid diagnostic phenotypes.73
How can we define endophenotypes for anxiety?
As compared with other psychiatric disorders (eg, schizophrenia), only a few endophenotypes for anxiety have been proposed so far.74 Among them, two at least can be assessed in animal models. One is HPA axis activation and other parameters associated with an inhibited (fearful) temperament.76 The other one is also linked to personality characteristics: trait anxiety (anxious temperament) and behavioral inhibition.77 These endophenotypes emphasize the major role played by coping strategies in individual vulnerability or resilience to anxiety (and other) disorders. Other, more psychophysiological endophenotypes that have been suggested are C02 sensitivity for panic disorder,78 stress-induced hyperthermia (SIH), which is found across numerous species, including humans, and reflects SAM activation,79 and the startle response, which is also found in humans and various species.80
Further progress in the field of animal models of anxiety will certainly rely heavily on discovering and validating more endophenotypes, in particular those related to individual brain and behavioral plasticity, and the capacity to adapt to stressful experiences.
What are the current trends in animal models?
Although there is still a need for better and more reliable animal models for screening potential therapeutic agents, there is also an increased interest in developing “face and construct validity” models to study the etiology and underlying neurobiological mechanisms of anxiety disorders, in particular for psychiatric genetic research. Trait anxiety (the “anxious temperament”), supposed to be a major risk factor for anxiety disorders,81 is found in a number of individuals in a normal rat or mice population, but is easier to study in lines obtained by (psycho)genetic selection, where expression of this trait is enhanced. A number of rat lines have been proposed as models of trait anxiety: The HAB rats,82 selected on the basis of their behavior in the EPM; the Syracuse rats83; the Maudsley reactive/nonreactive strains84 ; the Tsukuba85 and Floripa86 lines; and two lines selected on the basis of pups' ultrasonic vocalizations.87 The Roman Low-Avoidance (RLA) rats, selected on the basis of poor acquisition of a two-way avoidance response in the shuttle box, can also be considered as a model of high trait anxiety-emotionality.14,88-91 A number of mouse lines are also available.72 Selective breeding of rats and mice improves the probability of discovering anxiety-related neurobiological correlates,92 including genetic determinants, and allows the study of gene-environment interactions. Finding out how these gene-environment interactions (and associated epigenetic and psychophysiological/behavioral mechanisms) determine each individual's capacity to cope with fear, threat and stressful situations appears to be a major goal of animal models in the years to come.
As regards the genetic bases of vulnerability to anxiety disorders, many different approaches are being used, apart from using selected lines. These include targeted manipulation of candidate genes (eg, generation of knockout or transgenic animals), siRNA and viral transfection, quantitative trait loci (QTL) analysis, and the use of gene expression arrays, among others.72 A relatively new field in animal models of anxiety disorders is the study of structural brain plasticity and adaptive neurogenesis, which appears to be involved in anxiety-related behaviors.93
Summary and conclusions
In 2001, the National Institute of Mental Health (NIMH) organized a workshop to discuss the relationship between existing behavioral models of anxiety and the clinical profile of anxiety disorders. The conclusions were not too optimistic:
The probability of developing comprehensive animal models that, accurately reflect the relative influences of factors contributing to anxiety disorder syndromes is quite low. However, ample opportunity remains to better define and extend existing models and behavioral measures related to specific processes that may be disrupted in anxiety disorders, and to develop new models that consider the impact of combined factors in determining anxious behaviors.94
What is the situation like almost 10 years later?
Indeed, the last decade has seen some major conceptual progress. First, the primary importance of individual differences in personality, temperament, or coping style as regards vulnerability to various anxiety disorders has been recognized, not only in psychiatry, but also in animal models. This means taking into account genetic and epigenetic factors, life events, and response and adaptation to stressful situations (“life trajectories”) in future models. Second, some recent discoveries have also indicated an important role for behavioral flexibility and adaptive (neural) plasticity. This suggests that some disorders may result from a deficit in various forms of brain and behavioral plasticity and perhaps depend, at least in part, on altered neurodevelopmental processes. Application of new, stricter validation criteria, such as the “population validity” criterion, will be required to ensure the “face validity” of these future models and help discriminating between extreme forms of anxiety and truly pathological ones.
One issue that remains unsettled is the following: how accurately can existing or future animal models predict the efficacy of pharmacological or other kinds of treatments, and help in the development of new therapeutic approaches? It is likely that the answer will depend not only on the intrinsic validity of the models, but also on refining diagnostic criteria for anxiety disorders, which will have to be based at least in part on the description of relevant endophenotypes. This implies a bidirectional exchange of information and hypotheses between clinicians and neurobiologists, which is after all the true essence of translational research.
In conclusion, we believe that the future lies in the development of models based on individual vulnerability to anxiety disorders, particularly in relation to gene tic/epigene tic determinants, life events and conditioned fear responses, and coping strategies.
REFERENCES
- 1.Barros M., Totnaz C. Non-human primate models for investigating fear and anxiety. Neurosci Biobehav Rev. 2002;26:187–201. doi: 10.1016/s0149-7634(01)00064-1. [DOI] [PubMed] [Google Scholar]
- 2.Despret V. Penser comme un rat. Versailles, France: Editions QUAE. 2009 [Google Scholar]
- 3.Stein DJ., Bouwer C. A neuro-evolutionary approach to the anxiety disorders. J Anxiety Disord. 1997;11:409–429. doi: 10.1016/s0887-6185(97)00019-4. [DOI] [PubMed] [Google Scholar]
- 4.Stein DJ., Nesse RM. Threat detection, precautionary responses, and anxiety disorders. Neurosci Biobehav Rev. 2011;35:1075–1079. doi: 10.1016/j.neubiorev.2010.11.012. [DOI] [PubMed] [Google Scholar]
- 5.Norton W., Bally-Cuif L. Adult zebrafish as a model organism for behavioural genetics. BMC Neurosci. 2010;11:90. doi: 10.1186/1471-2202-11-90. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Gerlai R. Zebrafish antipredatory responses: a future for translational research? Behav Brain Res. 2010;207:223–231. doi: 10.1016/j.bbr.2009.10.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mathur P., Guo S. Use of zebrafish as a model to understand mechanisms of addiction and complex neurobehavioral phenotypes. Neurobiol Dis. 2010;40:66–72. doi: 10.1016/j.nbd.2010.05.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Maximino C., de Brito TM., Silva Batista AW., Herculano AM., Morato S., Gouveia A, Jr. Measuring anxiety in zebrafish: a critical review. Behav Brain Res. 2010;214:157–171. doi: 10.1016/j.bbr.2010.05.031. [DOI] [PubMed] [Google Scholar]
- 9.Belzung C., Philippot P. Anxiety from a phylogenetic perspective: is there a qualitative difference between human and animal anxiety? Neural Plast. 2007;2007:59676. doi: 10.1155/2007/59676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Steimer T. The biology of fear - and anxiety - related behaviors. Dialogues Clin Neurosci. 2002;4:123–137. doi: 10.31887/DCNS.2002.4.3/tsteimer. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Koolhaas JM., Korte SM., De Boer SF., et al. Coping styles in animals: current status in behavior and stress-physiology. Neurosci Biobehav Rev. 1999;23:925–935. doi: 10.1016/s0149-7634(99)00026-3. [DOI] [PubMed] [Google Scholar]
- 12.Blanchard DC., Hynd AL., Minke KA., Monemoto T., Blanchard RJ. Human defensive behaviors to threat scenarios show parallels to fear - and anxiety - related defense patterns of non-human mammals. Neurosci Biobehav Rev. 2001;25:761–770. doi: 10.1016/s0149-7634(01)00056-2. [DOI] [PubMed] [Google Scholar]
- 13.Overli O., Sorensen C., Pulman KGT., et al. Evolutionary background for stress-coping styles: Relationships between physiological, behavioral, and cognitive traits in non-mammalian vertebrates. Neurosci Biobehav Rev. 2007;31:396–412. doi: 10.1016/j.neubiorev.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 14.Steimer T., Driscoll P. Inter-individual vs line/strain differences in psychogenetically selected Roman High-(RHA) and Low-(RLA) Avoidance rats: neuroendocrine and behavioural aspects. Neurosci Biobehav Rev. 2005;29:99–112. doi: 10.1016/j.neubiorev.2004.07.002. [DOI] [PubMed] [Google Scholar]
- 15.Driscoll P., Fernandez-Teruel A., Corda MG., Giorgi O., Steimer T. Some guidelines for defining personality differences in rats. In: Kim Y, ed. Handbook of Behavior Genetics. Berlin, Germany: Springer. 2009:281–300. [Google Scholar]
- 16.Steimer T., Driscoll P. Divergent stress responses and coping styles in psychogenetically selected Roman high-(RHA) and low-(RLA) avoidance rats: behavioural, neuroendocrine and developmental aspects. Stress. 2003;6:87–100. doi: 10.1080/1025389031000111320. [DOI] [PubMed] [Google Scholar]
- 17.Coppens CM., De Boer SF., Koolhaas JM. Coping styles and behavioural flexibility: towards underlying mechanisms. Philos Trans R Soc Lond B Biol Sci. 2010;365:4021–4028. doi: 10.1098/rstb.2010.0217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Henry JP., Stephens PM. Health and the Social Environment: a Sociobioiogical Approach To Medicine. Berlin, Germany: Springer. 1997 [Google Scholar]
- 19.Engel GL., Schmale AH. Conservation Withdrawal: a Primary Regulatory Process for Organic Homeostasis. Physiology, Emotions and Psychosomatic Illness. New York; NY: Elsevier. 1972:57–95. doi: 10.1002/9780470719916.ch5. [DOI] [PubMed] [Google Scholar]
- 20.Cannon WB. Bodily Changes in Pain, Hunger, Fear and Rage. New York, NY: Appleton. 1915 [Google Scholar]
- 21.Henry JP., Wang S. Effects of early stress on adult affiliative behavior. Psychoneuroendocrinology. 1998;23:863–875. doi: 10.1016/s0306-4530(98)00058-4. [DOI] [PubMed] [Google Scholar]
- 22.Taylor PJ., Gooding P., Wood AM., Tarrier N. The role of defeat and entrapment in depression, anxiety, and suicide. Psychol Bull. 2011;137:391–420. doi: 10.1037/a0022935. [DOI] [PubMed] [Google Scholar]
- 23.Dixon AK. Ethological strategies for defence in animals and humans: their role in some psychiatric disorders. Br J Med Psychol. 1998;71:417–445. doi: 10.1111/j.2044-8341.1998.tb01001.x. [DOI] [PubMed] [Google Scholar]
- 24.Hori N., Yuyama N., Tamura K. Biting suppresses stress-induced expression of corticotropin-releasing factor (CRF) in the rat hypothalamus. J Dent Res. 2004;83:124–128. doi: 10.1177/154405910408300208. [DOI] [PubMed] [Google Scholar]
- 25.Overli O., Korzan WJ., Larson ET., et al. Behavioral and neuroendocrine correlates of displaced aggression in trout. Horm Behav. 2004;45:324–329. doi: 10.1016/j.yhbeh.2004.01.001. [DOI] [PubMed] [Google Scholar]
- 26.Ledoux JE., Gorman JM. A call to action: overcoming anxiety through active coping. Am J Psychiatry. 2001;158:1953–1955. doi: 10.1176/appi.ajp.158.12.1953. [DOI] [PubMed] [Google Scholar]
- 27.Gray JA. The structure of the emotions and the limbic system. Physiology, Emotions and Psychosomatic Illness. Amsterdam, the Netherlands: Elsevier; 1972:87–129. [Google Scholar]
- 28.Gray JA. The Neuropsychology of Anxiety An Enquiry into yhe Functions of the Septo-Hippocampal System. Oxford, UK: Clarendon Press. 1987 [Google Scholar]
- 29.Bijttebier P., Beck I., Claes L., Vandereycken W. Gray's Reinforcement Sensitivity Theory as a framework for research on personality-psychopathology associations. Clin Psychol Rev. 2009;29:421–430. doi: 10.1016/j.cpr.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 30.Corr PJ., Perkins AM. The role of theory in the psychophysiology of personality: from Ivan Pavlov to Jeffrey Gray. Int J Psychophysiol. 2006;62:367–376. doi: 10.1016/j.ijpsycho.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 31.Laborit H. Inhibition of action: Interdisciplinary approach to its mechanisms and physiopathology. In: Traue HC, Pennebaker JW, eds. Emotion, Inhibition and Health. Seattle, WA: Hogrefe & Huber Publishers. 1993: 57–79. [Google Scholar]
- 32.Selye H. The Stress of Life. 2nd, revised paperback ed. New-York: McGraw Hill. 1984 [Google Scholar]
- 33.Panksepp J. Affective Neuroscience. The Foundations of Human and Animal Emotions. 1st ed. New York, NY; Oxford, UK: Oxford University Press. 1998 [Google Scholar]
- 34.Panksepp J. The psychoneurology of fear:evolutionary perspectives and the role of animal models in understanding human anxiety. In: Burrows GD, Roth M, Noyes Jr R, eds. Handbook of Anxiety Vol. 3, The Neurobiology of Anxiety. Amsterdam, the Netherlands: Elsevier Science Publishers BV. 1990:3–58. [Google Scholar]
- 35.Maren S. Neurobiology of Pavlovian fear conditioning. Annu Rev Neurosci. 2001;24:897–931. doi: 10.1146/annurev.neuro.24.1.897. [DOI] [PubMed] [Google Scholar]
- 36.Le Doux J. Fear and the brain: where have we been, and where are we going. Biol Psychiatry. 1998;44:1229–1238. doi: 10.1016/s0006-3223(98)00282-0. [DOI] [PubMed] [Google Scholar]
- 37.Morgan MA., Romanski LM., Ledoux JE. Extinction of emotional learning: contribution of medial prefrontal cortex. Neurosci Lett. 1993;163:109–113. doi: 10.1016/0304-3940(93)90241-c. [DOI] [PubMed] [Google Scholar]
- 38.Cohen JA., Price EO. Grooming in the Norway rat: displacement activity or “boundary-shift”? Behav Neural Biol. 1979;26:177–188. doi: 10.1016/s0163-1047(79)92563-9. [DOI] [PubMed] [Google Scholar]
- 39.LeDoux J. The amygdala and emotion: a view through fear. In: Aggleton JP, ed. The Amygdala. Oxford, UK: Oxford University Press. 2000:289–310. [Google Scholar]
- 40.LeDoux J. The Emotional Brain. New York, NY: Simon & Schuster. 1998 [Google Scholar]
- 41.Dudley KJ., Li X., Kobor MS., Kippin TE., Bredy TW. Epigenetic mechanisms mediating vulnerability and resilience to psychiatric disorders. Neurosci Biobehav Rev. 2011;35:1544–1551. doi: 10.1016/j.neubiorev.2010.12.016. [DOI] [PubMed] [Google Scholar]
- 42.Beuzen A., Belzung C. Link between emotional memory and anxiety states: a study by principal component analysis. Physiol Behav. 1995;58:111–118. doi: 10.1016/0031-9384(95)00013-9. [DOI] [PubMed] [Google Scholar]
- 43.Lister RG. Ethologically-based animal models of anxiety disorders. Pharmacol Ther. 1990;46:321–340. doi: 10.1016/0163-7258(90)90021-s. [DOI] [PubMed] [Google Scholar]
- 44.Schmidt MV. Animal models for depression and the mismatch hypothesis of disease. Psychoneuroendocrinology. 2011;36:330–338. doi: 10.1016/j.psyneuen.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 45.Weinstock M. The long-term behavioural consequences of prenatal stress. Neurosci Biobehav Rev. 2008;32:1073–1086. doi: 10.1016/j.neubiorev.2008.03.002. [DOI] [PubMed] [Google Scholar]
- 46.Champagne FA., Francis DD., Mar A., Meaney MJ. Variations in maternal care in the rat as a mediating influence for the effects of environment on development. Physiol Behav. 2003;79:359–371. doi: 10.1016/s0031-9384(03)00149-5. [DOI] [PubMed] [Google Scholar]
- 47.Macri S., Zoratto F., Laviola G. Early-stress regulates resilience, vulnerability and experimental validity in laboratory rodents through mother-offspring hormonal transfer. Neurosci Biobehav Rev. 2011;35:1534–1543. doi: 10.1016/j.neubiorev.2010.12.014. [DOI] [PubMed] [Google Scholar]
- 48.Dingemanse NJ., Kazern AJ., Reale D., Wright J. Behavioural reaction norms: animal personality meets individual plasticity. Trends Ecol Evol. 2010;25:81–89. doi: 10.1016/j.tree.2009.07.013. [DOI] [PubMed] [Google Scholar]
- 49.West-Eberhard MJ. Developmental plasticity and the origin of species differences. Proc Natl Acad Sci U S A. 2005;102(suppl 1):6543–6549. doi: 10.1073/pnas.0501844102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Beery AK., Francis DD. Adaptive significance of natural variations in maternal care in rats: a translational perspective. Neurosci Biobehav Rev. 2011;35:1552–1561. doi: 10.1016/j.neubiorev.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Belsky J., Jonassaint C., Pluess M., Stanton M., Brummett B., Williams R. Vulnerability genes or plasticity genes? Mol Psychiatry. 2009;14:746–754. doi: 10.1038/mp.2009.44. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Stedman's Medical Dictionary. 28th ed. Philadelphia, PA: Lippincott Williams &Wilkins. 2006 [Google Scholar]
- 53.McKinney WT. Basis of development of animal models in psychiatry: an overview. In: Koob GF, Ehlers CL, Kupfer DJ, eds. Animal Models of Depression. Boston, MA: Birkhàuser. 1989:3–17. [Google Scholar]
- 54.Rodgers RJ. Animal models of 'anxiety': where next? Behav Pharmacol. 1997;8:477–496. doi: 10.1097/00008877-199711000-00003. [DOI] [PubMed] [Google Scholar]
- 55.Hart PC., Bergner CL., Smolinsky AL., et al. Experimental models of anxiety for drug discovery and brain research. In: Proetzel M, Wiles MV, eds. Mouse. Models for Drug Discovery. Clifton, NJ: Humana Press. 2010:299–321. doi: 10.1007/978-1-60761-058-8_18. [DOI] [PubMed] [Google Scholar]
- 56.Sanger DJ. Animal models of anxiety and the screening and development of novel anxiolytic drugs. In: Boulton A, Baker G, Martin-lverson M, eds. Animal Models in Psychiatry, II. Clifton, NJ: Humana Press. 1991:147–198. [Google Scholar]
- 57.Sahgal A. Behavioural Neuroscience. A Practical Approach. Oxford, UK: Oxford University Press. 1993 [Google Scholar]
- 58.Claassen V. Neglected Factors in Pharmacology and Neuroscience Research. Amsterdam, the Netherlands: Elsevier. 1994 [Google Scholar]
- 59.van Gaalen MM., Steckler T. Behavioural analysis of four mouse strains in an anxiety test battery. Behav Brain Res. 2000;115:95–106. doi: 10.1016/s0166-4328(00)00240-0. [DOI] [PubMed] [Google Scholar]
- 60.Aguilar C., Gil L., Flint J., et al. Learned fear, emotional reactivity and fear of heights: a factor analytic map from a large F2 intercross of Roman rat strains. Brain Res Bull. 2002;57:17–26. doi: 10.1016/s0361-9230(01)00632-3. [DOI] [PubMed] [Google Scholar]
- 61.Cruz APM., Frei F., Graeff FG. Ethopharmacological analysis of rat behavior on the elevated plus-maze. Pharmacol Biochem Behav. 1994;49:171–176. doi: 10.1016/0091-3057(94)90472-3. [DOI] [PubMed] [Google Scholar]
- 62.Calatayud F., Belzung C., Aubert A. Ethological validation and the assessment of anxiety-like behaviours: methodological comparison of classical analyses and structural approaches. Behav Processes. 2004;67:195–206. doi: 10.1016/j.beproc.2004.04.002. [DOI] [PubMed] [Google Scholar]
- 63.Roman E., Meyerson BJ., Hyytia P., Nylander I. The multivariate concentric square field test reveals different behavioural profiles in male AA and ANA rats with regard to risk taking and environmental reactivity. Behav Brain Res. 2007;183:195–205. doi: 10.1016/j.bbr.2007.06.009. [DOI] [PubMed] [Google Scholar]
- 64.Tecott LH., Nestler EJ. Neurobehavioral assessment in the information age. Nat Neurosci. 2004;7:462–466. doi: 10.1038/nn1225. [DOI] [PubMed] [Google Scholar]
- 65.Willner P. Validation criteria for animal models of human mental disorders: learned helplessness as a paradigm case. Prog Neuropsychopharmacol Biol Psychiatry. 1986;10:677–690. doi: 10.1016/0278-5846(86)90051-5. [DOI] [PubMed] [Google Scholar]
- 66.Willner P. Methods for assessing the validity of animal models of human psychopathology. In: Boulton AA, Baker GB, Martin-lverson MT, eds. Animal Models in Psychiatry I. Clifton, NJ: Humana Press. 1991:1–23. [Google Scholar]
- 67.Kessler RC., Berglund P., Demle O., Jin R., Merikangas KR., Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Arch Gen Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- 68.Alonso J., Lepine JP. Overview of key data from the European Study of the Epidemiology of Mental Disorders (ESEMeD). J Clin Psychiatry. 2007;68(suppl 2):3–9. [PubMed] [Google Scholar]
- 69.Cohen H., Zohar J., Matar MA., Zeev K., Loewenthal U., Richter-Levin G. Setting apart the affected: the use of behavioral criteria in animal models of post traumatic stress disorder. Neuropsychopharmacoiogy. 2004;29:1962–1970. doi: 10.1038/sj.npp.1300523. [DOI] [PubMed] [Google Scholar]
- 70.Diagnostic and Statistical Manual of Mental Disorders. 4th ed. Washington, DC: American Psychiatric Association. 1994 [Google Scholar]
- 71.World Health Organization. The ICD-10 Classification of Mental and Behavioral Disorders. Clinical descriptions and diagnostic guidelines. Geneva, Switzerland: World Health Organization. 1992 [Google Scholar]
- 72.Jacobson LH., Cryan JF. Genetic approaches to modeling anxiety in animals. In: Stein MB, Steckler T, eds. Behavioral Neurobiology of Anxiety and Its Treatment. Berlin, Heidelberg, Germany: Springer-Verlag. 2009:161–201. [Google Scholar]
- 73.Gottesman II., Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiatry. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 74.Flint J., Munafo MR. The endophenotype concept in psychiatric genetics. Psychol Med. 2007;37:163–180. doi: 10.1017/S0033291706008750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.John B., Lewis KR. Chromosome variability and geographic distribution in insects. Science. 1966; 152:711–721. doi: 10.1126/science.152.3723.711. [DOI] [PubMed] [Google Scholar]
- 76.Goldsmith HH., Lemery KS. Linking temperamental tearfulness and anxiety symptoms: a behavior-genetic perspective. Biol Psychiatry. 2000;48:1199–1209. doi: 10.1016/s0006-3223(00)01003-9. [DOI] [PubMed] [Google Scholar]
- 77.Smoller JW., Tsuang MT. Panic and phobic anxiety: defining phenotypes for genetic studies. Am J Psychiatry. 1998;155:1152–1162. doi: 10.1176/ajp.155.9.1152. [DOI] [PubMed] [Google Scholar]
- 78.Battaglia M., Ogliari A. Anxiety and panic: from human studies to animal research and back. Neurosci Biobehav Rev. 2005;29:169–179. doi: 10.1016/j.neubiorev.2004.06.013. [DOI] [PubMed] [Google Scholar]
- 79.Bouwknecht JA., Olivier B., Paylor RE. The stress-induced hyperthermia paradigm as a physiological animal model for anxiety: a review of pharmacological and genetic studies in the mouse. Neurosci Biobehav Rev. 2007;31:41–59. doi: 10.1016/j.neubiorev.2006.02.002. [DOI] [PubMed] [Google Scholar]
- 80.Grillon C. Startle reactivity and anxiety disorders: aversive conditioning, context, and neurobiology. Biol Psychiatry. 2002;52:958–975. doi: 10.1016/s0006-3223(02)01665-7. [DOI] [PubMed] [Google Scholar]
- 81.Nyman E., Miettunen J., Freimer N., et al. Impact of temperament on depression and anxiety symptoms and depressive disorder in a populationbased birth cohort. J Affect Disord. 2011;131:393–397. doi: 10.1016/j.jad.2010.12.008. [DOI] [PubMed] [Google Scholar]
- 82.Landgraf R., Wigger A. Born to be anxious: neuroendocrine and genetic correlates of trait anxiety in HAB rats. Stress. 2003;6:111–119. doi: 10.1080/1025389031000104193. [DOI] [PubMed] [Google Scholar]
- 83.Brush FR. The Syracuse strains, selectively bred for differences in active avoidance learning, may be models of genetic differences in trait and state anxiety. Stress. 2003;6:77–85. doi: 10.1080/1025389031000104670. [DOI] [PubMed] [Google Scholar]
- 84.Blizard DA., Adams N. The Maudsley Reactive and Nonreactive strains: a new perspective. Behav Genet. 2002;32:277–299. doi: 10.1023/a:1020206120248. [DOI] [PubMed] [Google Scholar]
- 85.Kitaoka A., Fujita O. Behavioral comparisons of the Tsukuba emotional strains of rats (Rattus norvegicus) in three types of novel situations. Behav Genet. 1991;21:317–325. doi: 10.1007/BF01065823. [DOI] [PubMed] [Google Scholar]
- 86.Ramos A., Correia EC., Izidio GS., Bruske GR. Genetic selection of two new rat lines displaying different levels of anxiety-related behaviors. Behav Genet. 2003;33:657–668. doi: 10.1023/a:1026131130686. [DOI] [PubMed] [Google Scholar]
- 87.Brunelli SA. Selective breeding for an infant phenotype: rat pup ultrasonic vocalization (USV). Behav Genet. 2005;35:53–65. doi: 10.1007/s10519-004-0855-6. [DOI] [PubMed] [Google Scholar]
- 88.Driscoll P., Bâttig K. Behavioral, emotional and neurochemical profiles of rats selected for extreme differences in active, two-way avoidance. In: Lieblich I, ed. Genetics of the Brain. Amsterdam, the Netherlands: Elsevier. 1982:95–123. [Google Scholar]
- 89.Fernandez-Teruel A., Escorihuela RM., et al. A quantitative trait locus influencing anxiety in the laboratory rat. Genome. Res. 2002;12:618–626. doi: 10.1101/gr.203402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Lopez-Aumatell R., Vicens-Costa E., Guitart-Masip M., et al. Unlearned anxiety predicts learned fear: a comparison among heterogeneous rats and the Roman rat strains. Behav Brain Res. 2009;202:92–101. doi: 10.1016/j.bbr.2009.03.024. [DOI] [PubMed] [Google Scholar]
- 91.Escorihuela RM., Fernandez-Teruel A., Gil L., Aguilar R., Tobena A., Driscoll P. Inbred roman high- and low - avoidance rats: differences in anxiety, novelty-seeking, and shuttlebox behaviors. Physiol Behav. 1999;67:19–26. doi: 10.1016/s0031-9384(99)00064-5. [DOI] [PubMed] [Google Scholar]
- 92.Singewald N. Altered brain activity processing in high-anxiety rodents revealed by challenge paradigms and functional mapping. Neurosci Biobehav Rev. 2007;31:18–40. doi: 10.1016/j.neubiorev.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 93.Revest JM., Dupret D., Koehl M., et al. Adult hippocampal neurogenesis is involved in anxiety-related behaviors. Mol Psychiatry. 2009;14:959–967. doi: 10.1038/mp.2009.15. [DOI] [PubMed] [Google Scholar]
- 94.Shekhar A., McCann UD., Meaney MJ., et al. Summary of a National Institute of Mental Health workshop: developing animal models of anxiety disorders. Psychopharmacology (Berl). 2001;157:327–339. doi: 10.1007/s002130100859. [DOI] [PubMed] [Google Scholar]
- 95.Crawley JN. What Is Wrong V/ith My Mouse? Behavioral Phenotyping of Transgenic and Knockout mice. New York, NY: Wiley-Liss. 2000 [Google Scholar]
- 96.Belzung C. Measuring rodent exploratory behavior. In: Crusio WE, Gerlai R, eds. Handbook of Molecular-Genetic Techniques for Brain and Behavior Research. Amsterdam, the Netherlands: Elsevier. 1999:738–749. [Google Scholar]
- 97.Prut L., Belzung C. The open field as a paradigm to measure the effects of drugs on anxiety-like behaviors: a review. Eur J Pharmacol. 2003;463:3–33. doi: 10.1016/s0014-2999(03)01272-x. [DOI] [PubMed] [Google Scholar]
- 98.Geller I., Kulak JT. Jr., Seifter J. The effects of chlordiazepoxide and chlorpromazine on a punishment discrimination. Psychopharmacoiogia. 1962;3:374–385. doi: 10.1007/BF00408322. [DOI] [PubMed] [Google Scholar]
- 99.Pellow S., File SE. Anxiolytic and anxiogenic drug effects on exloratory activity in an elevated plus-maze: a novel test of anxiety in the rat. Pharmacol Biochem Behav. 1986;24:525–529. doi: 10.1016/0091-3057(86)90552-6. [DOI] [PubMed] [Google Scholar]
- 100.Rodgers RJ., Dalvi A. Anxiety, defence and the elevated plus-maze. Neurosci Biobehav Rev. 1997;21:801–81 0. doi: 10.1016/s0149-7634(96)00058-9. [DOI] [PubMed] [Google Scholar]
- 101.Vogel JR., Beer B., Clody DE. A simple and reliable conflict procedure for testing anti-anxiety agents. Psychopharmacoiogia. 1971;21:1–7. doi: 10.1007/BF00403989. [DOI] [PubMed] [Google Scholar]
- 102.Millan MJ., Brocco M. The Vogel conflict test: procedural aspects, [gamma]-aminobutyric acid, glutamate and monoamines. Eur J Pharmacol. 2003;463:67–96. doi: 10.1016/s0014-2999(03)01275-5. [DOI] [PubMed] [Google Scholar]
- 103.Viana MB., Tomaz C., Graeff FG. The elevated T-maze: a new animal model of anxiety and memory. Pharmacol Biochem Behav. 1994;49:549–554. doi: 10.1016/0091-3057(94)90067-1. [DOI] [PubMed] [Google Scholar]
- 104.Shepherd JK., Grewal SS., Fletcher A., Bill DJ., Dourish CT. Behavioural and pharmacological characterisation of the elevated “zero-maze” as an animal model of anxiety. Psychopharmacology (Berl). 1994;116:56–64. doi: 10.1007/BF02244871. [DOI] [PubMed] [Google Scholar]
- 105.File SE. Factors controlling measures of anxiety and responses to novelty in the mouse. Behav Brain Res. 2001;125:151–157. doi: 10.1016/s0166-4328(01)00292-3. [DOI] [PubMed] [Google Scholar]
- 106.Griebel G., Belzung C., Misslin R., Vogel E. The free-exploratory paradigm: an effective method for measuring neophobic behaviour in mice and testing potential neophobia-reducing drugs. Behav Pharmacol. 1993;4:637–644. [PubMed] [Google Scholar]
- 107.Steimer T., Driscoll P., Schulz P. Brain metabolism of progesterone, coping behaviour and emotional reactivity in male rats from two psychogenetically selected lines. J Neuroendocrinol. 1997;9:169–175. doi: 10.1046/j.1365-2826.1997.t01-1-00571.x. [DOI] [PubMed] [Google Scholar]
- 108.Bourin M., Hascoët M. The mouse light/dark box test. Eur J Pharmacol. 2003;463:55–65. doi: 10.1016/s0014-2999(03)01274-3. [DOI] [PubMed] [Google Scholar]
- 109.Sahgal A. Passive avoidance procedures. In: Sahgal A, ed. Behavioural Neuroscience. Vol. 1. Oxford, UK: IRL Press. 1993:49–56. [Google Scholar]
- 110.Fernandez-Teruel A., Escorihuela RM., Nunez JF., et al. The early acquisition of two-way (shuttle-box) avoidance as an anxiety-mediated behavior:psychopharmacological validation. Brain Res Bull. 1991;26:173–176. doi: 10.1016/0361-9230(91)90205-x. [DOI] [PubMed] [Google Scholar]
- 111.Graeff FG., Netto CF., Zangrossi H. Jr. The elevated T-maze as an experimental model of anxiety. Neurosci Biobehav Rev. 1998;23:237–246. doi: 10.1016/s0149-7634(98)00024-4. [DOI] [PubMed] [Google Scholar]
- 112.Carvalho-Netto EF., Nunes-de-Souza RL. Use of the elevated T-maze to study anxiety in mice. Behav Brain Res. 2004;148:119–132. doi: 10.1016/s0166-4328(03)00184-0. [DOI] [PubMed] [Google Scholar]
- 113.Gardner CR. Distress vocalization in rat pups. A simple screening method for anxiolytic drugs. J Pharmacol Methods. 1985;14:181–187. doi: 10.1016/0160-5402(85)90031-2. [DOI] [PubMed] [Google Scholar]
- 114.Sanchez C. Stress-induced vocalisation in adult animals. A valid model of anxiety? Eur J Pharmacol. 2003;463:133–143. doi: 10.1016/s0014-2999(03)01277-9. [DOI] [PubMed] [Google Scholar]
- 115.File SE. The use of social interaction as a method for detecting anxiolytic activity of chlordiazepoxide-like drugs. J Neurosci Methods. 1980;2:219–238. doi: 10.1016/0165-0270(80)90012-6. [DOI] [PubMed] [Google Scholar]
- 116.Korte SM., De Boer SF. A robust animal model of state anxiety: fearpotentiated behaviour in the elevated plus-maze. Eur J Pharmacol. 2003;463:163–175. doi: 10.1016/s0014-2999(03)01279-2. [DOI] [PubMed] [Google Scholar]
- 117.Treit D., Pinel JP., Fibiger HC. Conditioned defensive burying: a new paradigm for the study of anxiolytic agents. Pharmacol Biochem Behav. 1981;15:619–626. doi: 10.1016/0091-3057(81)90219-7. [DOI] [PubMed] [Google Scholar]
- 118.De Boer SF., Koolhaas JM. Defensive burying in rodents: ethology, neurobiology and psychopharmacology. Eur J Pharmacol. 2003;463:145–161. doi: 10.1016/s0014-2999(03)01278-0. [DOI] [PubMed] [Google Scholar]
- 119.Blanchard DC., Griebel G., Blanchard RJ. The Mouse Defense Test Battery: pharmacological and behavioral assays for anxiety and panic. Eur J Pharmacol. 2003;463:97–1 1 6. doi: 10.1016/s0014-2999(03)01276-7. [DOI] [PubMed] [Google Scholar]
- 120.Staples LG. Predator odor avoidance as a rodent model of anxiety: learning-mediated consequences beyond the initial exposure. Neurobiol Learn Mem. 2010;94:435–445. doi: 10.1016/j.nlm.2010.09.009. [DOI] [PubMed] [Google Scholar]
- 121.ritton DR., Britton KT. A sensitive open field measure of anxiolytic drug activity. Pharmacol Biochem Behav. 1981;15:577–582. doi: 10.1016/0091-3057(81)90212-4. [DOI] [PubMed] [Google Scholar]
- 122.Stout JC., Weiss JM. An animal model for measuring behavioral responses to anxiogenic and anxiolytic manipulations. Pharmacol Biochem Behav. 1994;47:459–465. doi: 10.1016/0091-3057(94)90143-0. [DOI] [PubMed] [Google Scholar]
- 123.Schwegler H., Pilz PKD., Koch M., Fendt M., Linke R., Driscoll P. The acoustic startle response in inbred Roman High- and Low-Avoidance rats. Behav Genet. 1997;27:579–581. doi: 10.1023/a:1021465217299. [DOI] [PubMed] [Google Scholar]
- 124.Steimer T. Psychogenetic selection in the Roman rat lines: Short and long freezing responses in a Sudden Noise Test (SNT) as an alternative to differences in shuttle box (SB) avoidance. Behav Genet. 2007;37:796–797. [Google Scholar]