Abstract
Neurofibromatosis type 1 (NF1), the most common genetic disorder affecting the human nervous system, is characterized by the development of multiple benign Schwann cell tumors in skin and large peripheral nerves. These neoplasms, which are termed dermal and plexiform neurofibromas respectively, have distinct clinical courses; of particular note, plexiform, but not dermal, neurofibromas often undergo malignant progression to form malignant peripheral nerve sheath tumors (MPNSTs), the most common malignancy occurring in NF1 patients. In recent years, a number of genetically engineered mouse models have been created to investigate the molecular mechanisms driving the pathogenesis of these tumors. These models have been designed to address key questions including: 1) whether NF1 loss in the Schwann cell lineage is essential for tumorigenesis; 2) what cell type(s) in the Schwann cell lineage gives rise to dermal neurofibromas, plexiform neurofibromas and MPNSTs; 3) how the tumor microenvironment contributes to neoplasia; 4) what additional mutations contribute to neurofibroma-MPNST progression; 5) what role different neurofibromin-regulated Ras proteins play in this process and 6) how dysregulated growth factor signaling facilitates PNS tumorigenesis. In this review, we summarize the major findings from each of these models and their limitations as well as how discrepancies between these models may be reconciled. We also discuss how information gleaned from these models can be synthesized to into a comprehensive model of tumor formation in peripheral nervous system and consider several of the major questions that remain unanswered about this process.
Keywords: Neurofibromatosis, Schwann cell, tumor suppressor gene, tumor microenvironment, aberrant growth factor signaling
1. Introduction
Tumors of the peripheral nervous system (PNS)—neurofibromas, schwannomas and malignant peripheral nerve sheath tumors (MPNSTs)—cause considerable morbidity and mortality in afflicted individuals. This class of tumors is also common, representing 8.9% of the nervous system neoplasms resected in the United States between 2004 and 2006 [13]. While these tumors do occur sporadically, they are also often seen in association with the genetic disorders neurofibromatosis type I (NF1), neurofibromatosis type 2 (NF2), schwannomatosis, and Carney complex. Early transgenic modeling of these tumors thus focused on replicating the genetic defects seen in human patients with these disorders. This work provided insights into the role these mutated genes play in key signaling cascades, how they interact with other intratumoral abnormalities (e.g., aberrant growth factor signaling) and how their mutation enhances tumorigenesis via effects on the tumor microenvironment. These findings enabled the production of a second generation of genetically engineered murine (GEM) models that have further refined our understanding of tumorigenesis in the peripheral nervous system.
As the pathogenesis of NF1-related neoplasms (neurofibromas and MPNSTs) has been most extensively studied, we will focus on NF1-related GEM models in this review. We will first discuss the pathology of human NF1-related peripheral nerve sheath tumors, the genetic syndrome with which they are associated and our current understanding of the function(s) of the NF1 gene. We will then consider the mouse models that have been developed to investigate the mechanisms underlying NF1-related PNS tumorigenesis and the fundamental new insights that resulted from these models.
2. Pathology of Human Peripheral Nerve Sheath Tumors and Their Association with NF1
2.1 The Anatomy of Peripheral Nerve and Its Implications for the Pathogenesis of Peripheral Nerve Sheath Tumors
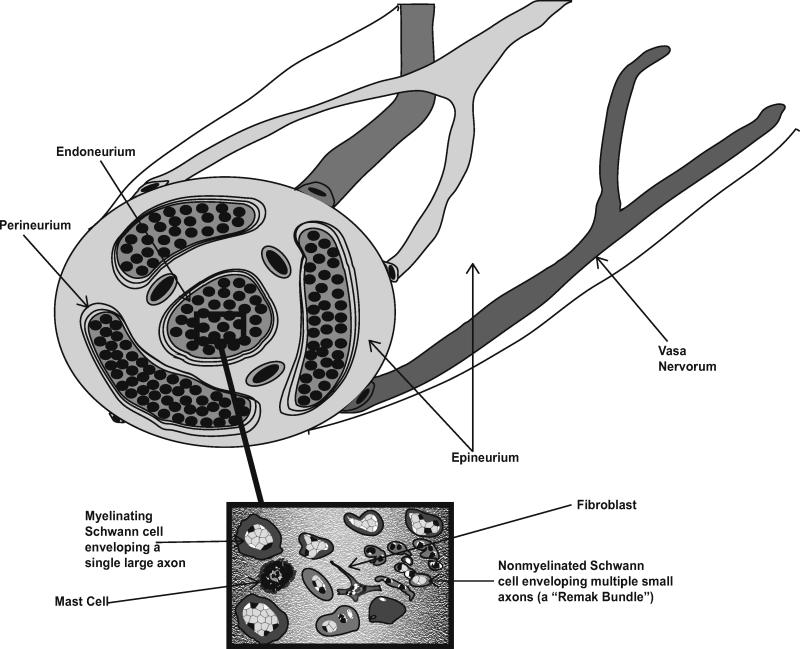
As peripheral nerve sheath tumors are derived from cells normally found in peripheral nerve, it is useful to first consider the composition and architecture of this complex tissue. The outermost layer of the nerve, the epineurium (Fig. 1), is composed of dense connective tissue and contains the highly anastamotic vascular supply of the nerve (the vasa nervorum). Within the epineurium, fascicles of nerve fibers are ensheathed by the perineurium, a dense concentric layer of specialized cells. Although perineurial cells were initially thought to be Schwann cell variants, it is now evident that these cells are not even of neural crest origin [36], being instead derived from the central nervous system (CNS) [44]. These perineurial cells, together with the neural vasculature, form a diffusion barrier (the “blood-nerve” barrier) that maintains endoneurial homeostasis. The compartment within the perineurium, the endoneurium, contains axons projecting into the periphery and their investing glia, the Schwann cells. The endoneurial space between axon-Schwann cell units contains collagen, fibroblasts, resident tissue macrophages and mast cells.
Fig. 1. Schematic illustrating the anatomy of normal peripheral nerve.
Indicated are the outmost layer of nerve (the epineurium) and the vasa nervorum, the perineurium (which ensheathes bundles of nerve fibers and forms the blood-nerve barrier) and the endoneurium. The inset highlights the mixture of cell types present in the endoneurium including axons, myelinating and nonmyelinating Schwann cells, fibroblasts and mast cells.
Neurofibromas are benign tumors that arise within peripheral nerve. Consistent with this origin, the cellular composition of a neurofibroma arising within a large nerve or nerve plexus (a plexiform neurofibroma) resembles a disordered version of the endoneurium (Fig. 2). These lesions contain large numbers of Schwann cell-like elements (referred to below as Schwann cells for simplicity’s sake; however, see Section 3.3 for a discussion of the origin of these cells) and fibroblasts that diffusely infiltrate along the length of the nerve, separating and spreading apart entrapped axons. Large numbers of mast cells are also typically present in neurofibromas. This cellular composition, considered together with evidence indicating that Schwann cells are the neoplastic cell type within neurofibromas (see below), implies that the initial steps in neurofibroma formation occur within the endoneurium and that interactions with other cell types found in this microenvironment shape the course of tumor formation. Further, the early stages of neurofibroma growth are likely constrained and shaped by the perineurium.
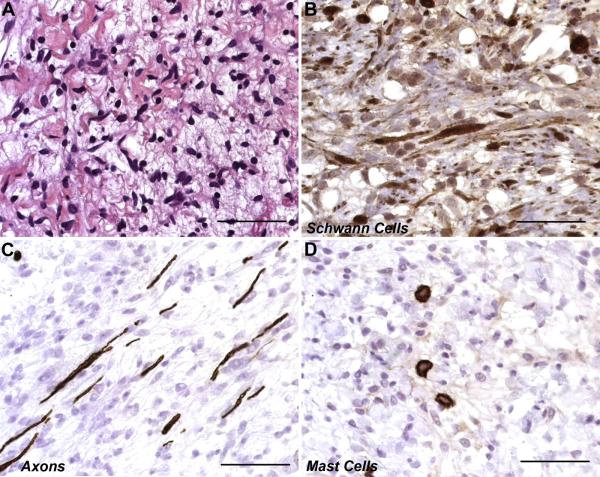
Fig. 2. Photomicrographs demonstrating the presence of multiple cell types in neurofibromas.
Unlike other types of peripheral nerve sheath tumors, neurofibromas are composed of a complex mixture of multiple cell types normally present in peripheral nerve. (A) Hematoxylin and eosin stained section of a plexiform neurofibroma showing the typical loosely packed collection of spindled cells characteristic of these tumors. (B) Immunostains for S100β label the cytoplasm and nuclei of Schwann cell-like elements within a plexiform neurofibroma. (C) Axons, which are visualized here by their immunoreactivity for neurofilaments, are separated by neoplastic Schwann cells and other cellular elements recruited into the tumor. This demonstrates the infiltrative nature of these lesions. (D) Numerous mast cells, identifiable by their immunoreactivity for CD117 (c-Kit), are also present in this plexiform neurofibroma. Scale bars, 50 μm.
In contrast, MPNSTs, the highly aggressive sarcomas that develop from plexiform neurofibromas, are overwhelmingly composed of cells with the morphologic, immunohistochemical (Fig. 3A, B) and ultrastructural characteristics of Schwann cells. Indeed, these observations, considered together with the observation that NF1 loss of heterozygosity (LOH) is found in Schwann cells but not other cell types intrinsic to neurofibromas, provide strong evidence that Schwann cells are the primary neoplastic cell type in both neurofibromas and MPNSTs. Interestingly, the conventional schwannomas arising in patients with schwannomatosis and NF2 (Fig. 3C) as well as the melanotic schwannomas occurring in Carney complex (Fig. 3D-F) are also composed of neoplastic Schwann cells. However, these benign lesions are composed almost exclusively of mature Schwann cells and lack other cellular components found in peripheral nerve. It is also exceedingly uncommon for schwannomas to undergo malignant progression. Considered together, these observations suggest that the mechanisms responsible for neurofibroma and MPNST pathogenesis are distinct from those driving the development of schwannomas.
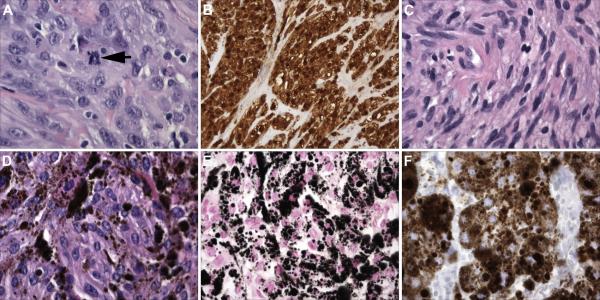
Fig. 3. A comparison of the pathology of MPNSTs with that of conventional schwannomas and melanotic schwannomas, two other tumor types composed predominantly of neoplastic Schwann cells.
(A) Hematoxylin and eosin stained section of an MPNST showing the high degree of cellularity and nuclear atypia characteristic of these neoplasms. The arrow indicates a mitotic figure. (B) Unlike plexiform neurofibromas, the benign precursors from which they arise, MPNSTs are overwhelmingly composed of neoplastic cells which in this panel are highlighted by their S100β immunoreactivity. (C) In contrast, this schwannoma resected from the VIIIth cranial nerve of an NF2 patient shows a lower degree of cellularity and relatively uniform “cigar-shaped” tumor cell nuclei. (D) Hematoxylin and eosin stained section of a melanotic schwannoma, a tumor type associated with Carney complex. Note the abundant deposits of brown pigment. (E) A Fontana stain highlights the melanin in a melanotic schwannoma as black deposits. (F) Unlike conventional schwannomas, melanotic schwannomas express antigens characteristic of melanocytes and melanomas. Shown is an immunostain for the melanoma marker HMB45 in this melanotic schwannoma. Magnification: A, C, D, E and F; 40x; B, 20x.
Histologically, the GEM PNS tumors described below closely resemble their human counterparts. Neurofibromas from early GEM models (Nf1flox/−; Krox20-Cre and Nf1+/−; Nf1−/− chimeras; see section 3.1 Initial Nf1 Knockout Models) contain long spindle-shaped cells on a myxoid background with extensive mast cell infiltration and collagen deposition while lacking marked hypercellularity, nuclear atypia or frequent mitotic figures [15, 73, 97]. Staining for S100β, a marker of Schwann cells, was observed in some but not all of these tumors; electron microscopy was required to establish the presence of cells with morphologic features characteristic of Schwann cells in S100β- tumors. Based on these features, a panel of pathologists classified these tumors as GEM grade I neurofibromas [73]. Although not reviewed by this panel, the neurofibromas formed by the more recently developed GEM models discussed in this review appear to share these same histologic traits. Initial investigator-assigned designations of these GEM neurofibromas as plexiform, however, could not be confirmed by the panel [73].
This panel of pathologists was also cautious in their classification of the higher grade PNS lesions observed in early models (cis-linked Nf1+/−/p53+/− mice). Although these GEM lesions share a number of histologic traits with human MPNSTs – including an association with a peripheral nerve or neurofibroma, high cellularity, brisk mitotic activity, nuclear pleomorphism and anaplasia – the panel recommended that they be classified as GEM grade III peripheral nerve sheath tumors (PNSTs) rather than as MPNSTs. The reason for this is that the panel felt that the term “malignant” was inappropriate due to a lack of information about the clinical course of these tumors at the time of their report [73]. While we recognize this point, we will refer to these GEM tumors as MPNSTs below for simplicity’s sake.
2.2 Clinical Characteristics of NF1 and Function of the Gene Mutated in this Disorder
NF1 is the most common genetic disease affecting the human nervous system, occurring in 1 in 3500 newborn infants. Manifestations of this autosomal dominant disease include learning disabilities, bony dysplasias, pigmentary lesions of the skin (café-au-lait macules, axillary freckling) and iris (Lisch nodules), and the development of a variety of tumor types (optic gliomas, glioblastomas, pheochromocytomas and juvenile myelomonocytic leukemia). As implied by the name of the disorder, however, neurofibromas are the hallmark lesion of NF1. It is widely recognized that distinct neurofibroma subtypes occur in NF1 patients and several classification schemes are currently in use for defining these subtypes. However, many investigators prefer to simply classify neurofibromas as dermal or plexiform variants as this has important clinical implications. Dermal neurofibromas arise in skin, typically developing in NF1 patients entering puberty or in women with NF1 that have become pregnant. Interestingly, dermal neurofibromas virtually never undergo malignant progression. In contrast, plexiform neurofibromas are often congenital. Individuals with plexiform neurofibromas also have an 8-13% lifetime risk that their tumors will progress to become MPNSTs, the most common malignancy developing in NF1 patients.
Linkage analyses were initially used to establish that the gene mutated in NF1 patients was localized to the long arm of human chromosome 17 [5, 70]. Transcription of this gene gives rise to a 13 kilobase mRNA [12, 85, 91] which encodes the 220 kDa (2,818 amino acid) tumor suppressor protein neurofibromin. Neurofibromin contains a domain homologous to the yeast GTPase activating proteins (GAPs) IRA1 and IRA2, which function as negative regulators of the yeast RAS1 and RAS2 proteins. As predicted by this homology, neurofibromin’s GAP-related domain (GRD) stimulates the intrinsic GTPase activity of the mammalian Ras homologues, catalyzing the hydrolysis of Ras-GTP to Ras-GDP, which inactivates these small growth-promoting G-proteins (Fig. 4). In keeping with these in vitro observations, Ras proteins are hyperactivated in nerve sheath tumors that have lost NF1, and reintroduction of the NF1 GRD into tumor cells decreases Ras activation and slows tumor cell proliferation [4, 54, 91].
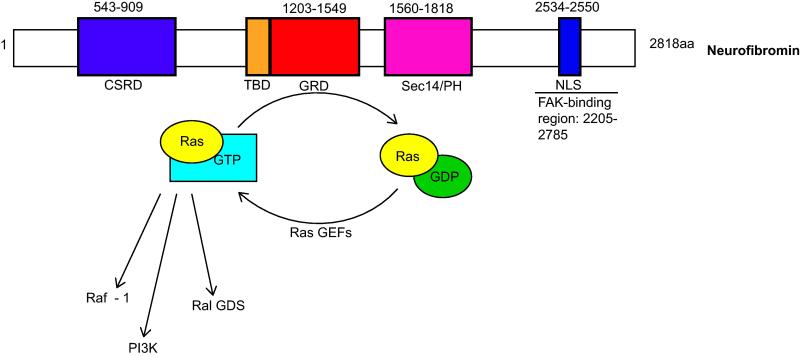
Fig. 4. Schematic illustrating key subdomains within neurofibromin, the tumor suppressor protein encoded by the NF1 gene.
Subdomains are indicated as follows: CSRD, cysteine/serine-rich domain; TBD, tubulin-binding domain; GRD, GAP-related domain; Sec14/PH, Sec14-homologous domain and pleckstrin homology domain; NLS, nuclear localization sequence. Numbers above each subdomain indicate the positions of the corresponding amino acids within the 2818 amino acid length of the neurofibromin protein. The amino acid position of the TBD is not indicated, as this is an 80 amino acid region found within the N-terminal GRD domain.
Although most studies of neurofibromin action have focused on its GRD domain, this domain is only a small portion of the protein (Fig. 4). Several other domains have been identified within neurofibromin, including a tubulin-binding domain (TBD) [7], a cysteine/serine-rich domain (CSRD) [32], a Sec14-homology domain (Sec14) [3], a pleckstrin homology domain (PH) [17] and a nuclear localization sequence (NLS) [79]. The TBD and CSRD may modulate neurofibromin’s ability to regulate Ras, as the GAP activity of neurofibromin is substantially reduced upon binding to tubulin [7] and is increased by CSRD phosphorylation [52]. At present, the function of the bipartite lipid binding and exchange motif created by the adjacent Sec14 and PH domains [17, 88] is unclear, as is the significance of the NLS. Neurofibromin also interacts with focal adhesion kinase (FAK) through a C-terminal region surrounding the NLS and can regulate FAK-mediated substrate adherence in serum deprived cells via an unknown mechanism [45]. Finally, neurofibromin modulates cAMP levels. Curiously, neurofibromin effects on cAMP levels are cell-type dependent, as neurofibromin loss elevates cAMP levels in Schwann cells [18, 38] and reduces them in astrocytes [20]. The mechanism responsible for these effects is unknown. However, since the neurofibromin CSRD contains cAMP-dependent kinase (PKA) phosphorylation sites, it is reasonable to postulate that these differential effects result from interruption of a feedback loop.
3. Mouse Models of Peripheral Nerve Sheath Tumors
3.1 Initial Nf1 Knockout Models
In 1994, the Copeland and Weinberg labs independently described knockout mice with null mutations of Nf1 exon 31, a region that is often mutated in human NF1 patients. Both groups found that homozygous Nf1Δ31/Δ31 mice died by embryonic day 13.5 (E13.5) due to cardiac failure [8, 33]. Defects in renal, hepatic, and skeletal muscle development were also observed [8]. However, nervous system pathology was limited to enlargement of sympathetic ganglia secondary to neuronal hyperplasia [8] and, less commonly, exencephaly. The former phenomenon may reflect the prolonged proliferation [82] and enhanced neurotropin-independent survival [81] that occurs in embryonic sympathetic neurons following Nf1 loss.
Interestingly, complete Nf1 loss had quite different effects on Schwann cells. In keeping with neurofibromin’s role as a negative regulator of Ras, Ras activation was increased in cultured Nf1Δ31/Δ31 Schwann cells. As activated Ras generally promotes cell growth, the initial expectation was that these cells would show enhanced mitogenesis. However, Nf1Δ31/Δ31 Schwann cells instead demonstrated reduced proliferation in response to neuregulin-1 (NRG-1) stimulation and axonal contact [39], two classic Schwann cell mitogenic stimuli. It was thus proposed that Ras hyperactivation in these cells led to oncogene-induced senescence, similar to that previously observed when activated Ras mutants were introduced into Schwann cells in the absence of other oncogenic signals [64]. However, it was unclear why Nf1 loss would produce senescence in Schwann cells but hyperplasia in sympathetic neurons, particularly given the absence of the latter finding in NF1 patients.
The phenotype of heterozygous Nf1Δ31/+ mice only created more questions. Contrary to expectations, these mice did not develop neurofibromas, pigmentation defects, or Lisch nodules [8, 33]. However, approximately 15% of Nf1Δ31/+ mice did develop adrenal tumors, many of which showed Nf1 LOH. These lesions were pheochromocytomas [33], a tumor type which is often observed in human NF1 patients but is very rare in wild-type mice. Interestingly, their pathogenesis was strain-specific, as the elevated pheochromocytoma incidence observed in Nf1Δ31/+ mice on a mixed sv/129 × C57BL/6 genetic background disappeared when the Nf1Δ31/+ allele was bred onto a sv/129 background [76]. Otherwise, the Nf1Δ31/+ mice were indistinguishable from wild-type mice until after 12 months in age, when their survival declined sharply due to the development of a lymphomas, leukemias, lung adenocarcinomas, hepatomas, fibrosarcomas and adrenal tumors [33]. As these malignancies are also normally observed in older (>24 months) wild-type mice [9], this suggested that germline loss of a single Nf1 allele merely accelerated the development of tumors to which the mice were already predisposed.
One possible explanation for the lack of neurofibroma formation in the Nf1Δ31/+ mice was that the acquisition of a second-hit mutation within one or more cell types in peripheral nerve was the rate limiting step for neurofibroma generation. To test this hypothesis, chimeric mice were generated by injecting Nf1−/− embryonic stem cells into Nf1+/− C57BL/6 blastocysts [15]. Although those animals with the highest degree of chimerism died by one month of unknown causes, mice with an intermediate degree of chimerism developed multiple plexiform neurofibromas. Consistent with the hypothesis that Nf1 LOH in Schwann cells was required for neurofibroma development, the tumors were composed largely of Nf1−/− Schwann cells.
The presence of Nf1−/− Schwann cells in the plexiform neurofibromas formed in the Nf1−/−;Nf1+/− chimeric mice suggested that Schwann cell Nf1 LOH was required for neurofibroma generation. However, it did not establish that such LOH was sufficient for plexiform neurofibroma pathogenesis. To address that question, Nf1flox/flox mice were bred to mice expressing Cre recombinase under the control of a Schwann cell-active promoter (Krox20-Cre mice) [97]. Peripheral nerves from these mice showed only mild Schwann cell hyperplasia with no evidence of neurofibroma formation, despite confirmation of Cre expression and Nf1 loss in Schwann cells from these animals. However, when conditional Nf1 ablation in Schwann cells occurred on an Nf1 heterozygous background (Nf1flox/−; Krox20-Cre mice), lesions with the histologic features of human neurofibromas developed in all of the animals by 1year of age [97]. These findings painted a much more complex picture of the neurofibroma pathogenesis than had been previously appreciated. In particular, the discovery that Nf1 loss in Schwann cells only resulted in neurofibroma formation when all other cell types were Nf1 haploinsufficient suggested for the first time that the presence of susceptible non-neoplastic cell types in the tumor microenvironment was critical for neurofibroma development.
3.2 Mouse Models Probing the Role of the Tumor Microenvironment in Neurofibroma Formation
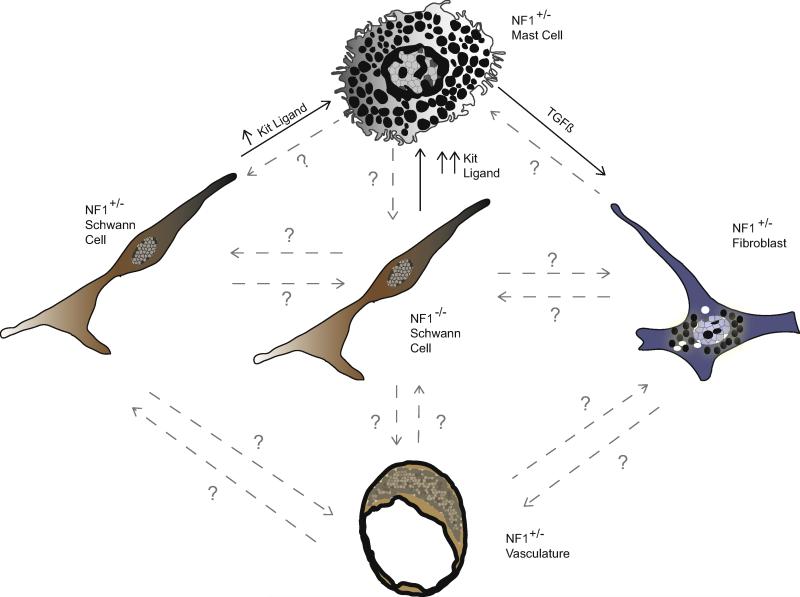
A key question that is still incompletely answered is precisely which cell types in the tumor microenvironment interact with neoplastic Schwann cells to promote neurofibroma pathogenesis. Although Nf1 haploinsufficiency in multiple cell types – including mast cells, fibroblasts and vascular elements – could contribute to this process, the most convincing work to date has focused on mast cells. These cells appear to function as critical intermediaries between Nf1−/− Schwann cells and other Nf1+/− cell types in the microenvironment. Nf1−/− Schwann cells secrete elevated levels of Kit ligand, a growth factor which activates the c-Kit membrane tyrosine kinase. Nf1+/− mast cells show increased c-Kit expression [19] and an enhanced chemotactic response to Kit ligand relative to wild-type mast cells [93], leading to increased recruitment of these cells into the nascent tumor. Kit ligand also induces enhanced activation, degranulation [14], and TGF-β secretion in Nf1+/− mast cells [92]. TGF-β in turn acts upon Nf1+/− fibroblasts and promotes increased production of collagen [92], a molecule which is found in abundance in neurofibromas.
An essential role for mast cells in neurofibroma formation has been elegantly demonstrated using bone marrow transplantation in knockout mice. As noted above, Nf1flox/flox;Krox20-Cre mice do not develop neurofibromas. However, when Nf1flox/flox;Krox20-Cre mice were lethally irradiated and transplanted with Nf1+/− bone marrow, they developed multiple plexiform neurofibromas that were infiltrated by donor mast cells [94]. In contrast, when donor marrow from Nf1+/− mice with hypoactive c-Kit receptors (Nf1+/−; c-KitW41/W41 mice) was used, no tumors formed, indicating that c-Kit signaling in bone marrow-derived elements was critical for neurofibroma formation. Consistent with the hypothesis that Nf1+/− mast cells are critically important for neurofibroma pathogenesis, no neurofibromas formed in lethally irradiated Nf1flox/−;Krox20-cre mice transplanted with wild-type bone marrow, despite the presence of Nf1 haploinsufficient fibroblasts and endothelial cells in the peripheral nerve [94].
Mast cell recruitment is also apparently important for the continued growth of existing neurofibromas. Treating eight-to-nine month old Nf1flox/−; Krox20-Cre animals with established plexiform neurofibromas with 200mg/kg/day of the c-Kit inhibitor imatinib mesylate substantially reduced the volume of their dorsal root ganglia as well as mast cell recruitment and hypercellularity in nerve segments proximal to the dorsal root. This treatment also decreased proliferation and increased apoptosis within the plexiform neurofibromas [94]. Following this demonstration, 350mg/m2 imatinib mesylate was administered to a child with life-threatening airway compression produced by an unresectable plexiform neurofibroma. This treatment produced a 70% reduction in tumor volume [94], consistent with the hypothesis that recruitment of Nf1+/− mast cells is critical for neurofibroma maintenance as well as formation.
Although it is clear that mast cell recruitment is essential for neurofibroma formation and that Nf1 haploinsufficiency in other cell types cannot overcome this requirement, it remains to be determined whether Nf1 haploinsufficiency in these other cell types promotes neurofibroma growth. It is also unclear what protumorigenic function(s) are performed by the recruited mast cells after their arrival in the nascent neurofibroma and whether these effects are directed at the neoplastic Schwann cells or other intratumoral cell types. At present, an entire series of protumorigenic interactions between the various cell types composing a neurofibroma can be envisioned (Fig. 5). However, the existence and functional significance of most of these interactions remains to be determined.
Fig. 5. Schematic illustrating established and potential interactions between NF1−/− Schwann cells and other NF1 haploinsufficient cell types intrinsic to peripheral nerve.
Established interactions are depicted with solid black arrows and font, while potential interactions are depicted with gray dashed arrows labeled with question marks. Established interactions include the elevated secretion of Kit ligand from NF1+/− Schwann cells, which is further increased upon loss of the remaining NF1 allele and which acts as a chemoattractant and activating factor for NF1+/− mast cells. Activated NF1+/− mast cells have also been shown to secrete elevated levels of TGFβ, which stimulates increased collagen deposition from NF1+/− fibroblasts.
3.3 The Neurofibroma Cell-of-Origin Debate
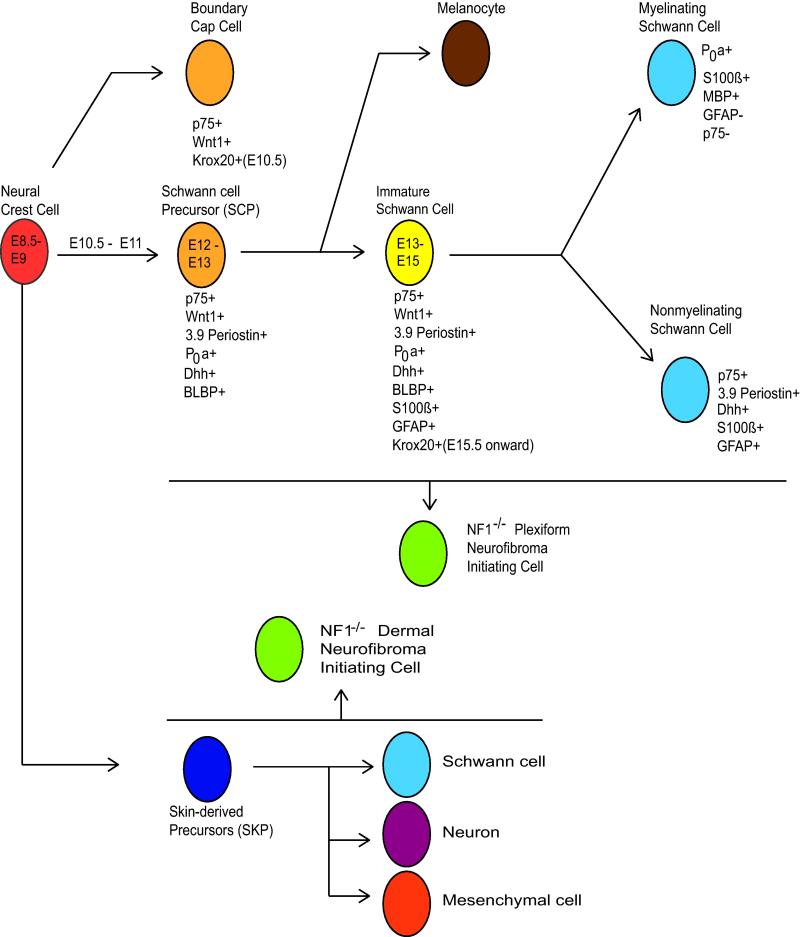
Although the neoplastic cells in plexiform neurofibromas clearly have schwannian characteristics, the initial Nf1 knockout models did not establish whether these neoplastic cells were derived from mature Schwann cells or a more primitive precursor (see Fig. 6 for an illustration of the stages of Schwann cell differentiation). To address this question, Nf1flox/− mice have been crossed to animals in which Cre expression was directed by promoters active at different stages in Schwann cell development. Elimination of Nf1 expression in neural crest cells, the earliest stage in Schwannian differentiation, was achieved by mating Nf1flox/− mice with Wnt1-Cre, Mpz-Cre, and Pax3-Cre animals. Although these mice had abnormal sympathetic ganglia and adrenal glands and died at birth, they did not develop neurofibromas [26]. Given the early death of these animals, it is conceivable that, had they survived, Nf1 ablation in neural crest cells would have ultimately resulted in the development of neurofibromas. However, Nf1flox/−; Krox20-Cre mice do develop neurofibromas, and Krox20 is not expressed in neural crest cells, which argues that Nf1 loss in neural crest cells is not required for neurofibroma pathogenesis.
Fig. 6. Schwann cell development.
Neural crest cells give rise to a series of cell types in the Schwann cell lineage, one or more of which can become a neurofibroma initiating cell (NIC) following biallelic NF1 loss. Plexiform NICs are thought to be derived from Schwann cell precursors or their more differentiated progeny in deep peripheral nerves; alternative origins such as boundary cap cell or satellite cells have also been proposed. Another progenitor population arising from the neural crest has been located in the dermis, and dermal NICs may arise instead from these skin-derived precursors (SKPs) or their progeny. Times indicated are the embryonic days (E) at which specific Schwann cell precursors appear in the mouse. Listed below each cell type are useful markers of specific developmental stages and promoters which are active at these stages (see text for further discussion). Note that promoters used to drive Cre-mediated recombination are often fragments of whole gene promoters and may have a separate designation (i.e. P0a is a 1.1kb fragment of the P0 promoter, and 3.9Periostin is a 3.9kb fragment of the periostin promoter).
Mouse models in which Nf1 was ablated in Schwann cell precursors (SCPs; also known as neural crest stem cells) were more informative. For these experiments, 3.9Periostin-Cre (which is active in SCPs by E11) and P0a-Cre (expressed in SCPs beginning at E12.5) driver lines were created and bred to Nf1flox/ − mice. While the majority of Nf1flox/−; 3.9Periosin-Cre animals died by the 4th postnatal week [35] (possibly due to activation of the 3.9Periostin promoter in cardiac fibroblasts [61, 72]), Nf1flox/−; P0a-Cre animals survived and formed neurofibromas by 15-20 months of age [35, 96]. Curiously, however, SCPs could not be isolated from the peripheral nerves of adult Nf1flox/−; P0a-Cre mice, and SCPs from E13 Nf1−/− mice did not generate tumors when transplanted into Nf1+/− sciatic nerves [35]. Further, the proliferating cells in these neurofibromas were p75+, GFAP+ and BLBP− [35, 96], suggesting that mature non-myelinating Schwann cells rather than SCPs were the cell type giving rise to neurofibromas in this model. In keeping with this idea, hyperproliferative non-myelinating Schwann cells were found in the postnatal sciatic nerves of Nf1flox/−; P0a-Cre mice prior to neurofibroma development [96].
However, this conclusion was inconsistent with the phenotype of a mouse model in which the Desert Hedgehog promoter drives Cre-mediated Nf1 ablation in SCPs at E12.5 (Nf1flox/flox; Dhh-Cre mice) [89]. Unlike the tumors arising in Nf1flox/−; P0a-Cre mice, neurofibromas developing in Nf1flox/flox; Dhh-Cre mice contained numerous BLBP+ cells [89], suggesting that immature Schwann cells were the progenitors for these tumors. Interestingly, the development of neurofibromas in Nf1flox/flox; Dhh-Cre mice occurred despite the presence of a wild-type Nf1 microenvironment; no evidence was found for Cre-mediated recombination in mast cells, endothelial cells or endoneurial fibroblasts [89], despite the fact that Dhh-expressing progenitors capable of differentiating into both Schwann cells and endoneurial fibroblasts have been found in peripheral nerve [36]. Given these contradictory results, it is not yet clear whether the neoplastic Schwann cells within plexiform neurofibromas are derived from mature nonmyelinating Schwann cells, immature Schwann cells or both cell types. The possibility that these neoplastic Schwann cells arise from another source such as boundary cap cells also has not yet been ruled out.
Given the marked differences in the clinical behavior of dermal and plexiform neurofibromas, it is possible that the neoplastic cells in these neurofibroma subtypes are derived from distinct progenitors. Neural-crest derived precursor cells capable of both Schwannian and melanocytic differentiation, termed skin-derived precursors (SKPs) [46], are present in the dermis of adult mice. Consistent with the hypothesis that SKPs give rise to dermal neurofibromas, topical administration of tamoxifen to neonatal Nf1flox/− ; CMV-CreERT2; Rosa26-LacZ(stop) mice results in dermal neurofibroma formation at the site of tamoxifen administration [46]. Further, SKPs isolated from these animals and treated ex vivo with tamoxifen to inactivate Nf1 were also capable of generating neurofibromas upon autologous subcutaneous transplantion into pregnant mice, indicating that these progenitors (and not other cell types residing in the dermis) were the cell of origin of the dermal neurofibromas.
Interestingly, Nf1−/− SKPs were also capable of forming plexiform neurofibromas when autografted into sciatic nerves (Le 2009). Thus, while SKPs residing in the dermis may be the cell of origin of dermal neurofibromas, these cells are apparently highly similar to the neurofibroma-initiating cells in peripheral nerve. Transcriptional profiling does not distinguish dermal and plexiform neurofibromas [56], which suggests that the distinct clinical behavior of these tumors primarily reflects differences in their microenvironment rather than their cell of origin. If this hypothesis is correct, the expansion of dermal neurofibromas during pregnancy, for example, may be due to hormonally-altered paracrine signaling from fibroblasts, melanocytes, or other cell types within the dermis feeding back on the Nf1−/− progenitor cells rather than major differences in the Nf1−/− progenitors themselves. This is consistent with the fact that pregnancy has a well-established effect on skin pigmentation and elasticity [77, 84].
However, if the cell of origin for dermal and plexiform neurofibromas is virtually identical save for their microenvironment niche, why do dermal neurofibromas not arise in the various Nf1flox/− models discussed above? The experiments described above clearly demonstrate that mouse dermis is capable of giving rise to neurofibromas, so species-specific differences in skin susceptibility to neurofibroma formation are unlikely to explain this phenomenon. Clearly, more work is needed to understand these cells and their true relationship with the progenitor cells in the peripheral nerve.
3.5 Mouse Models of Malignant Peripheral Nerve Sheath Tumor Pathogenesis
Although most mouse models of NF1 developed to date have focused on plexiform neurofibroma pathogenesis, the transformation of these benign tumors into MPNSTs is of far greater concern to NF1 patients [55], especially given the lack of effective treatment options for these malignancies. However, modeling MPNST formation in mice has proven challenging, as the plexiform neurofibromas developing in mice with Nf1 mutations only rarely progress to become MPNSTs. This may be due in part to the relatively short lifespan of mice, which could prevent murine Nf1−/− Schwann cells from having adequate time to accumulate the additional tumor suppressor mutations driving MPNST pathogenesis. Alternatively, cells that acquire secondary mutations may persist in the latent phase of malignancy progression until the mice die from other causes.
Consequently, mice with null alleles of both Nf1 and p53 were generated to accelerate this process. As these genes are both located on mouse chromosome 11, tumorigenesis was compared in mice with mutant Nf1 and p53 alleles on opposite chromosomes (trans Nf1+/−/p53+/− mice) and in mice with mutant Nf1 and p53 alleles on the same copy of chromosome 11 (cis-linked Nf1+/−/p53+/− mice). Perhaps not surprisingly, these animals showed discordant phenotypes. Trans Nf1+/−/p53+/− animals died by 10 months of non-MPNST soft-tissue sarcoma types typically associated with p53 loss of function. In contrast, 30% of the cis-linked Nf1+/−/p53+/− animals dying by 5 months of age were found to have MPNSTs [15]. Interestingly, these MPNSTs did not appear to arise in preexisting plexiform neurofibromas, suggesting that combined loss of Nf1 and p53 allowed MPNSTs to develop de novo.
MPNST formation also occurs in mice carrying a mutated Nf1 gene in combination with other tumor suppressor mutations. In keeping with the observation that CDKN2A is commonly mutated in human MPNSTs [1, 6, 43, 58, 63, 67], 26% of Nf1+/− mice with simultaneous homozygous deletion of the CDKN2A locus (Nf1+/−; p16Ink4a−/−/p19Arf−/− mice) developed MPNSTs, while those with heterozygous deletions of both loci (Nf1+/−; p16Ink4a/p19Arf+/− mice) developed MPNSTs at a much lower frequency [35]. As with MPNSTs arising in cis-linked Nf1+/−/p53+/− animals, the MPNSTs developing in Nf1+/−; p16Ink4a−/−/p19Arf−/− mice did not appear to arise from a precursor neurofibroma. Neither Nf1+/−; p19Arf−/− [41] or Nf1+/−; p16Ink4a−/− animals [35] showed a predisposition to MPNST development, indicating that deletion of both products encoded by the CDKN2A locus (and thus, dysregulation of both the p53 and pRb pathways) is necessary to promote MPNST formation in the setting of Nf1 heterozygosity.
The cell-of-origin debate was also revisited in MPNSTs derived from these models, as the absence of a benign precursor tumor raised the question of whether these malignancies were derived from a progenitor population distinct from that giving rise to neurofibromas. As at least some MPNST cells isolated from Nf1+/−; p16Ink4a−/−/p19Arf−/− and cis-linked Nf1+/−/p53+/− animals can grow as neurospheres capable of self-renewal [35], this work focused on the Schwann cell precursor stage of Schwannian development. However, SCPs did not show abnormal persistence or impaired differentiation in these animals and did not form tumors when transplanted into sciatic nerves. Moreover, MPNST cells from these animals, unlike SCPs, were not capable of differentiation along multiple lineages [35] and showed decreased levels of Sox10, a HMG-box factor critically important for maintaining the ability of precursor cells to give rise to glia and neurons [40].
Reduced Sox10 expression is also evident in human MPNSTs [47, 56, 57], together with a general downregulation of genes important for Schwannian differentiation [56]. At the same time, MPNSTs upregulate the expression of molecules characteristic of migrating neural crest cells such as Sox9 and Twist1 [56, 57]. In contrast, neurofibromas express a gene signature more characteristic of Schwann cell precursors or immature Schwann cells [56]. As human NF1-associated MPNSTs develop via malignant progression from neurofibromas, it is unlikely these differences in gene expression are due to neurofibromas and MPNSTs arising from distinct cell populations. Consequently, loss or suppression of Schwannian differentiation signals is apparently an important step in the progression to MPNSTs.
3.6 Neurofibroma and MPNST Formation in Conditional Ras Activation Mutants
As noted above, one of the best understood functions of neurofibromin is its ability to negatively regulate Ras action. What is not as widely appreciated is that neurofibromin regulates the activity of multiple Ras proteins from both the classic Ras (H-Ras, N-Ras, K-Ras) and R-Ras (R-Ras, R-Ras2/TC21, R-Ras3/M-Ras) families of small G-proteins. Further, it has not yet been established which Ras isoforms are expressed in neoplastic Schwann cells within human neurofibromas or MPNSTs or which of these molecules are critically important for tumorigenesis. However, mouse models in which constitutively active mutants of N-Ras or K-Ras are expressed in the Schwann cell lineage have been constructed for the purpose of determining whether this results in neurofibroma and/or MPNST pathogenesis.
Interestingly, distinct phenotypes were observed when activated N-Ras and K-Ras were expressed in the Schwann cell lineage. Mice expressing activated N-Ras in neural-crest derived cells (LSLNrasG12V/+; CAMK2-Cre) developed diffuse dermal neurofibromas [68] and hyperpigmented skin lesions similar to café-au-lait macules. Curiously, these skin lesions showed enhanced accumulation of pigment in melanocytes and large numbers of pigment-laden macrophages rather than an increase in melanocyte numbers [68], suggesting that pigment production rather than melanocyte proliferation was promoted by activated N-Ras. No plexiform neurofibromas or MPNSTs were observed in these animals.
In contrast, LSLKras2BG12D/+; mGFAP-Cre mice did not develop tumors on a wild-type background. However, when bred onto a Ptenflox/+ background, they all developed multiple plexiform neurofibromas by 4 months of age [28]. Interestingly, neurofibroma progression to MPNSTs, which was associated with loss of the remaining functional Pten allele, also occurred in all animals by 7 months. This pattern of tumor incidence was not evident in Nf1flox/+ Ptenflox/+; mGFAP-Cre mice, likely due to a requirement for LOH for both Nf1 and Pten in the same cell; as these genes are located on separate mouse chromosomes, this is probably a highly uncommon event. Considered together, these findings suggest a role for PTEN in MPNST progression, consistent with previous reports that PTEN deletion [30] or silencing by promoter methylation [37] is present in a subset of human MPNST samples.
So why does activation of N-Ras induce dermal neurofibroma formation directly, while plexiform neurofibroma generation in K-Ras2B-activated cells requires concomitant PTEN haploinsufficiency? One possible explanation is the Cre-driver lines used. CAMK2-Cre mediates recombination in cells derived from the neural crest [68]. Consequently, CAMK2-Cre will activate expression of the mutated Ras allele earlier in development and in more cell types than will the mGFAP-Cre driver, which is not active until the immature Schwann cell stage [28]. This would increase the pool of cells capable of transformation, leading to more frequent development of neurofibromas in CAMK2-Cre mice. Alternatively, cells at different stages in Schwannian development may differentially regulate the signaling pathways that are affected in these models. PTEN is a negative regulator of class I phosphoinositide-3-kinase (PI3K) signaling [51], a pro-survival cascade which can be activated by GTP-bound Ras [42, 65, 66, 80, 90]. In many cell types, transformation by oncogenic Ras requires coordinate activation of PI3K and other Ras effectors such as Raf and RalGDS [53, 83]. However, it is possible that Ras-induced transformation requires unrestrained PI3K activation in immature Schwann cells but not in neural crest cells and their immediate derivatives. Alternatively, cells at earlier developmental stages may downregulate PTEN, leading to heightened Ras-induced PI3K activation without the need for additional mutations. Finally, intrinsic differences in the ability of oncogenic K-Ras and N-Ras mutants to activate the PI3K pathway may also be responsible for the differential requirement for PTEN haploinsufficiency in these models. Although N-Ras and K-Ras activate PI3K signaling with approximately equal magnitude when overexpressed [29, 48, 65], studies with Ras molecules expressed at physiological levels indicate that K-Ras but not N-Ras is the major mediator of PI3K activation downstream of growth factors [49] and cytokines [95].
3.7 Manifestations of NF1 in Dysregulated Growth Factor Signaling Models
As noted above, when neurofibromin is lost in neoplastic Schwann cells, the rate of Ras inactivation is dramatically reduced, leading to accumulation of activated Ras. However, this begs the question of precisely what activates these Ras proteins in the first place. One likely possibility is that aberrant growth factor signaling performs this function in neoplastic Schwann cells. Indeed, aberrant expression of several growth factors and growth factor receptors has been identified in human neurofibromas and MPNSTs [11]. However, this hypothesis has been tested in transgenic mouse models only for the EGF receptor (EGFR, erbB1), the prototype of the erbB family of membrane tyrosine kinase receptors, and neuregulin-1 (NRG1), a growth factor that activates the other three members of the erbB kinase family (erbB2, erbB3, erbB4). Interestingly, these mouse models produced quite different outcomes.
A chemical carcinogenesis model of MPNST formation provided the first evidence implicating the erbB kinases in PNS tumor formation. Beginning more than four decades ago, investigators noted that rats [23, 34], mice [2] and hamsters [10, 22] exposed in utero to the chemical carcinogen N-ethyl-N-nitrosourea (EtNU) developed peripheral nerve sheath tumors that satisfy modern diagnostic criteria for MPNSTs. These tumors frequently carried activating mutations of the ErbB2 (HER2, c-neu) membrane tyrosine kinase [59, 60]. Although erbB2 does not directly bind growth factors, it is the preferred heterodimerization partner for the other erbB receptors [27, 78] and facilitates ErbB heterodimer signal transduction [71]; mutated erbB2 can also homodimerize [69, 86, 87]. Thus, ErbB2 activation could trigger MPNST formation by dysregulating signaling pathways downstream of erbB2 homodimers, downstream of EGF or NRG1 or all three. The potential importance of the observations in these rodent models was reinforced by the subsequent demonstration that amplification of ErbB2 [75] and EGFR [62, 63] occurs in at least some human MPNSTs.
To examine the impact of neuregulin signaling on PNS tumorigenesis, a transgenic mouse model was produced in which expression of the secreted NRG1 isoform GGFβ3 was directed by the Schwann cell-specific myelin protein zero (P0) promoter [31]. These animals developed Schwann cell hyperplasia that was evident by 1 month of age, followed by neurofibroma formation (unpublished observations) which progressed to MPNSTs by 6-10 months of age [31]. Importantly, this is one of only two reported transgenic models in which neurofibromas frequently progress to MPNSTs. Further, this malignant progression is associated with the mutation of additional tumor suppressor genes as is observed in human MPNSTs (unpublished data). Supporting the relevance of NRG signaling to human NF1-associated neurofibromas and MPNSTs, human neurofibromas, MPNSTs and MPNST cell lines express ErbB2, ErbB3, and/or ErbB4 together with multiple NRG-1 isoforms [74]. The erbB receptors expressed in these MPNST cell lines are constitutively activated and MPNST mitogenesis is profoundly inhibited by the pan-ErbB inhibitor PD168393 [74]. Further, stimulation of human MPNST cells with NRG-1β increases the migration and invasion of these cells [24].
The role of EGF receptor signaling in NF1-associated peripheral nerve sheath tumors has also been examined. EGFR is aberrantly expressed in many human neurofibromas and MPNSTs as shown by the fact that this molecule is not found in normal neonatal Schwann cells [21, 25]. In addition, the EGFR gene is amplified in a subset of human MPNSTs [62, 63] and EGF stimulation of serum-starved MPNST cells enhances their growth and survival [21]. To examine the contribution of this membrane tyrosine kinase to PNS tumorigenesis, transgenic mice were produced which over-expressed human EGFR in Schwann cells under the control of the CNPase promoter (CNP-hEGFR mice). These animals developed a hyperproliferative nerve phenotype with evidence of mast cell accumulation and fibrosis [50]. However, frank neurofibroma formation was exceedingly rare in these mice and was only observed at a very advanced age. Crossing CNP-hEGFR mice to Nf1+/− animals did not worsen the phenotype. However, Nf1+/−p53+/− mice did show enhanced survival on an EGFRwa-2 (EGFR hypomorphic mutation) background as compared to a wild-type background. As these animals develop many malignancies other than MPNSTs, it was unclear whether this increase in survival was due to a decrease in MPNST incidence or inhibition of the development of other tumor types that occur in these animals [50].
So why would NRG1 but not EGFR over-expression cause neurofibroma and MPNST formation? One possibility is that secreted NRG1 may affect multiple cell types within the peripheral nerve, potentially invoking paracrine signaling analogous to that occurring in human neurofibromas, while EGFR over-expression has action that is limited to Schwann cells. Another possibility is that EGFR expression is primarily required for tumor progression rather than promoting the initial formation of neurofibromas. Finally, it is possible that different signaling pathways are activated downstream of the NRG1 and EGF receptors, allowing the former but not the latter to mimic Nf1 loss.
4. Summary
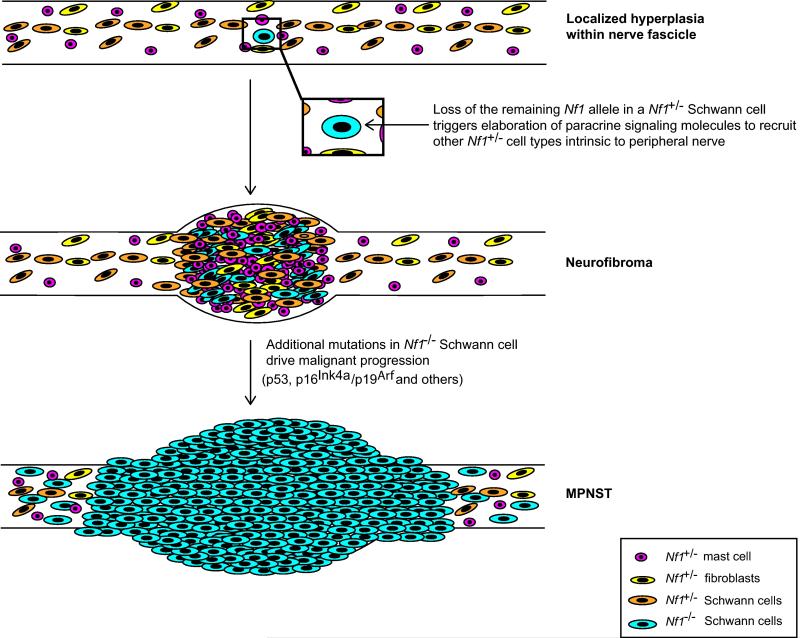
The findings from the mouse models described above together with observations from human tumors suggest that neurofibroma pathogenesis and subsequent progression to become MPNSTs results from the accumulation of a series of molecular abnormalities (Fig. 7). In this scenario, the initial step in neurofibroma pathogenesis is loss of the remaining functional NF1 allele in a Schwann cell. It is likely that Ras surveillance mechanisms as well as additional tumor suppressors such as p53, p16INK4a, p19Arf, and pRb at least transiently maintain the NF1−/− Schwann cell in a quiescent state, a suggestion which is consistent with the previous demonstration that senescent regions are present in neurofibromas [16]. Eventually, however, some unknown factor or confluence of factors stimulates a brief hyperproliferative period that is associated with paracrine recruitment of NF1+/− mast cells and fibroblasts. Paracrine signaling during this period may also facilitate inactivation of the tumor suppressor proteins noted above via mechanisms that promote cellular growth in untransformed cells. As this would probably occur in individual cells or in small regions of the benign tumor, tumor expansion could still occur regionally while the bulk of the tumor was maintained in a growth arrested state. Loss or mutation of tumor suppressor genes such as p53, CDKN2A and Pten would allow the neoplastic Schwann cells to stably evade this senescence response, resulting in progression to MPNSTs. At this point, paracrine signaling would no longer be required to support the growth of the tumor, resulting in gradual overgrowth of the malignant cells until the untransformed mast cells and fibroblasts were no longer evident in the growing mass.
Fig. 7. Major events in the pathogenesis of a neurofibroma and its subsequent progression to become a MPNST.
Illustrated are the changes leading to neurofibroma and MPNST development in GEM models of NF1. Transient hyperplasia is often observed in the peripheral nerves of these animals prior to neurofibroma development; it is unclear whether an Nf1+/− background is sufficient to generate this phenotype or whether biallelic loss of Nf1 in Schwann cells must also occur. Neurofibroma development, however, does depend upon biallelic loss of Nf1 in Schwann cells or their less differentiated precursors. Loss of the remaining functional Nf1 allele in the Schwann cell lineage triggers elaboration of paracrine signaling molecules that recruit other Nf1+/− cell types (including mast cells, fibroblasts, and other Schwann cells) from the peripheral nerve to the nascent tumor site. As not all Nf1−/− Schwann cells generate neurofibromas, there are likely other as-yet-undetermined events that must occur to the Nf1−/− Schwann cell or its microenvironment to trigger tumor formation. Once the neurofibroma has been established, additional mutations in tumor suppressor genes such as TP53 and p16Ink4a/p19Arf in Nf1−/− Schwann cells drive malignant progression. Comparisons with human neurofibromas and MPNSTs indicate that most of these events are relevant to the pathogenesis of the human counterparts of these murine tumors. However, the relevance of some changes seen in the murine tumor models (e.g., the initial phase of intraneural hyperplasia) remains to be determined. It should also be noted that other events associated with malignant progression in human MPNSTs (e.g., suppression of Sox10 expression and upregulation of Sox9 expression, altered epigenetic modification or miRNA regulation programs) have not yet been thoroughly explored in genetically engineered mouse models of peripheral nerve sheath tumors.
Although this scenario is consistent with the findings described in this review, it is clear that several key steps in this process remain poorly understood. Understanding these steps will require that we answer many questions such as how exactly how senescence fail-safes are evaded in neoplastic Schwann cells, what paracrine signaling molecules facilitate neurofibroma pathogenesis, what roles these paracrine signaling molecules play in this process and what additional mutations are responsible for neurofibroma-MPNST progression. Given the insights they have yielded thus far, new genetically engineered mouse models will undoubtedly play an important role in our efforts to answer these questions.
Table 1.
Genetically Engineered Mouse Models of PNS Neoplasia
| Transgenic Mouse Model |
Phenotype | Limitations | ||
|---|---|---|---|---|
| Early Models | Nf1Δ31/Δ31 | die by E13.5 due to cardiac failure |
early death prevents observation of tumorigenic effects of Nf1 loss |
|
| Nf1Δ31/+ | develop pheochromocytomas (15% incidence); show accelerated development of other non-NF1 tumors as compared to wild-type mice |
no neurofibromas or MPNSTs observed |
||
|
Nf1−/−;Nf1+/− chimeras |
multiple plexiform neurofibromas present in animals with intermediate level of chimerism |
cannot control which cell types are Nf1+/− and which are Nf1−/− |
||
|
Nf1flox/flox;Krox20 -Cre |
Schwann cell hyperplasia | no neurofibromas or MPNSTs observed |
||
|
Nf1flox/−;Krox20 -Cre |
plexiform neurofibroma development by 1yr of age (demonstrating importance of both Nf1+/− and Nf1−/− cells in neurofibroma formation) |
Krox20 promoter is expressed in Schwann cells and boundary cap cells, making it hard to identify a clear progenitor |
||
| Tumor Microenvironment Models |
Nf1flox/flox; Krox2- Cre transplanted with Nf1+/− bone marrow |
developed plexiform neurofibromas infiltrated by donor mast cells (demonstrating importance of Nf1 haploinsufficiency in hematopoeitic lineage for neurofibroma formation) |
Other Nf1+/− cell types within the hematopoietic lineage may contribute to neurofibroma development |
|
|
Nf1flox/flox; Krox2- Cre transplanted with Nf1+/−;c- KitW41/W41 bone marrow |
no neurofibromas developed (demonstrating importance of c-Kit signaling in Nf1 haploinsufficient cells in the hematopoeitic lineage) |
|||
|
Nf1flox/+; Krox2- Cre transplanted with Nf1+/+ bone marrow |
no neurofibromas developed (demonstrating importance of Nf1 haploinsufficiency in hematopoeitic lineage for neurofibroma formation) |
|||
| Tumor Cell of Origin Models |
Nf1 ablation in Migrating Neural Crest |
Nf1flox/−; Wnt1- Cre, |
died at birth; no neurofibromas developed |
early death prevents observation of tumorigenic effects of Nf1 loss |
| Nf1flox/-; Mpz-Cre | ||||
|
Nf1flox/−; Pax3- Cre | ||||
|
ablation in Schwann Cell |
Nf1flox/−; 3.9Periostin-Cre | died by 4 weeks after birth; no neurofibroma development observed |
early death prevents observation of tumorigenic effects of Nf1 loss |
|
| Nf1flox/−; P0a-Cre | plexiform neurofibroma formation observed by 15-20 months |
due to broad P0a promoter expression in the Schwann cell lineage, a definitive cell of origin still could not be identified |
||
|
Nf1flox/flox; Dhh- Cre |
plexiform and subcutaneous neurofibroma development (demonstrating that an Nf1+/− microenvironment might not be strictly required for neurofibroma formation) |
Dhh promoter expression in progenitor cells capable of differentiation into both Schwann cells and endoneurial fibroblasts |
||
|
Nf1 ablation in SKPs |
Nf1flox/−; CMV- CreERT2; Rosa26 |
dermal neurofibromas generated ~6 months following topical tamoxifen administration |
likely that ablation of Nf1 in non-SKP cells in dermis contributes to neurofibroma formation |
|
|
MPNST Formation Driven by Dual Tumor Suppressor Loss |
trans-linked Nf1+/−;p53+/− |
developed non-MPNST sarcomas characteristic of p53 LOH |
no MPNST formation | |
| cis-linked Nf1+/− ;p53+/− |
developed MPNSTs (~30% incidence) |
no neurofibroma precursor lesion | ||
| Nf1+/−p16Ink4a−/− | accelerated development of tumors characteristic of p16INK4a loss |
no MPNST formation | ||
| Nf1+/−p19Arf−/− | accelerated development of tumors characteristic of p19Arf loss |
no MPNST formation | ||
|
Nf1+/− p16Ink4a/p19Arf−/− |
developed MPNSTs (~30% incidence) |
no neurofibroma precursor lesion | ||
|
PNS Tumor Formation Driven by Ras Activation |
LSLNrasG12V/+; CAMK2-Cre |
pigmentary abnormalities of skin and dermal neurofibromas observed |
expression of the CAMK2 promoter in the Schwann cell lineage has not been clearly defined |
|
|
LSLKras2BG12D/+; mGFAP-Cre |
no obvious phenotype | no neurofibromas observed | ||
|
LSLKras2BG12D/+ Ptenflox/+; mGFAP-Cre |
plexiform neurofibroma development by 4 months of age with progression to MPNSTs by 7 months |
unclear how necessary Pten loss of function is to neurofibroma formation in the context of Nf1 loss |
||
|
PNS Tumor Formation Driven by Dysregulated Growth Factor Signaling |
P0-GGF β 3 | neurofibroma formation with progression to MPNSTs by 6- 10 months |
not yet clear whether and how dysregulated NRG1 signaling interacts with neurofibromin loss |
|
| CNPase-EGFR | Schwann cell hyperplasia with mast cell recruitment and fibrosis |
neurofibroma formation exceedingly rare at very advanced age; no MPNSTs observed |
||
Acknowledgements
This work was supported by the National Institute of Neurological Diseases and Stroke (R01 NS048353 to S.L.C.; F30 NS063626 to N.M.B.), the National Cancer Institute (R01 CA122804 to S.L.C.; R01 CA134773 to Kevin A. Roth and S.L.C.) and the Department of Defense (X81XWH-09-1-0086 to S.L.C.). We thank the Alabama Neuroscience Blueprint Core Center (P30 NS57098) and the UAB Neuroscience Core Center (P30 NS47466) for technical assistance with studies from our laboratory that are described in this review. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Department of Defense.
References
- [1].Agesen TH, Florenes VA, Molenaar WM, Lind GE, Berner JM, Plaat BE, Komdeur R, Myklebost O, van den Berg E, Lothe RA. Expression patterns of cell cycle components in sporadic and neurofibromatosis type 1-related malignant peripheral nerve sheath tumors. J Neuropathol Exp Neurol. 2005;64:74–81. doi: 10.1093/jnen/64.1.74. [DOI] [PubMed] [Google Scholar]
- [2].Anderson LM, Hagiwara A, Kovatch RM, Rehm S, Rice JM. Transplacental initiation of liver, lung, neurogenic, and connective tissue tumors by N-nitroso compounds in mice. Fundam Appl Toxicol. 1989;12:604–620. doi: 10.1016/0272-0590(89)90033-x. [DOI] [PubMed] [Google Scholar]
- [3].Aravind L, Neuwald AF, Ponting CP. Sec14p-like domains in NF1 and Dbl-like proteins indicate lipid regulation of Ras and Rho signaling. Curr Biol. 1999;9:R195–197. doi: 10.1016/s0960-9822(99)80127-4. [DOI] [PubMed] [Google Scholar]
- [4].Ballester R, Marchuk D, Boguski M, Saulino A, Letcher R, Wigler M, Collins F. The NF1 locus encodes a protein functionally related to mammalian GAP and yeast IRA proteins. Cell. 1990;63:851–859. doi: 10.1016/0092-8674(90)90151-4. [DOI] [PubMed] [Google Scholar]
- [5].Barker D, Wright E, Nguyen K, Cannon L, Fain P, Goldgar D, Bishop DT, Carey J, Baty B, Kivlin J, et al. Gene for von Recklinghausen neurofibromatosis is in the pericentromeric region of chromosome 17. Science. 1987;236:1100–1102. doi: 10.1126/science.3107130. [DOI] [PubMed] [Google Scholar]
- [6].Birindelli S, Perrone F, Oggionni M, Lavarino C, Pasini B, Vergani B, Ranzani GN, Pierotti MA, Pilotti S. Rb and TP53 pathway alterations in sporadic and NF1-related malignant peripheral nerve sheath tumors. Lab Invest. 2001;81:833–844. doi: 10.1038/labinvest.3780293. [DOI] [PubMed] [Google Scholar]
- [7].Bollag G, McCormick F, Clark R. Characterization of full-length neurofibromin: tubulin inhibits Ras GAP activity. Embo J. 1993;12:1923–1927. doi: 10.1002/j.1460-2075.1993.tb05841.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [8].Brannan CI, Perkins AS, Vogel KS, Ratner N, Nordlund ML, Reid SW, Buchberg AM, Jenkins NA, Parada LF, Copeland NG. Targeted disruption of the neurofibromatosis type-1 gene leads to developmental abnormalities in heart and various neural crest-derived tissues. Genes Dev. 1994;8:1019–1029. doi: 10.1101/gad.8.9.1019. [DOI] [PubMed] [Google Scholar]
- [9].Bronson RT. Rate of occurence of lesions in 20 inbred and hybrid genotypes of rats and mice sacrificed at 6 month intervals during the first years of life. In: Harrison DE, editor. Genetics of Aging II, Telford. 1990. pp. 280–358. [Google Scholar]
- [10].Buzard GS, Enomoto T, Hongyo T, Perantoni AO, Diwan BA, Devor DE, Reed CD, Dove LF, Rice JM. neu mutation in schwannomas induced transplacentally in Syrian golden hamsters by N-nitrosoethylurea: high incidence but low allelic representation. J Cancer Res Clin Oncol. 1999;125:529–540. doi: 10.1007/s004320050313. [DOI] [PubMed] [Google Scholar]
- [11].Carroll SL, Stonecypher MS. Tumor suppressor mutations and growth factor signaling in the pathogenesis of NF1-associated peripheral nerve sheath tumors: II. The role of dysregulated growth factor signaling. J Neuropathol Exp Neurol. 2005;64:1–9. doi: 10.1093/jnen/64.1.1. [DOI] [PubMed] [Google Scholar]
- [12].Cawthon RM, Weiss R, Xu GF, Viskochil D, Culver M, Stevens J, Robertson M, Dunn D, Gesteland R, O’Connell P, et al. A major segment of the neurofibromatosis type 1 gene: cDNA sequence, genomic structure, and point mutations. Cell. 1990;62:193–201. doi: 10.1016/0092-8674(90)90253-b. [DOI] [PubMed] [Google Scholar]
- [13].CBTRUS . In: CBTRUS Statistical Report: Primary Brain and Central Nervous System Tumors Diagnosed in the United States in 2004-2006. C.B.T.R.o.t.U. States, editor. Hinsdale, IL: 2010. [Google Scholar]
- [14].Chen S, Burgin S, McDaniel A, Li X, Yuan J, Chen M, Khalaf W, Clapp DW, Yang FC. Nf1−/− Schwann cell-conditioned medium modulates mast cell degranulation by c-Kit-mediated hyperactivation of phosphatidylinositol 3-kinase. Am J Pathol. 2010;177:3125–3132. doi: 10.2353/ajpath.2010.100369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [15].Cichowski K, Shih TS, Schmitt E, Santiago S, Reilly K, McLaughlin ME, Bronson RT, Jacks T. Mouse models of tumor development in neurofibromatosis type 1. Science. 1999;286:2172–2176. doi: 10.1126/science.286.5447.2172. [DOI] [PubMed] [Google Scholar]
- [16].Courtois-Cox S, Williams S.M. Genther, Reczek EE, Johnson BW, McGillicuddy LT, Johannessen CM, Hollstein PE, MacCollin M, Cichowski K. A negative feedback signaling network underlies oncogene-induced senescence. Cancer cell. 2006;10:459–472. doi: 10.1016/j.ccr.2006.10.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [17].D’Angelo I, Welti S, Bonneau F, Scheffzek K. A novel bipartite phospholipid-binding module in the neurofibromatosis type 1 protein. EMBO Rep. 2006;7:174–179. doi: 10.1038/sj.embor.7400602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [18].Dang I, De Vries GH. Aberrant cAMP Metabolism in NF1 Malignant Peripheral Nerve Sheath Tumor Cells. Neurochem Res. 2011 doi: 10.1007/s11064-011-0433-2. [DOI] [PubMed] [Google Scholar]
- [19].Dang I, Nelson JK, DeVries GH. c-Kit receptor expression in normal human Schwann cells and Schwann cell lines derived from neurofibromatosis type 1 tumors. J.Neurosci.Res. 2005;82:465–471. doi: 10.1002/jnr.20648. [DOI] [PubMed] [Google Scholar]
- [20].Dasgupta B, Dugan LL, Gutmann DH. The neurofibromatosis 1 gene product neurofibromin regulates pituitary adenylate cyclase-activating polypeptide-mediated signaling in astrocytes. J Neurosci. 2003;23:8949–8954. doi: 10.1523/JNEUROSCI.23-26-08949.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [21].DeClue JE, Heffelfinger S, Benvenuto G, Ling B, Li S, Rui W, Vass WC, Viskochil D, Ratner N. Epidermal growth factor receptor expression in neurofibromatosis type 1-related tumors and NF1 animal models. J Clin Invest. 2000;105:1233–1241. doi: 10.1172/JCI7610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [22].Diwan BA, Rehm S, Rice JM. Age- and dose-dependent transplacental carcinogenesis by N-nitrosoethylurea in Syrian golden hamsters. J Cancer Res Clin Oncol. 1996;122:643–652. doi: 10.1007/BF01209026. [DOI] [PubMed] [Google Scholar]
- [23].Druckrey H, Ivankovic S, Preussmann R. Teratogenic and carcinogenic effects in the offspring after single injection of ethylnitrosourea to pregnant rats. Nature. 1966;210:1378–1379. doi: 10.1038/2101378a0. [DOI] [PubMed] [Google Scholar]
- [24].Eckert JM, Byer SJ, Clodfelder-Miller BJ, Carroll SL. Neuregulin-1 beta and neuregulin-1 alpha differentially affect the migration and invasion of malignant peripheral nerve sheath tumor cells. Glia. 2009;57:1501–1520. doi: 10.1002/glia.20866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [25].Frohnert PW, Stonecypher MS, Carroll SL. Constitutive activation of the neuregulin-1/ErbB receptor signaling pathway is essential for the proliferation of a neoplastic Schwann cell line. Glia. 2003;43:104–118. doi: 10.1002/glia.10232. [DOI] [PubMed] [Google Scholar]
- [26].Gitler AD, Zhu Y, Ismat FA, Lu MM, Yamauchi Y, Parada LF, Epstein JA. Nf1 has an essential role in endothelial cells. Nat Genet. 2003;33:75–79. doi: 10.1038/ng1059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Graus-Porta D, Beerli RR, Daly JM, Hynes NE. ErbB-2, the preferred heterodimerization partner of all ErbB receptors, is a mediator of lateral signaling. Embo J. 1997;16:1647–1655. doi: 10.1093/emboj/16.7.1647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [28].Gregorian C, Nakashima J, Dry SM, Nghiemphu PL, Smith KB, Ao Y, Dang J, Lawson G, Mellinghoff IK, Mischel PS, Phelps M, Parada LF, Liu X, Sofroniew MV, Eilber FC, Wu H. PTEN dosage is essential for neurofibroma development and malignant transformation. Proc Natl Acad Sci U S A. 2009;106:19479–19484. doi: 10.1073/pnas.0910398106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Haigis KM, Kendall KR, Wang Y, Cheung A, Haigis MC, Glickman JN, Niwa-Kawakita M, Sweet-Cordero A, Sebolt-Leopold J, Shannon KM, Settleman J, Giovannini M, Jacks T. Differential effects of oncogenic K-Ras and N-Ras on proliferation, differentiation and tumor progression in the colon. Nat Genet. 2008;40:600–608. doi: 10.1038/ngXXXX. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Holtkamp N, Malzer E, Zietsch J, Okuducu AF, Mucha J, Mawrin C, Mautner VF, Schildhaus HU, von Deimling A. EGFR and erbB2 in malignant peripheral nerve sheath tumors and implications for targeted therapy. Neuro Oncol. 2008;10:946–957. doi: 10.1215/15228517-2008-053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Huijbregts RP, Roth KA, Schmidt RE, Carroll SL. Hypertrophic neuropathies and malignant peripheral nerve sheath tumors in transgenic mice overexpressing glial growth factor beta3 in myelinating Schwann cells. J Neurosci. 2003;23:7269–7280. doi: 10.1523/JNEUROSCI.23-19-07269.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [32].Izawa I, Tamaki N, Saya H. Phosphorylation of neurofibromatosis type 1 gene product (neurofibromin) by cAMP-dependent protein kinase. FEBS Lett. 1996;382:53–59. doi: 10.1016/0014-5793(96)00137-8. [DOI] [PubMed] [Google Scholar]
- [33].Jacks T, Shih TS, Schmitt EM, Bronson RT, Bernards A, Weinberg RA. Tumour predisposition in mice heterozygous for a targeted mutation in Nf1. Nat Genet. 1994;7:353–361. doi: 10.1038/ng0794-353. [DOI] [PubMed] [Google Scholar]
- [34].Jones EL, Searle CE, Smith WT. Tumours of the nervous system induced in rats by the neonatal administration of N-ethyl-N-nitrosourea. J Pathol. 1973;109:123–139. doi: 10.1002/path.1711090206. [DOI] [PubMed] [Google Scholar]
- [35].Joseph NM, Mosher JT, Buchstaller J, Snider P, McKeever PE, Lim M, Conway SJ, Parada LF, Zhu Y, Morrison SJ. The loss of Nf1 transiently promotes self-renewal but not tumorigenesis by neural crest stem cells. Cancer cell. 2008;13:129–140. doi: 10.1016/j.ccr.2008.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [36].Joseph NM, Mukouyama YS, Mosher JT, Jaegle M, Crone SA, Dormand EL, Lee KF, Meijer D, Anderson DJ, Morrison SJ. Neural crest stem cells undergo multilineage differentiation in developing peripheral nerves to generate endoneurial fibroblasts in addition to Schwann cells. Development. 2004;131:5599–5612. doi: 10.1242/dev.01429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [37].Kawaguchi K, Oda Y, Saito T, Takahira T, Yamamoto H, Tamiya S, Iwamoto Y, Tsuneyoshi M. Genetic and epigenetic alterations of the PTEN gene in soft tissue sarcomas. Hum.Pathol. 2005;36:357–363. doi: 10.1016/j.humpath.2005.01.017. [DOI] [PubMed] [Google Scholar]
- [38].Kim HA, Ratner N, Roberts TM, Stiles CD. Schwann cell proliferative responses to cAMP and Nf1 are mediated by cyclin D1. J Neurosci. 2001;21:1110–1116. doi: 10.1523/JNEUROSCI.21-04-01110.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [39].Kim HA, Rosenbaum T, Marchionni MA, Ratner N, DeClue JE. Schwann cells from neurofibromin deficient mice exhibit activation of p21ras, inhibition of cell proliferation and morphological changes. Oncogene. 1995;11:325–335. [PubMed] [Google Scholar]
- [40].Kim J, Lo L, Dormand E, Anderson DJ. SOX10 maintains multipotency and inhibits neuronal differentiation of neural crest stem cells. Neuron. 2003;38:17–31. doi: 10.1016/s0896-6273(03)00163-6. [DOI] [PubMed] [Google Scholar]
- [41].King D, Yang G, Thompson MA, Hiebert SW. Loss of neurofibromatosis-1 and p19(ARF) cooperate to induce a multiple tumor phenotype. Oncogene. 2002;21:4978–4982. doi: 10.1038/sj.onc.1205632. [DOI] [PubMed] [Google Scholar]
- [42].Kodaki T, Woscholski R, Hallberg B, Rodriguez-Viciana P, Downward J, Parker PJ. The activation of phosphatidylinositol 3-kinase by Ras. Curr Biol. 1994;4:798–806. doi: 10.1016/s0960-9822(00)00177-9. [DOI] [PubMed] [Google Scholar]
- [43].Kourea HP, Orlow I, Scheithauer BW, Cordon-Cardo C, Woodruff JM. Deletions of the INK4A gene occur in malignant peripheral nerve sheath tumors but not in neurofibromas. Am J Pathol. 1999;155:1855–1860. doi: 10.1016/S0002-9440(10)65504-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [44].Kucenas S, Takada N, Park HC, Woodruff E, Broadie K, Appel B. CNS-derived glia ensheath peripheral nerves and mediate motor root development. Nat Neurosci. 2008;11:143–151. doi: 10.1038/nn2025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [45].Kweh F, Zheng M, Kurenova E, Wallace M, Golubovskaya V, Cance WG. Neurofibromin physically interacts with the N-terminal domain of focal adhesion kinase. Mol Carcinog. 2009;48:1005–1017. doi: 10.1002/mc.20552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [46].Le LQ, Shipman T, Burns DK, Parada LF. Cell of origin and microenvironment contribution for NF1-associated dermal neurofibromas. Cell Stem Cell. 2009;4:453–463. doi: 10.1016/j.stem.2009.03.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [47].Levy P, Vidaud D, Leroy K, Laurendeau I, Wechsler J, Bolasco G, Parfait B, Wolkenstein P, Vidaud M, Bieche I. Molecular profiling of malignant peripheral nerve sheath tumors associated with neurofibromatosis type 1, based on large-scale real-time RT-PCR. Mol Cancer. 2004;3:20. doi: 10.1186/1476-4598-3-20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [48].Li W, Zhu T, Guan KL. Transformation potential of Ras isoforms correlates with activation of phosphatidylinositol 3-kinase but not ERK. J Biol Chem. 2004;279:37398–37406. doi: 10.1074/jbc.M405730200. [DOI] [PubMed] [Google Scholar]
- [49].Liao J, Planchon SM, Wolfman JC, Wolfman A. Growth factor-dependent AKT activation and cell migration requires the function of c-K(B)-Ras versus other cellular ras isoforms. J Biol Chem. 2006;281:29730–29738. doi: 10.1074/jbc.M600668200. [DOI] [PubMed] [Google Scholar]
- [50].Ling BC, Wu J, Miller SJ, Monk KR, Shamekh R, Rizvi TA, Decourten-Myers G, Vogel KS, DeClue JE, Ratner N. Role for the epidermal growth factor receptor in neurofibromatosis-related peripheral nerve tumorigenesis. Cancer cell. 2005;7:65–75. doi: 10.1016/j.ccr.2004.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [51].Maehama T, Dixon JE. The tumor suppressor, PTEN/MMAC1, dephosphorylates the lipid second messenger, phosphatidylinositol 3,4,5-trisphosphate. J Biol Chem. 1998;273:13375–13378. doi: 10.1074/jbc.273.22.13375. [DOI] [PubMed] [Google Scholar]
- [52].Mangoura D, Sun Y, Li C, Singh D, Gutmann DH, Flores A, Ahmed M, Vallianatos G. Phosphorylation of neurofibromin by PKC is a possible molecular switch in EGF receptor signaling in neural cells. Oncogene. 2006;25:735–745. doi: 10.1038/sj.onc.1209113. [DOI] [PubMed] [Google Scholar]
- [53].Marshall CJ. Ras effectors. Curr Opin Cell Biol. 1996;8:197–204. doi: 10.1016/s0955-0674(96)80066-4. [DOI] [PubMed] [Google Scholar]
- [54].Martin GA, Viskochil D, Bollag G, McCabe PC, Crosier WJ, Haubruck H, Conroy L, Clark R, O’Connell P, Cawthon RM, et al. The GAP-related domain of the neurofibromatosis type 1 gene product interacts with ras p21. Cell. 1990;63:843–849. doi: 10.1016/0092-8674(90)90150-d. [DOI] [PubMed] [Google Scholar]
- [55].McQueen M, MacCollin M, Gusella J, Plotkin SR. Patient and physician attitudes regarding clinical trials in neurofibromatosis 1. J Neurosci Nurs. 2008;40:341–345. doi: 10.1097/01376517-200812000-00005. [DOI] [PubMed] [Google Scholar]
- [56].Miller SJ, Jessen WJ, Mehta T, Hardiman A, Sites E, Kaiser S, Jegga AG, Li H, Upadhyaya M, Giovannini M, Muir D, Wallace MR, Lopez E, Serra E, Nielsen GP, Lazaro C, Stemmer-Rachamimov A, Page G, Aronow BJ, Ratner N. Integrative genomic analyses of neurofibromatosis tumours identify SOX9 as a biomarker and survival gene. EMBO Mol Med. 2009;1:236–248. doi: 10.1002/emmm.200900027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [57].Miller SJ, Rangwala F, Williams J, Ackerman P, Kong S, Jegga AG, Kaiser S, Aronow BJ, Frahm S, Kluwe L, Mautner V, Upadhyaya M, Muir D, Wallace M, Hagen J, Quelle DE, Watson MA, Perry A, Gutmann DH, Ratner N. Large-scale molecular comparison of human schwann cells to malignant peripheral nerve sheath tumor cell lines and tissues. Cancer Res. 2006;66:2584–2591. doi: 10.1158/0008-5472.CAN-05-3330. [DOI] [PubMed] [Google Scholar]
- [58].Nielsen GP, Stemmer-Rachamimov AO, Ino Y, Moller MB, Rosenberg AE, Louis DN. Malignant transformation of neurofibromas in neurofibromatosis 1 is associated with CDKN2A/p16 inactivation. Am J Pathol. 1999;155:1879–1884. doi: 10.1016/S0002-9440(10)65507-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [59].Nikitin A, Ballering LA, Lyons J, Rajewsky MF. Early mutation of the neu (erbB-2) gene during ethylnitrosourea-induced oncogenesis in the rat Schwann cell lineage. Proc Natl Acad Sci U S A. 1991;88:9939–9943. doi: 10.1073/pnas.88.22.9939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [60].Ohgaki H, Vogeley KT, Kleihues P, Wechsler W. neu mutations and loss of normal allele in schwannomas induced by N-ethyl-N-nitrosourea in rats. Cancer Lett. 1993;70:45–50. doi: 10.1016/0304-3835(93)90073-i. [DOI] [PubMed] [Google Scholar]
- [61].Oka T, Xu J, Kaiser RA, Melendez J, Hambleton M, Sargent MA, Lorts A, Brunskill EW, Dorn GW, 2nd, Conway SJ, Aronow BJ, Robbins J, Molkentin JD. Genetic manipulation of periostin expression reveals a role in cardiac hypertrophy and ventricular remodeling. Circ Res. 2007;101:313–321. doi: 10.1161/CIRCRESAHA.107.149047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [62].Perrone F, Da Riva L, Orsenigo M, Losa M, Jocolle G, Millefanti C, Pastore E, Gronchi A, Pierotti MA, Pilotti S. PDGFRA, PDGFRB, EGFR, and downstream signaling activation in malignant peripheral nerve sheath tumor. Neuro Oncol. 2009;11:725–736. doi: 10.1215/15228517-2009-003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [63].Perry A, Kunz SN, Fuller CE, Banerjee R, Marley EF, Liapis H, Watson MA, Gutmann DH. Differential NF1, p16, and EGFR patterns by interphase cytogenetics (FISH) in malignant peripheral nerve sheath tumor (MPNST) and morphologically similar spindle cell neoplasms. J Neuropathol Exp Neurol. 2002;61:702–709. doi: 10.1093/jnen/61.8.702. [DOI] [PubMed] [Google Scholar]
- [64].Ridley AJ, Paterson HF, Noble M, Land H. Ras-mediated cell cycle arrest is altered by nuclear oncogenes to induce Schwann cell transformation. Embo J. 1988;7:1635–1645. doi: 10.1002/j.1460-2075.1988.tb02990.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [65].Rodriguez-Viciana P, Warne PH, Dhand R, Vanhaesebroeck B, Gout I, Fry MJ, Waterfield MD, Downward J. Phosphatidylinositol-3-OH kinase as a direct target of Ras. Nature. 1994;370:527–532. doi: 10.1038/370527a0. [DOI] [PubMed] [Google Scholar]
- [66].Rodriguez-Viciana P, Warne PH, Vanhaesebroeck B, Waterfield MD, Downward J. Activation of phosphoinositide 3-kinase by interaction with Ras and by point mutation. Embo J. 1996;15:2442–2451. [PMC free article] [PubMed] [Google Scholar]
- [67].Sabah M, Cummins R, Leader M, Kay E. Loss of p16 (INK4A) expression is associated with allelic imbalance/loss of heterozygosity of chromosome 9p21 in microdissected malignant peripheral nerve sheath tumors. Appl Immunohistochem Mol Morphol. 2006;14:97–102. doi: 10.1097/01.pai.0000143787.80564.f5. [DOI] [PubMed] [Google Scholar]
- [68].Saito H, Yoshida T, Yamazaki H, Suzuki N. Conditional N-rasG12V expression promotes manifestations of neurofibromatosis in a mouse model. Oncogene. 2007;26:4714–4719. doi: 10.1038/sj.onc.1210250. [DOI] [PubMed] [Google Scholar]
- [69].Segatto O, King CR, Pierce JH, Di Fiore PP, Aaronson SA. Different structural alterations upregulate in vitro tyrosine kinase activity and transforming potency of the erbB-2 gene. Mol Cell Biol. 1988;8:5570–5574. doi: 10.1128/mcb.8.12.5570. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [70].Seizinger BR, Rouleau GA, Ozelius LJ, Lane AH, Faryniarz AG, Chao MV, Huson S, Korf BR, Parry DM, Pericak-Vance MA, et al. Genetic linkage of von Recklinghausen neurofibromatosis to the nerve growth factor receptor gene. Cell. 1987;49:589–594. doi: 10.1016/0092-8674(87)90534-4. [DOI] [PubMed] [Google Scholar]
- [71].Sliwkowski MX, Schaefer G, Akita RW, Lofgren JA, Fitzpatrick VD, Nuijens A, Fendly BM, Cerione RA, Vandlen RL, Carraway KL., 3rd Coexpression of erbB2 and erbB3 proteins reconstitutes a high affinity receptor for heregulin. J Biol Chem. 1994;269:14661–14665. [PubMed] [Google Scholar]
- [72].Snider P, Hinton RB, Moreno-Rodriguez RA, Wang J, Rogers R, Lindsley A, Li F, Ingram DA, Menick D, Field L, Firulli AB, Molkentin JD, Markwald R, Conway SJ. Periostin is required for maturation and extracellular matrix stabilization of noncardiomyocyte lineages of the heart. Circ Res. 2008;102:752–760. doi: 10.1161/CIRCRESAHA.107.159517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [73].Stemmer-Rachamimov AO, Louis DN, Nielsen GP, Antonescu CR, Borowsky AD, Bronson RT, Burns DK, Cervera P, McLaughlin ME, Reifenberger G, Schmale MC, MacCollin M, Chao RC, Cichowski K, Kalamarides M, Messerli SM, McClatchey AI, Niwa-Kawakita M, Ratner N, Reilly KM, Zhu Y, Giovannini M. Comparative pathology of nerve sheath tumors in mouse models and humans. Cancer Res. 2004;64:3718–3724. doi: 10.1158/0008-5472.CAN-03-4079. [DOI] [PubMed] [Google Scholar]
- [74].Stonecypher MS, Byer SJ, Grizzle WE, Carroll SL. Activation of the neuregulin-1/ErbB signaling pathway promotes the proliferation of neoplastic Schwann cells in human malignant peripheral nerve sheath tumors. Oncogene. 2005;24:5589–5605. doi: 10.1038/sj.onc.1208730. [DOI] [PubMed] [Google Scholar]
- [75].Storlazzi CT, Brekke HR, Mandahl N, Brosjo O, Smeland S, Lothe RA, Mertens F. Identification of a novel amplicon at distal 17q containing the BIRC5/SURVIVIN gene in malignant peripheral nerve sheath tumours. J Pathol. 2006;209:492–500. doi: 10.1002/path.1998. [DOI] [PubMed] [Google Scholar]
- [76].Tischler AS, Shih TS, Williams BO, Jacks T. Characterization of Pheochromocytomas in a Mouse Strain with a Targeted Disruptive Mutation of the Neurofibromatosis Gene Nf1. Endocr Pathol. 1995;6:323–335. doi: 10.1007/BF02738732. [DOI] [PubMed] [Google Scholar]
- [77].Tunzi M, Gray GR. Common skin conditions during pregnancy. Am Fam Physician. 2007;75:211–218. [PubMed] [Google Scholar]
- [78].Tzahar E, Waterman H, Chen X, Levkowitz G, Karunagaran D, Lavi S, Ratzkin BJ, Yarden Y. A hierarchical network of interreceptor interactions determines signal transduction by Neu differentiation factor/neuregulin and epidermal growth factor. Mol Cell Biol. 1996;16:5276–5287. doi: 10.1128/mcb.16.10.5276. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [79].Vandenbroucke I, Van Oostveldt P, Coene E, De Paepe A, Messiaen L. Neurofibromin is actively transported to the nucleus. FEBS Lett. 2004;560:98–102. doi: 10.1016/S0014-5793(04)00078-X. [DOI] [PubMed] [Google Scholar]
- [80].Vanhaesebroeck B, Welham MJ, Kotani K, Stein R, Warne PH, Zvelebil MJ, Higashi K, Volinia S, Downward J, Waterfield MD. P110delta, a novel phosphoinositide 3-kinase in leukocytes. Proc Natl Acad Sci U S A. 1997;94:4330–4335. doi: 10.1073/pnas.94.9.4330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [81].Vogel KS, Brannan CI, Jenkins NA, Copeland NG, Parada LF. Loss of neurofibromin results in neurotrophin-independent survival of embryonic sensory and sympathetic neurons. Cell. 1995;82:733–742. doi: 10.1016/0092-8674(95)90470-0. [DOI] [PubMed] [Google Scholar]
- [82].Vogel KS, Parada LF. Sympathetic neuron survival and proliferation are prolonged by loss of p53 and neurofibromin. Mol Cell Neurosci. 1998;11:19–28. doi: 10.1006/mcne.1998.0670. [DOI] [PubMed] [Google Scholar]
- [83].Vojtek AB, Der CJ. Increasing complexity of the Ras signaling pathway. J Biol Chem. 1998;273:19925–19928. doi: 10.1074/jbc.273.32.19925. [DOI] [PubMed] [Google Scholar]
- [84].Wade TR, Wade SL, Jones HE. Skin changes and diseases associated with pregnancy. Obstet Gynecol. 1978;52:233–242. [PubMed] [Google Scholar]
- [85].Wallace MR, Marchuk DA, Andersen LB, Letcher R, Odeh HM, Saulino AM, Fountain JW, Brereton A, Nicholson J, Mitchell AL, et al. Type 1 neurofibromatosis gene: identification of a large transcript disrupted in three NF1 patients. Science. 1990;249:181–186. doi: 10.1126/science.2134734. [DOI] [PubMed] [Google Scholar]
- [86].Weiner DB, Kokai Y, Wada T, Cohen JA, Williams WV, Greene MI. Linkage of tyrosine kinase activity with transforming ability of the p185neu oncoprotein. Oncogene. 1989;4:1175–1183. [PubMed] [Google Scholar]
- [87].Weiner DB, Liu J, Cohen JA, Williams WV, Greene MI. A point mutation in the neu oncogene mimics ligand induction of receptor aggregation. Nature. 1989;339:230–231. doi: 10.1038/339230a0. [DOI] [PubMed] [Google Scholar]
- [88].Welti S, Fraterman S, D’Angelo I, Wilm M, Scheffzek K. The sec14 homology module of neurofibromin binds cellular glycerophospholipids: mass spectrometry and structure of a lipid complex. J Mol Biol. 2007;366:551–562. doi: 10.1016/j.jmb.2006.11.055. [DOI] [PubMed] [Google Scholar]
- [89].Wu J, Williams JP, Rizvi TA, Kordich JJ, Witte D, Meijer D, Stemmer-Rachamimov AO, Cancelas JA, Ratner N. Plexiform and dermal neurofibromas and pigmentation are caused by Nf1 loss in desert hedgehog-expressing cells. Cancer cell. 2008;13:105–116. doi: 10.1016/j.ccr.2007.12.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [90].Wymann MP, Pirola L. Structure and function of phosphoinositide 3-kinases. Biochim Biophys Acta. 1998;1436:127–150. doi: 10.1016/s0005-2760(98)00139-8. [DOI] [PubMed] [Google Scholar]
- [91].Xu GF, O’Connell P, Viskochil D, Cawthon R, Robertson M, Culver M, Dunn D, Stevens J, Gesteland R, White R, et al. The neurofibromatosis type 1 gene encodes a protein related to GAP. Cell. 1990;62:599–608. doi: 10.1016/0092-8674(90)90024-9. [DOI] [PubMed] [Google Scholar]
- [92].Yang FC, Chen S, Clegg T, Li X, Morgan T, Estwick SA, Yuan J, Khalaf W, Burgin S, Travers J, Parada LF, Ingram DA, Clapp DW. Nf1+/− mast cells induce neurofibroma like phenotypes through secreted TGF-beta signaling. Hum Mol Genet. 2006;15:2421–2437. doi: 10.1093/hmg/ddl165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [93].Yang FC, Ingram DA, Chen S, Hingtgen CM, Ratner N, Monk KR, Clegg T, White H, Mead L, Wenning MJ, Williams DA, Kapur R, Atkinson SJ, Clapp DW. Neurofibromin-deficient Schwann cells secrete a potent migratory stimulus for Nf1+/− mast cells. J Clin Invest. 2003;112:1851–1861. doi: 10.1172/JCI19195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [94].Yang FC, Ingram DA, Chen S, Zhu Y, Yuan J, Li X, Yang X, Knowles S, Horn W, Li Y, Zhang S, Yang Y, Vakili ST, Yu M, Burns D, Robertson K, Hutchins G, Parada LF, Clapp DW. Nf1-dependent tumors require a microenvironment containing Nf1+/−- and c-kit-dependent bone marrow. Cell. 2008;135:437–448. doi: 10.1016/j.cell.2008.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [95].Zhang J, Lodish HF. Identification of K-ras as the major regulator for cytokine-dependent Akt activation in erythroid progenitors in vivo. Proc Natl Acad Sci U S A. 2005;102:14605–14610. doi: 10.1073/pnas.0507446102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [96].Zheng H, Chang L, Patel N, Yang J, Lowe L, Burns DK, Zhu Y. Induction of abnormal proliferation by nonmyelinating schwann cells triggers neurofibroma formation. Cancer cell. 2008;13:117–128. doi: 10.1016/j.ccr.2008.01.002. [DOI] [PubMed] [Google Scholar]
- [97].Zhu Y, Ghosh P, Charnay P, Burns DK, Parada LF. Neurofibromas in NF1: Schwann cell origin and role of tumor environment. Science. 2002;296:920–922. doi: 10.1126/science.1068452. [DOI] [PMC free article] [PubMed] [Google Scholar]