Abstract
The heat shock protein (Hsp) family is an evolutionarily conserved system that is charged with preventing unfolded or misfolded proteins in the cell from aggregating. In Alzheimer’s disease, extracellular accumulation of the amyloid β peptide (Aβ) and intracellular aggregation of the microtubule associated protein tau may result from mechanisms involving chaperone proteins like the Hsps. Due to the ability of Hsps to regulate aberrantly accumulating proteins like Aβ and tau, therapeutic strategies are emerging that target this family of chaperones to modulate their pathobiology. This article focuses on the use of Hsp-based therapeutics for treating primary and secondary tauopathies like Alzheimer’s disease. It will particularly focus on the pharmacological targeting of the Hsp70/90 system and the value of manipulating Hsp27 for treating Alzheimer’s disease.
Keywords: Heat shock proteins, chaperones, neurodegeneration, alzheimer, Hsp27, Tau.
INTRODUCTION
The chaperone system of heat shock proteins (Hsps) is an ancient and evolutionarily conserved protein family that regulates nascent folding as well as modulates the fate of unstructured or pathologically misfolded proteins, termed clients [1]. Misfolded or intrinsically unfolded clients of the chaperone network expose hydrophobic signatures that Hsps recognize and bind. Hsps attach to clients in these hydrophobic regions and, via a well-coordinated system of co-chaperone interactions, are routed for renaturation (if suitable) or degradation.
Neurodegenerative disorders such as tauopathies are characterized by the pathological aggregation of misfolded proteins. Thus, Hsps have become a promising system that may serve as a platform to modulate disease processes where protein misfolding is a common feature. Insofar, there have been several promising attempts to manipulate Hsps by pharmacological interventions [2, 3] and gene-delivery approaches [4-10].
The aim of this review is to chronicle strides in the investigation of Hsps from the perspective of Alzheimer’s disease (AD), the most common tauopathy, where intracellular tangles and extracellular amyloid plaques are pathological hallmarks. In particular, this review will focus on pharmacological therapeutics and genetic interventions to modulate Hsp70 and Hsp90. Finally, the current findings on research involving both Hsp27 in AD will be presented.
ALZHEIMER’S DISEASE
Alzheimer’s disease is a chronic neurodegenerative disorder characterized by progressive memory loss. Currently,5.3 million people in the U.S. have AD. There are an estimated 4% of AD cases in persons younger than 65. Fifty percent of the U.S. population older than 85 has AD. Alzheimer’s is the most common form of dementia and, with diabetes, it is the 7th leading cause of death in the U.S. Between 2000 and 2006, deaths because of AD increased by over 46%. It is projected that by midcentury, the number of AD patients will rise to 11-16 million [11].
Pathologically, AD hallmarks consist of aggregates of two abnormally folded proteins [12, 13]: beta-amyloid (Aβ) [14] and tau [15-17]. Recent data have suggested that large aggregates of these two proteins do not induce neurotoxicity, but rather small protofibrils of each may be responsible for disease pathogenesis [18-20]. In the case of amyloid, (Aβ, mostly its 42-amino acid version) accumulation occurs in the extracellular space into senile plaques. It is thought that internalization of Aβ oligomers by the neuron may be responsible for the cascade of detrimental events to the cell including tau tangle formation [21, 22].
CHAPERONE-BASED THERAPIES FOR ALZHEIMER’S DISEASE AND OTHER TAUOPATHIES
Chaperones are important in tau processing and likely participate in abnormal tau accumulation [23-31]. A major component of the chaperone network is the Hsp70 family of proteins (Hsp70) that folds and degrades nascent and misfolded polypeptides [32, 33]. These can operate alone or in concert with another major chaperone protein, Hsp90. Hsp70 functions as a monomer with a single ATP-binding pocket and interacts with DnaJ proteins (Hsp40s). DnaJ proteins alter the rate of ATP consumption and play an important role in client delivery to Hsp70. Conversely, Hsp90 functions as a homo-dimer with four ATP-binding pockets and does not interact with DnaJ proteins. The higher consumption of ATP by Hsp90 has enabled more robust screening efforts that have identified inhibitors such as geldanamycin. Inhibitors of Hsp90 have been implemented in the clinic and are the first-in-class for chaperone-based therapeutics [34]. Modulators of Hsp70 have been difficult to identify because of its low intrinsic consumption of ATP. Recent work has consistently demonstrated that genetic manipulation of Hsp70, rather than Hsp90, has a considerably greater impact on the stability of proteins associated with neurodegenerative disease, including tau [26, 28, 35-38]. With this in mind, we recently worked to identify the impact of chemical modulators of Hsp70 ATPase function on tau stability [39].
THE PARADOX OF CHAPERONE-BASED THERAPIES
We recently identified several compounds that either stimulate or inhibit the ATPase function of Hsp70 without inducing its expression [39-42]. These compounds generically bind to all Hsp70 family members and allosterically affect their rate of ATP consumption, which is tied to opening and closing of the protein lid over the substrate-binding domain [40]. Normally, chaperones primarily assist in protein folding and stabilization [43, 44]. But in neurodegenerative disease, where abnormal proteins accumulate, the role of chaperones may be much more diverse. Chaperones may preserve abnormal proteins rather than get rid of them, in an attempt to restore their function. Chaperones may also facilitate the degradation of misfolded disease-associated proteins they cannot repair. More recently it has been suggested that chaperones actually facilitate aggregation of disease-related proteins to prevent toxic aggregates from forming [2, 10, 45-47]. Based on these findings, much of the effort in chaperone research has focused on altering the expression levels of these heat shock proteins by activating the heat shock transcription factor (HSF1), and lead compounds have been identified that can cause this global response [48, 49]. Increasing heat shock protein levels might be expected to allow more abnormal protein clients to be bounded by the heat shock network and triaged for degradation; however, by overexpressing Hsps in several mammalian cell models of tauopathy, we have found that such increases may not be sufficient to facilitate client degradation [2]. In fact, increasing the levels of some heat shock proteins may actually preserve clients. This phenomenon suggests that there is a degree of antagonism that is built into the chaperone system [50]. Instead, inhibiting the ATPase activity of Hsp70 and Hsp90 proteins may be a much more effective strategy for dictating client fate [2, 39]. Perhaps the most effective strategy to reduce target clients would be to first increase levels of heat shock proteins, which would form more Hsp/client complexes, and then inhibit the ATPase activity of the chaperones to force client degradation, subverting any attempts at client repair.
HSP27
Another subset of chaperones that has received limited attention from a drug discovery perspective is the small heat shock protein (sHsp) family, which consists of Hsps of molecular weight less than 30 kDa. These sHsps are conserved throughout all phyla [1], and despite structural differences, their primary role is to bind unfolded proteins and prevent them from aggregating, which creates a reservoir of intermediates for reactivation [51]. It is thought that these proteins can prevent pathological aggregation of tau, and other amyloidogenic clients [23, 52, 53]. They are unique molecular chaperones in that they function independently of ATPase activity [54]; instead, sHsps perform chaperone functions by cycling between phosphorylation-dependent oligomers and smaller-order states [55]. Among some of their functions, sHsps participate in cell survival, cytoskeletal motility, and disruption of protein aggregation [56-59]. It is the latter function that makes sHsps of great interest as a therapeutic intervention for diseases of intracellular protein aggregation like tauopathies and other neurodegenerative disorders.
Due to its particular function as a disrupter of protein aggregation [60], the Hsp of 27 kDa, Hsp27, is a recent target of interest to the field of tauopathy research. However, unlike more classical chaperones like Hsp70 and Hsp90, the current knowledge of Hsp27 function is scarce. This may be due in part to the unique nature of Hsp27 as an ATPase-independent chaperone [54], which inherently makes it difficult to establish functional assays. Furthermore, Hsp27’s function is determined by a dramatic, phosphorylation-dependent change in quaternary structure; this dynamism makes the elucidation of structure-function relationships very challenging. During quiescent conditions, Hsp27 mostly exists as a large oligomeric conformer of 200kDa-800kDa [61, 62]. Upon a stress response, it becomes phosphorylated at three serine sites (S15, S78, and S82). As a result, phosphorylated Hsp27 breaks apart into a smaller conformation of monomers, dimers, and tetramers that allow scavenging of misfolded polypeptides favorable. It is presumed that clients interact with the smaller assemblies of Hsp27 [51, 60, 63, 64]. Next, the client-bound Hsp27 resurges into a large oligomeric complex while still bound to client, as suggested by studies in other sHsps [60, 65, 66]. Experimental evidence using Hsp27 mutants retained in either pseudo-phosphory-lated or perpetually dephosphorylated conformations suggests that it is the latter assembly that results in chaperone function. Albeit these results, recent evidence from our laboratory suggest that the processes are more complex in that the dynamic cycling from small to large structures is necessary for proper Hsp27 chaperone function in the dissolution of toxic protein aggregates [10].
Hsp27 is the human heat shock protein of 27 kilodaltons, which is encoded by a single intronless gene termed HSPB1. The mouse homolog is Hsp25. The gene resides on chromosome 7q11.23, and mutations in it have been associated with Charcot-Marie Tooth syndrome, a distal motor and sensory neuropathy that is caused by mis-aggregation of the Hsp27 protein itself [67]. Both the amino- and carboxy-termini of Hsp27 allow it to interact with other proteins [62]. The carboxy-terminus of all sHsps, including Hsp27, is characterized by having a conserved region that is related to the vertebrate lens protein α-crystallin [68, 69]. Hsp27’s α-crystallin domain is located between residues 87 and 167 [68]. In 1982, Ingolia and Craig hypothesized that since α-crystallins in the lens form soluble multimers then this shared domain would also facilitate Hsp27 oligomerization Ingolia and Craig 1982 PNAS). Indeed, experimental evidence using site-directed spin labeling indicated that the α-crystallin domain of Hsp27 is critical for the formation of discreet oligomeric structures with symmetrical orientation [70]. A second, less conserved region of all sHsps is the WDPF domain. It is characterized by a conserved tryptophan-aspartate-proline-phenylalanine sequence, and it lies at the N-terminus. Unlike the α-crystallin domain, the WDPF domain is sensitive to phosphorylation, and this process is critically linked to the multimerization of Hsp27 [62].
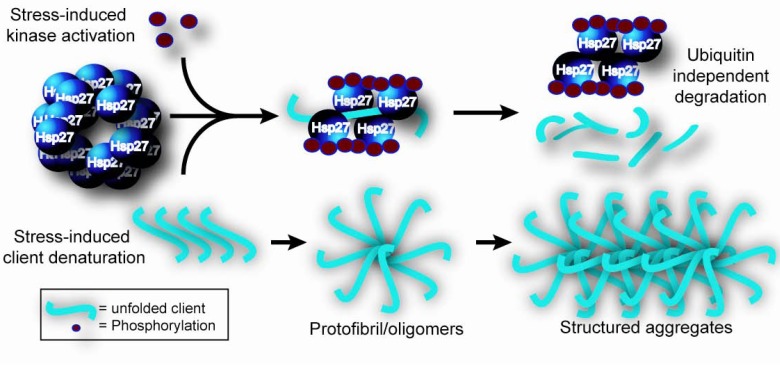
Functional modulation of human Hsp27 is triggered by phosphorylation at three key sites (S15, S78, and S82) by the p38 mitogen-activated protein kinase mitogen-activated protein kinase (MAPK)-activated protein kinase 2 and Akt pathway (for a thorough review see [71]). Phosphorylation of Hsp27 is reversible and dephosphorylation is mainly attributed to protein phosphatase 2A [72]. The result of cycling between phosphorylated and dephosphorylated states causes Hsp27 to rearrange from smaller assemblies to large self-aggregated rearrangements. This dynamic process results in a dual and independent function of Hsp27, both as a regulator of actin microfilament dynamics and as a molecular chaperone that prevents unfolded protein aggregation [62] Fig. (1).
Fig. (1).
The necessity of phosphorylation-dependent Hsp27 function for preventing protein aggregation of unfolded intermediates. Large molecular weight Hsp27 multimers are constitutively present in the cell under basal conditions. Upon extracellular stress, the p38 MAP kinase pathway is activated, phosphorylating Hsp27 (red circles). Consequently, the Hsp27 multimeric complex disassembles into smaller complexes that can bind the denatured client and prevent its subsequent aggregation. The client can then be targeted for degradation in a mechanism that is independent of ubiquitin. Without Hsp27, folding intermediates of the client would be prone to aggregate.
Before its identification, Hsp27 had been functionally defined as an inhibitor of actin polymerization in smooth muscle of turkey gizzards [73, 74]. It was later found that stress-induced actin depolymerization was partially blocked in CHO cells over-expressing Hsp27 [75]. This relationship was further evaluated using CHO cells over-expressing either wild-type Hsp27 or an Hsp27 mutant that is phosphorylation incompetent: only wild-type Hsp27 could stabilize actin filaments under stress [76], Thus it can be suggested that Hsp27 phosphorylation is a critical process for Hsp27 function. This finding was also corroborated by a time-course study in thrombin-activated platelets showing that unbound, cytoplasmic Hsp27 must be phosphorylated before it can attach to actin [77]. Phosphorylation kinetics are not responsible for Hsp27 cellular localization, but they are required for regulating microfilament formation [78, 79]. It was determined that only unphosphorylated, monomeric Hsp27 is able to block actin polymerization [80], and therefore manipulation of Hsp27 phosphorylation state enables genetic regulation of pinocytosis [76], cell migration [81-83], and muscle contraction [73, 74], among many others.
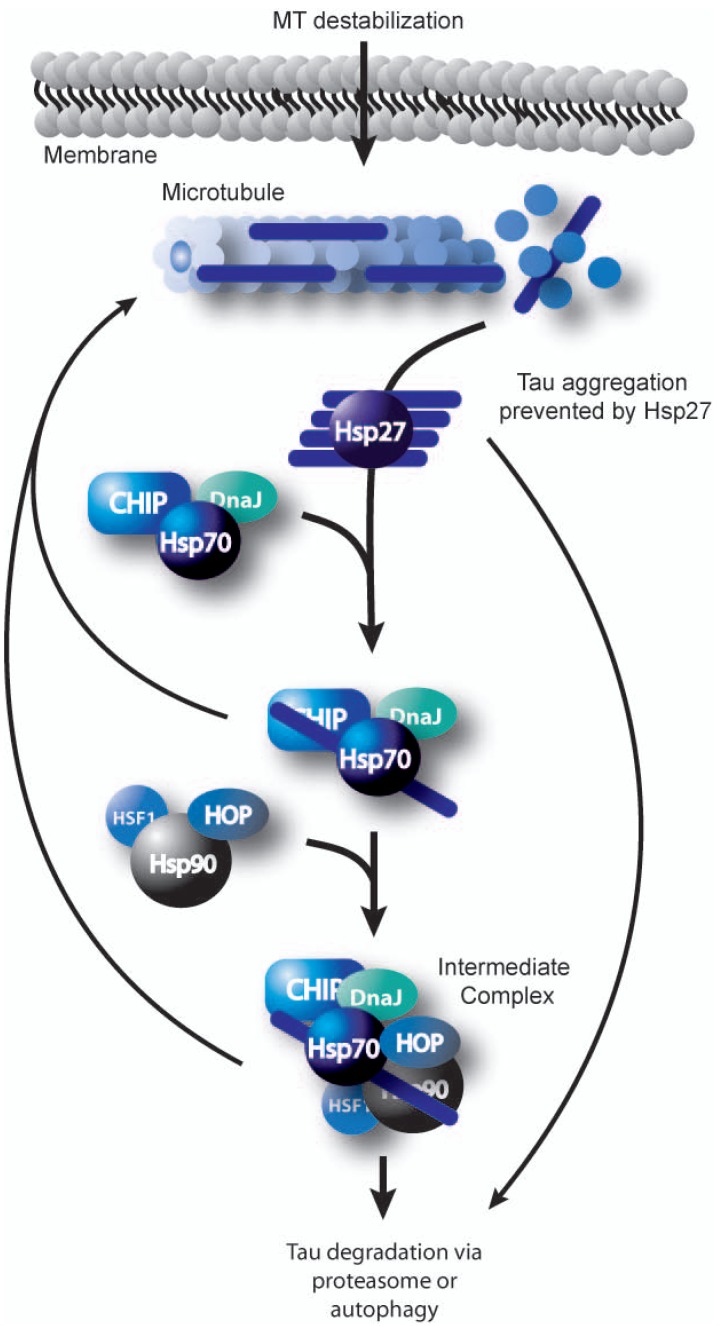
The other major role of Hsp27 is that of a molecular chaperone; however, the mechanism contributing to this activity is less clear. To date, it is thought that only large multimeric Hsp27 complexes are capable of chaperone activity [55]. This is largely based on evidence that substrates/clients have previously been detected attached to Hsp27 oligomers [51, 60, 63, 64]. The ultimate fate of the bound client is likely driven by interactions with specific co-chaperones and the extent of damage to the misfolded client. Data from in vivo and in vitro experiments show that Hsp27 can prevent self-association of aggregation prone polypeptides like the insulin B chain upon disulphide reduction, malate dehydrogenase, and citrate synthase, among others [51, 63, 84]. In addition, Hsp27 can also hold misfolded or intrinsically disordered clients such as tau in a conformation that is stable for subsequent transfer to Hsp70, culminating in client refolding at the expense of ATP [51]. Hsp27 can also deliver clients for ubiquitin-independent clearance via the proteasome in a mechanism that is not well characterized [23], but it likely involves other chaperones such as Hsp90 and the ubiquitin ligase CHIP [24] Fig. (2). It is this capacity of Hsp27 to hold and prevent aggregation of unfolded clients that is interesting for neurodegenerative disorders like tauopathies, synucleinopathies, and poly-glutamine diseases, where aggregation of misfolded proteins is the confounding pathogenic hallmark. Thus, enhancing Hsp27 levels or function to prevent aggregation of these toxic elements, may serve as a potential therapeutic strategy in neurodegenerative diseases.
Fig. (2).
Coordination of substrate transport through the chaperone network. Upon stress induced microtubule disassembly, Hsp27 and perhaps other small Hsps, bind tau to prevent its toxic aggregation. The Hsp27/tau complex is stable, and tau can then be transferred to the Hsp70/DnaJ chaperone complex. If refolding is not possible, the tau-bound Hsp70 complex will associate with the Hsp90 complex for further attempts at refolding. Alternatively, the client is processed for degradation. The decisions are made based on coordinate regulation by the chaperones and cochaperones associating with the complex, such as Hsp90, CHIP and Hop.
HSP27 EXPRESSION AND LOCALIZATION IN THE CNS IS SITE- AND CELL-SPECIFIC
Hsp27 is detectable under basal conditions in both a site and cell-specific manner in the central nervous system (CNS). The patterns of Hsp27 expression following stress and during development also adhere to specific anatomical patterns. Moreover, differentiating cells are Hsp27 immuno-reactive, while apoptotic cells lack Hsp27 expression [85]. This suggests that Hsp27 participates in cell survival during development and into adulthood [4, 6, 86, 87].
Under basal conditions, Hsp27 immuno-reactivity is scarce but detectable in motor and sensory neurons of the spinal cord and brain stem [88], most ocular neural structures [89], and a small set of Purkinje cells in mouse cerebellum [90-92]. Levels of constitutive Hsp27 are highest in astrocytes surrounding leptomeningeal and parenchymal blood vessels [93]. Meanwhile, other brain regions are virtually devoid of constitutive Hsp27 immuno-reactivity, and if found, it’s mostly in glial populations [93-99].
Site-specific up-regulation of Hsp27 is detected upon stimulation by various stress triggers. For instance, while Hsp27 is barely detectable in the cortex and hippocampus of un-injured rats [88, 98], it is induced in these regions upon stressors like hyperthermia and ischemia [96-98]. Hyperthermia also stimulates Hsp27 expression in large glial populations and some neurons of forebrain, brain stem, hypothalamus, and cerebellum [100-102]. Other examples of stress-related Hsp27 inducers include; middle cerebral artery occlusion [97, 100, 101], traumatic nerve injury [103-106], and kainic acid-induced epilepsy [95, 99, 100, 102].
HSP27 AND AD
In addition to the stressors described above, Hsp27 is also increased in the brains of AD patients [107-109]. This phenomenon could be explained by the convergence of a variety of stress insults that occur in the AD brain, including oxidation, inflammation, or aberrant calcium influx [110, 111]. This up-regulation of Hsp27 appears to be site and cell-specific. For instance, using immuno-histochemical, sandwich ELISA, and dot blot analyses, several groups determined that AD brains express significantly increased levels of Hsp27 in the frontal, parietal, and temporal cortices, but not in occipital cortex when compared to age-matched non-demented controls; specifically, Hsp27 co-localized with areas affected by senile plaques and cerebral amyloid angiopathy [53, 93, 107, 109]. Constitutive Hsp27 expression in control brains was mostly found in glia surrounding leptomeningeal and parenchymal blood vessels and occasionally in a scarce number of astrocytes throughout the cortex of control brains, as described above [107].
This distribution suggests that efforts to increase the levels of Hsp27 in neurons may be a neuro-protective strategy in AD [95-99]. For example, one family of compounds that specifically upregulates Hsp27 expression without affecting the expression of other Hsps is the statins [112-118]. Indeed statins are proposed to have therapeutic efficacy for AD [119-123] and increasing Hsp27 levels may be one of several mechanisms that are beneficial for tau-bearing neurons. Thus identifying therapeutics that specifically upregulate Hsp27 in neurons may reduce the extent of neuronal damage, prevent neuronal death, and increase neuronal function despite neurotoxic stressors. As a counterpoint to this approach, it is important to consider the possible negative consequences to the neuron that result from sustained up-regulation of Hsp27 protein levels. Perpetual induction of Hsp27 may ultimately be detrimental over time. Perhaps intermittent delivery strategies that temporally upregulate Hsps like Hsp27 may be the most sustainable and effective way to target these proteins therapeutically.
HSP27 AND Aβ
The capacity of Hsp27 to ameliorate amyloid β (Aβ)-induced toxicity has been elegantly evaluated in a series of in vitro experiments [94]. Primary cortical neurons of P1 rats over-expressing Hsp27 showed reduction in Aß-mediated cell death, increased neurite outgrowth, and mitochondrial protection from Aß-induced damage [94]. Importantly, only neuronal Hsp27 conferred survival; changes to glial or endothelial Hsp27 levels were not protective. These results corroborate previous work showing that Hsp27 could abrogate Aβ aggregation and toxicity [124-126]. Three independent studies show that Hsp27 binds Aβ, whether it be in the form of Aβ40 [126], Aβ42 [94], or a particular variant of Aß40 that carries the Dutch mutation (D-Aβ40) [124]. Aggregation of the different Aß subtypes was also decreased upon incubation with Hsp27, except when Aß42 fibrils formed [125]. Together, these results suggest that Hsp27 binds aggregation-prone Aβ, reduces its ability to assemble into fibrils, and consequently confers protection from cell death.
HSP27 AND TAU
In addition to Aß, Hsp27 also associates with tau tangles in AD, as well as other tauopathies including progressive supranuclear palsy, corticobasal degeneration and fronto-temporal dementia [53, 127, 128]. Interestingly, while biochemical assays show increased total levels of Hsp27 in AD, immuno-staining assays reveal that the bulk of Hsp27 (apart from that found in tangles) is found in glia, particularly astrocytes.
In 2004, Shimura et al. more thoroughly evaluated the interaction of Hsp27 with tau [23]. Using human brain lysates from both AD and age-matched control individuals, they identified Hsp27 as a direct binding partner of phosphorylated tau. These results were later corroborated in larger studies of AD brains, where Hsp27 levels were shown to correlate with neurofibrillary tangle pathology progression [53, 127, 128]. The interaction between Hsp27 and tau was enhanced by phosphorylation of either protein [23]. While these findings suggest that Hsp27 preferentially interacts with aberrant tau, the persistence of tangles in the brain of AD patients despite this interaction suggests that Hsp27 alone is not sufficient to prevent tau pathogenesis.
Subsequent experiments in human neuronal-like cells revealed that both Hsp27 and its phosphorylated form, pHsp27, participated in the dephosphorylation of tau and perhaps facilitated tau degradation [23]. Cell culture studies showed that both wildtype and phosphorylated Hsp27 were able to prevent tau aggregation and prevent apoptosis [23]. These results further suggest that Hsp27 is capable of successfully preventing pathogenic protein aggregation and attenuating toxicity. But this protection is only imparted during stress. Therefore, chemical or genetic induction of neuronal Hsp27 could be exploited to protect neurons from the adverse consequences of intra-neuronal tangle accumulation.
CONCLUDING REMARKS
Although in vitro and in vivo experimental efforts have yielded successful abrogation of AD pathology by inhibiting Hsp70 and Hsp90, the translation of these approaches to human therapeutics may lead to complications in many other biological processes linked to the normal functioning of these proteins, and therefore lead to undesirable outcomes. As such, Hsp70 and Hsp90 inhibition become unattractive therapeutic targets. However, such experimental success paves the road to focus on Hsps as powerful tools to combat diseases rooted in the abnormal aggregation of proteins in cells. A shift of attention to explore the family of sHsps has procured data highlighting the impact of Hsp27 on preventing disease progression [10].
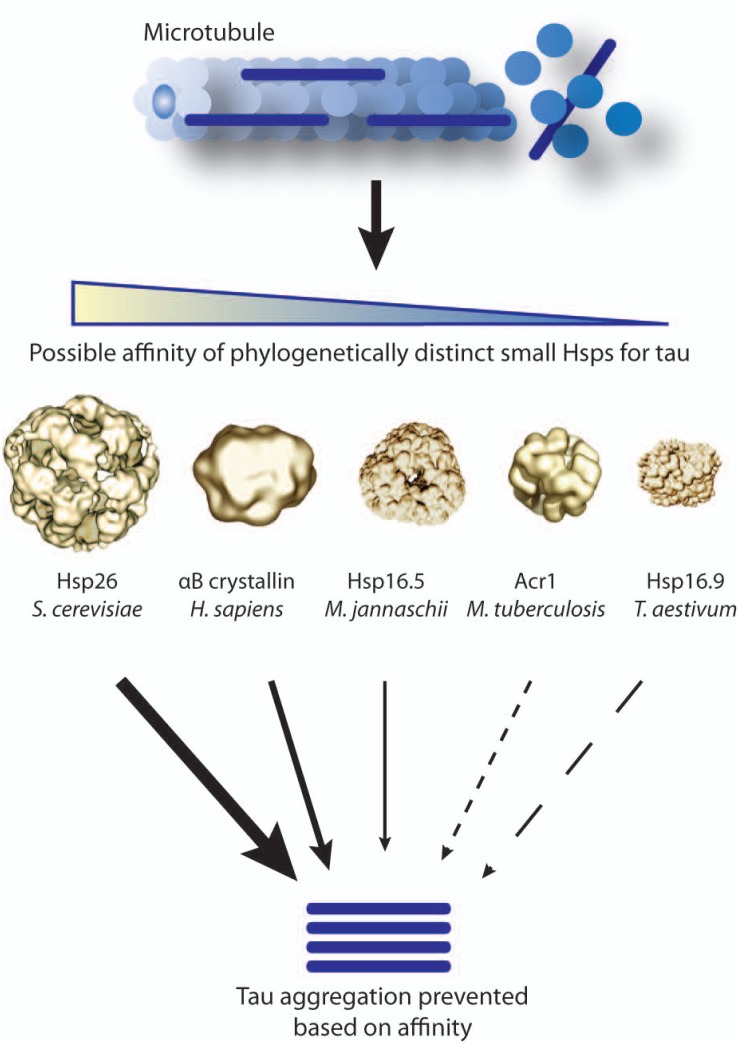
Any therapeutic strategy aimed at up-regulating Hsp27 levels must override the cell’s natural tendency to express low levels of endogenous Hsp27. For instance, the murine leukemic cell line P388 is incapable of expressing endogenous Hsp25 despite heat shock stimulation [129]. It is possible that P388 cells have an Hsp25 gene silencing mechanism since HeLa cells transfected with the P388 Hsp25 promoter were capable of inducing expression. Whether neurons are under similar regulatory constraints that subdue Hsp27 expression remains to be determined. However, this regulation of Hsp27 is not confined to all neurons. In fact, Hsp27 induction is observed after ischemic stress in rat neurons of the fourth and fifth cortical layers and pyramidal neurons in the hippocampus [97]. Besides endogenous production as a primary means for Hsp27 upregulation, it would be interesting to determine if these neurons are capable of internalizing glial-Hsp27 therefore exploring a secondary mechanism for increased neuronal Hsp27. Other possible means to enhance Hsp27 levels could include slowing neuronal Hsp27 turnover or even extrinsically delivering recombinant sHsps derived from other organisms. It is possible that these homologous versions of Hsp27 may have even more potent activity for reducing aggregation than human Hsp27 itself Fig. (3).
Fig. (3).
The variable affinity of phylogenetically diverse sHsps for tau may be therapeutically relevant. Client specificity of sHsps is not well characterized. Small Hsp-mediated prevention of toxic tangle formation can be further enhanced by selecting sHsps, perhaps even from other species, with higher affinity for tau and driving their expression. Therefore certain sHsps will likely bind tau quicker and more stably, intervening before tau can begin to aggregate.
Further understanding of the mechanisms regulating neuronal Hsp27 expression will provide insight for the development of new therapies. A neuron-specific strategy may be the most effective for treating neurodegenerative tauopathies, the most common of which is AD. Furthermore, this review has focused on only one of the many members of the large and conserved family of sHsps. The complex regulation of this phylogenetically conserved group of proteins may coordinate their activities to clear the pathological inclusions associated with AD. Defining these mechanisms will likely lead to improved rational drug design for targeting proteotoxic diseases.
ACKNOWLEDGEMENTS
We thank Dr. Johannes Buchner for allowing us to use his illustration within Fig. (3). This work was supported by the Rosalinde and Arthur Gilbert Foundation/American Federation for Aging Research, CurePSP, the Alzheimer’s Association grant IIRG-09-130689 and NIA grant R00AG031291.
ABBREVIATIONS
- Hsp
= Heat shock proteins
- sHsp
= Small Hsp
- AD
= Alzheimer’s disease
REFERENCES
- 1.Richter-Landsberg C. Expression and functional roles in nerve cells and glia. In: Richter-Landsberg C, editor. Heat Shock Proteins in neural cells. New York: Landes Bioscience; 2007. pp. 1–12. [Google Scholar]
- 2.Dickey CA, Kamal A, Lundgren K, Klosak N, Bailey RM, Dunmore J, Ash P, Shoraka S, Zlatkovic J, Eckman CB, Pat-terson C, Dickson DW, Nahman NS, Jr., Hutton M, Burrows F, Petrucelli L. The high-affinity HSP90-CHIP complex recognizes and selectively degrades phosphorylated tau client proteins. J. Clin. Invest. 2007;117:648–658. doi: 10.1172/JCI29715. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Liu GT. Bicyclol: a novel drug for treating chronic viral hepatitis B and C. Med. Chem. 2009;5:29–43. doi: 10.2174/157340609787049316. [DOI] [PubMed] [Google Scholar]
- 4.Benn SC, Perrelet D, Kato AC, Scholz J, Decosterd I, Mannion RJ, Bakowska JC, Woolf CJ. Hsp27 upregulation and phosphorylation is required for injured sensory and motor neuron survival. Neuron. 2002;36:45–56. doi: 10.1016/s0896-6273(02)00941-8. [DOI] [PubMed] [Google Scholar]
- 5.Wagstaff MJ, Collaco-Moraes Y, Smith J, de Belleroche JS, Coffin RS, Latchman DS. Protection of neuronal cells from apoptosis by Hsp27 delivered with a herpes simplex virus-based vector. J. Biol. Chem. 1999;274:5061–5069. doi: 10.1074/jbc.274.8.5061. [DOI] [PubMed] [Google Scholar]
- 6.Kalwy SA, Akbar MT, Coffin RS, de Belleroche J, Latchman DS. Heat shock protein 27 delivered via a herpes simplex virus vector can protect neurons of the hippocampus against kainic-acid-induced cell loss. Brain Res. Mol. Brain Res. 2003;111:91–103. doi: 10.1016/s0169-328x(02)00692-7. [DOI] [PubMed] [Google Scholar]
- 7.Badin RA, Lythgoe MF, van der Weerd L, Thomas DL, Gadian DG, Latchman DS. Neuroprotective effects of virally delivered HSPs in experimental stroke. J. Cereb. Blood Flow Metab. 2006;26:371–381. doi: 10.1038/sj.jcbfm.9600190. [DOI] [PubMed] [Google Scholar]
- 8.Stetler RA, Cao G, Gao Y, Zhang F, Wang S, Weng Z, Vosler P, Zhang L, Signore A, Graham SH, Chen J. Hsp27 protects against ischemic brain injury via attenuation of a novel stress-response cascade upstream of mitochondrial cell death sig-naling. J. Neurosci. 2008;28:13038–13055. doi: 10.1523/JNEUROSCI.4407-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.An JJ, Lee YP, Kim SY, Lee SH, Lee MJ, Jeong MS, Kim DW, Jang SH, Yoo KY, Won MH, Kang TC, Kwon OS, Cho SW, Lee KS, Park J, Eum WS, Choi SY. Trans-duced human PEP-1-heat shock protein 27 efficiently protects against brain ischemic insult. FEBS J. 2008;275:1296–1308. doi: 10.1111/j.1742-4658.2008.06291.x. [DOI] [PubMed] [Google Scholar]
- 10.Abisambra JF, Blair LJ, Hill SE, Jones J, Kraft C, Rogers J, Koren J, Jinwal UK, Lawson LY, Johnson AG, Wilcock D, O'Leary J, Jansen K, Muschol M, Golde TE, Weeber EJ, Banko J, Dickey CA. Phosphorylation dynamics regulate Hsp27-mediated rescue of neuronal plasticity deficits in tau transgenic mice. J. Neurosci. 2010;30(46 ):15374–82. doi: 10.1523/JNEUROSCI.3155-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Colton CA, Mott RT, Sharpe H, Xu Q, Van Nostrand WE, Vitek MP. Expression profiles for macrophage alternative activa-tion genes in AD and in mouse models of AD. J. Neuroinflamm. 2006;3:27–0. doi: 10.1186/1742-2094-3-27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Pike CJ, Burdick D, Walencewicz AJ, Glabe CG, Cotman CW. Neurodegeneration induced by beta-amyloid peptides in vitro: the role of peptide assembly state. J. Neurosci. 1993;13:1676–1687. doi: 10.1523/JNEUROSCI.13-04-01676.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kelly JW. The alternative conformations of amyloidogenic pro-teins and their multi-step assembly pathways. Curr. Opin. Struct. Biol. 1998;8:101–106. doi: 10.1016/s0959-440x(98)80016-x. [DOI] [PubMed] [Google Scholar]
- 14.Kang J, Lemaire HG, Unterbeck A, Salbaum JM, Masters CL, Grzeschik KH, Multhaup G, Beyreuther K, Muller-Hill B. The precursor of Alzheimer's disease amyloid A4 protein resembles a cell-surface receptor. Nature. 1987;325:733–736. doi: 10.1038/325733a0. [DOI] [PubMed] [Google Scholar]
- 15.Kosik KS, Joachim CL, Selkoe DJ. Microtubule-associated protein tau (tau) is a major antigenic component of paired helical filaments in Alzheimer disease. Proc. Natl. Acad. Sci. U. S. A. 1986;83:4044–4048. doi: 10.1073/pnas.83.11.4044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Iqbal K, Wisniewski HM, Shelanski ML, Brostoff S, Liwnicz BH, Terry RD. Protein changes in senile dementia. Brain Res. 1974;77:337–343. doi: 10.1016/0006-8993(74)90798-7. [DOI] [PubMed] [Google Scholar]
- 17.Grundke-Iqbal I, Iqbal K, Quinlan M, Tung YC, Zaidi MS, Wisniewski HM. Microtubule-associated protein tau.A component of Alzheimer paired helical filaments. J. Biol. Chem. 1986;261:6084–6089. [PubMed] [Google Scholar]
- 18.Shankar GM, Li S, Mehta TH, Garcia-Munoz A, Shepardson NE, Smith I, Brett FM, Farrell MA, Rowan MJ, Lemere CA, Regan CM, Walsh DM, Sabatini BL, Selkoe DJ. Amyloid-beta protein dimers isolated directly from Alzheimer's brains impair synaptic plasticity and memory. Nat. Med. 2008;14:837–42. doi: 10.1038/nm1782. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Walsh DM, Klyubin I, Fadeeva JV, Cullen WK, Anwyl R, Wolfe MS, Rowan MJ, Selkoe DJ. Naturally secreted oli-gomers of amyloid beta protein potently inhibit hippocampal long-term potentiation in vivo. Nature. 2002;416:535–539. doi: 10.1038/416535a. [DOI] [PubMed] [Google Scholar]
- 20.Santacruz K, Lewis J, Spires T, Paulson J, Kotilinek L, Ingelsson M, Guimaraes A, DeTure M, Ramsden M, McGowan E, Forster C, Yue M, Orne J, Janus C, Mariash A, Kuskowski M, Hyman B, Hutton M, Ashe KH. Tau sup-pression in a neurodegenerative mouse model improves memory function. Science. 2005;309:476–481. doi: 10.1126/science.1113694. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Selkoe DJ. Alzheimer's disease: genotypes, phenotypes, and treatments. Science. 1997;275:630–631. doi: 10.1126/science.275.5300.630. [DOI] [PubMed] [Google Scholar]
- 22.Hardy J, Duff K, Hardy KG, Perez-Tur J, Hutton M. Genetic dissection of Alzheimer's disease and related dementias: amyloid and its relationship to tau. Nat. Neurosci. 1998;1:355–358. doi: 10.1038/1565. [DOI] [PubMed] [Google Scholar]
- 23.Shimura H, Miura-Shimura Y, Kosik KS. Binding of tau to heat shock protein 27 leads to decreased concentration of hyperphosphorylated tau and enhanced cell survival. J. Biol. Chem. 2004;279:17957–17962. doi: 10.1074/jbc.M400351200. [DOI] [PubMed] [Google Scholar]
- 24.Shimura H, Schwartz D, Gygi SP, Kosik KS. CHIP-Hsc70 complex ubiquitinates phosphorylated tau and enhances cell survival. J. Biol. Chem. 2004;279:4869–4876. doi: 10.1074/jbc.M305838200. [DOI] [PubMed] [Google Scholar]
- 25.Carrettiero DC, Hernandez I, Neveu P, Papagiannakopoulos T, Kosik KS. The cochaperone BAG2 sweeps paired helical fila-ment- insoluble tau from the microtubule. J. Neurosci. 2009;29:2151–2161. doi: 10.1523/JNEUROSCI.4660-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Petrucelli L, Dickson D, Kehoe K, Taylor J, Snyder H, Grover A, De Lucia M, McGowan E, Lewis J, Prihar G, Kim J, Dillmann WH, Browne SE, Hall A, Voellmy R, Tsuboi Y, Dawson TM, Wolozin B, Hardy J, Hutton M. CHIP and Hsp70 regulate tau ubiquitination, degradation and aggregation. Hum. Mol. Genet. 2004;13:703–714. doi: 10.1093/hmg/ddh083. [DOI] [PubMed] [Google Scholar]
- 27.Dickey CA, Koren J, Zhang YJ, Xu YF, Jinwal UK, Birnbaum MJ, Monks B, Sun M, Cheng JQ, Patterson C, Bailey RM, Dunmore J, Soresh S, Leon C, Morgan D, Petrucelli L. Akt and CHIP coregulate tau degradation through coordinated interactions. Proc. Natl. Acad. Sci. U. S. A. 2008;05:3622–3627. doi: 10.1073/pnas.0709180105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Dou F, Netzer WJ, Tanemura K, Li F, Hartl FU, Takashima A, Gouras GK, Greengard P, Xu H. Chaperones increase association of tau protein with microtubules. Proc. Natl. Acad. Sci. U. S. A. 2003;100:721–726. doi: 10.1073/pnas.242720499. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo W, Dou F, Rodina A, Chip S, Kim J, Zhao Q, Moulick K, Aguirre J, Wu N, Greengard P, Chiosis G. Roles of heat-shock protein 90 in maintaining and facilitating the neurodegenerative phenotype in tauopathies. Proc. Natl. Acad. Sci. U. S. A. 2007;104:9511–9516. doi: 10.1073/pnas.0701055104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Dickey CA, Dunmore J, Lu B, Wang JW, Lee WC, Kamal A, Burrows F, Eckman C, Hutton M, Petrucelli L. HSP induction mediates selective clearance of tau phosphorylated at proline-directed Ser/Thr sites but not KXGS (MARK) sites. FASEB J. 2006;20:753–755. doi: 10.1096/fj.05-5343fje. [DOI] [PubMed] [Google Scholar]
- 31.Dickey CA, Ash P, Klosak N, Lee WC, Petrucelli L, Hutton M, Eckman CB. Pharmacologic reductions of total tau levels; implications for the role of microtubule dynamics in regulating tau expression. Mol. Neurodegener. 2006;1:6–0. doi: 10.1186/1750-1326-1-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Bukau B, Horwich AL. The Hsp70 and Hsp60 chaperone ma-chines. Cell. 1998;92:351–366. doi: 10.1016/s0092-8674(00)80928-9. [DOI] [PubMed] [Google Scholar]
- 33.Walter S, Buchner J. Molecular chaperones--cellular machines for protein folding. Angew. Chem. Int. Ed. Engl. 2002;41:1098–1113. doi: 10.1002/1521-3773(20020402)41:7<1098::aid-anie1098>3.0.co;2-9. [DOI] [PubMed] [Google Scholar]
- 34.Neckers L, Neckers K. Heat-shock protein 90 inhibitors as novel cancer chemotherapeutic agents. Expert Opin. Emerg. Drugs. 2002;7:277–288. doi: 10.1517/14728214.7.2.277. [DOI] [PubMed] [Google Scholar]
- 35.Klucken J, Shin Y, Masliah E, Hyman BT, McLean PJ. Hsp70 Reduces alpha-Synuclein aggregation and toxicity. J. Biol. Chem. 2004;279:25497–25502. doi: 10.1074/jbc.M400255200. [DOI] [PubMed] [Google Scholar]
- 36.Elliott E, Tsvetkov P, Ginzburg I. BAG-1 associates with Hsc70.Tau complex and regulates the proteasomal degradation of Tau protein. J. Biol. Chem. 2007;282:37276–37284. doi: 10.1074/jbc.M706379200. [DOI] [PubMed] [Google Scholar]
- 37.Sarkar M, Kuret J, Lee G. Two motifs within the tau micro-tubule-binding domain mediate its association with the hsc70 molecular chaperone. J. Neurosci. Res. 2008;86:2763–2773. doi: 10.1002/jnr.21721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Oddo S, Caccamo A, Tseng B, Cheng D, Vasilevko V, Cribbs DH, LaFerla FM. Blocking Abeta42 accumulation delays the onset and progression of tau pathology via the C terminus of heat shock protein70-interacting protein: a mechanistic link between Abeta and tau pathology. J. Neurosci. 2008;28:12163–12175. doi: 10.1523/JNEUROSCI.2464-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Jinwal UK, Miyata Y, Koren J 3rd, Jones JR, Trotter JH, Chang L, O'Leary J, Morgan D, Lee DC, Shults CL, Rousaki A, Weeber EJ, Zuiderweg ER, Gestwicki JE, Dickey CA. Chemical manipulation of hsp70 ATPase activity regulates tau stability. J. Neurosci. 2009;29:12079–12088. doi: 10.1523/JNEUROSCI.3345-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Jinwal UK, O'Leary IIIJ, Borysov SI, Jones JR, Li Q, Koren IIIJ, Abisambra JF, Vestal GD, Lawson LY, Johnson AG, Blair LJ, Jin Y, Miyata Y, Gestwicki JE, Dickey CA. Hsc70 rapidly engages tau after microtubule destabilization. J. Biol. Chem. 2010;285:16798–805. doi: 10.1074/jbc.M110.113753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Koren J 3rd, Jinwal UK, Jin Y, O'Leary J, Jones JR, Johnson AG, Blair LJ, Abisambra JF, Chang L, Miyata Y, Cheng AM, Guo J, Cheng JQ, Gestwicki JE, Dickey CA. Facilitating Akt clearance via manipulation of Hsp70 activity and levels. J. Biol. Chem. 2010;285:2498–2505. doi: 10.1074/jbc.M109.057208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Koren J, Jinwal UK, Jin Y, O'Leary J, Jones JR, Johnson AG, Blair LJ, Abisambra JF, Chang L, Miyata Y, Cheng AM, Guo J, Cheng JQ, Gestwicki JE, Dickey CA. Facilitating Akt clearance via manipulation of Hsp70 activity and levels. J. Biol. Chem. 2009;285:2498–505. doi: 10.1074/jbc.M109.057208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ellis J. Proteins as molecular chaperones. Nature. 1987;328:378–379. doi: 10.1038/328378a0. [DOI] [PubMed] [Google Scholar]
- 44.Hemmingsen SM, Woolford C, van der Vies SM, Tilly K, Dennis DT, Georgopoulos CP, Hendrix RW, Ellis RJ. Homologous plant and bacterial proteins chaperone oligomeric protein assembly. Nature. 1988;333:330–334. doi: 10.1038/333330a0. [DOI] [PubMed] [Google Scholar]
- 45.Cyr DM, Hohfeld J, Patterson C. Protein quality control: U-box-containing E3 ubiquitin ligases join the fold. Trends Biochem. Sci. 2002;27:368–375. doi: 10.1016/s0968-0004(02)02125-4. [DOI] [PubMed] [Google Scholar]
- 46.Muchowski PJ, Schaffar G, Sittler A, Wanker EE, Hayer-Hartl MK, Hartl FU. Hsp70 and hsp40 chaperones can inhibit self-assembly of polyglutamine proteins into amyloid-like fibrils. Proc. Natl. Acad. Sci. U.S.A. 2000;97:7841–7846. doi: 10.1073/pnas.140202897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Cohen E, Bieschke J, Perciavalle RM, Kelly JW, Dillin A. Opposing activities protect against age-onset proteotoxicity. Science. 2006;313:1604–1610. doi: 10.1126/science.1124646. [DOI] [PubMed] [Google Scholar]
- 48.Westerheide SD, Bosman JD, Mbadugha BN, Kawahara TL, Matsumoto G, Kim S, Gu W, Devlin JP, Silverman RB, Morimoto RI. Celastrols as inducers of the heat shock response and cytoprotection. J. Biol. Chem. 2004;279:56053–56060. doi: 10.1074/jbc.M409267200. [DOI] [PubMed] [Google Scholar]
- 49.Zou J, Guo Y, Guettouche T, Smith DF, Voellmy R. Repression of heat shock transcription factor HSF1 activation by HSP90 (HSP90 complex) that forms a stress-sensitive complex with HSF1. Cell. 1998;94:471–480. doi: 10.1016/s0092-8674(00)81588-3. [DOI] [PubMed] [Google Scholar]
- 50.Jinwal UK, O'Leary JC, 3rd Borysov, S.I. Jones, J.R. Li, Q. Koren, J. 3rd, Abisambra JF, Vestal GD, Lawson LY, Johnson AG, Blair LJ, Jin Y, Miyata Y, Gestwicki JE, Dickey CA. Hsc70 rapidly engages tau after microtubule destabilization. J. Biol. Chem. 2010;285:16798–16805. doi: 10.1074/jbc.M110.113753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Ehrnsperger M, Graber S, Gaestel M, Buchner J. Binding of non-native protein to Hsp25 during heat shock creates a reservoir of folding intermediates for reactivation. EMBO J. 1997;16:221–229. doi: 10.1093/emboj/16.2.221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Kumar P, Ambasta RK, Veereshwarayya V, Rosen KM, Kosik KS, Band H, Mestril R, Patterson C, Querfurth HW. CHIP and HSPs interact with beta-APP in a proteasome-dependent manner and influence Abeta metabolism. Hum. Mol. Genet. 2007;16:848–864. doi: 10.1093/hmg/ddm030. [DOI] [PubMed] [Google Scholar]
- 53.Bjorkdahl C, Sjogren MJ, Zhou X, Concha H, Avila J, Win-blad B, Pei JJ. Small heat shock proteins Hsp27 or alphaB-crystallin and the protein components of neurofibrillary tangles: tau and neurofilaments. J. Neurosci. Res. 2008;86:1343–1352. doi: 10.1002/jnr.21589. [DOI] [PubMed] [Google Scholar]
- 54.Jakob U, Gaestel M, Engel K, Buchner J. Small heat shock proteins are molecular chaperones. J. Biol. Chem. 1993;268:1517–1520. [PubMed] [Google Scholar]
- 55.Ehrnsperger M, Lilie H, Gaestel M, Buchner J. The dynamics of Hsp25 quaternary structure.Structure and function of different oligomeric species. J. Biol. Chem. 1999;274:14867–14874. doi: 10.1074/jbc.274.21.14867. [DOI] [PubMed] [Google Scholar]
- 56.Liang P, Amons R, Clegg JS, MacRae TH. Molecular characterization of a small heat shock/alpha-crystallin protein in encysted Artemia embryos. J. Biol. Chem. 1997;272:19051–19058. doi: 10.1074/jbc.272.30.19051. [DOI] [PubMed] [Google Scholar]
- 57.Head MW, Goldman JE. Small heat shock proteins, the cytoskeleton, and inclusion body formation. Neuropathol. Appl. Neurobiol. 2000;26:304–312. doi: 10.1046/j.1365-2990.2000.00269.x. [DOI] [PubMed] [Google Scholar]
- 58.Richter-Landsberg C, Bauer NG. Tau-inclusion body formation in oligodendroglia: the role of stress proteins and proteasome inhibition. Int. J. Dev. Neurosci. 2004;22:443–451. doi: 10.1016/j.ijdevneu.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 59.Goldbaum O, Richter-Landsberg C. Proteolytic stress causes heat shock protein induction, tau ubiquitination, and the re-cruitment of ubiquitin to tau-positive aggregates in oligodendrocytes in culture. J. Neurosci. 2004;24:5748–5757. doi: 10.1523/JNEUROSCI.1307-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Stromer T, Ehrnsperger M, Gaestel M, Buchner J. Analysis of the interaction of small heat shock proteins with unfolding proteins. J. Biol. Chem. 2003;278:18015–18021. doi: 10.1074/jbc.M301640200. [DOI] [PubMed] [Google Scholar]
- 61.Arrigo AP, Suhan JP, Welch WJ. Dynamic changes in the structure and intracellular locale of the mammalian low-molecular-weight heat shock protein. Mol. Cell Biol. 1988;8:5059–5071. doi: 10.1128/mcb.8.12.5059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Lambert H, Charette SJ, Bernier AF, Guimond A, Landry J. HSP27 multimerization mediated by phosphorylation-sensitive intermolecular interactions at the amino terminus. J. Biol. Chem. 1999;274:9378–9385. doi: 10.1074/jbc.274.14.9378. [DOI] [PubMed] [Google Scholar]
- 63.Lee GJ, Roseman AM, Saibil HR, Vierling E. A small heat shock protein stably binds heat-denatured model substrates and can maintain a substrate in a folding-competent state. EMBO J. 1997;16:659–671. doi: 10.1093/emboj/16.3.659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Haslbeck M, Franzmann T, Weinfurtner D, Buchner J. Some like it hot: the structure and function of small heat-shock proteins. Nat. Struct. Mol. Biol. 2005;12:842–846. doi: 10.1038/nsmb993. [DOI] [PubMed] [Google Scholar]
- 65.Basha E, Lee GJ, Demeler B, Vierling E. Chaperone activity of cytosolic small heat shock proteins from wheat. Eur. J. Biochem. 2004;271:1426–1436. doi: 10.1111/j.1432-1033.2004.04033.x. [DOI] [PubMed] [Google Scholar]
- 66.Nover L, Scharf KD, Neumann D. Formation of cytoplasmic heat shock granules in tomato cell cultures and leaves. Mol. Cell Biol. 1983;3:1648–1655. doi: 10.1128/mcb.3.9.1648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Evgrafov OV, Mersiyanova I, Irobi J, Van Den Bosch L, Dierick I, Leung CL, Schagina O, Verpoorten N, Van Impe K, Fedotov V, Dadali E, Auer-Grumbach M, Windpassinger C, Wagner K, Mitrovic Z, Hilton-Jones D, Talbot K, Martin JJ, Vasserman N, Tverskaya S, Polyakov A, Liem RK, Gettemans J, Robberecht W, De Jonghe P, Timmerman V. Mutant small heat-shock protein 27 causes axonal Charcot-Marie-Tooth disease and distal hereditary motor neuropathy. Nat. Genet. 2004;36:602–606. doi: 10.1038/ng1354. [DOI] [PubMed] [Google Scholar]
- 68.Ingolia TD, Craig EA. Four small Drosophila heat shock pro-teins are related to each other and to mammalian alpha-crystallin. Proc. Natl. Acad. Sci. U. S .A. 1982;79:2360–2364. doi: 10.1073/pnas.79.7.2360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.McHaourab HS, Berengian AR, Koteiche HA. Site-directed spin-labeling study of the structure and subunit interactions along a conserved sequence in the alpha-crystallin domain of heat-shock protein 27.Evidence of a conserved subunit interface. . Biochemistry. 1997;36:14627–14634. doi: 10.1021/bi971700s. [DOI] [PubMed] [Google Scholar]
- 70.Berengian AR, Parfenova M, McHaourab HS. Site-directed spin labeling study of subunit interactions in the alpha-crystallin domain of small heat-shock proteins. Comparison of the oligomer symmetry in alphaA-crystallin, HSP 27, and HSP 16.3. J. Biol. Chem. 1999;274:6305–6314. doi: 10.1074/jbc.274.10.6305. [DOI] [PubMed] [Google Scholar]
- 71.Kostenko S, Moens U. Heat shock protein 27 phosphorylation: kinases, phosphatases, functions and pathology. Cell Mol. Life Sci. 2009;66:3289–3307. doi: 10.1007/s00018-009-0086-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Cairns J, Qin S, Philp R, Tan YH, Guy GR. Dephosphoryla-tion of the small heat shock protein Hsp27 in vivo by protein phosphatase 2A. J. Biol. Chem. 1994;269:9176–9183. [PubMed] [Google Scholar]
- 73.Miron T, Wilchek M, Geiger B. Characterization of an inhibitor of actin polymerization in vinculin-rich fraction of turkey gizzard smooth muscle. Eur. J. Biochem. 1988;178:543–553. doi: 10.1111/j.1432-1033.1988.tb14481.x. [DOI] [PubMed] [Google Scholar]
- 74.Miron T, Vancompernolle K, Vandekerckhove J, Wilchek M, Geiger B. A 25-kD inhibitor of actin polymerization is a low molecular mass heat shock protein. J. Cell Biol. 1991;114:255–261. doi: 10.1083/jcb.114.2.255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Lavoie JN, Gingras-Breton G, Tanguay RM, Landry J. Induction of Chinese hamster HSP27 gene expression in mouse cells confers resistance to heat shock.HSP27 stabilization of the microfilament organization. J. Biol. Chem. 1993;268:3420–3429. [PubMed] [Google Scholar]
- 76.Lavoie JN, Hickey E, Weber LA, Landry J. Modulation of actin microfilament dynamics and fluid phase pinocytosis by phos-phorylation of heat shock protein 27. J. Biol. Chem. 1993;268:24210–24214. [PubMed] [Google Scholar]
- 77.Zhu Y, O'Neill S, Saklatvala J, Tassi L, Mendelsohn ME. Phosphorylated HSP27 associates with the activation-dependent cytoskeleton in human platelets. Blood. 1994;84:3715–3723. [PubMed] [Google Scholar]
- 78.Piotrowicz RS, Levin EG. Basolateral membrane-associated 27-kDa heat shock protein and microfilament polymerization. J. Biol. Chem. 1997;272:25920–25927. doi: 10.1074/jbc.272.41.25920. [DOI] [PubMed] [Google Scholar]
- 79.Lavoie JN, Lambert H, Hickey E, Weber LA, Landry J. Modulation of cellular thermoresistance and actin filament stability accompanies phosphorylation-induced changes in the oligomeric structure of heat shock protein 27. Mol. Cell Biol. 1995;15:505–516. doi: 10.1128/mcb.15.1.505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Benndorf R, Hayess K, Ryazantsev S, Wieske M, Behlke J, Lutsch G. Phosphorylation and supramolecular organization of murine small heat shock protein HSP25 abolish its actin polymerization-inhibiting activity. J. Biol. Chem. 1994;269:20780–20784. [PubMed] [Google Scholar]
- 81.Hedges JC, Dechert MA, Yamboliev IA, Martin JL, Hickey E, Weber LA, Gerthoffer WT. A role for p38(MAPK)/HSP27 pathway in smooth muscle cell migration. J. Biol. Chem. 1999;274:24211–24219. doi: 10.1074/jbc.274.34.24211. [DOI] [PubMed] [Google Scholar]
- 82.Rousseau S, Houle F, Landry J, Huot J. p38 MAP kinase activation by vascular endothelial growth factor mediates actin reorganization and cell migration in human endothelial cells. Oncogene. 1997;15:2169–2177. doi: 10.1038/sj.onc.1201380. [DOI] [PubMed] [Google Scholar]
- 83.Hong Z, Zhang QY, Liu J, Wang ZQ, Zhang Y, Xiao Q, Lu J, Zhou HY, Chen SD. Phosphoproteome study reveals Hsp27 as a novel signaling molecule involved in GDNF-induced neurite outgrowth. J. Proteome Res. 2009;8:2768–2787. doi: 10.1021/pr801052v. [DOI] [PubMed] [Google Scholar]
- 84.Kampinga HH, Brunsting JF, Stege GJ, Konings AW, Landry J. Cells overexpressing Hsp27 show accelerated recovery from heat-induced nuclear protein aggregation. Biochem. Biophys. Res. Commun. 1994;204:1170–1177. doi: 10.1006/bbrc.1994.2586. [DOI] [PubMed] [Google Scholar]
- 85.Mehlen P, Coronas V, Ljubic-Thibal V, Ducasse C, Granger L, Jourdan F, Arrigo AP. Small stress protein Hsp27 accumulation during dopamine-mediated differentiation of rat olfactory neurons counteracts apoptosis. Cell Death Differ. 1999;6:227–233. doi: 10.1038/sj.cdd.4400483. [DOI] [PubMed] [Google Scholar]
- 86.Franklin TB, Krueger-Naug AM, Clarke DB, Arrigo AP, Currie RW. The role of heat shock proteins Hsp70 and Hsp27 in cellular protection of the central nervous system. Int. J. Hyperther-mia. 2005;21:379–392. doi: 10.1080/02656730500069955. [DOI] [PubMed] [Google Scholar]
- 87.Mehlen P, Schulze-Osthoff K, Arrigo AP. Small stress proteins as novel regulators of apoptosis.Heat shock protein 27 blocks Fas/APO-1- and staurosporine-induced cell death. J. Biol. Chem. 1996;271:16510–16514. doi: 10.1074/jbc.271.28.16510. [DOI] [PubMed] [Google Scholar]
- 88.Plumier JC, Hopkins DA, Robertson HA, Currie RW. Con-stitutive expression of the 27-kDa heat shock protein (Hsp27) in sensory and motor neurons of the rat nervous system. J. Comp. Neurol. 1997;384:409–428. [PubMed] [Google Scholar]
- 89.Dean DO, Tytell M. Hsp25 and -90 immunoreactivity in the normal rat eye. Invest. Ophthalmol. Vis. Sci. 2001;42:3031–3040. [PubMed] [Google Scholar]
- 90.Armstrong CL, Krueger-Naug AM, Currie RW, Hawkes R. Constitutive expression of the 25-kDa heat shock protein Hsp25 reveals novel parasagittal bands of purkinje cells in the adult mouse cerebellar cortex. J. Comp. Neurol. 2000;416:383–397. doi: 10.1002/(sici)1096-9861(20000117)416:3<383::aid-cne9>3.0.co;2-m. [DOI] [PubMed] [Google Scholar]
- 91.Armstrong CL, Krueger-Naug AM, Currie RW, Hawkes R. Expression of heat-shock protein Hsp25 in mouse Purkinje cells during development reveals novel features of cerebellar compart-mentation. J. Comp. Neurol. 2001;429:7–21. doi: 10.1002/1096-9861(20000101)429:1<7::aid-cne2>3.0.co;2-q. [DOI] [PubMed] [Google Scholar]
- 92.Chen S, Brown IR. Neuronal expression of constitutive heat shock proteins: implications for neurodegenerative diseases. Cell Stress Chaperones. 2007;12:51–58. doi: 10.1379/CSC-236R.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Wilhelmus MM, Otte-Holler I, Wesseling P, de Waal RM, Boelens WC, Verbeek MM. Specific association of small heat shock proteins with the pathological hallmarks of Alzheimer's dis-ease brains. Neuropathol. Appl. Neurobiol. 2006;32:119–130. doi: 10.1111/j.1365-2990.2006.00689.x. [DOI] [PubMed] [Google Scholar]
- 94.King M, Nafar F, Clarke J, Mearow K. The small heat shock protein Hsp27 protects cortical neurons against the toxic effects of beta-amyloid peptide. J. Neurosci. Res. 2009;87:3161–3175. doi: 10.1002/jnr.22145. [DOI] [PubMed] [Google Scholar]
- 95.Akbar MT, Wells DJ, Latchman DS, de Belleroche J. Heat shock protein 27 shows a distinctive widespread spatial and tempo-ral pattern of induction in CNS glial and neuronal cells compared to heat shock protein 70 and caspase 3 following kainate admini-stration. Brain Res. Mol. Brain Res. 2001;93:148–163. doi: 10.1016/s0169-328x(01)00199-1. [DOI] [PubMed] [Google Scholar]
- 96.Bechtold DA, Brown IR. Induction of Hsp27 and Hsp32 stress proteins and vimentin in glial cells of the rat hippocampus follow-ing hyperthermia. Neurochem. Res. 2003;28:1163–1173. doi: 10.1023/a:1024268126310. [DOI] [PubMed] [Google Scholar]
- 97.Kato H, Liu Y, Kogure K, Kato K. Induction of 27-kDa heat shock protein following cerebral ischemia in a rat model of ischemic tolerance. Brain Res. 1994;634:235–244. doi: 10.1016/0006-8993(94)91926-7. [DOI] [PubMed] [Google Scholar]
- 98.Krueger-Naug AM, Hopkins DA, Armstrong JN, Plumier JC, Currie RW. Hyperthermic induction of the 27-kDa heat shock protein (Hsp27) in neuroglia and neurons of the rat central nervous system. J. Comp. Neurol. 2000;428:495–510. doi: 10.1002/1096-9861(20001218)428:3<495::aid-cne7>3.0.co;2-4. [DOI] [PubMed] [Google Scholar]
- 99.Plumier JC, Armstrong JN, Landry J, Babity JM, Robertson HA, Currie RW. Expression of the 27,000 mol.wt heat shock protein following kainic acid-induced status epilepticus in the rat. Neuroscience. 1996;75:849–856. doi: 10.1016/0306-4522(96)00317-x. [DOI] [PubMed] [Google Scholar]
- 100.Currie RW, Ellison JA, White RF, Feuerstein GZ, Wang X, Barone FC. Benign focal ischemic preconditioning induces neuronal Hsp70 and prolonged astrogliosis with expression of Hsp27. Brain Res. 2000;863:169–181. doi: 10.1016/s0006-8993(00)02133-8. [DOI] [PubMed] [Google Scholar]
- 101.Plumier JC, David JC, Robertson HA, Currie RW. Cortical application of potassium chloride induces the low-molecular weight heat shock protein (Hsp27) in astrocytes. J. Cereb. Blood Flow Metab. 1997;17:781–790. doi: 10.1097/00004647-199707000-00008. [DOI] [PubMed] [Google Scholar]
- 102.Bechtold DA, Brown IR. Heat shock proteins Hsp27 and Hsp32 localize to synaptic sites in the rat cerebellum following hyper-thermia. Brain Res. Mol. Brain Res. 2000;75:309–320. doi: 10.1016/s0169-328x(99)00323-x. [DOI] [PubMed] [Google Scholar]
- 103.Krueger-Naug AM, Emsley JG, Myers TL, Currie RW, Clarke DB. Injury to retinal ganglion cells induces expression of the small heat shock protein Hsp27 in the rat visual system. Neuroscience. 2002;110:653–665. doi: 10.1016/s0306-4522(01)00453-5. [DOI] [PubMed] [Google Scholar]
- 104.Hopkins DA, Plumier JC, Currie RW. Induction of the 27-kDa heat shock protein (Hsp27) in the rat medulla oblongata after vagus nerve injury. Exp. Neurol. 1998;153:173–183. doi: 10.1006/exnr.1998.6870. [DOI] [PubMed] [Google Scholar]
- 105.Lewis SE, Mannion RJ, White FA, Coggeshall RE, Beggs S, Costigan M, Martin JL, Dillmann WH, Woolf CJ. A role for HSP27 in sensory neuron survival. J. Neurosci. 1999;19:8945–8953. doi: 10.1523/JNEUROSCI.19-20-08945.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Krueger-Naug AM, Emsley JG, Myers TL, Currie RW, Clarke DB. Administration of brain-derived neurotrophic factor suppresses the expression of heat shock protein 27 in rat retinal ganglion cells following axotomy. Neuroscience. 2003;16:49–58. doi: 10.1016/s0306-4522(02)00582-1. [DOI] [PubMed] [Google Scholar]
- 107.Renkawek K, Bosman GJ, de Jong WW. Expression of small heat-shock protein hsp 27 in reactive gliosis in Alzheimer disease and other types of dementia. Acta Neuropathol. 1994;87:511–519. doi: 10.1007/BF00294178. [DOI] [PubMed] [Google Scholar]
- 108.Renkawek K, Bosman GJ, Gaestel M. Increased expression of heat-shock protein 27 kDa in Alzheimer disease: a preliminary study. Neuroreport. 1993;5:14–16. doi: 10.1097/00001756-199310000-00003. [DOI] [PubMed] [Google Scholar]
- 109.Shinohara H, Inaguma Y, Goto S, Inagaki T, Kato K. Alpha B crystallin and HSP28 are enhanced in the cerebral cortex of patients with Alzheimer's disease. J. Neurol. Sci. 1993;119:203–208. doi: 10.1016/0022-510x(93)90135-l. [DOI] [PubMed] [Google Scholar]
- 110.Lukiw WJ. Gene expression profiling in fetal, aged, and Alzheimer hippocampus: a continuum of stress-related signaling. Neurochem. Res. 2004;29:1287–1297. doi: 10.1023/b:nere.0000023615.89699.63. [DOI] [PubMed] [Google Scholar]
- 111.Pardon MC, Rattray I. What do we know about the long-term consequences of stress on ageing and the progression of age-related neurodegenerative disorders? Neurosci. Biobehav. Rev. 2008;32:1103–1120. doi: 10.1016/j.neubiorev.2008.03.005. [DOI] [PubMed] [Google Scholar]
- 112.Efthymiou CA, Mocanu MM, Yellon DM. Atorvastatin and myocardial reperfusion injury: new pleiotropic effect implicating multiple prosurvival signaling. J. Cardiovasc. Pharmacol. 2005;45:247–252. doi: 10.1097/01.fjc.0000154376.82445.06. [DOI] [PubMed] [Google Scholar]
- 113.Negre-Aminou P, van Leeuwen RE, van Thiel GC, van den IP, de Jong WW, Quinlan RA, Cohen LH. Differential effect of simvastatin on activation of Rac(1) vs. activation of the heat shock protein 27-mediated pathway upon oxidative stress, in human smooth muscle cells. Biochem. Pharmacol. 2002;64:1483–1491. doi: 10.1016/s0006-2952(02)01388-6. [DOI] [PubMed] [Google Scholar]
- 114.Ciocca DR, Rozados VR, Cuello Carrion FD, Gervasoni SI, Matar P, Scharovsky OG. Hsp25 and Hsp70 in rodent tumors treated with doxorubicin and lovastatin. Cell Stress Chaperones. 2003;8:26–36. doi: 10.1379/1466-1268(2003)8<26:hahirt>2.0.co;2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Tartakover-Matalon S, Cherepnin N, Kuchuk M, Drucker L, Kenis I, Fishman A, Pomeranz M, Lishner M. Impaired migration of trophoblast cells caused by simvastatin is associated with decreased membrane IGF-I receptor, MMP2 activity and HSP27 expression. Hum. Reprod. 2007;22:1161–1167. doi: 10.1093/humrep/del464. [DOI] [PubMed] [Google Scholar]
- 116.Wang X, Tokuda H, Hatakeyama D, Hirade K, Niwa M, Ito H, Kato K, Kozawa O. Mechanism of simvastatin on induction of heat shock protein in osteoblasts. Arch. Biochem. Biophys. 2003;415:6–13. doi: 10.1016/s0003-9861(03)00213-3. [DOI] [PubMed] [Google Scholar]
- 117.Kretz A, Schmeer C, Tausch S, Isenmann S. Simvastatin promotes heat shock protein 27 expression and Akt activation in the rat retina and protects axotomized retinal ganglion cells in vivo. Neurobiol. Dis. 2006;21:421–430. doi: 10.1016/j.nbd.2005.08.003. [DOI] [PubMed] [Google Scholar]
- 118.Schmeer C, Gamez A, Tausch S, Witte OW, Isenmann S. Statins modulate heat shock protein expression and enhance retinal ganglion cell survival after transient retinal ischemia/reperfusion in vivo. Invest. Ophthalmol. Vis. Sci. 2008;49:4971–4981. doi: 10.1167/iovs.07-1597. [DOI] [PubMed] [Google Scholar]
- 119.Jick H, Zornberg GL, Jick SS, Seshadri S, Drachman DA. Statins and the risk of dementia. Lancet. 2000;356:1627–1631. doi: 10.1016/s0140-6736(00)03155-x. [DOI] [PubMed] [Google Scholar]
- 120.Hajjar I, Schumpert J, Hirth V, Wieland D, Eleazer GP. The impact of the use of statins on the prevalence of dementia and the progression of cognitive impairment. J. Gerontol. A Biol. Sci. Med. Sci. 2002;57:M414–418. doi: 10.1093/gerona/57.7.m414. [DOI] [PubMed] [Google Scholar]
- 121.Rockwood K, Kirkland S, Hogan DB, MacKnight C, Merry H, Verreault R, Wolfson C, McDowell I. Use of lipid-lowering agents, indication bias, and the risk of dementia in community-dwelling elderly people. Arch. Neurol. 2002;59:223–227. doi: 10.1001/archneur.59.2.223. [DOI] [PubMed] [Google Scholar]
- 122.Rea TD, Breitner JC, Psaty BM, Fitzpatrick AL, Lopez OL, Newman AB, Hazzard WR, Zandi PP, Burke GL, Lyketsos CG, Bernick C, Kuller LH. Statin use and the risk of incident dementia: the Cardiovascular Health Study. Arch. Neurol. 2005;62:1047–1051. doi: 10.1001/archneur.62.7.1047. [DOI] [PubMed] [Google Scholar]
- 123.Li G, Higdon R, Kukull WA, Peskind E, Van Valen Moore K, Tsuang D, van Belle G, McCormick W, Bowen JD, Teri L, Schellenberg GD, Larson EB. Statin therapy and risk of dementia in the elderly: a community-based prospective cohort study. Neurology. 2004;63:1624–1628. doi: 10.1212/01.wnl.0000142963.90204.58. [DOI] [PubMed] [Google Scholar]
- 124.Wilhelmus MM, Boelens WC, Otte-Holler I, Kamps B, de Waal RM, Verbeek MM. Small heat shock proteins inhibit amyloid-beta protein aggregation and cerebrovascular amyloid-beta protein toxicity. Brain Res. 2006;1089:67–78. doi: 10.1016/j.brainres.2006.03.058. [DOI] [PubMed] [Google Scholar]
- 125.Kudva YC, Hiddinga HJ, Butler PC, Mueske CS, Eberhardt NL. Small heat shock proteins inhibit in vitro A beta(1-42) amyloidogenesis. FEBS Lett. 1997;416:117–121. doi: 10.1016/s0014-5793(97)01180-0. [DOI] [PubMed] [Google Scholar]
- 126.Lee S, Carson K, Rice-Ficht A, Good T. Small heat shock proteins differentially affect Abeta aggregation and toxicity. Biochem. Res. Commun. 2006;347:527–533. doi: 10.1016/j.bbrc.2006.06.128. [DOI] [PubMed] [Google Scholar]
- 127.Dabir DV, Trojanowski JQ, Richter-Landsberg C, Lee VM, Forman MS. Expression of the small heat-shock protein alphaB-crystallin in tauopathies with glial pathology. Am. J. Pathol. 2004;64:155–166. doi: 10.1016/s0002-9440(10)63106-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Sahara N, Maeda S, Yoshiike Y, Mizoroki T, Yamashita S, Murayama M, Park JM, Saito Y, Murayama S, Takashima A. Molecular chaperone-mediated tau protein metabolism counter-acts the formation of granular tau oligomers in human brain. J. Neurosci. Res. 2007;85:3098–3108. doi: 10.1002/jnr.21417. [DOI] [PubMed] [Google Scholar]
- 129.Neininger A, Gaestel M. Evidence for a hsp25-specific mecha-nism involved in transcriptional activation by heat shock. Exp. Cell Res. 1998;242:285–293. doi: 10.1006/excr.1998.4099. [DOI] [PubMed] [Google Scholar]