Abstract
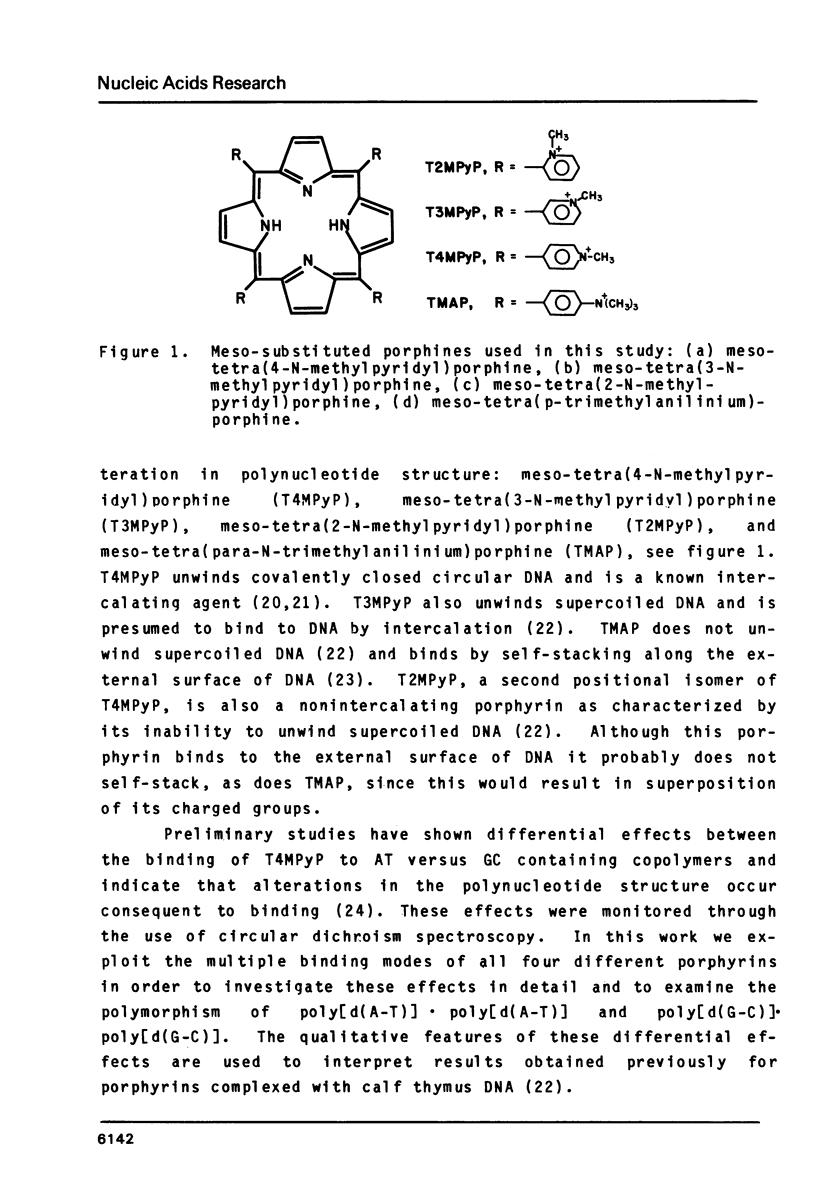
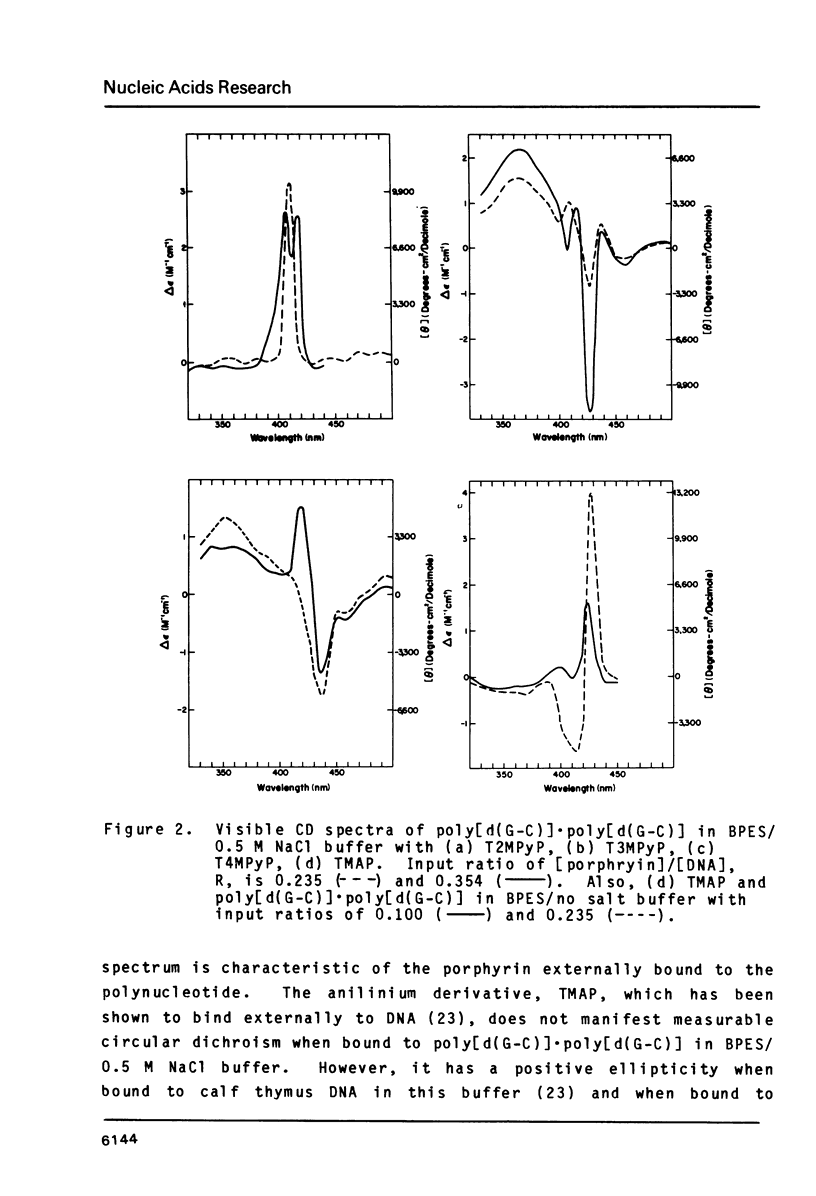
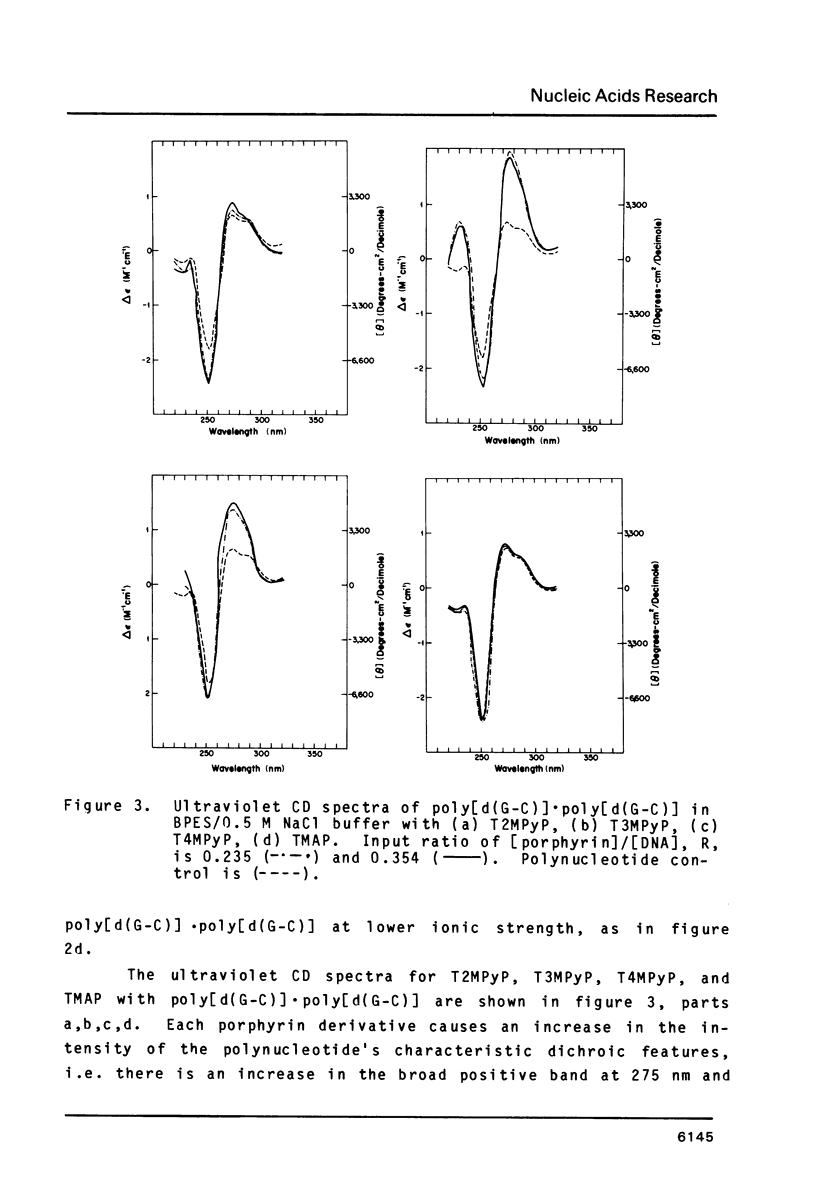
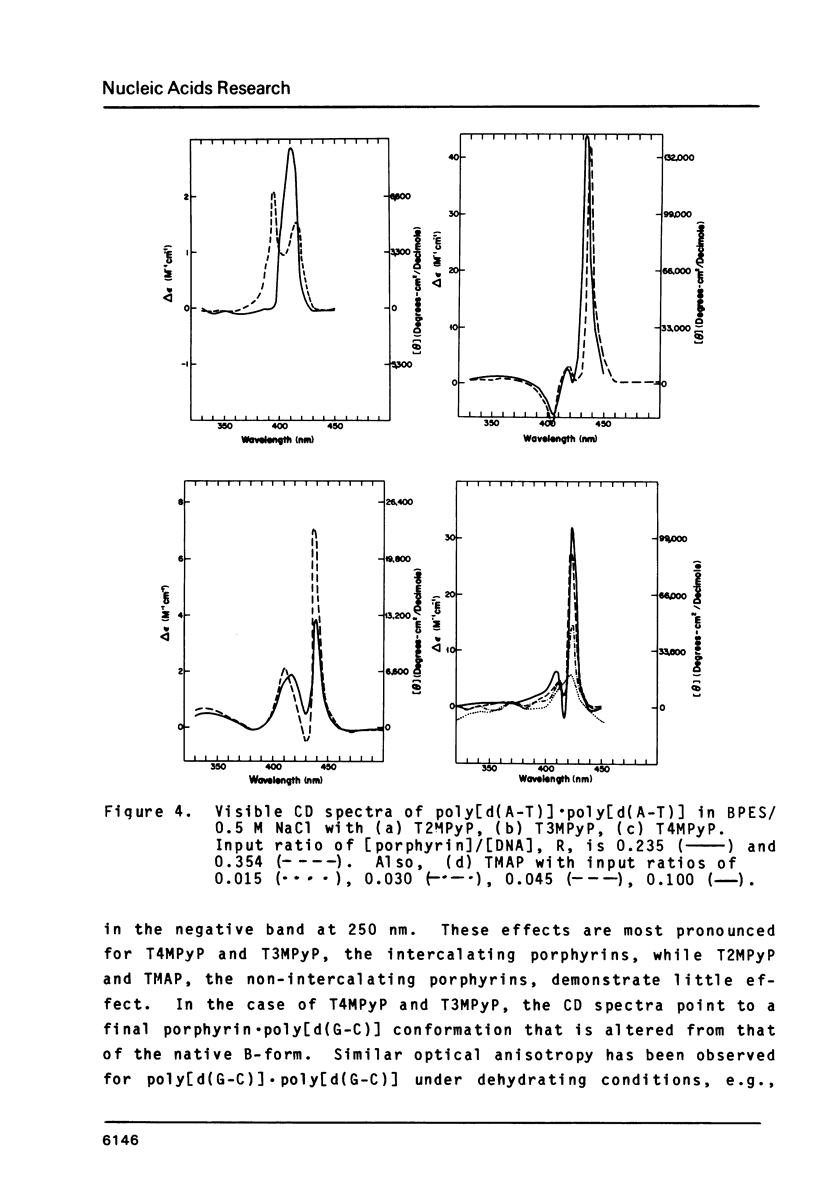
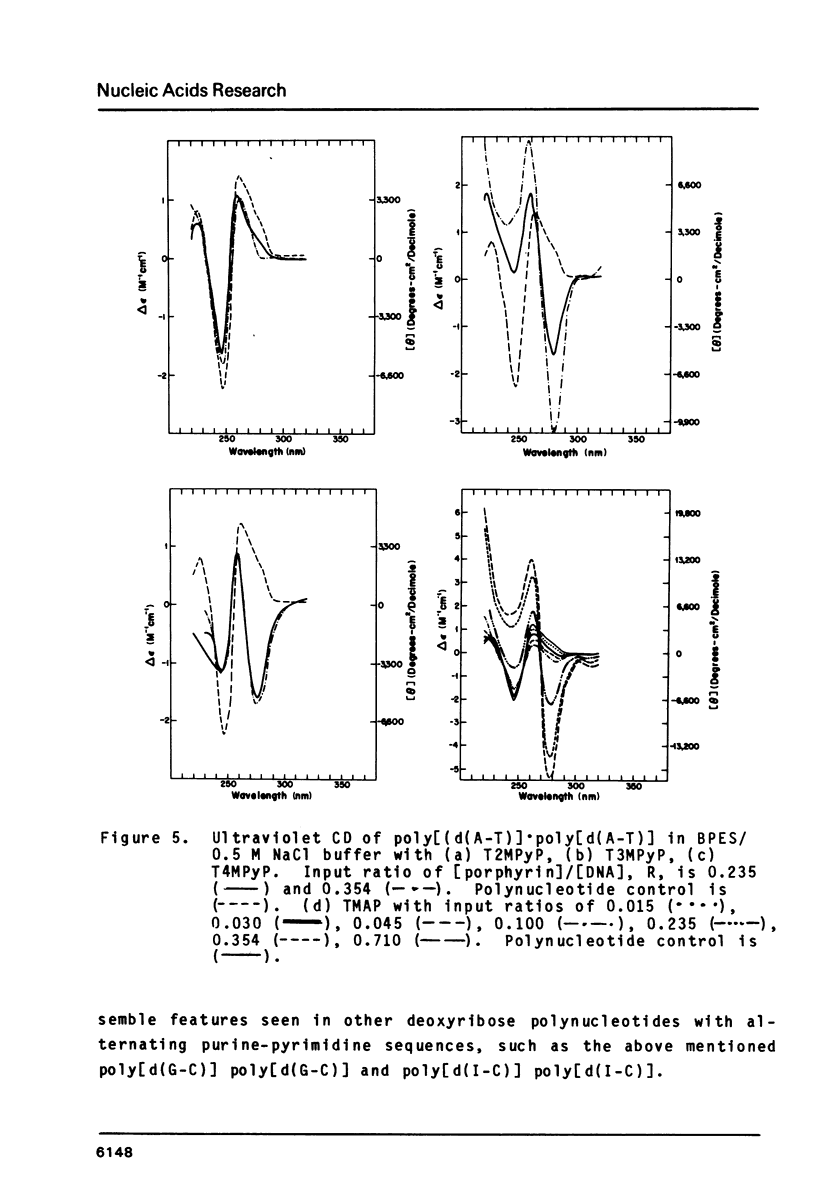
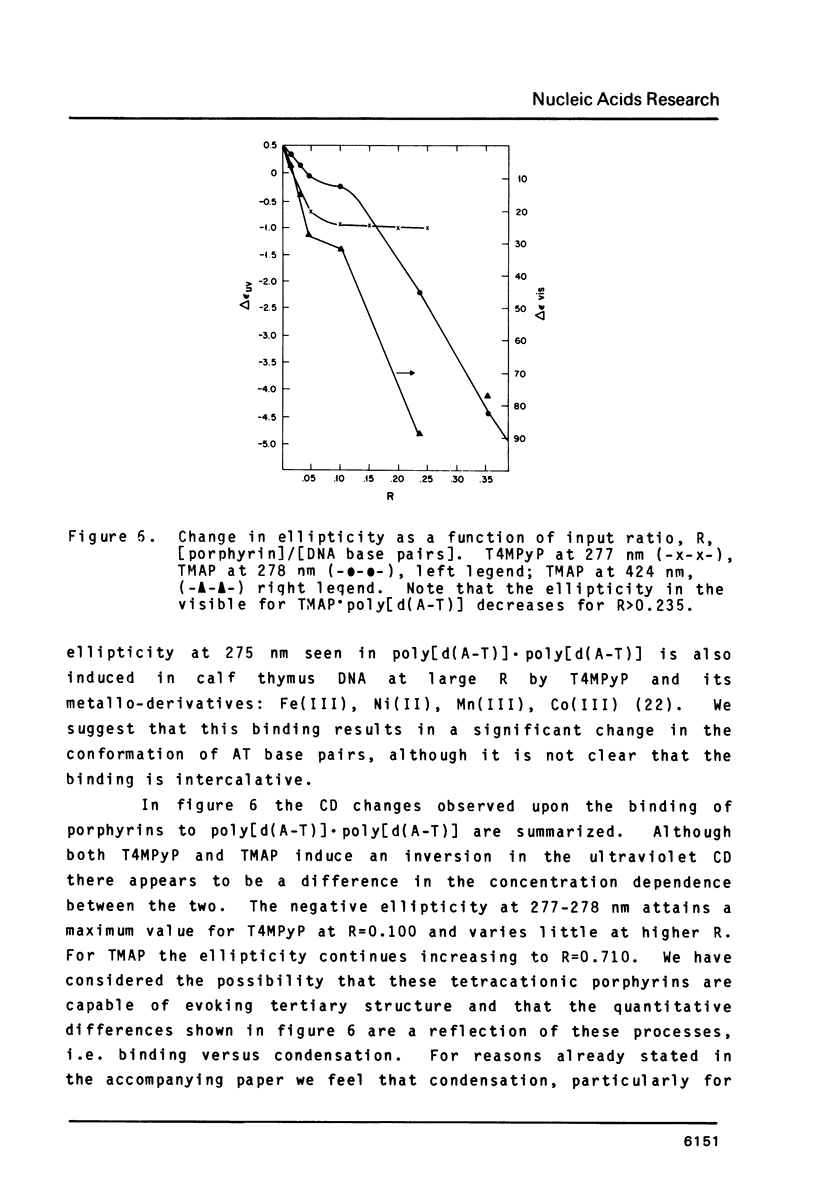
The interactions of two positional isomers and one analogue of meso-tetra (4-N-methylpyridyl) porphine, with the synthetic polynucleotides poly[d(A-T)] . poly[d(A-T)] and poly[d(G-C)] . poly[d(G-C)] have been investigated by circular dichroism. All four porphyrins were found to bind to the polynucleotides as shown by the induction of circular dichroism in their Soret bands. Furthermore, the sign of the induced ellipticity reflects selective occupation of binding sites by the porphyrin ligands. The conformational lability of poly[d(A-T)] X poly[d(A-T)] was found to be appreciable as micromolar amounts of meso-substituted 4-N-methylpyridyl, 3-N-methylpyridyl, and p-N-trimethylanilinium porphines induced a CD spectrum similar but not identical to that of DNA in the Z-form, i.e. a negative band at 280 nm and a positive band at 259 nm. The effect of porphyrin binding to poly[d(G-C)] X poly[d(G-C)] was less pronounced and dissimilar to that seen in the AT polymer.
Full text
PDF







Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Behe M., Felsenfeld G. Effects of methylation on a synthetic polynucleotide: the B--Z transition in poly(dG-m5dC).poly(dG-m5dC). Proc Natl Acad Sci U S A. 1981 Mar;78(3):1619–1623. doi: 10.1073/pnas.78.3.1619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carvlin M. J., Datta-Gupta N., Fiel R. J. Circular dichroism spectroscopy of a cationic porphyrin bound to DNA. Biochem Biophys Res Commun. 1982 Sep 16;108(1):66–73. doi: 10.1016/0006-291x(82)91832-0. [DOI] [PubMed] [Google Scholar]
- Chen H. H., Charney E., Rau D. C. Length changes in solution accompanying the B-Z transition of poly (dG-m5dC) induced by Co(NH3)63+. Nucleic Acids Res. 1982 Jun 11;10(11):3561–3571. doi: 10.1093/nar/10.11.3561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R., Conner B. N., Wing R. M., Fratini A. V., Kopka M. L. The anatomy of A-, B-, and Z-DNA. Science. 1982 Apr 30;216(4545):475–485. doi: 10.1126/science.7071593. [DOI] [PubMed] [Google Scholar]
- Dickerson R. E., Drew H. R. Structure of a B-DNA dodecamer. II. Influence of base sequence on helix structure. J Mol Biol. 1981 Jul 15;149(4):761–786. doi: 10.1016/0022-2836(81)90357-0. [DOI] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. A new model for DNA containing A.T and I.C base pairs. EMBO J. 1982;1(6):663–667. doi: 10.1002/j.1460-2075.1982.tb01227.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drew H. R., Dickerson R. E. Structure of a B-DNA dodecamer. III. Geometry of hydration. J Mol Biol. 1981 Sep 25;151(3):535–556. doi: 10.1016/0022-2836(81)90009-7. [DOI] [PubMed] [Google Scholar]
- Drew H., Takano T., Tanaka S., Itakura K., Dickerson R. E. High-salt d(CpGpCpG), a left-handed Z' DNA double helix. Nature. 1980 Aug 7;286(5773):567–573. doi: 10.1038/286567a0. [DOI] [PubMed] [Google Scholar]
- Early T. A., Kearns D. R., Hillen W., Wells R. D. A 300- and 600-MHz proton nuclear magnetic resonance investigation of a 12 base pair deoxyribonucleic acid restriction fragment: relaxation behavior of the low-field resonances in water. Biochemistry. 1981 Jun 23;20(13):3756–3764. doi: 10.1021/bi00516a014. [DOI] [PubMed] [Google Scholar]
- Early T. A., Kearns D. R., Hillen W., Wells R. D. A 300-MHz proton nuclear magnetic resonance investigation of deoxyribonucleic acid restriction fragments: dynamic properties. Biochemistry. 1981 Jun 23;20(13):3764–3769. doi: 10.1021/bi00516a015. [DOI] [PubMed] [Google Scholar]
- Fiel R. J., Howard J. C., Mark E. H., Datta Gupta N. Interaction of DNA with a porphyrin ligand: evidence for intercalation. Nucleic Acids Res. 1979 Jul 11;6(9):3093–3118. doi: 10.1093/nar/6.9.3093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiel R. J., Munson B. R. Binding of meso-tetra (4-N-methylpyridyl) porphine to DNA. Nucleic Acids Res. 1980 Jun 25;8(12):2835–2842. doi: 10.1093/nar/8.12.2835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanlon S., Brudno S., Wu T. T., Wolf B. Structural transitions of deoxyribonucleic acid in aqueous electrolyte solutions. I. Reference spectra of conformational limits. Biochemistry. 1975 Apr 22;14(8):1648–1660. doi: 10.1021/bi00679a017. [DOI] [PubMed] [Google Scholar]
- Ivanov V. I., Minyat E. E. The transitions between left- and right-handed forms of poly(dG-dC). Nucleic Acids Res. 1981 Sep 25;9(18):4783–4798. doi: 10.1093/nar/9.18.4783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamiya M. The induced circular dichroism of proflavine intercalated to DNA. Dye-polymer exciton interactions. Biochim Biophys Acta. 1979 Mar 28;562(1):70–79. doi: 10.1016/0005-2787(79)90127-8. [DOI] [PubMed] [Google Scholar]
- Kypr J., Vorlícková M., Budesinský M., Sklenár V. Strange double helix of poly (dA-dT) in high-salt solution. Biochem Biophys Res Commun. 1981 Apr 30;99(4):1257–1264. doi: 10.1016/0006-291x(81)90755-5. [DOI] [PubMed] [Google Scholar]
- Malenkov G., Minchenkova L., Minyat E., Schyolkina A., Ivanov V. The nature of the B-A transition of DNA in solution. FEBS Lett. 1975 Mar 1;51(1):38–42. doi: 10.1016/0014-5793(75)80850-7. [DOI] [PubMed] [Google Scholar]
- Malfoy B., Hartmann B., Leng M. The B goes to Z transition of poly(dG-dC) . poly(dG-dC) modified by some platinum derivatives. Nucleic Acids Res. 1981 Nov 11;9(21):5659–5669. doi: 10.1093/nar/9.21.5659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mercado C. M., Tomasz M. Circular dichroism of mitomycin-DNA complexes. Evidence for a conformational change in DNA. Biochemistry. 1977 May 3;16(9):2040–2046. doi: 10.1021/bi00628a044. [DOI] [PubMed] [Google Scholar]
- Mitsui Y., Langridge R., Shortle B. E., Cantor C. R., Grant R. C., Kodama M., Wells R. D. Physical and enzymatic studies on poly d(I-C)-poly d(I-C), an unusual double-helical DNA. Nature. 1970 Dec 19;228(5277):1166–1169. doi: 10.1038/2281166a0. [DOI] [PubMed] [Google Scholar]
- Nastasi M., Morris J. M., Rayner D. M., Seligy V. L., Szabo A. G., Williams D. F., Williams R. E., Yip R. W. Structural implications of the electronic spectra of quinacrine-deoxyribonucleic acid complexes in the ultraviolet region (250-300 nm). J Am Chem Soc. 1976 Jun 23;98(13):3979–3986. doi: 10.1021/ja00429a039. [DOI] [PubMed] [Google Scholar]
- Pohl F. M., Jovin T. M. Salt-induced co-operative conformational change of a synthetic DNA: equilibrium and kinetic studies with poly (dG-dC). J Mol Biol. 1972 Jun 28;67(3):375–396. doi: 10.1016/0022-2836(72)90457-3. [DOI] [PubMed] [Google Scholar]
- SPATZ H., BALDWIN R. L. STUDY OF THE FOLDING OF THE DAT COPOLYMER BY KINETIC MEASUREMENTS OF MELTING. J Mol Biol. 1965 Feb;11:213–222. doi: 10.1016/s0022-2836(65)80052-3. [DOI] [PubMed] [Google Scholar]
- Sage E., Leng M. Conformation of poly(dG-dC) . poly(dG-dC) modified by the carcinogens N-acetoxy-N-acetyl-2-aminofluorene and N-hydroxy-N-2-aminofluorene. Proc Natl Acad Sci U S A. 1980 Aug;77(8):4597–4601. doi: 10.1073/pnas.77.8.4597. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Santella R. M., Grunberger D., Nordheim A., Rich A. N-2-Acetylaminofluorene modification of poly(dG-m5dC) . poly(dG-m5dC) induces the Z-DNA conformation. Biochem Biophys Res Commun. 1982 Jun 30;106(4):1226–1232. doi: 10.1016/0006-291x(82)91243-8. [DOI] [PubMed] [Google Scholar]
- Santella R. M., Grunberger D., Weinstein I. B., Rich A. Induction of the Z conformation in poly(dG-dC).poly(dG-dC) by binding of N-2-acetylaminofluorene to guanine residues. Proc Natl Acad Sci U S A. 1981 Mar;78(3):1451–1455. doi: 10.1073/pnas.78.3.1451. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szabo A. G., Seligy V. L., Nastasi M., Yip R. W. The requirement of intact double helical structure for primary binding in quinacrine-nucleic acid complexes. Biochem Biophys Res Commun. 1975 Feb 17;62(4):830–836. doi: 10.1016/0006-291x(75)90397-6. [DOI] [PubMed] [Google Scholar]
- Ushay H. M., Santella R. M., Caradonna J. P., Grunberger D., Lippard S. J. Binding of [(dien)PtCl] Cl to poly(dG-dC)-poly(dG-dC) facilitates the B goes to Z conformational transition. Nucleic Acids Res. 1982 Jun 11;10(11):3573–3588. doi: 10.1093/nar/10.11.3573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorlícková M., Kypr J., Kleinwächter V., Palecek E. Salt-induced conformational changes of poly(dA-dT). Nucleic Acids Res. 1980 Sep 11;8(17):3965–3973. doi: 10.1093/nar/8.17.3965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorlícková M., Sedlácek P., Kypr J., Sponar J. Conformational transitions of poly(dA-dT)poly(dA-dT) in ethanolic solutions. Nucleic Acids Res. 1982 Nov 11;10(21):6969–6979. doi: 10.1093/nar/10.21.6969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorlíckovă M., Kypr J., Stokrová S., Sponar J. A Z-like form of poly(dA-dC).poly(dG-dT) in solution? Nucleic Acids Res. 1982 Feb 11;10(3):1071–1080. doi: 10.1093/nar/10.3.1071. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang A. H., Quigley G. J., Kolpak F. J., Crawford J. L., van Boom J. H., van der Marel G., Rich A. Molecular structure of a left-handed double helical DNA fragment at atomic resolution. Nature. 1979 Dec 13;282(5740):680–686. doi: 10.1038/282680a0. [DOI] [PubMed] [Google Scholar]
- Wells R. D., Miglietta J. J., Kłysik J., Larson J. E., Stirdivant S. M., Zacharias W. Spectroscopic studies on acetylaminofluorene-modified (dT-dG)n . (dC-dA)n suggest a left-handed conformation. J Biol Chem. 1982 Sep 10;257(17):10166–10171. [PubMed] [Google Scholar]
- Zimmer C., Tymen S., Marck C., Guschlbauer W. Conformational transitions of poly(dA-dC).poly(dG-dT) induced by high salt or in ethanolic solution. Nucleic Acids Res. 1982 Feb 11;10(3):1081–1091. doi: 10.1093/nar/10.3.1081. [DOI] [PMC free article] [PubMed] [Google Scholar]



