Abstract
Background
Belinostat (PXD 101) is a novel inhibitor of class I and II histone deacetylases. This class of compounds has demonstrated anti-cancer activity in malignant mesothelioma. We conducted a phase II study of belinostat in patients with relapsed malignant pleural mesothelioma.
Methods
Patients with advanced mesothelioma, progression with one prior chemotherapy regimen and ECOG PS 0-2 were eligible. Belinostat was administered at 1000 mg/m2 intravenously over 30 minutes on days 1–5 of every 3 week cycle. The primary endpoint was response rate. The Simon two-stage design was utilized. Disease assessments were performed every 2 cycles.
Results
Thirteen patients were enrolled. Baseline characteristics were: median age of 73 years; ECOG performance status 0 (n=4), 1 (8) and 2 (1). A median of 2 cycles of therapy were administered. Disease stabilization was seen in 2 patients. No objective responses were noted and the study did not meet criteria to proceed to the second stage of accrual. Median survival was 5 months with a median progression-free survival of 1 month. Salient toxicities included nausea, emesis, fatigue and constipation. One patient died as a consequence of cardiac arrhythmia which was deemed ‘possibly’ related to therapy.
Conclusions
Belinostat is not active as monotherapy against recurrent malignant pleural mesothelioma. Evaluation of combination strategies or alternate dosing schedules may be necessary for further development of this novel agent in mesothelioma.
Keywords: Mesothelioma, Histone deacetylase inhibition, belinostat, PXD 101
Introduction
Approximately 2000 cases of malignant pleural mesothelioma (MPM) are diagnosed annually in the United States 1. It is a locally invasive tumor that is associated with a 5-year survival rate of less than 15% 2. Complete surgical resection is the only treatment modality that is associated with a curative potential. However, majority of the patients present with advanced stage disease and therefore are not candidates for aggressive curative surgical resection. The median survival for such patients is approximately 6 months with supportive care measures alone 3. For patients with unresectable disease, systemic chemotherapy is the treatment of choice. The combination of pemetrexed and cisplatin is the FDA-approved regimen for the treatment of patients with advanced MPM. The median survival of 13 months and a 1-year survival rate of 56% with the combination were superior when compared to monotherapy with cisplatin in a randomized phase III study 4. There is currently no approved therapy for patients who experience disease progression following therapy with pemetrexed-platinum combination.
Histone deacetylase (HDAC) inhibitors are a novel class of agents that have demonstrated promising anticancer activity against a variety of malignancies. Histones, the core nucleosomal proteins, exist in either a transcriptionally active acetylated form or an inactive deacetylated state. The dynamic equilibrium between the acetylated and non-acetylated states is mediated by histone acetyl transferase and histone deacetylase. HDAC inhibition results in histone acetylation, which leads to the expression of genes associated with cell cycle arrest and tumor suppression 5–7. In addition to inhibition of histone deacetylation, HDAC inhibitors also mediate anti-cancer effects by acetylation of a number of non-histone proteins. Vorinostat, a HDAC inhibitor, has been approved by the FDA for the treatment of refractory cutaneous T-cell lymphoma.
Early phase clinical trials with vorinostat have demonstrated promising anti-cancer activity in patients with malignant mesothelioma 8. This has prompted a large randomized study that compares the efficacy of vorinostat with best supportive care in mesothelioma patients who have progressed following prior standard chemotherapy. HDAC inhibitors have been documented to enhance the susceptibility of mesothelioma cell lines to tumor necrosis factor-related apoptosis-inducing ligand (TRAIL) 9. In addition, HDAC inhibitors block angiogenic signaling mediated by vascular endothelial growth factor (VEGF) as a result of their effect on acetylation of hypoxia inducing factor 1 alpha 10. Notably, patients with mesothelioma have higher levels of circulating VEGF compared to other solid tumors 11.
Belinostat is a novel, low molecular weight hydroxamic acid HDAC inhibitor. It exerts anti-cancer activity against a variety of human tumor cell lines 13–15. Preclinical studies have also documented a strong association between the anti-cancer effects of belinostat with increased histone acetylation 15. Based on these data, belinostat is now currently under evaluation for the treatment of a variety of solid organ and hematological malignancies. The recommended dose of belinostat for phase II studies is 1000 mg/m2 administered iv over 30 minutes for five consecutive days of each 21-day cycle 16. The dose-limiting toxicities are diarrhea and fatigue. In the phase I study of belinostat, disease stabilization was noted in a variety of solid tumors. We conducted this phase II study to evaluate the anti-cancer activity of belinostat in patients with malignant mesothelioma following progressive disease with standard chemotherapy.
Patients and methods
Patient eligibility
Patients with histologic confirmation of malignant pleural mesothelioma, age ≥ 18 years, Eastern Cooperative Oncology Group (ECOG) performance status (PS) of 0–2, and measurable disease were eligible. No more than 1 prior systemic chemotherapy regimen was allowed for study entry. Qualifying laboratory criteria were: leukocytes ≥3,000/μL; absolute neutrophil count (ANC) ≥1,500/μL; platelet count ≥100,000/μL; serum total bilirubin ≤ institutional upper limit of normal (ULN); serum transminases ≤ 2.5 X ULN; and serum creatinine ≤ ULN. Patients with serum creatinine levels ≥ ULN were eligible if their creatinine clearance was ≥ 50 mL/min/1.73 m2. Other pertinent exclusion criteria were: history of long QT syndrome; concomitant medications that might prolong QT interval; significant cardiac disease; need for active anti-arrhythmic therapy; use of valprioc acid, a known HDAC inhibitor and the presence of active intercurrent infections. Pregnant women were excluded, and subjects with reproductive potential were required to use contraception. At least 3 weeks should have elapsed since any prior chemotherapy or radiotherapy (6 weeks for nitrosoureas or mitomycin C) for study eligibility. All patients provided written informed consent before enrollment. The study was approved by the Institutional Review Board at each participating site.
Treatment plan
Belinostat was administered as a 30-minute iv infusion on days 1 to 5 of each 21-day treatment cycle. All treatments were given in the outpatient setting. No pre-medications were necessary on a routine basis. All qualifying laboratory criteria were to be met before initiation of each cycle of therapy. Toxicities related to therapy should have recovered to grade 1 or less before each new cycle. Treatment cycles could be delayed for a maximum to 2 weeks to allow for recovery from toxicity. An electrocardiogram was obtained at the end of infusion of belinostat on day 5 of each cycle to evaluate for changes in QTc interval.
Treatment cycles were continued for a maximum of 6 cycles and were discontinued earlier for disease progression, unacceptable toxicity, withdrawal of informed consent, or other intercurrent illness that altered the risk-benefit ratio of study therapy.
Toxicity was graded using the National Cancer Institute Common Terminology Criteria version 3.0. All patients who received at least one dose of the experimental agent were considered evaluable for toxicity. The dose of belinostat was modified for patients who experienced grade 3 or higher non-hematological toxicity in 25% decrements. Similar dose reduction was done for grade 4 hematological toxicities or grade 3 neutropenia associated with fever. For patients who experience grade 4 QTc prolongation, belinostat was to be discontinued permanently. All dose reductions were permanent and no more than 2 dose reductions were allowed for each patient. Those who required more than 2 dose reductions were to be removed from the study.
Patient evaluation
Pretreatment evaluations were: history and physical examination (H&P), assessment of PS, complete blood count (CBC), hepatic and renal function tests. An EKG was obtained at baseline. Women of reproductive age underwent a serum pregnancy test. Patients were evaluated weekly with CBC, hepatic and renal function tests during the first cycle. Serum chemistry tests and CBC were done on day 1 of each cycle from cycle 2 onwards. Radiological studies to assess response were performed after every 2 cycles of therapy. H & P and assessment of PS were done before initiation of each cycle. Responses were assessed with RECIST criteria 17.
Statistical considerations
The primary endpoint of the study was to determine the response rate associated with belinostat in patients with recurrent or refractory malignant pleural mesothelioma. Secondary endpoints included the assessment of safety profile, median progression-free survival and overall survival associated with belinostat monotherapy.
Since there are no proven treatment options for patients with mesothelioma in the second line setting, a response rate of ≥ 20% with belinostat in this study would warrant further evaluation, where as a response rate of < 5% would be considered uninteresting. The two stage optimum design by Simon was utilized 18. In the first stage, 12 patients were to be accrued. At least 1 objective response out of 12 was necessary to proceed to the full accrual of 37 total patients. Four (4) or more responses out of 37 patients would be considered evidence warranting further study of the regimen providing other factors, such as toxicity and survival, also appeared favorable. With this sample size, the probability of falsely declaring a regimen with a 5% response rate as warranting further study is 0.10 (alpha) and the probability of correctly declaring an agent with a 20% response rate as warranting further study is 0.90 (power). Patients who completed 1 cycle of therapy, terminated treatment for reasons of toxicity, or progressed prior to completion of 1 cycle of therapy, were included in the analysis of tumor response, and in any decision to terminate the study early. With the total sample size of 37 evaluable patients, the 95% confidence interval for the response rate had a half-width of ± 17 % or less.
Results
Baseline characteristics
A total of 13 patients were enrolled to the study between June 2006 and June 2007 (Table 1). The median age was 74 years (range 27–82). Five out of the 13 patients were females. All patients were of Caucasian ethnicity. The performance status at baseline was as follows: ECOG 0: 4 pts; 1 – 8 pts and 2 - 1 pt. One patient had prior radiotherapy to his chest and 12 patients had received prior chemotherapy. One treatment-naïve patient who refused chemotherapy was enrolled to the study as allowed by the protocol. Seven patients had the epithelial subtype of mesothelioma. Seven patients had received the combination of cisplatin and pemetrexed as front-line therapy and four patients had been treated with a pemetrexed-containing regimen without cisplatin. Two patients had achieved a partial response to first line therapy and four had stable disease as the best response. The remainder of the patients was refractory to frontline chemotherapy.
Table 1.
Patient baseline characteristics
| Patient Characteristic | N |
|---|---|
| Patients enrolled | 13 |
|
| |
| Male | 8 |
|
| |
| Median age | 73 (27–82) |
|
| |
| Performance status (ECOG) | |
| 0 | 4 |
| 1 | 8 |
| 2 | 1 |
|
| |
| Prior therapy | |
| Chemotherapy | 12 |
| Cisplatin-pemetrexed | 7 |
| Carboplatin-pemetrexed | 4 |
| Cisplatin-gemcitabine | 1 |
| Radiotherapy | 1 |
|
| |
| Histology | |
| Epithelial subtype | 7 |
| Sarcomatoid subtype | 1 |
| NOS | 5 |
Treatment delivery and toxicity
A median of 2 cycles of treatment were administered (range 1–6). Disease progression was the most common reason for discontinuation of treatment (n=7). One patient completed the protocol-specified maximum of 6 cycles of therapy. A 81 years old male patient with prior history of valvular heart disease was hospitalized for rapid atrial fibrillation after day # 2 of belinostat infusion during cycle 1. He was noted to have a non-Q wave myocardial infarction and required mechanical ventilation for heart failure. He subsequently developed ventilator-associated pneumonia and had a prolonged hospital course. The family opted to discontinue mechanical ventilation and the patient died shortly there after. One patient who received 4 cycles of therapy decided to discontinue therapy for non-toxicity-related personal reasons. The commonly noted toxicities related to therapy were anorexia, nausea, and fatigue (Table 2).
Table 2.
*. Toxicity
| Toxicity** | Grade 2 (n) | Grade 3 (n) | Grade 4 (n) |
|---|---|---|---|
| Hematological | |||
| Hemoglobin | 6 | ||
| Lymphopenia | 1 | ||
| Non-hematological | |||
| Albumin | 1 | ||
| Allergic reaction | 1 | ||
| Anorexia | 2 | ||
| AST | 1 | ||
| Constipation | 2 | ||
| Creatinine | 1 | ||
| Sweating | 1 | ||
| Dyspnea | 3 | 1 | 1 |
| Fatigue | 2 | ||
| Glucose (hyperglycemia) | 6 | ||
| Hypoxia | 1 | ||
| Hypotension | 1 | ||
| Infection | 2 | ||
| Nausea | 2 | ||
| Pain | 1 | ||
| Sodium (Hyponatremia) | 3 | ||
| Supraventricular arrhythmia NOS | 2 | 1 | |
Represents the number of patients with toxicity of grade > 1 experienced during the course of study. Only those adverse events those were definite, probable, possible or likely related to treatment are included in the table.
Toxicity was graded by NCI CTC version 3.0
Efficacy
All thirteen patients were evaluable for response assessment. Two patients had stable disease, but there were no partial responses. Based on the first stage response analysis, the study did not meet the criteria to continue to the next stage of accrual. The median progression-free survival was 1 month and the median overall survival was 5 months. Three patients only received one cycle of therapy and were removed from study due to symptomatic or radiological progression. Of the two patients with stable disease, one was refractory to prior chemotherapy and the other had stable disease as the best response.
Discussion
HDAC inhibitors are a new class of anti-cancer agents and are being evaluated for the treatment of a variety of malignancies. Malignant mesothelioma is a refractory neoplasm that does not have any proven treatment options for progressive disease following combination chemotherapy with cisplatin and pemetrexed. The outcome for these patients is poor and is therefore an unmet medical need. The promising single agent activity noted with vorinostat in this setting prompted our phase II study to evaluate the anti-cancer activity of belinostat, another novel HDAC inhibitor, for second line therapy of malignant mesothelioma. Our study failed to note objective tumor response in any of the 13 patients enrolled to the first stage of accrual and was therefore closed for lack of efficacy. Encouraging disease stabilization was noted in two patients who received therapy for 4 and 6 cycles respectively. Belinostat was tolerated well at the dose and schedule used for the study. Some of the common adverse events noted in our study such as anemia, hyperglycemia and hyponatremia have all been documented with the use of belinostat. Three patients had supraventricular tachycardia during the course of the study. While this toxicity has also been reported with the use of belinostat, other factors such as hypoxia, concomitant medications and prior cardiac disease could have also been the causal factors.
Since the time of initiation of our study, new data have become available with HDAC inhibitor monotherapy in various solid organ tumors. In a phase II study for patients with metastatic breast cancer, vorinostat was not associated with single agent activity (personal communication, Luu et al). Similarly, in a study for patients with advanced head and neck cancer, no confirmed responses were noted in a cohort of 12 patients who were treated with vorinostat as monotherapy 19. Vorinostat also failed to demonstrate single agent activity in 14 patients with advanced stage non-small cell lung cancer that had progressed following standard treatment options 20. Taken together, these results demonstrate that HDAC inhibitors are not active as monotherapy in the treatment of solid tumors.
Despite this, certain aspects of belinostat and our study patient characteristics warrant further mention as potential reasons behind the negative results of our study. Our study included a patient group with aggressive disease as evidenced by the fact that half the patients had progressive disease within 1 month of initiation of study treatment. The biological aggressiveness of the disease could have rendered this patient population to be resistant to any therapy. Furthermore, vorinostat and other HDAC inhibitors that are administered orally are being given on a continuous daily schedule as monotherapy. Belinostat is an intravenously administered agent and has been developed on an intermittent schedule (5 continuous days of therapy every 3 weeks). Pharmacodynamic modulation of the targets with belinostat has been documented to last for approximately 24 hours after exposure to the drug 15. Therefore, under the current schedule, the tumors are exposed to the drug for only a third of the duration of each treatment cycle. This could also have contributed to the lack of efficacy noted with belinostat in our study. An oral formulation of the agent is under development and will be conducive for continuous administration in future studies.
Though inactive as monotherapy, HDAC inhibitors have demonstrated promising activity when given in combination with cytotoxic or other targeted agents. In a phase I study, the combination of vorinostat with carboplatin and paclitaxel resulted in robust anti-cancer activity in patients with advanced NSCLC. An ongoing randomized phase II/III study will evaluate if the addition of vorinostat improves the survival of NSCLC patients treated with carboplatin and paclitaxel. Belinostat has also demonstrated promising activity in combination with carboplatin and paclitaxel in solid tumors. A randomized clinical trial is currently underway for patients with carcinoma of unknown primary to determine if the addition of belinostat to carboplatin and paclitaxel results in improved survival.
Our study included collection of archived tumor tissues to evaluate for potential biomarker evaluation. However, the planned exploratory studies were not conducted in light of the lack of single agent activity. Sequential pre- and post-treatment biopsies could have been helpful to establish if the experimental agent effected the changes on target protein as anticipated.
In summary, belinostat is not active as monotherapy when given on the present schedule for second line therapy of malignant pleural mesothelioma. Further studies should evaluate novel combinations of HDAC inhibitors with either cytotoxic agents or other molecularly targeted agents for the treatment of this disease.
Figure 1.
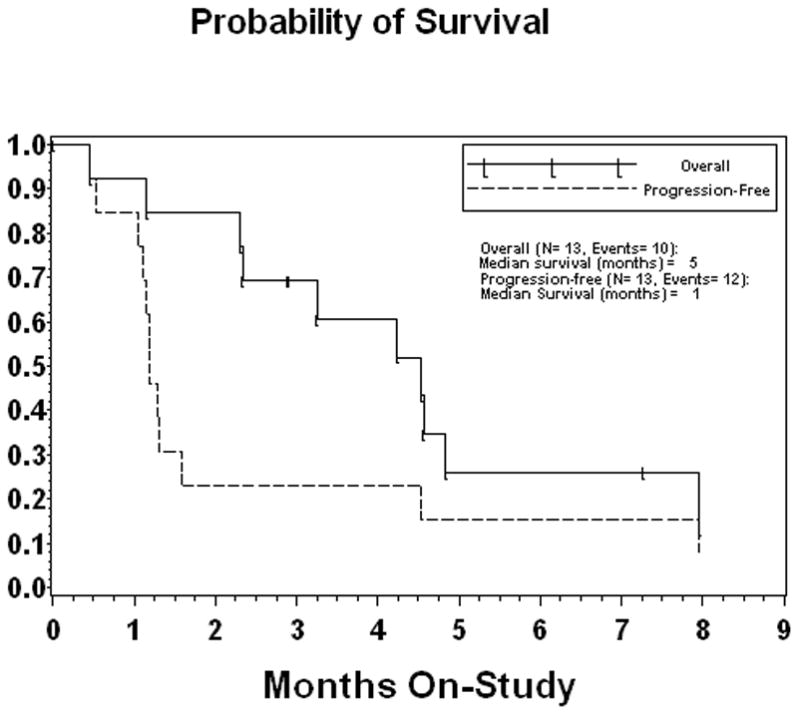
Kaplan Meier curve for overall and progression-free survival
Acknowledgments
Supported by NCI NO1 CM-62209
References
- 1.Connelly RR, Spirtas R, Myers MH, et al. Demographic patterns for mesothelioma in the United States. J Natl Cancer Inst. 1987;78:1053–60. [PubMed] [Google Scholar]
- 2.Zellos LS, Sugarbaker DJ. Multimodality treatment of diffuse malignant pleural mesothelioma. Semin Oncol. 2002;29:41–50. doi: 10.1053/sonc.2002.30230. [DOI] [PubMed] [Google Scholar]
- 3.Antman K, Shemin R, Ryan L, et al. Malignant mesothelioma: prognostic variables in a registry of 180 patients, the Dana-Farber Cancer Institute and Brigham and Women's Hospital experience over two decades, 1965–1985. J Clin Oncol. 1988;6:147–53. doi: 10.1200/JCO.1988.6.1.147. [DOI] [PubMed] [Google Scholar]
- 4.Vogelzang NJ, Rusthoven JJ, Symanowski J, et al. Phase III study of pemetrexed in combination with cisplatin versus cisplatin alone in patients with malignant pleural mesothelioma. J Clin Oncol. 2003;21:2636–44. doi: 10.1200/JCO.2003.11.136. [DOI] [PubMed] [Google Scholar]
- 5.Kelly WK, Richon V, Troso-Sandoval T. Suberoylanilide hydroxamic acid (SAHA), a Histone Deacetylase inhibitor: Biologic activity without toxicity. Proc Am Soc Clin Oncol. 2001;20:A344. [Google Scholar]
- 6.Richon VM, Emiliani S, Verdin E, et al. A class of hybrid polar inducers of transformed cell differentiation inhibits histone deacetylases. Proc Natl Acad Sci U S A. 1998;95:3003–7. doi: 10.1073/pnas.95.6.3003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Yoshida M, Kijima M, Akita M, et al. Potent and specific inhibition of mammalian histone deacetylase both in vivo and in vitro by trichostatin A. J Biol Chem. 1990;265:17174–9. [PubMed] [Google Scholar]
- 8.Krug LM, Curley T, Schwartz L, et al. Potential role of histone deacetylase inhibitors in mesothelioma: clinical experience with suberoylanilide hydroxamic acid. Clin Lung Cancer. 2006;7:257–61. doi: 10.3816/CLC.2006.n.003. [DOI] [PubMed] [Google Scholar]
- 9.Neuzil J, Swettenham E, Gellert N. Sensitization of mesothelioma to TRAIL apoptosis by inhibition of histone deacetylase: role of Bcl-xL down-regulation. Biochem Biophys Res Commun. 2004;314:186–91. doi: 10.1016/j.bbrc.2003.12.074. [DOI] [PubMed] [Google Scholar]
- 10.Deroanne CF, Bonjean K, Servotte S, et al. Histone deacetylases inhibitors as anti-angiogenic agents altering vascular endothelial growth factor signaling. Oncogene. 2002;21:427–36. doi: 10.1038/sj.onc.1205108. [DOI] [PubMed] [Google Scholar]
- 11.Linder C, Linder S, Munck-Wikland E, et al. Independent expression of serum vascular endothelial growth factor (VEGF) and basic fibroblast growth factor (bFGF) in patients with carcinoma and sarcoma. Anticancer Res. 1998;18:2063–8. [PubMed] [Google Scholar]
- 12.Glaser KB, Staver MJ, Waring JF, et al. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol Cancer Ther. 2003;2:151–63. [PubMed] [Google Scholar]
- 13.Buckley MT, Yoon J, Yee H, et al. The histone deacetylase inhibitor belinostat (PXD101) suppresses bladder cancer cell growth in vitro and in vivo. J Transl Med. 2007;5:49. doi: 10.1186/1479-5876-5-49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qian X, Ara G, Mills E, et al. Activity of the histone deacetylase inhibitor belinostat (PXD101) in preclinical models of prostate cancer. Int J Cancer. 2008;122:1400–10. doi: 10.1002/ijc.23243. [DOI] [PubMed] [Google Scholar]
- 15.Plumb JA, Finn PW, Williams RJ, et al. Pharmacodynamic response and inhibition of growth of human tumor xenografts by the novel histone deacetylase inhibitor PXD101. Mol Cancer Ther. 2003;2:721–8. [PubMed] [Google Scholar]
- 16.Steele NL, Plumb JA, Vidal L, et al. A phase 1 pharmacokinetic and pharmacodynamic study of the histone deacetylase inhibitor belinostat in patients with advanced solid tumors. Clin Cancer Res. 2008;14:804–10. doi: 10.1158/1078-0432.CCR-07-1786. [DOI] [PubMed] [Google Scholar]
- 17.Therasse P, Arbuck SG, Eisenhauer EA, et al. New guidelines to evaluate the response to treatment in solid tumors. European Organization for Research and Treatment of Cancer, National Cancer Institute of the United States, National Cancer Institute of Canada. J Natl Cancer Inst. 2000;92:205–16. doi: 10.1093/jnci/92.3.205. [DOI] [PubMed] [Google Scholar]
- 18.Simon R. Optimal two-stage designs for phase II clinical trials. Control Clin Trials. 1989;10:1–10. doi: 10.1016/0197-2456(89)90015-9. [DOI] [PubMed] [Google Scholar]
- 19.Blumenschein GR, Jr, Kies MS, Papadimitrakopoulou VA, et al. Phase II trial of the histone deacetylase inhibitor vorinostat (Zolinza, suberoylanilide hydroxamic acid, SAHA) in patients with recurrent and/or metastatic head and neck cancer. Invest New Drugs. 2008;26:81–7. doi: 10.1007/s10637-007-9075-2. [DOI] [PubMed] [Google Scholar]
- 20.Traynor AM, Dubey S, Eickhoff J, et al. A phase II study of vorinostat (NSC 701852 ) in patients (pts) with relapsed non-small cell lung cancer (NSCLC) J Clin Oncol. 2007;25 doi: 10.1097/jto.0b013e3181952478. Abs # 18044. [DOI] [PMC free article] [PubMed] [Google Scholar]


