Abstract
Background
Having opioid dependence and HIV infection are associated with poor HIV-related treatment outcomes.
Methods
HIV-infected, opioid-dependent subjects (N = 295) recruited from 10 clinical sites initiated buprenorphine/naloxone (BUP/NX) and were assessed at baseline and quarterly for 12 months. Primary outcomes included receiving antiretroviral therapy (ART), HIV-1 RNA suppression, and mean changes in CD4 lymphocyte count. Analyses were stratified for the 119 subjects not on ART at baseline. Generalized estimating equations were deployed to examine time-dependent correlates for each outcome.
Results
At baseline, subjects on ART (N = 176) were more likely than those not on ART (N = 119) to be older, heterosexual, have lower alcohol addiction severity scores, and lower HIV-1 RNA levels; they were less likely to be homeless and report sexual risk behaviors. Subjects initiating BUP/NX (N = 295) were significantly more likely to initiate or remain on ART and improve CD4 counts over time compared with baseline; however, these improvements were not significantly improved by longer retention on BUP/NX. Retention on BUP/NX for three or more quarters was, however, significantly associated with increased likelihood of initiating ART (β = 1.34 [1.18, 1.53]) and achieve viral suppression (β = 1.25 [1.10, 1.42]) for the 64 of 119 (54%) subjects not on ART at baseline compared with the 55 subjects not retained on BUP/NX. In longitudinal analyses, being on ART was positively associated with increasing time of observation from baseline and higher mental health quality of life scores (β = 1.25 [1.06, 1.46]) and negatively associated with being homo- or bisexual (β = 0.55 [0.35, 0.97]), homeless (β = 0.58 [0.34, 0.98]), and increasing levels of alcohol addiction severity (β = 0.17 [0.03, 0.88]). The strongest correlate of achieving viral suppression was being on ART (β = 10.27 [5.79, 18.23]). Female gender (β = 1.91 [1.07, 3.41]), Hispanic ethnicity (β = 2.82 [1.44, 5.49]), and increased general health quality of life (β = 1.02 [1.00,1.04]) were also independently correlated with viral suppression. Improvements in CD4 lymphocyte count were significantly associated with being on ART and increased over time.
Conclusions
Initiating BUP/NX in HIV clinical care settings is feasible and correlated with initiation of ART and improved CD4 lymphocyte counts. Longer retention on BPN/NX was not associated with improved prescription of ART, viral suppression, or CD4 lymphocyte counts for the overall sample in which the majority was already prescribed ART at baseline. Among those retained on BUP/NX, HIV treatment outcomes did not worsen and were sustained. Increasing time on BUP/NX, however, was especially important for improving HIV treatment outcomes for those not on ART at baseline, the group at highest risk for clinical deterioration. Retaining subjects on BUP/NX is an important goal for sustaining HIV treatment outcomes for those on ART and improving them for those who are not. Comorbid substance use disorders (especially alcohol), mental health problems, and quality–of-life indicators independently contributed to HIV treatment outcomes among HIV-infected persons with opioid dependence, suggesting the need for multidisciplinary treatment strategies for this population.
Keywords: HIV, acquired immunodeficiency syndrome, buprenorphine, antiretroviral therapy, CD4, HIV-1 RNA, longitudinal cohort, substance use disorders, opioid dependence, healthcare integration, addiction severity, quality of life, alcohol dependence
Introduction
HIV and opioid dependence remain as intertwined epidemics in the United States, contributing to poor health outcomes.1,2 There are an estimated 2.4 million opioid-dependent Americans, of which approximately 900,000 are dependent on heroin; many are infected with or at risk for HIV infection.3–6 Injectable opioids (especially heroin) have figured prominently in the HIV epidemic in the United States and abroad, although opioids can also be taken orally, intranasally, and smoked. Injection drug users and their partners and children currently constitute 36% of the cumulative AIDS cases in the United States.7 Transmission of HIV through injection drug use remains significant, accounting for approximately 10,000 of the 56,000 new HIV infections each year and may be associated with increasing prevalence of resistant HIV.8–10
HIV clinicians can effectively reduce HIV transmission by counseling patients on the reduction of HIV risk-taking behaviors,11 treating12,13 and facilitating adherence to HIV therapies.14 The most effective treatment strategy for the management of opioid dependence remains pharmacotherapies such as prescription of methadone and buprenorphine.15,16 Methadone maintenance remains highly structured and is limited to licensed treatment settings, limiting access to treatment for providers and patients alike. Currently, methadone treatment is available to only 15% to 20% of opioid-dependent patients in the United States.17
The introduction of the partial mu opioid agonist buprenorphine in 2002 has expanded treatment options for opioid dependence; empiric evidence supports its use as an effective treatment.18–22 Buprenorphine is commonly prescribed in a coformulated tablet with naloxone (buprenorphine/naloxone) and administered sublingually. The unique pharmacology and regulatory status of buprenorphine/naloxone (BUP/NX) permits the medication's prescription in primary care practices by physicians who meet specific criteria. The Drug Abuse Treatment Act of 2000 (DATA 2000) allows for the provision of opioid agonist therapy beyond structured methadone clinics, thereby potentially expanding access to opioid dependence treatment from physicians experienced in providing HIV care.23
The availability of potent combination antiretroviral therapy (ART) is cost-effective and has resulted in impressive reductions in HIV-1 RNA levels, increases in CD4 lymphocyte counts, and reductions in both morbidity and mortality. Despite these gains in transforming HIV into a chronic and treatable condition, individuals with substance use disorders have benefited less from treatment than others.1 Reasons for decreased benefit from ART among drug users are complex and include decreased ART prescription24 and, once prescribed, decreased adherence to ART.25 Prescription of ART is critical to derive benefit for HIV treatment outcomes. Although BUP/NX maintenance has resulted in impressive drug treatment outcomes, similar to those found in methadone-treated patients, there are little data to examine its longitudinal and naturalistic impact on HIV treatment outcomes.26 Indeed, compared with those who received offsite referral to substance abuse treatment from HIV clinical care settings in a randomized controlled trial, those receiving BUP/NX on-site were more likely to remain engaged in HIV treatment, but other beneficial HIV treatment outcomes were not demonstrated (eg, initiation of ART and improvements in viral load and CD4).27 Therefore, the purpose of the current study was to assess the effect of BUP/NX maintenance on HIV treatment outcomes, including receipt of ART and changes in CD4 counts and viral suppression in a naturalistic observational study. Moreover, to determine those cofactors that may contribute to these outcomes, other patient characteristics were assessed for contribution to HIV treatment outcomes.
Methods
Setting
In September 2004, 10 geographically dispersed HIV clinical sites developed different models for integrating HIV primary care and BUP/NX treatment for opioid dependence as part of the Special Projects of National Significance initiative funded by the Health Resources and Services Administration.28 Although treatment models varied, all sites participated in a national, multisite evaluation using uniform eligibility criteria, cross-site assessments, and surveillance intervals. Each site and the national evaluation center at The New York Academy of Medicine obtained Institutional Review Board approval for conducting this evaluation.
Participants
Study participants were identified through provider referral, word of mouth, and community outreach. Eligible participants were HIV-infected, at least 18 years old, met Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition criteria for opioid dependence, and spoke English or Spanish. Potential participants were excluded if they had investigator-defined alcohol or benzodiazepine abuse or other uncontrolled medical or psychiatric conditions, aspartate aminotransferase or alanine transaminasae levels greater than five times normal, or were pregnant. All participants completed written informed consent before enrollment.
Data Collection Methods
Study participants completed baseline assessments that recorded demographic, social, substance use, and behavioral characteristics. Research assistants abstracted clinical data from patient medical records at baseline and at 3, 6, 9, and 12 months follow-up. Data were entered electronically at participating sites and transferred to the national evaluation center for collation and analysis.
Measures
Primary HIV treatment outcomes (being on antiretroviral therapy, HIV-1 RNA levels, and CD4 counts) were the dependent variables. Being on ART was defined by self-report and confirmed through chart review; all subjects who self-reported not being on ART were coded as such irrespective of their chart review information. Inclusion in the analysis required eligible subjects to receive a prescription for BUP/NX with the intent to maintain subjects on BUP/NX in accordance with Treatment Improvement Protocol 40 guidelines.29 Multiple independent variables were examined, including prescription of BUP/NX; a single prescription within a quarter was categorized as having received a prescription for that quarter, even if a single dose or prescription was provided. At several sites, laboratory testing, including HIV-1 RNA and CD4 lymphocyte counts, was performed as part of routine HIV care and not as part of a prespecified protocol; some sites had prespecified laboratory collection quarterly. Standardized instruments were administered at baseline and quarterly thereafter for 1 year and included baseline demographic and social circumstances and validated scales including the Addiction Severity Index,30,31 the Medical Outcomes Study Short Form Health Survey,32 and the Clinical Epidemiological Scale for Depression.33 These variables were used as covariates to address potential confounders such as retention on BUP/NX, substance use, quality of life, and depression.
To determine the observational cohort, 295 subjects who initiated a single dose of BUP/NX served as the sample for analysis for being on ART; 266 (90.1%) and 261 (88.5%) had baseline HIV-1 and CD4 data, respectively, and were ultimately included in the viral load and CD4 models. A number of time-dependent HIV treatment outcomes were assessed quarterly over a 12-month time period after BUP/NX induction. Primary outcomes included self-reported taking of antiretroviral therapy, achieving viral suppression (HIV-1 RNA levels less than 400 copies/mL), and changes in CD4 lymphocyte count analyzed at baseline and quarterly thereafter. Retention in HIV care was defined as having HIV-1 RNA, CD4 lymphocyte count, and a HIV clinical care visit measured within a 6-month period and did not include measurements from the first 30 days after study enrollment. Being retained on BUP/NX was defined as receiving a prescription at any time for three or four quarters.
Analyses
As a result of missing data, time-dependent outcomes were measured using generalized estimating equations (GEE). To assess the randomness of missing values, a retention analysis was conducted for each dependent variable and baseline characteristics, including demographic characteristics, employment status, homelessness, and addiction severity. Baseline fixed covariates included age, race/ethnicity, gender, education, employment, sexual orientation, housing status, substance use history, mental health history and prior treatment, and quality of life. Time-dependent independent variables included sexual and injection-related HIV risk-taking variables, incarceration within the previous 30 days, standardized addiction severity scores and current use, physical and mental health quality-of-life scores, and standardized depression scores. All models were built systematically. The first step examined the independent effect of each predictor by building bivariate GEE models in which only a single variable was entered. If the predictor remained significant in the full model, it was then entered into the parsimonious model, which also included the effect of time (ie, quarter). In accordance with GEE, cases were excluded from an analysis if the baseline dependent variable (eg, being on ART, HIV-1 RNA level, CD4) was missing. Independent variables associated with the dependent variable at the P < 0.10 level were included in final parsimonious model. Significance in the full model was assessed at P < 0.05.
Groups were further stratified based on whether subjects were on ART at baseline to determine the differential influence of BUP/NX on HIV treatment outcomes. Retention on BUP/NX was defined as being prescribed BUP/NX for three or four quarters, even if provided a single prescription during the quarter.
Results
Table 1 provides the baseline characteristics of the 295 subjects who initiated BUP/NX and were stratified by those who were on and not on ART. These individuals were mostly men (68%) in their mid-40s (mean, 45.2 years) and comprised mostly of people of color (73%). Nineteen percent identified as being homo- or bisexual and 25% were homeless at study entry. Baseline addiction severity scores were high (greater than two standard deviations above the general population)34 and Medical Outcomes Study Short Form Health Survey scores were relatively low. Although 62% were being prescribed psychiatric medications at baseline, only 55% acknowledged having a psychiatric diagnosis. Forty percent of subjects were not on ART at study entry. Those who were taking and not taking ART at baseline differed on several baseline characteristics (see Table 1). Compared with subjects not on ART, those on ART were older (46.1 versus 43.9 years, P = 0.022), and less likely to be homo- or bisexual (13.6% versus 26.9% P = 0.004), homeless (18.8% versus 33.6%, P = 0.004), and to have self-reported sexual risk in the past 30 days (17.6% versus 31.9%, P = 0.004). They also had lower mean alcohol addiction severity (7.4 versus 10.3, P = 0.048). Clinically, those on ART were significantly more likely to have lower log10 HIV-1 RNA levels (3.10 versus 4.03 log10 copies/mL, P < 0.001) but their mean CD4 counts did not differ significantly from subjects not on ART.
Table 1. Baseline Characteristics of Subjects Prescribed Buprenorphine (N = 295).
| Characteristic | All Subjects (N = 295) No. (%) | On ART (N = 176) (59.7%) No. (%) | Off ART (N = 119) (40.3%) No. (%) | P (on ART versus off HAART) |
|---|---|---|---|---|
| Mean age in years (standard deviation) | 45.2 (8.2) | 46.1 (7.8) | 43.9 (8.6) | 0.022 |
| Gender | ||||
| Male | 201 (68.1) | 121 (68.8) | 80 (67.2) | NS |
| Female | 94 (31.9) | 55 (31.3) | 39 (32.8) | |
| Race/ethnicity | ||||
| Black | 148 (50.9) | 90 (51.1) | 58 (48.7) | NS |
| Hispanic | 65 (22.0) | 39 (22.2) | 26 (21.8) | |
| White | 68 (23.1) | 37 (21.0) | 31 (26.0) | |
| Sexual orientation | ||||
| Heterosexual | 239 (81.0) | 152 (86.4) | 87 (73.1) | 0.004 |
| Homo- or bisexual | 56 (19.0) | 24 (13.6) | 32 (26.9) | |
| Homeless | 73 (24.7) | 33 (18.8) | 40 (33.6) | 0.004 |
| Buprenorphine prescription during study | 0.303 | |||
| One quarter only | 118 (40.0) | 63 (35.8) | 55 (46.2) | |
| Two quarters only | 29 (9.8) | 17 (9.7) | 12 (10.1) | |
| Three quarters only | 64 (21.7) | 41 (23.3) | 23 (19.3) | |
| All four quarters | 84 (28.5) | 55 (31.3) | 29 (24.4) | |
| Self-reported shared needle use in past 30 days | 27 (9.2) | 12 (6.8) | 15 (12.6) | NS |
| Self-reported sexual risk in past 30 days | 69 (23.4) | 31 (17.6) | 38 (31.9) | 0.004 |
| Used stimulants (cocaine or methamphetamine) within past 30 days | 171 (58.0) | 98 (55.7) | 73 (61.3) | NS |
| Self-reported diagnosis of mental illness | 161 (54.6) | 91 (51.7) | 70 (58.8) | NS |
| Self-reported prescription of psychiatric medications | 182 (61.7) | 103 (58.5) | 79 (66.4) | NS |
| Mean addiction severity—drugs (mean score, range 1.28–62.95) | 32.1 (13.0) | 31.7 (13.1) | 32.5 (12.9) | NS |
| Mean addiction severity—alcohol (mean score, range 0.0–66.95) | 8.6 (12.0) | 7.4 (10.5) | 10.3 (13.7) | 0.048 |
| Bodily pain quality of life (SF-12) (mean score, range 0–100) | 57.8 (33.9) | 56.4 (35.3) | 59.9 (31.6) | NS |
| Physical role quality of life (SF-12) (mean score, range 0–100) | 53.2 (28.1) | 53.0 (29.7) | 53.5 (25.8) | NS |
| General health quality of life (SF-12) (mean score, range 0–100) | 45.1 (29.5) | 46.0 (29.5) | 43.7 (29.7) | NS |
| Mental health quality of life (SF-12) (mean score, range 0–100) | 48.7 (25.5) | 49.9 (26.5) | 46.9 (23.8) | NS |
| HIV symptom distress (mean score, range 1.0–4.75) | 2.57 (0.8) | 2.53 (0.8) | 2.62 (0.8) | NS |
| Mean HIV-1 RNA level, copies/mL (range 50–750,000) N = 227 | 36,428 | 22,737 | 56,517 | 0.006 |
| Mean log10 HIV-1 RNA level, copies/mL HIV viral load (range 1.7–5.88) N = 266 | 3.47 (1.06) | 3.10 (0.94) | 4.03 (0.97) | < 0.001 |
| Mean CD4 lymphocyte count, cells/mL (range 2–1755) N = 261 | 354.9 | 375.8 | 325.2 | NS |
P = nonsignificant (NS) is P > 0.05.
ART, antiretroviral therapy.
Figure 1 depicts the proportion of subjects on ART and achieving virologic suppression at baseline and quarterly thereafter. Compared with baseline, there were significantly higher proportions of subjects on ART in subsequent quarters of observation. Although missing values during any given quarter were as high as 37% for being on ART and 49% for HIV-1 RNA levels, these data were missing at random and were not associated with any known variables previously associated with HIV poor treatment outcomes (eg, homelessness, addiction severity, presence of mental illness, etc; data not shown).
Figure 1.
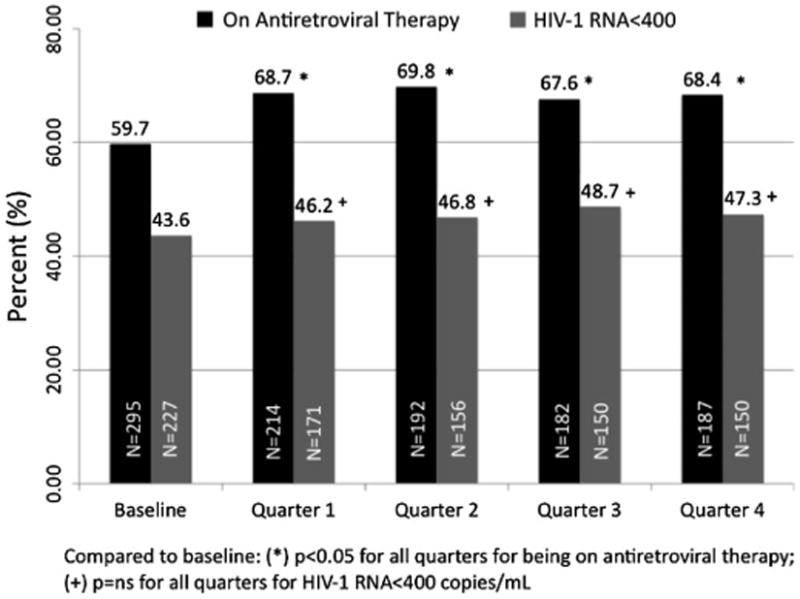
Proportion of all subjects on antiretroviral therapy and with viral suppression (HIV-1 RNA<400) over time.
The first outcome of interest was whether prescription (initiation) of BUP/NX was associated with an increased likelihood of being on ART. Most (60%) of the sample was already on ART at the time of initiating BUP/NX. Compared with baseline, subjects were significantly more likely to be prescribed ART during subsequent quarters of observation (see Fig. 1). Table 2 depicts the correlates of being on ART expressed over time in multiple regression analysis. Increasing time during follow-up and having higher levels of mental health quality of life were positively associated with being on ART, whereas factors that reduced the likelihood of being on ART included being homo- or bisexual, homelessness, and high addiction severity for alcohol.
Table 2. Time-Dependent Correlates of Being on Antiretroviral Therapy Among All Subjects.
| All Subjects (N = 295) | ||||||
|---|---|---|---|---|---|---|
| Full Model | Parsimonious Model | |||||
| Exp β | 95% Confidence Interval | ρ | Exp β | 95% Confidence Interval | ρ | |
| Intercept | 6.13 | (1.25–29.9) | 0.03 | 4.04 | (2.03–8.05) | <0.01 |
| Gay or bisexual | 0.59 | (0.33–1.04) | 0.07 | 0.55 | (0.31–0.97) | 0.04 |
| Homeless | 0.60 | (0.35–1.04) | 0.07 | 0.58 | (0.34–0.98) | 0.04 |
| Self-reported shared needle use in past 30 days | 0.83 | (0.44–1.55) | NS | |||
| Self-reported sexual risk in past 30 days | 0.72 | (0.47–1.10) | NS | |||
| Used stimulants (cocaine or methamphetamine) | 0.92 | (0.62–1.36) | NS | |||
| Self-reported diagnosis of mental illness | 0.87 | (0.62–1.21) | NS | |||
| Addiction severity—drugs | 0.44 | (0.11–1.86) | NS | |||
| Addiction severity—alcohol | 0.27 | (0.06–1.01) | 0.06 | 0.17 | (0.03–0.88) | 0.03 |
| Bodily pain quality of life (SF-12) | 0.98 | (0.97–1.00) | 0.02 | 0.99 | (0.97–1.00) | 0.06 |
| Physical role quality of life (SF-12) | 1.01 | (0.99–1.03) | NS | |||
| Mental health quality of life (SF-12) | 1.18 | (0.99–1.40) | 0.07 | 1.25 | (1.06–1.46) | <0.01 |
| HIV symptom distress | 0.98 | (0.70–1.38) | NS | |||
| Buprenorphine prescription during study | ||||||
| One quarter only | 1.00 | |||||
| Two quarters only | 0.98 | (0.46–2.12) | NS | |||
| Three quarters only | 1.22 | (0.68–2.20) | NS | |||
| All four quarters | 1.21 | (0.68–2.13) | NS | |||
| Time of observation | ||||||
| Baseline (N = 295) | 1.00 | |||||
| Quarter 1 (N = 206) | 1.54 | (1.15–2.07) | <0.01 | |||
| Quarter 2 (N = 189) | 1.52 | (1.13–2.04) | <0.01 | |||
| Quarter 3 (N = 176) | 1.41 | (1.01–1.95) | 0.04 | |||
| Quarter 4 (N = 181) | 1.49 | (1.07–2.07) | 0.02 | |||
P = nonsignificant (NS) is P > 0.05.
Table 3 depicts the correlates of achieving viral suppression among all study subjects. The strongest correlate of viral suppression was receiving ART, which was significantly associated increasing time after initiating BUP/NX (Fig. 1). Viral suppression for the entire sample (see Fig. 1), however, did not significantly increase over time or with increasing retention on BUP/NX (see Table 3) but was independently associated with female gender, Hispanic ethnicity, lower addiction severity to drugs, and improved general health quality–of-life indicators.
Table 3. Correlates of Achieving Viral Suppression (HIV-1 RNA Less Than 400 Copies/mL) Among the Full Buprenorphine Sample.
| All Subjects (N = 266) | ||||||
|---|---|---|---|---|---|---|
| Full Model | Parsimonious Model | |||||
| Exp β | CI | ρ | Exp β | CI | ρ | |
| Intercept | 0.14 | (0.02–0.95) | 0.04 | 0.10 | (0.03–0.38) | <0.01 |
| Female gender | 1.98 | (1.08–3.63) | 0.03 | 1.91 | (1.07–3.41) | 0.03 |
| Race/ethnicity | ||||||
| Other race | 2.16 | (0.56–8.41) | NS | 2.31 | (0.56,9.61) | NS |
| Asian | 3.56 | (0.31–40.7) | NS | 3.37 | (0.23–48.37) | NS |
| Hispanic | 2.71 | (1.37–5.37) | <0.01 | 2.82 | (1.44–5.49) | <0.01 |
| White | 1.49 | (0.77–2.89) | NS | 1.49 | (0.76–2.91) | NS |
| Black (referent) | 1.00 | 1.00 | ||||
| Gay/bisexual | 0.76 | (0.38–1.51) | NS | |||
| Used stimulants (cocaine or methamphetamine) | 0.83 | (0.50–1.38) | NS | |||
| Self-reported diagnosis of mental illness | 0.91 | (0.52–1.59) | NS | |||
| Prescribed psychiatric medications | 1.34 | (0.77–2.34) | NS | |||
| Addiction severity—drugs | 0.13 | (0.02–0.74) | 0.02 | 0.10 | (0.02–0.61) | 0.01 |
| Being on antiretroviral therapy | 9.78 | (5.49–17.43) | <0.001 | 10.27 | (5.79–18.23) | <0.001 |
| Bodily pain quality of life (SF-12) | 0.99 | (0.97–1.01) | NS | |||
| General health quality of life (SF-12) | 1.03 | (1.00–1.05) | 0.03 | 1.02 | (1.00–1.04) | 0.04 |
| HIV symptom index | 0.95 | (0.66–1.36) | NS | |||
| Buprenorphine prescription during study | ||||||
| One quarter only | 1.00 | |||||
| Two quarters only | 1.69 | (0.63–4.55) | NS | |||
| Three quarters only | 1.11 | (0.57–2.14) | NS | |||
| All four quarters | 1.32 | (0.67–2.59) | NS | |||
| Time of observation | ||||||
| Baseline (N = 266) | 1.00 | |||||
| Quarter 1 | 1.07 | (0.64–1.79) | NS | |||
| Quarter 2 | 0.86 | (0.50–1.48) | NS | |||
| Quarter 3 | 0.98 | (0.54–1.80) | NS | |||
| Quarter 4 | 0.89 | (0.48–1.65) | NS | |||
P = nonsignificant (NS) is P > 0.05.
CI, confidence interval.
CD4 lymphocyte count changes (Fig. 2), the final outcome for this analysis, significantly increased over subsequent quarters of observation but was not associated with retention on BUP/NX; this finding held true even when stratifying by whether subjects were on ART at baseline. CD4 lymphocyte counts also significantly increased among women, those who were prescribed psychiatric medications, and those whose general health quality of life was improved. CD4 lymphocyte counts decreased significantly, however, among those who had lower levels of mental health quality of life (see Table 4).
Figure 2.
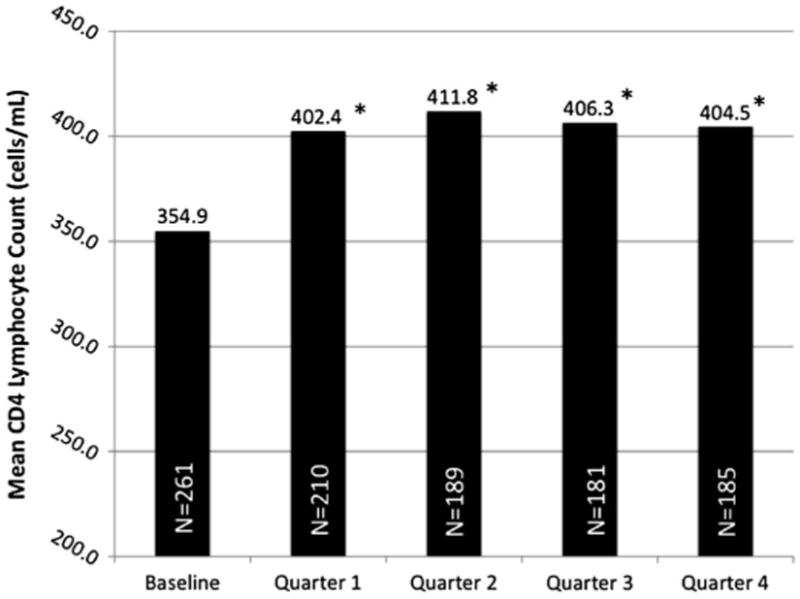
Mean CD4 count at baseline and throughout 12 months of observation. Compared with baseline, P < 0.05 for each subsequent quarter of follow-up observation.
Table 4. Factors Associated With Changes in Absolute CD4 Lymphocyte Count Among All Subjects.
| All Subjects (N = 261) | ||||||
|---|---|---|---|---|---|---|
| Full Model | Parsimonious Model | |||||
| β | 95% Confidence Interval | ρ | β | 95% Confidence Interval | ρ | |
| Intercept | 97.2 | (−143.6, 338.1) | NS | 53.9 | (−25.5, 133.3) | NS |
| Female gender | 67.3 | (5.1, 129.5) | 0.03 | 72.2 | (6.6, 137.8) | 0.03 |
| Self-reported sexual risk in past 30 days | 41.2 | (−27.2, 109.7) | NS | |||
| Self-reported Incarceration in past 30 days | 48.8 | (−21.3, 118.9) | NS | |||
| Used stimulants (cocaine or methamphetamine) | −19.5 | (−65.9, 26.9) | NS | |||
| Self-reported diagnosis of mental illness | −11.4 | (−60.5, 37.7) | NS | |||
| Prescribed psychiatric medications | 72.2 | (27.6, 116.9) | <0.01 | 83.5 | (36.6, 130.4) | <0.001 |
| HIV service use in each quarter | −7.5 | (−59.6, 44.5) | NS | |||
| Being on antiretroviral therapy | 31.5 | (−19.5, 82.5) | NS | |||
| Buprenorphine status during study | ||||||
| One quarter only | — | |||||
| Two quarters only | −33.8 | (−137.6, 70.1) | NS | |||
| Three quarters only | 35.1 | (−42.7, 113.0) | NS | |||
| All four quarters | 46.3 | (−19.9, 112.4) | NS | |||
| Addiction severity—drugs | −30.0 | (−211.5, 151.5) | NS | |||
| Physical role quality of life (SF-12) | −1.0 | (−4.1, 2.0) | NS | |||
| General health quality of life (SF-12) | 5.1 | (2.9, 7.3) | <0.01 | 5.1 | (3.4, 6.9) | <0.001 |
| Physical health quality of life (SF-12) | −3.6 | (−40.0, 32.9) | NS | |||
| Mental health quality of life (SF-12) | −26.3 | (−53.3, 0.8) | 0.06 | −28.5 | (−51.6, −5.4) | 0.02 |
| Time of observation | – | |||||
| Baseline (N = 261) | 1.00 | |||||
| Quarter 1 | 47.5 | (2.7, 77.7) | 0.04 | |||
| Quarter 2 | 56.9 | (10.6, 103.2) | 0.02 | |||
| Quarter 3 | 51.4 | (3.9, 98.8) | 0.03 | |||
| Quarter 4 | 49.6 | (3.8, 102.9) | 0.04 | |||
P = nonsignificant (NS) is P > 0.05.
Because being on ART was strongly correlated with viral suppression and the proportion of subjects on ART at baseline was high, a subanalysis was conducted to assess the impact of BUP/NX treatment among the 119 subjects who were not on ART at baseline; 109 of these subject had complete data for analysis (see Fig 3). This is a group of subjects who would be likely to gain the most clinical benefit from initiating and remaining on ART because doing so at nearly all CD4 strata is associated with improved morbidity and mortality.35–37 Among subjects not initially on BUP/NX, subjects who were prescribed BUP/NX for three or four quarters (N = 64 [58.7%]) were not only significantly more likely to receive ART, but were also more likely to suppress HIV-1 RNA levels to below the limits of detection compared with those prescribed BUP/NX for shorter time periods. This finding held true at each quarter of observation and throughout the study.
Figure 3.
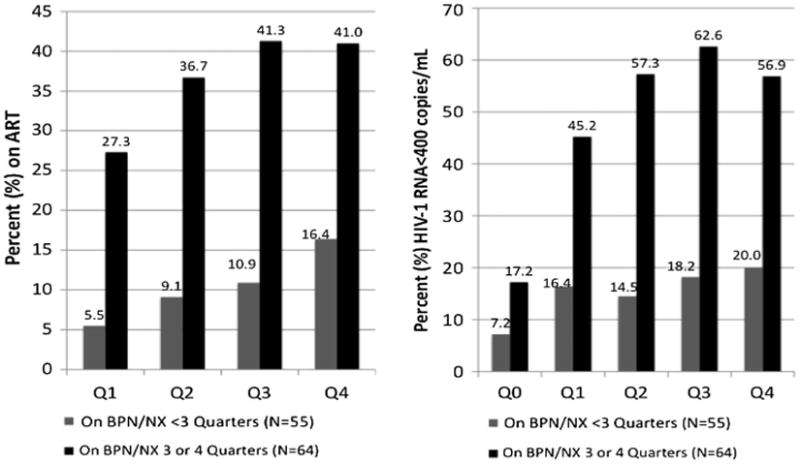
Comparison of initiating antiretroviral therapy and viral suppression outcomes among subjects not on antiretroviral therapy at baseline: stratified analysis among subjects retained on buprenorphine/naloxone for three or more quarters. Compared to baseline, P ≤ 0.05 for all comparisons.
Although not depicted in tables or figures, the proportion subjects on ART at baseline that remained on it by the fourth quarter decreased to 87.7%. The proportion of this subgroup who achieved viral suppression by the end of the fourth quarter, however, had increased from 67.0% to 76.9%, but this did not quite reach statistical significance (P = 0.09). Prescription of BUP/NX for three or four quarters among those on ART at baseline was not associated with significant improvements in viral suppression (β = 1.13; 95% confidence interval [CI], 0.95–1.35) or changes in CD4 counts (β = 9.30; 95% CI, −10.2 to 28.7), but did confirm sustained clinical benefit without evidence of clinical deterioration.
Discussion
This is the largest multisite, observational study of HIV-infected patients prescribed BUP/NX. Over time, the overall sample of subjects who initiated BUP/NX, irrespective of whether they were on ART or not at baseline, were more likely to be prescribed ART and have increases in CD4 counts. For this group, however, increasing retention on BUP/NX was not associated with these outcomes. The finding that the majority of the sample was already on ART at baseline likely explains the lack of association of increased retention on BUP/NX with improving HIV treatment outcomes because most were already optimally engaged in care. To test whether retention on BUP/NX is likely to result in improved HIV treatment outcomes, the stratified analysis of those off of ART at baseline provides considerable insight. Among the group who was at highest risk for an adverse clinical outcome—those not on ART at baseline—longer retention on BUP/NX was significantly associated with higher rates of ART initiation and viral suppression that approached parity with those already on ART at baseline. Achievement of parity in viral suppression over time in this group suggests the potential for a ceiling effect for this outcome. Although not associated with improved HIV treatment outcomes among those who were already on ART at baseline, retention on BUP/NX appears to sustain already maximized positive benefits and does not worsen HIV treatment outcomes among those already experiencing this benefit.
Being on ART is strongly associated with increases in CD4 count; an increase in CD4 count in clinical trials and observational studies is strongly associated with decreased morbidity and mortality.38,39 Although being prescribed BUP/NX did not demonstrate an independent effect on viral suppression over time for the entire sample, this likely reflects the large number of subjects already receiving ART and high levels of viral suppression at baseline and sustained throughout the study. Nevertheless, prescription of BUP/NX, irrespective of duration on treatment, was highly correlated with receiving ART and improved CD4 counts that increased over time. As such, just initiating BUP/NX treatment in an HIV clinical care setting is associated with more individuals receiving ART, although the greatest contribution to this outcome is among those who were not on ART at baseline and especially those who were retained on BUP/NX. The increased association of receiving ART may reflect the notion that initiating treatment for their opioid dependence was sufficient to get patients to take ART, clinicians to prescribe it because they felt the patients were motivated and engaged, or both. Moreover, ART use itself was the single most important individual contributor to viral suppression. These findings differ slightly from recent data from Roux et al from France that found that retention on opioid agonist treatment was associated with long-term virologic HIV suppression with slightly better outcomes for those on methadone compared to those on buprenorphine. This French study, however, had only 113 subjects of which only 53 were on buprenorphine (without naloxone).40 Subjects in the French study were followed for 5 years, considerably longer than the 1 year of follow-up in our study. Our data suggest that there is relatively high retention on ART among those who were already on it but that the greatest benefits are for those who were not on ART. This is particularly true for subjects who are retained on BUP/NX for longer (three or four quarters) time periods.
In a US study of 93 HIV-infected opioid-dependent patients randomized to BUP/NX treatment provided on-site at an HIV clinic versus referral to offsite substance abuse treatment, on-site BUP/NX-treated patients were more likely to remain engaged in HIV care. Different from our study, however, their subjects did not have an increased likelihood of being prescribed ART or increase their CD4 lymphocyte counts over time. Similar to our study was the finding that there was no significant improvement in their viral suppression rates.27 Unlike our study, however, only 46 subjects received BUP/NX, over half were on ART at baseline, and there was no assessment among those who were not on ART at baseline. In summary, our longitudinal cohort assessment confirms several positive HIV treatment benefits from being prescribed BUP/NX, especially if retained in treatment among those not on ART at baseline.
Importantly, there were several covariates that positively and negatively contributed to HIV treatment outcomes. Being homeless was associated with a 40% reduced likelihood of being on ART among all subjects. Multiple studies confirm this association,41 including the influence of homelessness on poor access to and delayed entry into HIV care,42,43 the decreased likelihood of receiving ART primarily because of physician concerns,43,44 and the decreased adherence to ART once inititated.43 Other studies, however, suggest that once prescribed ART, homeless persons are willing and able to successfully adhere to ART.45,46 The lack of association of homelessness with HIV-1 RNA and CD4 counts among those prescribed ART in our study confirms this latter finding, suggesting that homelessness itself should not preclude ART prescription.
This study also confirms that active use of alcohol and higher addiction severity contributes negatively to HIV treatment outcomes among patients prescribed BUP/NX treatment. First, alcohol use severity negatively impacted receipt of ART. This finding is consistent with findings from other studies that demonstrate the negative impact of alcohol use on receipt of ART.47,48 Alcohol use severity did not, however, negatively impact virologic or immunologic outcomes, but this may be attributed to its strong independent negative effect on receiving ART and therefore the sample size was insufficient to demonstrate a benefit for a group whose alcohol use disorders were not being treated. Stimulant use, primarily of cocaine, did not influence receipt of ART, viral suppression, or changes in CD4 lymphocyte count. Although cocaine use has been associated with poor antiretroviral adherence49 and increased HIV disease progression in some studies,50 it did not negatively impact surrogates of HIV treatment successes in this observational study.
Active alcohol and drug use have both been negatively correlated with HIV treatment outcomes.51 The current study appears to be the first to demonstrate that increasing levels of drug addiction severity, rather than just drug or alcohol use itself, are associated with poor HIV treatment outcomes. In this case, only alcohol use severity was associated with not receiving ART, whereas drug use severity was independently associated with poor viral suppression outcomes. There is insufficient information from this study, however, to speculate whether it was continuous alcohol drinking or binge drinking that contributed most to these findings. Nonetheless, there is much to suggest that even reductions in severity of alcohol or drug use, especially among these opioid-dependent patients with significant drug and alcohol comorbidity, is critical to improve HIV treatment outcomes.47 Both behavioral and pharmacologic treatment options for alcohol52 and drug dependence53 should be considered for those with the highest levels of addiction severity among HIV-infected persons.1
Improved mental health and general health quality of life were associated with being on ART. It is unclear from these data, however, whether it was receipt of ART that resulted in improved quality–of-life or whether being in a better mental and physical state was associated with access to and receipt of ART. These data do, however, support other studies that show an association of ART with improving quality of life54,55 that have been demonstrated in prospective observational studies.33,56 One potential explanation is that ART use is associated with decreased depressive symptoms33 and reductions in cognitive impairment,57 and as a result, subjects derived improved quality–of-life. Further insights into these associations are explored further within this supplement.58
It is not surprising that being on ART was the strongest correlate for virologic suppression. Similarly, being on ART was strongly correlated with increases in CD4 counts. It is unclear, however, why being Hispanic was associated with improved virologic outcomes. This observation was not replicated, however, when only subjects on ART were examined (data not shown). One explanation may be specialized Spanish-speaking and adherence education services were available at a few representative sites. The number of sites was too large to make inferences between sites given the existing sample size.
Improved general health quality of life was significantly associated not only with being on ART, but also with viral suppression. In contrast to previous studies, this study had the availability of contemporary regimens, including simplified, better-tolerated combinations and boosted protease inhibitors, which appear to reduce the likelihood of resistance and allow lower adherence levels to achieve robust virologic control.59–61 It is possible that contemporary ART regimens, which are tolerated better and more amenable to perturbations in adherence, resulted in improved quality-of-life measures and overrode the effects of cocaine use and mental illness that have been associated with poor adherence outcomes in other studies.
CD4 lymphocyte counts are the best predictor of HIV-related morbidity and mortality.62,63 It is therefore imperative to restore and retain immune function. Compared with baseline, BUP/NX- treated subjects steadily and significantly increased their CD4 counts over time (Fig. 2); therefore, efforts that retain individuals in HIV care, such as initiating BPN/NX when indicated, are likely to have the most optimal treatment outcomes and reduce HIV progression.64 Studies of BUP/NX treatment for longer durations, however, are needed because HIV is a chronic condition requiring a lifetime of treatment. Also contributing to improvements in CD4 count was the receipt of psychiatric medications. Untreated mental illness has been associated with decreased access to ART65–67 and decreased ART adherence once prescribed.68,69 In this study, over half of subjects met criteria for “triple diagnosis”— mental illness, substance use disorder, and HIV. Our study, similar to findings from Mellins et al,69 confirms that those individuals with triple diagnosis who receive treatment for all three conditions (psychiatric medications, BUP/NX, and ART) can achieve improved HIV treatment outcomes, particularly ones that are associated with reductions in HIV progression. Minimally, it now allows for a single HIV clinician to effectively treat the triply diagnosed client without requiring multiple referrals and provision of fragmented care.25,70
Although this study has important implications for HIV-infected persons who are dependent on opioids, it does have limitations. First, the study represents a naturalistic observation of HIV-infected patients who are initiated on BUP/NX. As a consequence of a naturalistic observational study rather than a longitudinal cohort with systematically consistent measures, there was considerable variation in the rigor with which CD4 counts, viral load measurements, and recording of retention on BUP/NX and ART were obtained, resulting in missing values. Although GEE is one of the most robust ways of overcoming concerns about missing values, there remains some possibility that those with missing values, despite the values being missing at random, did not contribute equally to positive health outcomes solely as a matter of retention. Retention in HIV care is a well-known obstacle for this population. Second, there is no reliable comparison group. Assumptions for the entire group are based solely on the initiation of a single prescription (in some cases a single dose) of BUP/NX. As such, we cannot assert that initiating BUP/NX is better than other available treatment modalities for opioid dependence for this population, such as prescription of methadone or extended release naltrexone. We do know, however, that those who were retained on BUP/NX at least three or four quarters did have improved HIV treatment outcomes compared with those who were not retained. This finding may in part be the result of the lack of specificity of our variable measuring retention on BUP/NX (receiving a single dose during any quarter) and the naturalistic setting of the study, which may contribute to missing data. Third, the models of treatment delivery varied considerably across sites71 and it is possible that the treatment delivery strategy resulted in differing outcomes. This is the largest, multisite evaluation of BUP/NX treatment on HIV treatment outcomes and despite these limitations, it appears that BUP/NX prescription for the entire sample was associated with several improved HIV treatment outcomes, especially time-dependent improvement in prescription of ART and increased CD4 counts. Retention on BUP/NX does have its greatest impact on those not already receiving ART, but it also appears to sustain the benefit already conferred by ART among those on it at baseline.
Buprenorphine/naloxone's availability has the potential to markedly increase access to opioid agonist treatment for HIV-infected patients.23 Although considerable challenges in the implementation of BUP/NX treatment in HIV clinical care settings remain, this multisite study confirms several of the positive HIV treatment outcomes associated with integration of BUP/NX treatment into HIV specialty care settings.72 As a result, if BUP/NX becomes routinely available in this setting, it has the potential to improve access to ART and reduce morbidity and mortality among HIV-infected opioid-dependent patients who have traditionally been less likely to access and adhere to ART. Further investigations remain, however, to assess the long-term benefits of BUP/NX treatment and to improve its assimilation into standard HIV medical care practices.
Acknowledgments
Funding for this research was provided by the Health Resources Services Agency and the Special Projects of National Significance as well as from the National Institutes on Drug Abuse for career development awards (Altice, K24 DA017072; Korthuis, K23 DA019809; and Bruce K23 DA022143).
Appendix 1: Bhives Collaborative
The CORE Center (Chicago, IL), El Rio Santa Cruz Neighborhood Health Center (Tucson, AZ), Johns Hopkins University (Baltimore, MD), Miriam Hospital (Providence, RI), Montefiore Medical Center (Bronx, NY), OASIS, (Oakland, CA), Oregon Health Sciences University (Portland, OR), University of California San Francisco Positive Health Program at San Francisco General Hospital (San Francisco, CA), University of Miami Medical School (Miami, FL), Yale University School of Medicine (New Haven, CT), and The New York Academy of Medicine (New York, NY).
Footnotes
The authors have no conflicts of interest to disclose.
References
- 1.Altice FL, Kamarulzaman A, Soriano VV, et al. Treatment of medical, psychiatric, and substance-use comorbidities in people infected with HIV who use drugs. Lancet. 2010;376:59–79. doi: 10.1016/S0140-6736(10)60829-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Masur H, Michelis MA, Greene JB, et al. An outbreak of community-acquired Pneumocystis carinii pneumonia: initial manifestation of cellular immune dysfunction. N Engl J Med. 1981;305:1431–1438. doi: 10.1056/NEJM198112103052402. [DOI] [PubMed] [Google Scholar]
- 3.Stancliff S. Buprenorphine and the treatment of opioid addiction. The PRN Notebook. 2004;9:28–32. [Google Scholar]
- 4.SAMHSA. Results From the 2003 National Survey on Drug Use and Health: National Findings. Rockville, MD: SAMHSA; 2004. (NSDUH Series H-25, DHHS Publication No. SMA 04-3964). [Google Scholar]
- 5.Fiellin DA, O'Connor PG. Clinical practice. Office-based treatment of opioid-dependent patients. N Engl J Med. 2002;347:817–823. doi: 10.1056/NEJMcp013579. [DOI] [PubMed] [Google Scholar]
- 6.Kreek MJ, Vocci FJ. History and current status of opioid maintenance treatments: blending conference session. J Subst Abuse Treat. 2002;23:93–105. doi: 10.1016/s0740-5472(02)00259-3. [DOI] [PubMed] [Google Scholar]
- 7.Centers for Disease Control and Prevention. HIV and AIDS in the United States. [Accessed July 23, 2010];2010 Available at: http://www.cdc.gov/hiv/resources/factsheets/PDF/us.pdf.
- 8.Centers for Disease Control and Prevention. Advancing HIV prevention: new strategies for a changing epidemic—United States, 2003. MMWR Morb Mortal Wkly Rep. 2003;52:329–332. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. HIV diagnoses among injection-drug users in states with HIV Surveillance—25 states, 1994– 2000. MMWR Morb Mortal Wkly Rep. 2003;52:634–636. [PubMed] [Google Scholar]
- 10.Kozal MJ, Amico KR, Chiarella J, et al. HIV drug resistance and HIV transmission risk behaviors among active injection drug users. J Acquir Immune Defic Syndr. 2005;40:106–109. doi: 10.1097/01.qai.0000159666.95455.d2. [DOI] [PubMed] [Google Scholar]
- 11.Fisher JD, Cornman DH, Osborn CY, et al. Clinician-initiated HIV risk reduction intervention for HIV-positive persons: formative research, acceptability, and fidelity of the Options Project. J Acquir Immune Defic Syndr. 2004;37(Suppl 2):S78–S87. doi: 10.1097/01.qai.0000140605.51640.5c. [DOI] [PubMed] [Google Scholar]
- 12.De Cock KM, Crowley SP, Lo YR, et al. Preventing HIV transmission with antiretrovirals. Bull World Health Organ. 2009;87:488–488A. doi: 10.2471/BLT.09.067330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Granich RM, Gilks CF, Dye C, et al. Universal voluntary HIV testing with immediate antiretroviral therapy as a strategy for elimination of HIV transmission: a mathematical model. Lancet. 2009;373:48–57. doi: 10.1016/S0140-6736(08)61697-9. [DOI] [PubMed] [Google Scholar]
- 14.Tuldra A, Wu AW. Interventions to improve adherence to antiretroviral therapy. J Acquir Immune Defic Syndr. 2002;31(Suppl 3):S154–S157. doi: 10.1097/00126334-200212153-00014. [DOI] [PubMed] [Google Scholar]
- 15.Mattick RP, Kimber J, Breen C, et al. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2002;4:CD002207. doi: 10.1002/14651858.CD002207. [DOI] [PubMed] [Google Scholar]
- 16.Yoast R, Williams MA, Deitchman SD, et al. Report of the Council on Scientific Affairs: methadone maintenance and needle-exchange programs to reduce the medical and public health consequences of drug abuse. J Addict Dis. 2001;20:15–40. doi: 10.1300/J069v20n02_03. [DOI] [PubMed] [Google Scholar]
- 17.Fiellin DA, Pantalon MV, Pakes JP, et al. Treatment of heroin dependence with buprenorphine in primary care. Am J Drug Alcohol Abuse. 2002;28:231–241. doi: 10.1081/ada-120002972. [DOI] [PubMed] [Google Scholar]
- 18.Johnson RE, Chutuape MA, Strain EC, et al. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 19.Johnson RE, Eissenberg T, Stitzer ML, et al. A placebo controlled clinical trial of buprenorphine as a treatment for opioid dependence. Drug Alcohol Depend. 1995;40:17. doi: 10.1016/0376-8716(95)01186-2. [DOI] [PubMed] [Google Scholar]
- 20.Fiellin DA, Rosenheck RA, Kosten TR. Office-based treatment for opioid dependence: reaching new patient populations. Am J Psychiatry. 2001;158:1200–1204. doi: 10.1176/appi.ajp.158.8.1200. [DOI] [PubMed] [Google Scholar]
- 21.Kosten TR, Morgan C, Kleber HD. Treatment of heroin addicts using buprenorphine. Am J Drug Alcohol Abuse. 1991;17:119–128. doi: 10.3109/00952999108992815. [DOI] [PubMed] [Google Scholar]
- 22.Fiellin DA, Kleber H, Trumble-Hejduk JG, et al. Consensus statement on office-based treatment of opioid dependence using buprenorphine. J Subst Abuse Treat. 2004;27:153–159. doi: 10.1016/j.jsat.2004.06.005. [DOI] [PubMed] [Google Scholar]
- 23.Altice FL, Sullivan LE, Smith-Rohrberg D, et al. The potential role of buprenorphine in the treatment of opioid dependence in HIV-infected individuals and in HIV infection prevention. Clin Infect Dis. 2006;43(Suppl 4):S178–S183. doi: 10.1086/508181. [DOI] [PubMed] [Google Scholar]
- 24.Melo AC, Caiaffa WT, Cesar CC, et al. Utilization of HIV/AIDS treatment services: comparing injecting drug users and other clients. Cad Saude Publica. 2006;22:803–813. doi: 10.1590/s0102-311x2006000400019. [DOI] [PubMed] [Google Scholar]
- 25.Bruce RD, Altice FL. Clinical care of the HIV-infected drug user. Infect Dis Clin North Am. 2007;21:149–179. doi: 10.1016/j.idc.2007.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Johnson RE, Chutuape MA, Strain EC, et al. A comparison of levomethadyl acetate, buprenorphine, and methadone for opioid dependence. N Engl J Med. 2000;343:1290–1297. doi: 10.1056/NEJM200011023431802. [DOI] [PubMed] [Google Scholar]
- 27.Lucas GM, Chaudhry A, Hsu J, et al. Clinic-based treatment of opioid-dependent HIV-infected patients versus referral to an opioid treatment program: a randomized trial. Ann Intern Med. 2010;152:704–711. doi: 10.1059/0003-4819-152-11-201006010-00003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Cheever LW, Kresina TF, Cajina A, et al. A model federal collaborative to increase patient access to buprenorphine treatment in HIV primary care. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S3–S6. doi: 10.1097/QAI.0b013e318209740f. [DOI] [PubMed] [Google Scholar]
- 29.Center for Substance Abuse Treatment. Substance Abuse and Mental Health Services Administration (SAMHSA) Rockville, MD: Department of Health and Human Services; 2004. Clinical Guidelines for the Use of Buprenorphine in the Treatment of Opioid Addiction Treatment Improvement Protocol (TIP) Series 40. [PubMed] [Google Scholar]
- 30.McLellan AT, Luborsky L, Woody GE, et al. An improved diagnostic evaluation instrument for substance abuse patients. The Addiction Severity Index. J Nerv Ment Dis. 1980;168:26–33. doi: 10.1097/00005053-198001000-00006. [DOI] [PubMed] [Google Scholar]
- 31.McLellan AT, Kushner H, Metzger D, et al. The fifth edition of the addiction severity index. J Subst Abuse Treat. 1992;9:199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- 32.Wu AW, Hays RD, Kelly S, et al. Applications of the Medical Outcomes Study health-related quality of life measures in HIV/AIDS. Qual Life Res. 1997;6:531–554. doi: 10.1023/a:1018460132567. [DOI] [PubMed] [Google Scholar]
- 33.Springer SA, Chen S, Altice FL. Depression and symptomatic response among HIV-infected drug users enrolled in a randomized controlled trial of directly administered antiretroviral therapy. AIDS Care. 2009;21:976–983. doi: 10.1080/09540120802657555. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leonhard C, Mulvey K, Gastfriend DR, et al. The Addiction Severity Index: a field study of internal consistency and validity. J Subst Abuse Treat. 2000;18:129–135. doi: 10.1016/s0740-5472(99)00025-2. [DOI] [PubMed] [Google Scholar]
- 35.Antiretroviral Therapy Cohort. Causes of death in HIV-1-infected patients treated with antiretroviral therapy, 1996–2006: collaborative analysis of 13 HIV cohort studies. Clin Infect Dis. 2010;50:1387–1396. doi: 10.1086/652283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Study Group on Death Rates at High, CD4 Count in Antiretroviral Naive Patients. Lodwick RK, Sabin CA, et al. Death rates in HIV-positive antiretroviral-naive patients with CD4 count greater than 350 cells per microL in Europe and North America: a pooled cohort observational study. Lancet. 2010;376:340–345. doi: 10.1016/S0140-6736(10)60932-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Tien PC, Choi AI, Zolopa AR, et al. Inflammation and mortality in HIV-infected adults: analysis of the FRAM Study Cohort. J Acquir Immune Defic Syndr. 2010;55:316–322. doi: 10.1097/QAI.0b013e3181e66216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Antiretroviral Therapy Cohort Collaboration Rates of disease progression according to initial highly active antiretroviral therapy regimen: a collaborative analysis of 12 prospective cohort studies. J Infect Dis. 2006;194:612–622. doi: 10.1086/506362. [DOI] [PubMed] [Google Scholar]
- 39.Baker JV, Peng G, Rapkin J, et al. CD4+ count and risk of non-AIDS diseases following initial treatment for HIV infection. AIDS. 2008;22:841–848. doi: 10.1097/QAD.0b013e3282f7cb76. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Roux P, Carrieri MP, Cohen J, et al. Retention in opioid substitution treatment: a major predictor of long-term virological success for HIV-infected injection drug users receiving antiretroviral treatment. Clin Infect Dis. 2009;49:1433–1440. doi: 10.1086/630209. [DOI] [PubMed] [Google Scholar]
- 41.Leaver CA, Bargh G, Dunn JR, et al. The effects of housing status on health-related outcomes in people living with HIV: a systematic review of the literature. AIDS Behav. 2007;11(Suppl):85–100. doi: 10.1007/s10461-007-9246-3. [DOI] [PubMed] [Google Scholar]
- 42.Aidala AA, Lee G, Abramson DM, et al. Housing need, housing assistance, and connection to HIV medical care. AIDS Behav. 2007;11(Suppl):101–115. doi: 10.1007/s10461-007-9276-x. [DOI] [PubMed] [Google Scholar]
- 43.Kidder DP, Wolitski RJ, Campsmith ML, et al. Health status, health care use, medication use, and medication adherence among homeless and housed people living with HIV/AIDS. Am J Public Health. 2007;97:2238–2245. doi: 10.2105/AJPH.2006.090209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Loughlin A, Metsch L, Gardner L, et al. Provider barriers to prescribing HAART to medically-eligible HIV-infected drug users. AIDS Care. 2004;16:485–500. doi: 10.1080/09540120410001683411. [DOI] [PubMed] [Google Scholar]
- 45.Petersen ML, Wang Y, van der Laan MJ, et al. Pillbox organizers are associated with improved adherence to HIV antiretroviral therapy and viral suppression: a marginal structural model analysis. Clin Infect Dis. 2007;45:908–915. doi: 10.1086/521250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Royal SW, Kidder DP, Patrabansh S, et al. Factors associated with adherence to highly active antiretroviral therapy in homeless or unstably housed adults living with HIV. AIDS Care. 2009;21:448–455. doi: 10.1080/09540120802270250. [DOI] [PubMed] [Google Scholar]
- 47.Azar MM, Springer SA, Meyer JP, et al. A systematic review of the impact of alcohol use disorders on HIV treatment outcomes, adherence to antiretroviral therapy and health care utilization. Drug Alcohol Depend. 2010;112:178–193. doi: 10.1016/j.drugalcdep.2010.06.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Pence BW, Ostermann J, Kumar V, et al. The influence of psychosocial characteristics and race/ethnicity on the use, duration, and success of antiretroviral therapy. J Acquir Immune Defic Syndr. 2008;47:194–201. doi: 10.1097/QAI.0b013e31815ace7e. [DOI] [PubMed] [Google Scholar]
- 49.Kim TW, Palepu A, Cheng DM, et al. Factors associated with discontinuation of antiretroviral therapy in HIV-infected patients with alcohol problems. AIDS Care. 2007;19:1039–1047. doi: 10.1080/09540120701294245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Baum MK, Rafie C, Lai S, et al. Crack-cocaine use accelerates HIV disease progression in a cohort of HIV-positive drug users. J Acquir Immune Defic Syndr. 2009;50:93–99. doi: 10.1097/QAI.0b013e3181900129. [DOI] [PubMed] [Google Scholar]
- 51.Lucas GM, Gebo KA, Chaisson RE, et al. Longitudinal assessment of the effects of drug and alcohol abuse on HIV-1 treatment outcomes in an urban clinic. AIDS. 2002;16:767–774. doi: 10.1097/00002030-200203290-00012. [DOI] [PubMed] [Google Scholar]
- 52.Bertholet N, Daeppen JB, Wietlisbach V, et al. Reduction of alcohol consumption by brief alcohol intervention in primary care: systematic review and meta-analysis. Arch Intern Med. 2005;165:986–995. doi: 10.1001/archinte.165.9.986. [DOI] [PubMed] [Google Scholar]
- 53.Amato L, Minozzi S, Davoli M, et al. Psychosocial combined with agonist maintenance treatments versus agonist maintenance treatments alone for treatment of opioid dependence. Cochrane Database Syst Rev. 2008;4:CD004147. doi: 10.1002/14651858.CD004147.pub2. [DOI] [PubMed] [Google Scholar]
- 54.del Castillo LS, Ruiz-Perez I, de Labry-Lima AO, et al. Influence of antiretroviral treatment on quality of life in seropositive inmates. Int J STD AIDS. 2008;19:172–177. doi: 10.1258/ijsa.2007.007093. [DOI] [PubMed] [Google Scholar]
- 55.Murri R, Fantoni M, Del Borgo C, et al. Determinants of health-related quality of life in HIV-infected patients. AIDS Care. 2003;15:581–590. doi: 10.1080/0954012031000134818. [DOI] [PubMed] [Google Scholar]
- 56.Mannheimer SB, Matts J, Telzak E, et al. Quality of life in HIV-infected individuals receiving antiretroviral therapy is related to adherence. AIDS Care. 2005;17:10–22. doi: 10.1080/09540120412331305098. [DOI] [PubMed] [Google Scholar]
- 57.Anand P, Springer SA, Copenhaver MM, et al. Neurocognitive Impairment and HIV risk factors: a reciprocal relationship. AIDS Behav. 2010;14:1213–1226. doi: 10.1007/s10461-010-9684-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Korthius PT, Tozzi MJ, Nandi V, et al. Improved quality of life for opioid-dependent patients receiving buprenorphine treatment in HIV clinics. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S39–S45. doi: 10.1097/QAI.0b013e318209754c. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Parienti JJ, Das-Douglas M, Massari V, et al. Not all missed doses are the same: sustained NNRTI treatment interruptions predict HIV rebound at low-to-moderate adherence levels. PLoS One. 2008;3:e2783. doi: 10.1371/journal.pone.0002783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Gardner EM, Burman WJ, Steiner JF, et al. Antiretroviral medication adherence and the development of class-specific antiretroviral resistance. AIDS. 2009;23:1035–1046. doi: 10.1097/QAD.0b013e32832ba8ec. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lima VD, Harrigan R, Bangsberg DR, et al. The combined effect of modern highly active antiretroviral therapy regimens and adherence on mortality over time. J Acquir Immune Defic Syndr. 2009;50:529–536. doi: 10.1097/QAI.0b013e31819675e9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Egger M, May M, Chene G, et al. Prognosis of HIV-1-infected patients starting highly active antiretroviral therapy: a collaborative analysis of prospective studies. Lancet. 2002;360:119–129. doi: 10.1016/s0140-6736(02)09411-4. [DOI] [PubMed] [Google Scholar]
- 63.Kohli R, Lo Y, Howard AA, et al. Mortality in an urban cohort of HIV-infected and at-risk drug users in the era of highly active antiretroviral therapy. Clin Infect Dis. 2005;41:864–872. doi: 10.1086/432883. [DOI] [PubMed] [Google Scholar]
- 64.Kavasery R, Galai N, Astemborski J, et al. Nonstructured treatment interruptions among injection drug users in Baltimore, MD. J Acquir Immune Defic Syndr. 2009;50:360–366. doi: 10.1097/QAI.0b013e318198a800. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Maisels L, Steinberg J, Tobias C. An investigation of why eligible patients do not receive HAART. AIDS Patient Care STDs. 2001;15:185–191. doi: 10.1089/10872910151133701. [DOI] [PubMed] [Google Scholar]
- 66.Chander G, Himelhoch S, Moore RD. Substance abuse and psychiatric disorders in HIV-positive patients: epidemiology and impact on antiretroviral therapy. Drugs. 2006;66:769–789. doi: 10.2165/00003495-200666060-00004. [DOI] [PubMed] [Google Scholar]
- 67.Wong MD, Cunningham WE, Shapiro MF, et al. Disparities in HIV treatment and physician attitudes about delaying protease inhibitors for nonadherent patients. J Gen Intern Med. 2004;19:366–374. doi: 10.1111/j.1525-1497.2004.30429.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Wagner GJ, Kanouse DE, Koegel P, et al. Adherence to HIV antiretrovirals among persons with serious mental illness. AIDS Patient Care STDs. 2003;17:179–186. doi: 10.1089/108729103321619782. [DOI] [PubMed] [Google Scholar]
- 69.Mellins CA, Havens JF, McDonnell C, et al. Adherence to antiretroviral medications and medical care in HIV-infected adults diagnosed with mental and substance abuse disorders. AIDS Care. 2009;21:168–177. doi: 10.1080/09540120802001705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Bruce RD, Altice FL. Editorial comment: why treat three conditions when it is one patient? AIDS Read. 2003;13:378–379. [PubMed] [Google Scholar]
- 71.Weiss L, Netherland J, Egan JE, et al. Integration of buprenorphine/naloxone treatment into HIV clinical care: lessons from the BHIVES collaborative. J Acquir Immune Defic Syndr. 2011;56(Suppl 1):S68–S75. doi: 10.1097/QAI.0b013e31820a8226. [DOI] [PubMed] [Google Scholar]
- 72.Basu S, Smith-Rohrberg D, Bruce RD, et al. Models for integrating buprenorphine therapy into the primary HIV care setting. Clin Infect Dis. 2006;42:716–721. doi: 10.1086/500200. [DOI] [PubMed] [Google Scholar]


