Abstract
Grizzly bears (Ursus arctos horribilis) are inactive for up to 6 months during hibernation. They undergo profound seasonal changes in food intake, body mass, and energy expenditure. The circa-annual regulation of metabolism is poorly understood. In the present study, we measured plasma ghrelin, leptin, obestatin, and neuropeptide-Y (NPY) levels, hormones known to be involved in the regulation of energy homeostasis, in ten grizzly bears. Blood samples were collected during the active summer period, early hibernation and late hibernation. Plasma levels of leptin, obestatin, and NPY did not change between the active and the hibernation periods. Plasma total ghrelin and desacyl-ghrelin concentrations significantly decreased during the inactive winter period compared to summer levels. The elevated ghrelin levels may help enhance body mass during pre-hibernation, while the low plasma ghrelin concentrations during hibernation season may contribute to the maintenance of hypophagia, low energy utilization and behavioral inactivity. Our results suggest that ghrelin plays a potential role in the regulation of metabolic changes and energy homeostasis during hibernation in grizzly bears.
Keywords: ghrelin, leptin, hibernation, food intake, energy balance
1. Introduction
Many mammals undergo a unique state of energy conservation, hibernation, under harsh climatic conditions. Grizzly bears (Ursus arctos horribilis) do not eat, drink, urinate, or defecate and demonstrate minimal activity during their annual 4–6-month hibernation period. Energy expenditure is greatly reduced and body temperature drops to 32–34°C. Metabolic energy is derived from fat catabolism during this time. The bears loose 20–25 % of their body mass [7]. It is likely that hormones, such as ghrelin and leptin, which are involved in the regulation of energy homeostasis in non-hibernating species, also have a role in eliciting the characteristic hypo-metabolic state during hibernation. Ghrelin is produced predominantly by endocrine cells of the stomach, and it is also a neurotransmitter in hypothalamic neuronal circuits [reviewed in Ref. 6]. Leptin is released from white adipose tissue; circulating leptin levels are proportional to the size of the adipose tissue. The effects of the two hormones and their role in regulating energy homeostasis are the opposite. The major targets for both hormones are the neuropeptide-Y (NPY)-containing neurons in the arcuate nucleus where ghrelin and leptin receptors are co-localized [reviewed in Ref. 5]. Ghrelin stimulates these neurons, leading to increased NPY expression and release and elevated food intake; leptin elicits the opposite effects. Ghrelin decreases energy expenditure which leads to deposition of fat, whereas leptin promotes the mobilization of energy stores and increases energy expenditure. The role of ghrelin and leptin in the regulation of vigilance also appears to be opposite. Intracerebroventricular administration [10] or intrahypothalamic microinjections [11] of ghrelin strongly stimulate wakefulness in rats. Disrupted ghrelin signaling, on the other hand, abolishes the arousal-inducing effects of fasting and mild stress [2]. Central administration of leptin stimulates sleep in rats [1].
Besides ghrelin, there are at least two other peptides that originate from the same pre-proghrelin gene. Different post-translational processing leads to the formation of desacyl-ghrelin and obestatin. Desacyl-ghrelin does not bind to the known ghrelin receptor. Some, but not all [reviewed in Ref. 4], observations suggest an influence of desacyl-ghrelin on food intake and energy homeostasis. The effects of obestatin on feeding [14], metabolism and sleep [13] appear to be the opposite to those of ghrelin. The aim of our study was to determine the potential role of ghrelin, leptin, obestatin and NPY in the regulation of energy homeostasis during hibernation in grizzly bears.
2. Materials and Methods
2.1. Animals
Ten grizzly bears (7 females, 3 males) were used in this study. Their ages were between 1–12 years. The animals were housed at the Washington State University Bear Research, Education and Conservation Facility. The animals were maintained according to the Bear Care and Colony Health Standard Operating Procedures, and procedures were approved by the Washington State University (WSU) Institutional Animal Care Use Committee (IACUC) based on the National Institutes of Health (NIH) guidelines. In early October, food was gradually reduced until completely withdrawn in late October. Hibernation began during the last week of October when feeding ceased and ended at the second week of March when feeding resumed. Water was available ad libitum during the entire hibernation period. Bears hibernated in pairs in unheated pens (3 m×3 m×2.5 m) with straw provided for bedding. They had continuous access through a small door to an outdoor area (3 m×5 m×5 m) that was covered on all sides to minimize external noise and stimulation. Because the pens were open to the outside, the bears experienced daily light and temperature fluctuations.
2.2 Sample collection
Blood samples were collected during the active summer period (6 samples were collected in July 2005 and 4 samples were collected at the beginning of September 2007) and during early hibernation period (in the first 2 months of hibernation) from all animals. Additional blood samples were taken from 4 bears during the late hibernation period (about 4 months after the beginning of hibernation). Blood was drawn from the jugular or dorsal metatarsal vein while the bears were anesthetized (tiletamine HCl/zolazepam HCl, 2.5 mg/kg each, during the active and 1 mg/kg each, during the hibernation period, given intramuscularly). Blood was collected into EDTA-containing tubes, centrifuged and the plasma was stored at −80°C until assayed.
2.3. Hormone measurements
Commercially available radioimmunoassays (Phoenix Pharmaceuticals, Belmont, CA) were used to determine total ghrelin (developed for rat and mouse ghrelin measurements), obestatin (developed for human and monkey), NPY (developed for human, rat, and mouse NPY measurements, Phoenix Pharmaceuticals, Belmont, CA) and leptin (multi-species, LINCO Research, St. Charles, MO) concentrations. Desacyl-ghrelin was measured by enzyme immunoassay (developed for rat desacyl-ghrelin measurements, SPI-BIO, France). The hormones were measured in duplicate with a detection limit of 10 pg/ml for ghrelin, 30 pg/ml for obestatin, 10 pg/ml for NPY, 0.5 ng/ml for leptin and 10 pg/ml for desacyl-ghrelin. The intra- and interassay coefficients of variation were less than 8.2 % and 12.3 %, respectively.
2.4. Statistics
Paired t-tests were used to compare mean values in the active and early hibernation periods (n = 10). One-way repeated measures ANOVA was used to compare values of hormone concentrations among the active, early hibernation, and late hibernation periods (n = 4). When ANOVA indicated significant effects, the significantly different group(s) were identified by Holm-Sidak method. A p < 0.05 level was considered to be significant in all tests.
3. Results and Discussion
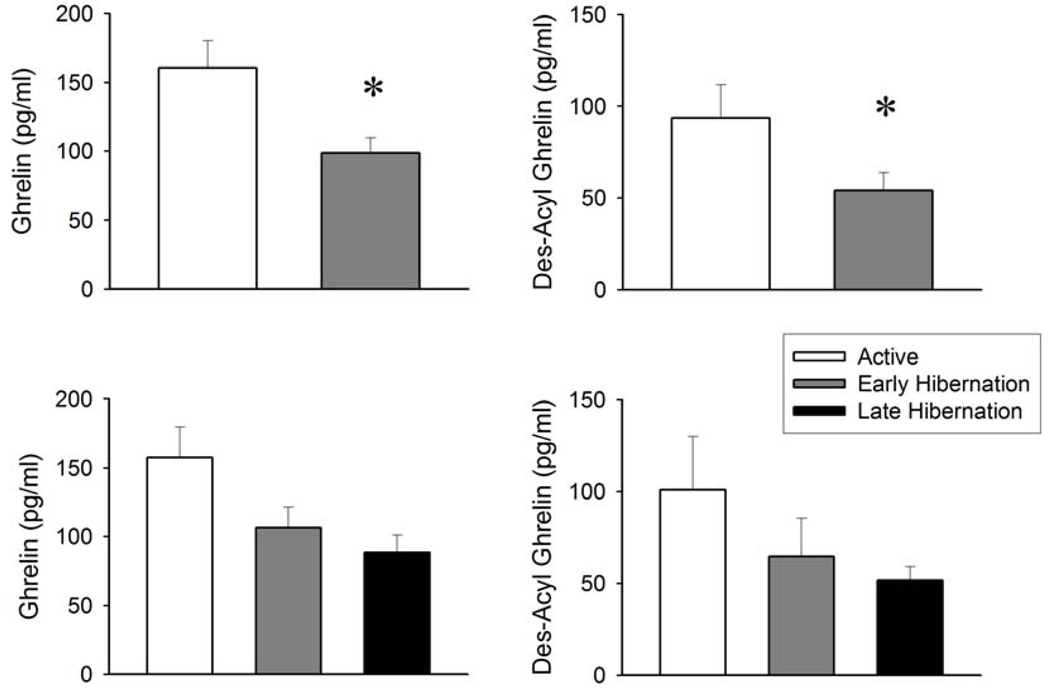
Plasma levels of obestatin and NPY did not differ between pre-hibernation and hibernation. Plasma leptin levels tended to decrease during late-hibernation relative to active and early-hibernation periods; these changes, however, did not reach the level of statistical significance (Table 1.). There were significant differences in plasma total ghrelin and desacyl-ghrelin levels between the active and the hibernation periods (Figure 1.). Plasma total ghrelin concentrations were significantly lower during hibernation than during summer. There was no additional decrease toward the end of the hibernation period. Desacyl-ghrelin plasma concentrations showed a similar pattern, they were reduced significantly during hibernation. The total ghrelin/desacyl-ghrelin ratio did not change during hibernation suggesting that plasma levels of the acylated ghrelin concentrations changed in a manner similar to those of total ghrelin and desacyl-ghrelin.
Table 1.
Mean ± SE values of plasma NPY, obestatin, and leptin concentrations during the active and hibernation periods in grizzly bears. Plasma levels of NPY and obestatin did not differ between pre-hibernation and hibernation. There was a tendency towards decreased plasma leptin levels during late-hibernation relative to active and early-hibernation periods
| NPY (pg/ml) | Obestatin (pg/ml) | Leptin (ng/ml) | |
|---|---|---|---|
| Active (n = 10) | 35.1 ± 5.21 | 159.0 ± 7.92 | 1.6 ± 0.19 |
| Hibernation (n = 10) | 44.9 ± 8.38 | 155.3 ± 9.79 | 2.0 ± 0.30 |
| Active (n = 4) | 43.5 ± 10.1 | 152.2 ± 9.24 | 2.0 ± 0.23 |
| Early-hibernation (n = 4) | 63.8 ± 14.3 | 142.4 ± 17.9 | 2.5 ± 0.55 |
| Late-hibernation (n = 4) | 48.7 ± 5.73 | 144.7 ± 9.43 | 1.0 ± 0.17 |
Figure 1.
Plasma total ghrelin and desacyl-ghrelin levels (mean ± SE) during the active and the hibernation periods (n = 10, top panels); and during the active, the early hibernation, and the late hibernation periods (n = 4, bottom panels). * Significant difference between the active and the hibernation periods (paired t-test, p < 0.05).
Ghrelin stimulates food intake, reduces lipolysis and suppresses energy expenditure. High ghrelin levels may facilitate the accumulation of fat during pre-hibernation, while the lower ghrelin concentrations during hibernation period may allow lipolysis and provide a satiety signal. The lower ghrelin levels may work in synergy with high leptin levels in this process, since plasma leptin levels remained high during early period of hibernation. The tendency towards decreased leptin levels during the late hibernation period may reflect the decreasing size of the fat depots. The raccoon dog is species with passive wintering strategy. In the fall, raccoon dog accumulates significant fat stores. Their body temperature remains close to normal in the winter, there are occasional periods of arousal, even food intake, but the raccoon dog usually fasts during this period. Our finding with leptin levels are similar to the data observed in the raccoon dog, that is leptin concentrations did not change during the winter fasting period [8,9]. In raccoon dog, plasma ghrelin levels increased during winter fasting in one study [9] and slightly decreased in another experiment [8]. Fasting is a strong stimulus for ghrelin secretion in rats, mice and humans [reviewed in Ref. 6]. In bears, however, plasma ghrelin levels were suppressed during hibernation as compared to the active, summer period. This suggests that fasting per se is less salient signal to stimulate ghrelin levels in this species and ghrelin levels are likely affected by the overall adiposity of the animal. Similarly low ghrelin levels were observed during hibernation in the golden-mantled ground squirrel which is a deep hibernator species [3]. Ghrelin is a potent arousal-stimulating hormone [10,11]. Suppressed ghrelin production in mice deepens fasting-induced torpor bouts in cold environment [12]. We posit that suppressed ghrelin levels may be important for maintaining the behavioral quiescence and lower metabolism during hibernation in the grizzly bear.
Research Highlights.
-
>
Metabolic hormone levels were determined in hibernating grizzly bears.
-
>
Ghrelin levels were lower during hibernation compared to the summer period.
-
>
Suppressed ghrelin may allow behavioral quiescence and low metabolism in hibernation.
Acknowledgements
This work was supported in part by the National Institutes of Health (USA) Grant Nos. NS027250 and NS031453 to JK.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
János Gardi, Email: jgardi@vetmed.wsu.edu.
O. Lynne Nelson, Email: olnelson@wsu.edu.
Charles T. Robbins, Email: ctrobbins@wsu.edu.
Éva Szentirmai, Email: eszentirmai@wsu.edu.
Levente Kapás, Email: kapas@wsu.edu.
James M. Krueger, Email: krueger@vetmed.wsu.edu.
References
- 1.Chang H-Y, Kapás L. The effects of leptin on sleep and brain temperature in rats. Soc. Neurosci. Abstr. 1997;23(Part 2):1846. [Google Scholar]
- 2.Esposito M, Pellinen J, Kapás L, Szentirmai É. Fasting-and novel environment-induced arousal is attenuated in mice lacking functional ghrelin receptor; Program No. 300.24.2010 Neuroscience Meeting Planner; San Diego, CA: Society for Neuroscience; 2010. Online. [Google Scholar]
- 3.Healy JE, Ostrom CE, Wilkerson GK, Florant GL. Plasma ghrelin concentrations change with physiological state in a sciurid hibernator (Spermophilus lateralis) Gen. Comp. Endocrinol. 2010;166:372–378. doi: 10.1016/j.ygcen.2009.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Inhoff T, Wiedenmann B, Klapp BF, Mönnikes H, Kobelt P. Is desacyl ghrelin a modulator of food intake. Peptides. 2009;30:991–994. doi: 10.1016/j.peptides.2009.01.019. [DOI] [PubMed] [Google Scholar]
- 5.Jovanovic Z, Yeo GS. Central leptin signalling: Beyond the arcuate nucleus. Auton. Neurosci. 2010;156:8–14. doi: 10.1016/j.autneu.2010.05.008. [DOI] [PubMed] [Google Scholar]
- 6.Kojima M, Kangawa K. Ghrelin: from gene to physiological function. Results Probl. Cell Differ. 2010;50:185–205. doi: 10.1007/400_2009_28. [DOI] [PubMed] [Google Scholar]
- 7.Nelson OL, Robbins CT. Cardiac function adaptations in hibernating grizzly bears (Ursus arctos horribilis) J. Comp. Physiol. B. 2010;180:465–473. doi: 10.1007/s00360-009-0421-x. [DOI] [PubMed] [Google Scholar]
- 8.Nieminen P, Mustonen AM, Asikainen J, Hyvärinen H. Seasonal weight regulation of the raccoon dog (Nyctereutes procyonoides): interactions between melatonin, leptin, ghrelin, and growth hormone. J. Biol. Rhythms. 2002;17:155–163. doi: 10.1177/074873040201700206. [DOI] [PubMed] [Google Scholar]
- 9.Nieminen P, Saarela S, Pyykönen T, Asikainen J, Mononen J, Mustonen AM. Endocrine response to fasting in the overwintering captive raccoon dog (Nyctereutes procyonoides) J. Exp. Zool. A Comp. Exp. Biol. 2004;301:919–929. doi: 10.1002/jez.a.126. [DOI] [PubMed] [Google Scholar]
- 10.Szentirmai É, Hajdú I, Obál F, Jr, Krueger JM. Ghrelin-induced sleep responses in ad libitum fed and food-restricted rats. Brain Res. 2006;1088:131–140. doi: 10.1016/j.brainres.2006.02.072. [DOI] [PubMed] [Google Scholar]
- 11.Szentirmai É, Kapás L, Krueger JM. Ghrelin microinjection into forebrain sites induces wakefulness and feeding in rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2007;292:R575–R585. doi: 10.1152/ajpregu.00448.2006. [DOI] [PubMed] [Google Scholar]
- 12.Szentirmai É, Kapás L, Sun Y, Smith RG, Krueger JM. The preproghrelin gene is required for the normal integration of thermoregulation and sleep in mice. Proc. Natl. Acad. Sci. USA. 2009;106:14069–14074. doi: 10.1073/pnas.0903090106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Szentirmai É, Krueger JM. Obestatin alters sleep in rats. Neurosci Lett. 2006;404:222–226. doi: 10.1016/j.neulet.2006.05.053. [DOI] [PubMed] [Google Scholar]
- 14.Zhang JV, Ren PG, Avsian-Kretchmer O, Luo CW, Rauch R, Klein C, Hsueh AJ. Obestatin, a peptide encoded by the ghrelin gene, opposes ghrelin's effects on food intake. Science. 2005;310:996–999. doi: 10.1126/science.1117255. [DOI] [PubMed] [Google Scholar]