Abstract
Most neuroimaging studies examining verb morphology have focused on verb tense, with fewer examining agreement morphology, and no previous fMRI studies have investigated distinctions between past and present tense inflection. However, models of language representation and processing suggest differences in where these inflections are instantiated in the phrase structure as well as differences in the linguistic functions they serve, suggesting unique neural networks for these forms. In addition, results of available neuroimaging studies of grammatical morphology vary considerably due to methodological differences. Some studies have used overt production tasks, whereas others have used covert tasks. In the present study we examined brain activation associated with past tense and present tense/agreement morphology under overt and covert production conditions in 13 healthy adults using an event-related functional magnetic resonance imaging (fMRI) design. Production of verbs inflected for past tense (V + −ed) and present tense/agreement (V −s) was elicited using temporal adverbs (i.e. Yesterday, Nowadays). Results showed that in healthy adults inflecting both past tense and agreement morphology (compared to a verb stem production condition) recruited not only left inferior frontal structures, but also motor and premotor cortices, and posterior parietal regions. Activation also was observed in the basal ganglia, thalamus, and the cingulate gyrus. Past tense and present tense/agreement recruited partially overlapping tissue in these regions, with distinctions observed for the two forms in frontal and parietal brain areas. We also found that activation varied with task demands, with more extensive frontal activation noted in the overt compared to the covert verb inflection task. These results are consistent with the hypothesis that the neural signatures for verb inflection differ from that for verb stems alone and involve a distributed frontal and parietal network of brain regions. Further, the neural tissue recruited for instantiation of past tense versus present tense/agreement morphology is distinct, supporting linguistic theories that differentiate the two forms.
Keywords: Verb inflection, Tense and agreement processing, Covert and overt production, fMRI
1. Introduction
In the past two decades verb inflection has been used extensively to investigate neural systems that underlie language processing. For example, the neurocognitive architecture of verb inflection (e.g., past tense, third person singular) has served as a testing ground for linguistic theories, which view language as involving both a mental dictionary or lexicon and a set of symbolic rules (Bybee & Slobin, 1982; Chomsky, 1972; Druks, 2006; Halle & Mohanan, 1985; Pinker, 1998; see Ullman, Corkin et al., 1997; Ullman et al., 2005), and connectionist theories, which suggest that all inflections are computed by an associative memory system (developed based on multiple simultaneous constraints developed through learning) (Bybee, 1985; Hay, 2002; Hay & Baayen, 2005; Joanisse & Seidenberg, 1999; McClelland & Patterson, 2002; Rumelhart & McClelland, 1986).
On the former theories production of morphologically complex forms engages morphophonological as well as syntactic processes that concatenate selected grammatical features. For example, it is assumed that past tense as well as singular present tense forms are computed by the rule-based morphological transformation that attaches the correct tense suffix to the base word (suffixes − ed and − s, respectively; e.g., he walk + ed, he walk + s). These theories make distinct predictions about neural correlates involved in processing of lexically stored vs. generated forms. In this framework, all inflected forms are generated in the left inferior frontal regions by grammatical combinatorial rules which concatenate stems and affixes, whereas uninflected stems are stored and retrieved from lexical memory, housed in the left temporo-parietal cortex. The results of neuroimaging studies support these distinctions and suggest that inflectional processes engage both frontal and posterior brain regions (Beretta et al., 2003; Dhond, Marinkovic, Dale, Witzel, & Halgren, 2003; Hagoort, 2003, 2005; Jaeger et al., 1996; Joanisse & Seidenberg, 2005; Sahin, Pinker, Cash, Schomer, & Halgren, 2009). Conversely, connectionist accounts abolish morphological rules of any sort, focusing instead on the organization of the lexicon as the source of grammatical inflection. Word formation arises from phonological, orthographic and semantic regularities in the organization of an associational lexical network. This latter idea is able to account for frequency effects and other graded, contextual effects in morphologically complex forms that would not be expected if such forms were concatenated or otherwise assembled by rules that operate without regard to contextual considerations.
Observations that some patients with aphasia show asymmetries in their ability to process past and present tense features, however, complicate these issues (Bastiaanse, 2008; Nilipour, 2000; Stavrakaki & Kouvava, 2003). For example, Jonkers (2009) showed that past tense forms were more difficult to produce and comprehend for patients with aphasia in a sentence-to-picture matching task. Specifically, reference to the past seems to be more difficult than reference to the present. It has been suggested that this difference arises as a result of the greater semantic complexity involved in representing events in reference to the past (Bastiaanse, 2008). The time frame for present tense includes the “here and now”; whereas, the temporal representation for the past includes two time points: “here and now, and a time before here and now” (Bastiaanse, 2008). These findings suggest that distinct neural processes may come into play even for these finer grained tense differences.
Interestingly, tense and subject-verb agreement also have been shown to dissociate in patients with aphasia (Arabatzi & Edwards, 2002; Burchert, Swoboda-Moll, & De Bleser, 2005; Clahsen & Ali, 2009; Friedmann & Grodzinsky, 1997; Lee, Milman, & Thompson, 2008). On some linguistic theories tense and agreement inflection are similarly instantiated. Principles and Parameters syntax (Chomsky, 1981), for example, holds that the Inflection Phrase (IP) houses all verb inflection, particularly in English. All tense features and agreement are, therefore, generated from the same node of the syntactic tree (Bobaljik & Thrainsson, 1998). Based on these models, one might expect that similar neurocognitive processes underlie tense and agreement morphology. However, other linguistic accounts of verb inflection suggest that tense and agreement may be distinguished by their location in the phrase structure. On these accounts IP is split, resulting in separate nodes for the two forms (Pollock, 1989). In addition, in the Minimalist Program of syntax (Chomsky, 1993; Marantz, 1995), tense and agreement are structurally distinct: tense phrases exist to check interpretable features (those with meaning outside the grammar), whereas agreement (Agree) is conceptualized as a matching operation between verbs and subjects. That is, when arriving at an inflected verb, the subject noun is checked for number (cf. Nicol, Forster, & Veres, 1997). Further, tense is referential, indicating an associated time interval via inter-sentential (or extrasentential) dependencies (Dickey, 2001; Partee, 1973; Varlokosta et al., 2006), whereas agreement is a local syntactic operation, which expresses intra-sentential structural relation between elements in the sentence, meaningless outside the grammatical system. These latter ideas, coupled with the aforementioned patient data, suggest that these two categories may recruit partially distinct neural processes. In the present study we set out to investigate the neural correlates of past tense (V + ed inflection) and present tense/agreement processing (V + s inflection).
Only a handful of studies have examined the neural mechanisms underlying agreement inflection, and no studies have directly compared the neural correlates of verb tense versus agreement processing. In addition, no previous neuroimaging studies have examined past vs. present tense distinction. Wassenaar, Brown, and Hagoort (2004), using event-related brain potentials, found a P600/SPS effect (associated with a posterior distribution) for agreement violations. Similarly, in an MMN study, Shtyrov and Pulvermüller (2002) found a more central maximum with a more posterior perisylvian distribution for the affixed verb (i.e., +−s) as compared to the noninflected verb stem condition. In that study, however, morphosyntactic processing was limited because stimuli were presented as single words (e.g., come, comes) devoid of context.
Another issue, relevant to neuroimaging studies examining verb inflection, is that the results vary considerably across studies, likely due to differences in task demands and other methodological details. Whereas, some studies have used scan tasks that require spoken responses (Desai, Conant, Waldron, & Binder, 2006; Jaeger et al., 1996; Sach, Seitz, & Indefrey, 2004), others have used tasks requiring covert responding (Beretta et al., 2003; Dhond et al., 2003; Joanisse & Seidenberg, 2005; Ullman, Bergida, & O'Craven, 1997). It is possible that the cognitive processes operating during covert tasks differ from those underlying overt verbal responding. In fact, some existing fMRI studies suggest that covert and overt verbal responses produce different patterns of neural activation (McCarthy, Blamire, Rothman, Gruetter, & Shulman, 1993; Zelkowicz, Herbster, Nebes, Mintun, & Becker, 1998). In addition to regions involved in motor planning and articulation, inhibition of inappropriate responses may come into play when overt responses are required (Barch et al., 1999). Covert tasks, on the other hand, precluded the direct collection of on-line RT and accuracy data, making it difficult to know whether the subjects performed the task correctly. A direct comparison of brain activity underlying overt and covert verb production can reveal the extent to which these tasks rely on similar neural processes.
We, therefore, undertook the present study to examine the neural mechanisms of verb inflection. In particular, we investigated past tense (i.e., Yesterday the cat followed the mouse) and present tense/agreement morphology (i.e., Nowadays the cat follows the mouse) in both overt and covert production fMRI tasks. In English the suffix – s expresses both singular present tense and agreement. Even through the participants were instructed to produce a singular agent in response to the prompt “Nowadays” (e.g., Nowadays he cooks), language-specific characteristics of English make it difficult to tease apart tense and agreement inflection. Thus, the V + s condition expresses both present tense and agreement.
We hypothesized that both inflected forms (as well as verb stem production) would recruit a distributed fronto-parietal neural network in keeping with that proposed for language perception and production in general (Hickok & Poeppel, 2000, 2004; Mesulam, 1990). The Memory, Unification and Control model proposed by Hagoort (2005) also suggests that frontal regions are involved in synthesis of morphological constituents that are stored in posterior regions (Hagoort, 2005). Thus, we expected that inflection production would engage this anterior and posterior network.
Based on models suggesting that inflected forms are generated using combinatorial mechanism, we anticipated distinct neural signatures for verb inflection generation compared to verb stem production. However, if stems, past tense and present tense/agreement inflections are computed by the same associative memory system, similar brain regions will be recruited for all forms. This pattern would also fit with linguistic theories, which suggest that both tense and agreement features are represented on the same node on the syntactic tree. Conversely, based on models suggesting that tense and agreement are structurally distinct we anticipated distinct neural signatures for these verb inflections. Furthermore, based on dissociations observed in aphasiac individuals it was expected that processing of past tense and present tense/agreement would recruit partially distinct brain regions.
2. Method
2.1. Participants
Thirteen native speakers of English (6 males) between the ages of 19 and 52 (M = 28.73, SD = 12.15) recruited from the greater Chicago region participated in the study. All were right handed normal or corrected to normal vision. None of the participants had a history of neurological/psychological disease or neurologic symptoms. The mean duration of their formal education was 16.44 years, SD = 3.1. The study was approved by the Internal Ethics Review Board (IRB) at Northwestern University and all participants gave their written informed consent prior to the study. All were compensated for their participation in the study.
2.2. Materials and procedure
An event-related fMRI design was used to examine verb inflection production. The stimuli consisted of 10 transitive verbs, matched for the log10 lemma frequency of occurrence per million (M = 1.52; SD = 0.88), using the CELEX database (Baayen, Piepenbrock, & Rijn, 1993), and syllable length (1–2 syllables). Mean imageability ratings were also obtained using the MRC psycholinguistic database (MRC database, Wilson, 1988). The resulting ratings for verbs were M = 469; SD = 80.38 (See Table 1).
Table 1.
List of verb characteristics used in the overt and covert production tasks.
| Syllable length | Imageabilitya | Total_log_frequencyb | |
|---|---|---|---|
| Call | 1 | 424 | 2.38 |
| Cover | 2 | 443 | 2.02 |
| Crown | 1 | 602 | 1.41 |
| Follow | 2 | n/a | 1.95 |
| Paint | 1 | 567 | 1.62 |
| Pull | 1 | 446 | 1.83 |
| Save | 1 | 365 | 1.83 |
| Shave | 1 | n/a | 0.84 |
| Tickle | 2 | 492 | 0.34 |
| Weigh | 1 | 411 | 0.99 |
| Mean | 1.3 | 468.75 | 1.521 |
| SD | 0.48 | 80.38 | 0.63 |
n/a = no entry in CELEX.
Imageability ratings (Based on the MRC database).
log10 frequency per million from CELEX.
We developed a task similar to that used in a recent study by Sahin et al. (2009). For each verb, participants produced three forms: the verb stem (e.g. `call'), a past tense inflected verb (`called'), and the present tense/agreement inflected verb (`calls'). For each item, a transitive verb and cue word signaling the correct verb form were presented on a single screen (see Fig. 1). Production of verbs inflected for past tense (V + −ed) and present tense/agreement (V + −s) was elicited using temporal adverbs (i.e. Yesterday, Nowadays, respectively), and the cue word “Say” was used to elicit production of verb stems. For example, the cue word `Yesterday' and the verb `crown_' were visually presented and the participants were expected to respond “crowned”. All verbs in the past tense condition had regular past tense endings. In the V + s condition participants were presented with the cue word “Nowadays” and the verb “cook” and were expected to respond “cooks”. This task was selected because it requires inflectional processes to be engaged with minimal demands placed on working memory and semantic integration processes.
Fig. 1.

Sample stimulus used in the fMRI production task for elicitation of (verb + −ed).
Two versions of the task were administered in sequence, counterbalanced across participants. The two versions were identical except that in the first participants were asked to respond overtly, whereas in the second version verbs were produced covertly. Stimulus materials were presented using SuperLab Pro experimental software (Abboud & Sugar, 1997). Shortened versions of the overt (15 items) and covert (15 items) tasks were administered in the testing room and again in a simulated scanner to familiarize participants with the procedures. No feedback regarding the accuracy of the verb form was provided during training sessions.
The overt and covert tasks were administered over four runs (2 runs per task), with each run lasting 7 min and 50 s. A total of 90 items were presented in each task (30 nowadays, 30 yesterday, and 30 say/stem verb forms). Each item was displayed for 6 s with an ISI of 4 s, which allowed detection of BOLD signal changes without significant motion artifact. Items were pseudo-randomly presented, with no more than two tokens of a particular verb form occurring consecutively.
Monitoring of auditory responses over the scanner noise was accomplished by placing a plastic tube close to participants' mouths, which was connected to the microphone in the scanner. Two researches simultaneously scored participants' overt responses on line. Responses were scored as accurate if they included the target verb with the correct inflectional form (+ed, +s, no inflection).
2.3. fMRI data acquisition and analysis
A 3T Trio Siemens scanner was used to obtain anatomical (T1-weighted) and functional (T2*-weighted) scans. The images were obtained in transaxial planes parallel to AC-PC line. T1 weighted volumes were acquired using an MP-RAGE sequence with a TR/TE of 2100 ms/2.4 ms, flip angle 8°, TI of 1100 ms, matrix size of 256 mm × 256 mm; FOV of 220 mm, and slice thickness of 1 mm. Functional scans were obtained in the same orientation as the anatomical, with a TR of 2000 ms used to acquire 32 slices 3 mm in thickness. To allow for image saturation, the first 6 volumes of each run were discarded.
Pre-processing and analysis of fMRI data were performed using SPM8 (Welcome Department of Imaging Neuroscience, Institute for Neurology, University College London) running in Matlab 7.8 environment (R2009a, The Math Works, Inc., Navick, MA). Functional scans were corrected for slice-acquisition timing and realigned to the first session image. The functional images were coregistered to the individual high resolution anatomical scan. Next, the anatomical image was used to determine the spatial normalization parameters, and the images were normalized to the Montreal Neurological Institute (MNI) 152-subject template brain (ICBM, NIH P-20 project). The functional volumes were normalized using the same transformations and were spatially smoothed using a 9 mm (FWHM) isotopic Gaussian kernel.
In the first level analysis, a high-pass filter of 256 s was used to eliminate scanner drift. For each run, six movement parameters obtained during pre-processing were entered as user-defined regressors in the design matrix, to co-vary out effects correlated with head movement in the scanner. Data were analyzed in the context of the general linear model and the conditions were modeled separately for stems, past tense and present tense/agreement inflections. Individual participants summary activation maps for the six main effects (stem-covert, past tense-covert, present tense/agreement-covert; stem-overt, past tense-overt, present tense/agreement-overt) were entered into a second level within-subjects analysis of variance (ANOVA) with production mode (overt, covert) and inflection condition (stem, past tense, present tense/agreement) as the two factors.
Second level (random-effects) statistics were used to evaluate voxel-wise significance using a threshold of p < .05, corrected for multiple comparisons per family wise error (FWE), with a minimum cluster size of 3 contiguous voxels. Comparisons of the inflection effects within the two production modes were examined using t-tests.
3. Results
3.1. Behavioral results
Errors on the production task consisted 4% of total responses, distributed evenly across inflection types. Because of this low number of errors it was decided not to remove these trials from the dataset.
3.2. fMRI results
3.2.1. Past tense and present tense/agreement effects: covert vs. overt production
Regions of significant activation are shown in Tables 2 and 3 for the covert and overt production tasks, respectively. Local maxima, MNI coordinates, with cluster sizes in anatomical regions, and corresponding Brodmann's areas are presented.
Table 2.
Regions of significant activation, local maxima, Brodmann's areas, MNI coordinates, and cluster sizes in the covert production task (FWE corrected, p < .05, k > 3).
| Location | Hem | BA | x | y | z | Cluster Size |
|---|---|---|---|---|---|---|
| Stem (main effects) | ||||||
| Angular gyrus | R | 39 | 33 | −70 | 22 | 28 |
| Past tense (main effects) | ||||||
| Precentral gyrus | L | 6 | −42 | −4 | 46 | 80 |
| L | 6 | −51 | 2 | 37 | ||
| Anerior cingulate | R | 32 | −3 | 8 | 52 | 104 |
| Posterior cingulate | R | 31 | 30 | −70 | 25 | 76 |
| Precuneus | L | 7 | −27 | −70 | 31 | 102 |
| L | 7 | −21 | −55 | 46 | ||
| Precuneus, posterior cingulate | L | 7/31 | −27 | −73 | 22 | |
| Present tense/agreement (main effects) | ||||||
| MFG, precentral gyrus | L | 6 | −6 | 5 | 52 | 82 |
| L | 6 | 6 | −1 | 58 | ||
| MFG | L | 6 | −48 | −1 | 46 | 60 |
| L | 9 | −51 | 2 | 34 | ||
| Precuneus | L | 7 | −27 | −70 | 31 | 16 |
| L | 7 | −21 | −52 | 43 | 64 | |
| Precuneus/cuneus | R | 7/19 | 30 | −70 | 28 | 50 |
| Past tense + present tense/agreement-stem | ||||||
| MFG, precentral gyrus | L | 6 | −42 | −4 | 52 | 19 |
| Anterior cingulate | L | 32 | −9 | 8 | 52 | 9 |
| Precuneus | L | 7 | −21 | −55 | 43 | 78 |
| L | 7 | −27 | −70 | 34 | ||
| Past tense-stem | ||||||
| MFG, precentral gyrus | L | 6 | −42 | −4 | 52 | 7 |
| Precuneus | L | 7 | −21 | −55 | 43 | 25 |
| L | 7 | −27 | −70 | 34 | 19 | |
| Present tense/agreement-stem | ||||||
| MFG, precentral gyrus | L | 6 | −39 | −4 | 56 | 6 |
| Precuneus | L | 7 | −21 | −55 | 43 | 28 |
| Precuneus, cuneus | L | 7/19 | −27 | −73 | 34 | 4 |
| Past tense-Present tense/agreement-stem | ||||||
| Precentral | L | 6 | −24 | 2 | 34 | 8 |
| Precuneus, posterior cingulate | L | 7/31 | −9 | −70 | 22 | 16 |
Note. MFG = middle frontal gyrus.
Table 3.
Regions of significant activation, local maxima, Brodmann's areas, MNI coordinates, and the cluster sizes in the overt production task (FWE corrected, p < .05, k > 3).
| Location | Hem | BA | x | y | z | Cluster Size |
|---|---|---|---|---|---|---|
| Stem (main effects) | ||||||
| Precentral gyrus | L | 6 | −51 | −10 | 31 | 699 |
| L | 6 | −54 | −7 | 22 | ||
| Insula | L | 13 | −33 | −25 | 10 | |
| Precentral gyrus | R | 6 | 0 | 5 | 55 | 300 |
| R | 6 | 54 | −7 | 40 | 707 | |
| Motor cortex | R | 4 | 57 | −7 | 19 | 17 |
| L | 4 | −18 | −28 | 58 | ||
| R | 4 | 18 | −25 | 58 | 8 | |
| Putamen | L | 21 | 2 | 1 | 107 | |
| Claustrum | R | 33 | 8 | 4 | ||
| Thalamus | L | −12 | −19 | 4 | 21 | |
| R | 15 | −16 | 4 | 17 | ||
| Past tense (main effects) | ||||||
| Precentral gyrus | L | 6 | −51 | −10 | 31 | 2411 |
| R | 6 | 54 | −7 | 40 | ||
| IFG | L | 44 | −54 | 7 | 22 | |
| SFG/precentral gyrus | R | 6 | 0 | 5 | 55 | 591 |
| Anterior cingulate | L | 24 | −6 | 20 | 25 | |
| Motor cortex | R | 4 | 18 | −28 | 58 | 11 |
| L | 4 | −21 | −28 | 55 | 10 | |
| Precuneus | R | 7 | −3 | −76 | 34 | 9 |
| Posterior cingulate | R | 30 | 12 | −67 | 10 | 161 |
| Present tense/agreement (main effects) | ||||||
| Precenral gyrus | L | 6 | −51 | −10 | 31 | 1180 |
| L | 6 | −54 | −7 | 22 | ||
| Motor cortex | L | 4 | −45 | −16 | 37 | |
| SFG/precentral | R | 6 | 0 | 5 | 55 | 576 |
| Precenral gyrus | R | 6 | 54 | −7 | 40 | 1125 |
| R | 6 | 51 | −4 | 49 | ||
| Motor cortex | R | 4 | 57 | −7 | 19 | |
| Motor cortex | L | 4 | −18 | −28 | 58 | 17 |
| R | 4 | 18 | −28 | 58 | 21 | |
| Posterior cingulate | R | 31 | 12 | −67 | 10 | 203 |
| Angular gyrus | R | 39 | 33 | −70 | 25 | 6 |
| Past tense + present tense/agreement-stem | ||||||
| SFG/IFG | L | 9/44 | −51 | 2 | 25 | 68 |
| SFG/precentral gyrus | R | 6 | 0 | 8 | 55 | 318 |
| Anterior cingulate | L | 32 | −6 | 14 | 40 | |
| R | 32 | 6 | 17 | 37 | ||
| Precentral gyrus | L | 6 | −39 | −4 | 55 | 52 |
| R | 6 | 51 | −4 | 49 | 5 | |
| Insula | L | 13 | −33 | 23 | 1 | 16 |
| Claustrum | L | 23 | −33 | 14 | 7 | |
| Medial globus pallidus | L | −12 | −4 | 1 | 10 | |
| SPL/precuneus | L | 7 | −24 | −58 | 43 | 54 |
| Posterior cingulate | L | 7 | −18 | −58 | 49 | |
| Past tense-stem | ||||||
| IFG | L | 44 | −51 | 5 | 19 | 37 |
| R | 44 | −45 | 5 | 13 | ||
| MFG | R | 6 | −51 | 2 | 31 | |
| SFG, precentral gyrus | R | 6 | 0 | 8 | 55 | 291 |
| Anterior cingulate | R | 32 | 6 | 17 | 34 | |
| MFG, precentral gyrus | L | 6 | −39 | −4 | 55 | 36 |
| R | 6 | 45 | 2 | 52 | 8 | |
| Precentral gyrus | R | 6 | 51 | −4 | 49 | |
| Claustrum | L | −33 | 14 | 7 | 27 | |
| Insula | L | 13 | −33 | 23 | 1 | |
| Thalamus | L | −12 | −4 | 7 | 31 | |
| Precuneus | L | 7 | −21 | −61 | 46 | 34 |
| Present tense/agreement-stem | ||||||
| Anterior cingulate | L | 32 | −6 | 11 | 40 | 206 |
| SFG | R | 6 | 3 | 8 | 55 | |
| Precentral gyrus | L | 6 | −42 | −7 | 49 | 24 |
| IFG | L | 9/44 | −51 | 2 | 25 | 34 |
| Precuneus/SPL | L | 7 | −24 | −58 | 43 | 28 |
| L | 7 | −18 | −58 | 49 | ||
| Past tense-present tense/agreement | ||||||
| SFG | L | 6 | −9 | 26 | 55 | 13 |
| MFG | R | 8 | 21 | 32 | 46 | 154 |
| R | 8 | 30 | 26 | 43 | ||
| R | 8 | 24 | 23 | 37 | ||
| SFG | L | 9 | −15 | 47 | 31 | 6 |
| MFG | L | 9 | −21 | 26 | 34 | 4 |
| L | 9 | −30 | 26 | 37 | 3 | |
| L | 10 | −15 | 50 | 13 | 13 | |
| R | 10 | 15 | 44 | 16 | 6 | |
| Posterior cingulate | R | 29/31 | 15 | −43 | 13 | 4 |
| Thalamus | R | 0 | −19 | 13 | 9 | |
| Present tense/agreement-past tenlse | ||||||
| Posterior cingulate | L | 31 | −15 | −28 | 49 | 38 |
| Paracentral lobule | R | 5 | 21 | −43 | 49 | 11 |
| Angular gyrus | R | 39 | 30 | −58 | 31 | 5 |
Note. STG = superior temporal gyrus; IFG = inferior frontal gyrus; SFG = superior frontal gyrus; SPL = superior parietal lobule; MFG = middle frontal gyrus.
3.2.1.1. Main effects
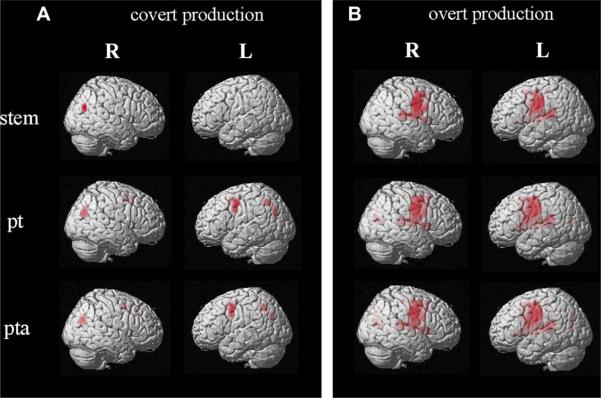
In the first level of analysis the main effects for stem, past tense, and present tense/agreement were investigated separately in the covert and overt production tasks (see Fig. 2). For the stem condition activation was found in the right angular gyrus (AG, BA 39) in the covert production condition, and in the precentral gyrus (BA 6) and the motor cortex (BA 4), bilaterally, in the overt condition. Significant activation also was found in the left insula (BA13) and, subcortically, in the basal ganglia and thalamus in both hemispheres.
Fig. 2.
Activation patterns for stem, past tense (pt), and present tense/agreement (pta) conditions (main effects) in covert (A) and overt (B) production tasks (corrected FWE, p < .05).
Main effects for past tense were found in the left precentral gyrus (BA 6), left precuneus (BA 7), and right anterior and posterior cingulate gyri (BAs 32, 31) in the covert production condition. In the overt production task, activation for past tense was observed in the posterior left inferior frontal gyrus (BA 44), and extended to the bilateral precentral gyri (BA 6) and motor cortex (BA 4). Activation also was found in the right precuneus (BA 7) and posterior cingulate (BA 30).
Finally, main effects for present tense/agreement were found in the left middle frontal and pre-central gyri (BA 9, 6), and left and right precuenus (BA 7) in the covert production condition. Whereas, in the overt task activation was observed in the bilateral precentral gyri (BA 6) and motor cortex (BA 4). Significant activation also was found in the right posterior cingulate gyrus (BA 31) and right angular gyrus (BA 39).
3.2.1.2. Inflected forms versus stem activation
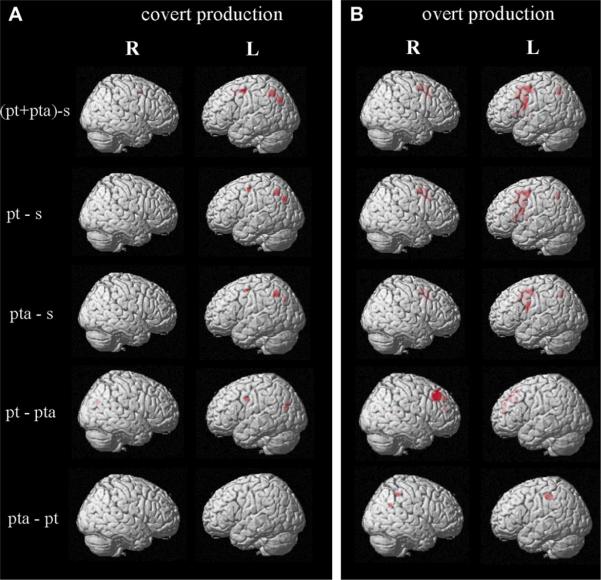
In subsequent analyses, activation found under both inflection conditions was contrasted with that of the stem condition (past tense + present tense/agreement-stem). In the covert task (see Fig. 3A) activation was found primarily in the left hemisphere and included the precentral gyrus (BA 6), anterior cingulate gyrus (BA 32), and the precuneus (BA 7). In the overt production task, activation was found in the bilateral precentral gyri (BA 6) and the left inferior frontal gyrus (BA 9, 44) for this contrast (see Fig. 3B). In addition, overt production was associated with activation in the bilateral anterior cingulate gyri (BA 32) and left insula (BA 13). In the parietal lobe activation was found in the left precuneus (BA 7). Recruitment of subcortical structures, the medial globus pallidus and claustrum, also was observed for overt production.
Fig. 3.
Activation patterns for contrasts of past tense (pt), present tense/agreement (pta), and stem (s in covert (A) and overt (B) production tasks (corrected FWE, p < .05; past tense-present tense/agr, present tense/agr-past tense: uncorrected, p < .001).
The next analysis compared past tense inflection to stem (past tense-stem) production. Activation clusters in the left middle frontal gyrus (LMFG) and precuneus (BA 7) were observed in both overt and covert production conditions (see Fig. 3). In addition in the overt task, generation of tense inflection was associated with increased activation, in the bilateral precentral gyri (BA 6) and the posterior inferior frontal gyri (BA 44). Activation was also observed in the left insula (BA 13), and the right anterior cingulate gyrus (BA 32).
Inflecting verbs for present tense/agreement compared to stem (present tense/agreement-stem) production in the covert task was correlated with left precentral (BA 6) and precuneus (BA 7) activation. Similarly, in the overt production condition, activation was found in the precentral gyri (BA 6), bilaterally, and in the left superior, middle and posterior inferior frontal gyri (BA 6, 9/44). Activation was also present in the left anterior cingulate gyrus (BA 32) and the left precuneus (BA 7).
Finally, comparison of past tense to present tense/agreement (past tense-present tense/agreement) in the covert task, revealed significant activation in the left precentral gyrus (BA 6). In the left hemisphere, there was additional activation in the precuneus (BA 7) that extended into the posterior cingulate gyrus (BA 31). In the overt task, inflecting for past tense compared to present tense/agreement included bilateral activation in the middle and superior frontal gyri (BAs 8, 9, 10). In the right hemisphere, activation was found in the posterior cingulate gyrus (BA 29/31), and subcortically in thalamus.
In the reverse contrast, comparison of present tense/agreement to past tense (present tense/agreement-past tense) production in the covert condition did not reveal areas of significant activation, even using an uncorrected threshold (p < .001). However, in the overt task, significant activation was observed in the left posterior cingulate gyrus (BA 31), right AG (BA 39) and right paracentral lobule (BA 5).
3.2.2. Covert vs. overt production
Table 4 lists regions of significant activation together with local maxima, MNI coordinates, cluster sizes, and Brodmann's areas for the covert and overt production conditions, computed across all verb production conditions. See Fig. 4 for corresponding activation maps.
Table 4.
Regions of significant activation, Brodmann's areas, MNI coordinates, and cluster sizes for covert and overt production tasks (FWE corrected, p < .05, k > 3).
| Location | Hem | BA | x | y | z | Cluster Size |
|---|---|---|---|---|---|---|
| Covert (main effects) | ||||||
| Precuneus/posterior cingulate | R | 31 | 30 | −70 | 25 | 66 |
| SFG/precentral | L | 6 | −3 | 5 | 52 | 60 |
| Precuneus | L | 7 | −21 | −52 | 43 | 24 |
| MFG/precentral | L | 6 | −45 | −1 | 46 | 40 |
| Precentral gyrus | L | 6 | −51 | 2 | 40 | |
| Precuneus | L | 7 | −27 | −70 | 31 | 7 |
| Overt (main effects) | ||||||
| Precentral gyrus | L | 6 | −51 | −10 | 31 | 2336 |
| R | 6 | 54 | −7 | 40 | ||
| L | 6 | −54 | −7 | 22 | ||
| SFG | R | 6 | 0 | 5 | 55 | 546 |
| Posterior cingulate | R | 31/30 | 12 | −67 | 10 | 152 |
| Motor cortex | L | 4 | −18 | −28 | 58 | 18 |
| R | 4 | 18 | −28 | 58 | 16 | |
| Precuneus | L | 7 | −3 | −76 | 34 | 5 |
| R | 7 | 12 | −79 | 34 | 4 | |
| Covert > overt | ||||||
| MFG | R | 6 | 21 | −10 | 43 | 104 |
| SFG | R | 8 | 18 | 23 | 49 | 72 |
| Postcentral gyrus | R | 2 | 33 | −28 | 40 | |
| R | 2 | 48 | −25 | 49 | ||
| Caudate | R | 6 | 8 | 19 | 6 | |
| Precuneus | R | 7 | 21 | −55 | 40 | 13 |
| vert > covert | ||||||
| Precentral gyrus | R | 6 | 54 | −7 | 37 | 506 |
| R | 6 | 54 | −10 | 28 | ||
| Precentral gyrus | R | 4 | 57 | −7 | 19 | |
| L | 6 | −54 | −7 | 22 | 540 | |
| Insula | L | 13 | −33 | −25 | 10 | |
| Claustrum | L | −36 | −10 | 10 | ||
| SFG/precentral gyrus | L | 6 | 3 | 8 | 55 | 99 |
| MFG/precentral gyrus | R | 6 | 0 | −4 | 55 | |
| Anterior cingulate gyrus | R | 23/24 | 0 | 2 | 43 | |
| Putamen | R | 21 | 2 | 1 | 36 | |
| R | 27 | 14 | 4 | |||
| Claustrum | R | 33 | 8 | 4 | ||
| Thalamus | L | −15 | −16 | 4 | 4 |
Note. MFG = middle frontal gyrus; SFG = superior frontal gyrus.
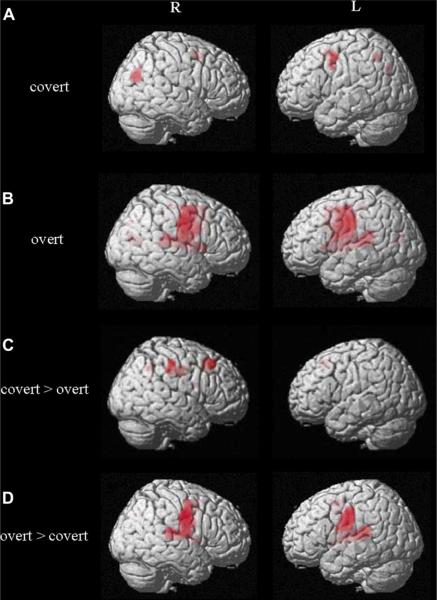
Fig. 4.
Activation patterns for covert and overt production across all verb conditions (corrected FWE, p < .05; covert > overt: uncorrected, p < .001).
Across all conditions, covert production was associated primarily with significant left hemisphere activation in the superior and middle frontal gyri and precentral gyri (BA 6), including the precuneus (BA 7). In the right hemisphere, activation was present in the precuneus and the posterior cingulate gyrus (BA 31).
Similar analysis for the overt production condition revealed bilateral activation in the precentral gyrus (BA 6) and the motor cortex (BA 4). As illustrated in the Fig. 4B, in the parietal cortex, activation was present in the bilateral precuneus (BA 7) and extended into the right posterior cingulate (BA 31).
In direct comparison of covert production to overt production (covert > overt), no voxels survived the corrected voxel-wise threshold. However, analysis using an uncorrected threshold (p < .001), yielded clusters of significant activation in the right middle frontal (BA 9) and superior frontal (BA 8) gyri. Covert production was also associated with activation in the right postcentral gyrus (BA 2) and precuneus (BA 7).
Finally, contrasting overt to covert production conditions (overt > covert) revealed significant bilateral activation in the superior/middle frontal gyri, precentral gyrus (BA 6). In the left hemisphere, activation included the motor cortex (BA 4), the insula (BA 13) and the precuneus (BA 7). In the right hemisphere, there was additional significant activation in the anterior cingulate gyrus (BA 32, 24). Subcortically, activation also was present in the right putamen and the left thalamus.
4. Discussion
4.1. Production of verb inflection
This study investigated the neural correlates of verb inflection, i.e., past tense and present tense/agreement affixation, using both covert and overt verb production tasks. As anticipated, production of both inflected forms (as well as verb stems) elicited a distributed fronto-parietal neural network (Hickok & Poeppel, 2000, 2004; Mesulam, 1990), including the left inferior, middle and precentral frontal gyri, cingulate gyrus, insula, inferior parietal lobule and precuneus. Notably, these regions of significant activation have been found in previous studies of verb generation (Binder et al., 1997; Perani et al., 1999; Petersen, Fox, Posner, Mintun, & Raichle, 1988; Tyler, Marslen-Wilson, & Stamatakis, 2005; Warburton et al., 1996). However, within this network several distinct regions of significant activation were found under the verb inflection conditions (i.e., past tense + present tense/agreement) compared to the verb stem condition. In both, overt and covert conditions production of inflected forms activated not only left inferior and middle frontal structures, but also we found robust activation in bilateral superior frontal and premotor regions as well as in the superior parietal region. These results are similar to those reported in a recent study by Sahin et al. (2009) focused on production of verb inflection (past tense and agreement). The present findings are consistent with the role of the frontal cortex for selection and unification of lexical information, stored in posterior brain regions that are involved in lexical processing, into syntactically complex structures (Hagoort, 2003, 2005). These results also support linguistic theories suggesting that inflected forms involve concatenation of verb stems, accessed via a look-up system in the mental dictionary (Clahsen, 1999; Pinker, 1998; Pinker & Ullman, 2002; Ullman et al., 2005). The opposite view, that verb inflection is instantiated in a manner similar to that of verb stems (Joanisse & Seidenberg, 1999; McClelland & Patterson, 2002; Rumelhart & McClelland, 1986) would putatively not engender quantifiable differences in neural activity for stem versus inflected forms as found in the present study.
Results from the present study also support observations that patients with agrammatic aphasia evince selective deficits involving verb inflection. That is, production of verbs stems or the infinitive form of verbs often is spared, but production of verbs inflected for tense and/or agreement often is markedly impaired (Arabatzi & Edwards, 2002; Benedet, Christiansen, & Goodglass, 1998; Faroqi-Shah & Thompson, 2004, 2007; Goodglass, Christainsen, & Gallagher, 1993 and many others). Many such patients present with lesions involving the network for verb inflection identified in this study, including the prefrontal cortex (PFC) and often tissue in the posterior perisylvian region also is infarcted (Hagoort, 2003; Vanier & Caplan, 1990; Wassenaar et al., 2004).
The bilateral (PFC) activation found in the present study for verb inflection corroborates findings derived from previous studies, indicating that this region plays an important role in production of grammatical morphology. In particular, both the left and right PFC have been found to be engaged for processing verb inflections, including regular past tense (V + -ed) and present progressive tense (i.e., V + -ing (aspectual ing)) in several studies using a variety of tasks (Beretta et al., 2003; Dhond et al., 2003; Joanisse & Seidenberg, 2005; Lavric, Pizzagalli, Forstmeier, & Rippon, 2001; Tyler, Bright, Fletcher, & Stamatakis, 2004; Sahin et al., 2009). This activation may reflect morphosyntactic and/or morphophonological processes involved in verb inflection, as well as processing demands associated with response selection.
Circuits in the left inferior frontal region (and other frontal regions) have been associated with morphosyntactic processes: that is, concatenating selected grammatical features (Tyler, Randall, & Marslen-Wilson, 2002; Ullman, 2001, 2004). Therefore, activation of this region for verb inflection is not surprising and supports the notion that it is crucial for instantiation of rule-based grammatical morphology. Interestingly, verb inflection does not involve syntactic movement or long-distance dependencies, processes thought to be controlled by the left IFG (on strong views, see Grodzinsky, 2000). The present findings support a more general linguistic function for these frontal regions, i.e., computing grammatical relationships, including verb inflection (Hagoort, 2005).
BOLD responses found in the frontal regions may also reflect morphophonological processes including selection and manipulation of phonological material associated with inflected forms. Verb inflection, as compared to verb stem, production involves increased phonological demands associated with retrieval of the correct inflectional allomorph, which can be instantiated differently for past tense (e.g.,/t/, /d/, /Id/) and present tense/agreement (/s/, /z/, /Iz/), depending on the phonological form of the stem. The opercular part of the IFG (BA 44) activated in the present study has been implicated in the analysis and synthesis of phonological information, and concatenation of sub-lexical speech segments into words (Bookheimer, 2002; Shalom & Poeppel, 2008). Together, the precentral (BA 6) and posterior inferior frontal gyri (BA 44) combine phonological units into larger segments and subsequently plan the articulatory phase of verbal responses (Gold & Buckner, 2002; Hagoort, 2005; Indefrey & Levelt, 2004).
It also has been suggested that the frontal lobes are involved in synthesizing information that is analyzed in more posterior parietal regions. For example, the left PFC has been associated with response selection: that is, selecting appropriate lexical representations from among competing alternatives across a variety of linguistic and non-linguistic domains (Gabrieli, Poldrack, & Desmond, 1998; Gold & Buckner, 2002; Thompson-Schill, D'Esposito, & Kan, 1999). With regard to grammatical morphology, frontal regions may serve to access, maintain, and manipulate lexical word representations stored in posterior brain regions (Buckner, Koutstaal, Schacter, & Rosen, 2000). In turn, this frontal, premotor, and parietal network activated for overt production of inflections may be involved in mapping of articulatory speech codes (Shalom & Poeppel, 2008). This interaction between frontal and posterior brain regions is consistent with the neural instantiation of the unification model (Hagoort, 2003). Specifically, the Memory, Unification and Control model (MUC) of language proposes that the left inferior frontal cortex recruits and selects task-relevant lexical and syntactic information that is stored in the temporal areas, and unifies them into larger structures (Hagoort, 2005). On this model, unification is defined as combining linguistic elements into larger units and these unification operations are proposed to occur in parallel to compute syntactic, semantic, and phonological information.
Activation of frontal areas together with posterior parietal regions found in the present study may also reflect increased working memory demands required in the grammatical inflection task. These regions are often activated by tasks that require verbal working memory and are thought to be part of a working memory loop that participates in sub-vocal rehearsal and short-term maintenance of phonological information (Hickok & Poeppel, 2000).
In fact, this fronto-parietal network seems to be engaged during a range of verb processing tasks (Binder et al., 1997; Thompson et al., 2007; Warburton et al., 1996). Functional models of speech perception indicate that parietal regions play an important role in language production by interacting with motor representations stored in the left frontal regions of the brain (Hickok & Poeppel, 2000, 2004). On this account, the left inferior parietal lobe forms an interface between auditory and articulatory aspects of speech. This region participates in speech production by interfacing sound based representations of speech with the motor speech system in the frontal cortex. This system also provides an articulatory mechanism that keeps phonemic representations active in working memory (Hickok & Poeppel, 2000). The activation observed in the posterior inferior frontal and parietal regions in the present study provides further evidence for the interaction between these regions for production of verb inflection.
Activation in the anterior cingulate gyrus observed in the present study is consistent with this interpretation. Integration of information between anterior and posterior brain regions may be mediated by the cingulate gyrus, which seems to be involved in monitoring of interactions between different brain pathways (Braver, Barch, Gray, Molfese, & Snyder, 2001; Bush, Luu, & Posner, 2000; Fletcher, McKenna, Friston, Frith, & Dolan, 1999). This structure appears to be a part of the attentional system involved in selection of actions and error detection and correction (Petersen et al., 1988). In grammatical production, the cingulate gyrus may be engaged in linking motor and cognitive aspects of behavior (Bush et al., 2000; Heun et al., 1999). During verb inflection generation, the role of the cingulate gyrus would be to modulate fronto-parietal interaction that is required for monitoring and selecting task-relevant responses. It has also been suggested that the anterior cingulate and dorsolateral PFC (BA 46/9) form a network of brain areas involved in verbal action planning and attentional control. These areas are thought to constitute the control component of language processing that links language to action (Hagoort, 2005). In fact, this fronto-parietal-cingulate network of brain regions has been found to activate in various verb generations tasks (Desai et al., 2006; Indefrey & Levelt, 2004).
4.2. Past tense vs. present tense/agreement production
When contrasted with stem production, generation of past tense and present tense/agreement inflection activated very similar frontal and parietal brain areas in both tasks. However, direct comparison of past tense and present tense/agreement production revealed several clusters of activation unique to each inflection type. In direct comparison to present tense/agreement, past tense inflection was associated with unique activation in the left inferior frontal cortex, as well as bilateral precentral, middle and superior frontal areas.
Recruitment of left inferior frontal regions most likely reflects morphosyntactic and morphophonological demands of past tense inflection processes as discussed above. In fact, it has been suggested that the posterior IFG (BA 44) specializes in phonological processing (Bookheimer, 2002; Gough, Nobre, & Devlin, 2005; Paulesu et al., 1997; Poldrack et al., 1999). Parts of the left IFG also have been implicated with selection of semantic knowledge (Thompson-Schill, D'Esposito, Aguirre, & Farah, 1997; Thompson-Schill et al., 1999). These findings are consistent with the idea that inflecting verbs for tense involves semantic processes (Bastiaanse, 2008). Past tense inflection was also associated with right middle frontal activation, which was larger in the overt production task. Right frontal recruitment has been associated with more complex executive demands and working memory manipulations in previous verb generation studies (Lavric et al., 2001).
The results of the present study indicate that there are differences in how the brain encodes reference to different temporal frames, i.e. past vs. present events, and support results from previous studies indicating that individuals with aphasia have more difficulty with past tense compared to present tense inflection (Bastiaanse, 2008; Jonkers, 2009; Nilipour, 2000; Stavrakaki & Kouvava, 2003). It has been suggested that this tense distinction may relate to semantic differences between the two forms, and that reference to the past might be more semantically complex because the connection must be made between two time periods (Bastiaanse, 2008; Jonkers, 2009). For past tense, the activity and the moment of speech refer to different points in time; whereas, for the present tense, the action and the moment of speech refer to the same point in time. Thus, it appears that temporal reference to the past is processed slightly differently from the temporal reference to the present. The greater activation for V + ed compared to V + s found in the present study support this distinction.
However, the present findings also could be related to differences between tense and agreement because both are expressed using one morpheme – s. Thus, the pattern of associations and dissociations observed in the present study also suggests that the neural representation of tense and agreement differ somewhat, reflecting partially unique computations and processing routines. In line with the present findings, dissociations between past tense and present tense/agreement observed among some patients with aphasia suggest that these verbal inflections may encode unique syntactic features and implement different morphosyntactic operations (Burchert et al., 2005; Faroqi-Shah & Thompson, 2004; Friedmann & Grodzinsky, 1997).
Tense and agreement differ in terms of the structural context in which these grammatical morphemes appear, and they play different functional roles in sentence structure. Tense is a grammatical category that denotes the temporal location of an event and contributes to the semantic interpretation of the sentence, whereas, agreement establishes structural relations between elements in the sentence. Whereas agreement expresses an intra-sentential relationship between subject and the verb, tense expresses the extrasentential relationship to a time frame (Bastiaanse, 2008). The present results support these linguistic distinctions at least to some extent and suggest that subtle differences in the syntactic representations and morphological processes may be involved in instantiation of these forms. This conclusion is partially consistent with interpretations of the hierarchical theories, such as the TPH and Split-Inflection hypothesis (Friedmann & Grodzinsky, 1997; Pollock, 1989), which propose functional independence between tense and agreement. However, the hierarchical ordering of tense and agreement nodes in the phrase structure representation cannot be determined from the present data.
That said, the pattern of activation observed for past tense and agreement in the present study may be better accounted for by the recent developments of syntactic theories. According to the Minimalist Program (MP; Chomsky, 2000), agreement is fundamentally different from tense from a syntactic point of view. MP theory treats tense and agreement as different features within a single tense node, with both categories projecting from this Inflection node. On this account, agreement is not treated as a separate functional element heading its own node, rather it is considered an operation by which certain uninterpretable features of tense, such as person and number, are checked or valued against the interpretable person and number features of the subject. Therefore, this node hosts both semantically interpretable tense feature and uninterpretable agreement features.
The present results are also consistent with the notion that dissociations observed between tense and agreement inflections are underlined by differences in the features that they encode. Although these morphemes appear to be similarly marked, tense (in English) is underspecified for person and number features, whereas agreement – s instantiates all three features: tense, person and number (Burchert et al., 2005; Wenzlaff & Clahsen, 2004). Accordingly, -s inflection in English would be more specified than past tense – ed. Perhaps these differences in feature specification underlie the distinct activation patterns found for these two functional categories. A related finding in the present study is that a slightly greater number of brain regions were activated for past tense inflection, as compared to present tense/agreement. These results suggest that computation of inflectional categories with underspecified features may be more demanding and require more neural resources. Bilateral frontal activation for past tense is consistent with this interpretation, as these regions are often recruited by more demanding tasks (Just, Carpenter, Keller, Eddy, & Thulborn,1996; Petersen et al.,1988; Seidenberg & Hoeffner,1998).
4.3. Overt vs. covert production
Another goal of the present study was to examine the activation patterns associated with covert and overt production. Analysis of covert and overt task across inflection types revealed activation in bilateral middle frontal gyri and precuneus that extended into the left posterior cingulate gyrus. The fronto-parietal-cingulate network has been implicated in the production of verb inflection in the previous studies (Desai et al., 2006; Sahin et al., 2009). In nonhuman primates these areas have been shown to have rich anatomical connections with each other (Petrides & Pandya, 1984). The fronto-parietal-cingulate system has been found to engage in tasks that require selective attention and working memory. Additionally, these regions have been implicated in resolution of stimulus response incompatibility in various modalities. The present inflection tasks required inhibition of competing responses to allow for relevant response selection.
Activation also was observed in the left supplementary motor areas (SMA, BA 6) in both production conditions. The potentially greater articulatory demands between stem production and generation of inflected forms may have resulted in stronger activation in these precentral areas. Activation in the SMA/precentral gyri in response to production of inflections can be attributed to the role of this region in the formulation and initiation of a strategy to identify appropriate verb forms from memory and prepare for articulation of responses. Together with activation in the inferior frontal cortex these areas may reflect attention to the task and engagement of short-term verbal working memory, even when the task is performed silently. Other studies have also found the SMA to activate in response to verb generation during both covert and overt production (Indefrey & Levelt, 2004; Palmer et al., 2001; Warburton et al., 1996).
In the overt task, additional activation was observed in the bilateral motor cortex. Involvement of motor areas together with extensive activation of the premotor cortex seem to be directly related to the processes of overt word generation (Barch et al., 1999; Kemeny et al., 2006; Palmer et al., 2001). These areas are most likely involved in the conversion of phoneme sequences into articulatory motor commands and formulation of the phonetic output (Zelkowicz et al., 1998).
Additionally, direct comparisons between covert and overt tasks, revealed activation unique for the overt production in the bilateral precentral and motor cortex that extended into the left insular cortex. The overt task also engaged bilateral basal ganglia and thalamic nuclei. The basal ganglia have strong connections to the frontal parts of the brain through several parallel circuits, and have been found to activate in conjunction with frontal areas in production (Desai et al., 2006; Kemeny et al., 2006). It is likely that the basal ganglia structures together with motor areas are involved in planning of articulatory movements, which is more demanding during overt as compared to covert responses. Left insular activation also has been reported in other word production tasks. It has been suggested that this region may be involved in the phonological code generation and motor planning of speech (Indefrey & Levelt, 2004; Kemeny et al., 2006; McCarthy et al., 1993; Warburton et al., 1996; Zelkowicz et al., 1998).
5. Conclusions
The results of this study indicate that in healthy adults inflecting verbs for grammatical morphology, as compared to producing verb stems, involves a number of areas in both the right and left hemispheres. These results are consistent with the hypothesis that frontal and parietal areas constitute a distributed network of brain regions involved in speech production and concatenation of speech segments into larger lexical units (Devlin, Jamison, Matthews, & Gonnerman, 2004; Hagoort, 2005; Hickok & Poeppel, 2000). The exact pattern of activation varies with the demands of the task at hand. Our results identified distinct neural mechanisms for inflecting verbs for past tense versus present tense/agreement morphology, supporting linguistic theories that distinguish between the two forms. However, whether our findings reflect difference between past and present tense processing, specifically, or between tense and agreement, more generally, requires additional research.
Acknowledgements
This research was supported by the NIH grants RO1-DC01948 (C.K. Thompson) and R01-DC007213 (C.K. Thompson). The authors wish to thank the NIH for its support over the past 20 years. We also thank members of the Aphasia and Neurolinguistics Research Laboratory at Northwestern University for assistance with the study, including Drs. Michael Walsh-Dickey, Miseon Lee, and Stephen Fix and Ms. Janet O'Connor.
References
- Abboud H, Sugar D. SuperLab pro experimental lab software. version 2.0 Cedrus Corporation; Phoenix, AZ: 1997. [Google Scholar]
- Arabatzi M, Edwards S. Tense and syntactic processes in agrammatic speech. Brain and Language. 2002;80:314–327. doi: 10.1006/brln.2001.2591. [DOI] [PubMed] [Google Scholar]
- Baayen RH, Piepenbrock R, Rijn H. The CELEX lexical database (Release 1) [CD-ROM] Linguistic Data Consortium, University of Pennsylvania; Philadelphia, PA: 1993. [Google Scholar]
- Barch DM, Sabb FW, Carter CS, Braver TS, Noll DC, Cohen JD. Overt verbal responding during fMRI scanning: empirical investigations of problems and potential solutions. Neuroimage. 1999;10:642–657. doi: 10.1006/nimg.1999.0500. [DOI] [PubMed] [Google Scholar]
- Bastiaanse R. Production of verbs in base position in Dutch agrammatic speakers: inflection versus finiteness. Journal of Neurolinguistics. 2008;21:104–119. [Google Scholar]
- Benedet MJ, Christiansen JA, Goodglass H. A cross-linguistic study of grammatical morphology in Spanish and English speaking agrammatic aphasics. Cortex. 1998;34:309–336. doi: 10.1016/s0010-9452(08)70758-5. [DOI] [PubMed] [Google Scholar]
- Beretta A, Campbell C, Carr TH, Huang J, Schmitt LM, Christianson K, et al. An ER-fMRI investigation of morphological inflection in German reveals that the brain makes a distinction between regular and irregular forms. Brain and Language. 2003;85:67–92. doi: 10.1016/s0093-934x(02)00560-6. [DOI] [PubMed] [Google Scholar]
- Binder JR, Frost JA, Hammeke TA, Cox RW, Rao SM, Prieto T. Human brain language areas identified by functional magnetic resonance imaging. Journal of Neuroscience. 1997;17:353–362. doi: 10.1523/JNEUROSCI.17-01-00353.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bobaljik J, Thrainsson H. Two heads aren't always better than one. Syntax. 1998;1:37–71. [Google Scholar]
- Bookheimer S. Functional MRI of language. New approaches to understanding the cortical organization of semantic processing. Annual Review of Neuroscience. 2002;25:151–188. doi: 10.1146/annurev.neuro.25.112701.142946. [DOI] [PubMed] [Google Scholar]
- Braver TS, Barch DM, Gray JR, Molfese DL, Snyder A. Anterior cingulate cortex and response conflict: effects of frequency, inhibition and errors. Cerebral Cortex. 2001;11:825–836. doi: 10.1093/cercor/11.9.825. [DOI] [PubMed] [Google Scholar]
- Buckner RL, Koutstaal W, Schacter DL, Rosen BR. Functional MRI evidence for a role of frontal and inferior temporal cortex in amodal components of priming. Brain. 2000;123:620–640. doi: 10.1093/brain/123.3.620. [DOI] [PubMed] [Google Scholar]
- Burchert F, Swoboda-Moll M, De Bleser R. Tense and agreement dissociations in German agrammatic speakers: underspecification vs. hierarchy. Brain and Language. 2005;94:188–199. doi: 10.1016/j.bandl.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Bush G, Luu P, Posner MI. Cognitive and emotional influences in anterior cingulate cortex. Trends in Cognitive Sciences. 2000;4:215–222. doi: 10.1016/s1364-6613(00)01483-2. [DOI] [PubMed] [Google Scholar]
- Bybee JL. Morphology: A study of the relation between meaning and form. Benjamins; Philadelphia: 1985. [Google Scholar]
- Bybee JL, Slobin DT. Rules and schemas in the development and use of the English past tense. Language. 1982;58:265–289. [Google Scholar]
- Chomsky N. Language and mind. Harcourt Brace Jovanovich; New York: 1972. [Google Scholar]
- Chomsky N. Lectures on government and binding. Foris; Dordrecht: 1981. [Google Scholar]
- Chomsky N. A minimalist program for linguistic theory. In: Hale K, Keyser SJ, editors. The view from building. Vol. 20. MIT Press; Cambridge, MA: 1993. pp. 1–52. [Google Scholar]
- Chomsky N. Minimalist inquires: the framework. In: Martin R, Michaelis D, Uriagereka J, editors. Step by step. MIT Press; Cambridge Mass: 2000. pp. 89–155. [Google Scholar]
- Clahsen H. Lexical entries and rules of language: a multidisciplinary study of German inflection. Behavioral and Brain Sciences. 1999;22:991–1060. doi: 10.1017/s0140525x99002228. [DOI] [PubMed] [Google Scholar]
- Clahsen H, Ali M. Formal features in aphasia: tense, agreement, and mood in English agrammatism. Journal of Neurolinguistics. 2009;22:436–450. [Google Scholar]
- Desai R, Conant LL, Waldron E, Binder JR. fMRI of past tense processing: the effects of phonological complexity and task difficulty. Journal of Cognitive Neuroscience. 2006;18:278–297. doi: 10.1162/089892906775783633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Devlin JT, Jamison HL, Matthews PM, Gonnerman LM. Morphology and the internal structure of words. Proceedings of the National Academy of Sciences. 2004;101:14984–14988. doi: 10.1073/pnas.0403766101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhond RP, Marinkovic K, Dale AM, Witzel T, Halgren E. Spatiotemporal maps of past tense verb inflection. Neuroimage. 2003;19:91–100. doi: 10.1016/s1053-8119(03)00047-8. [DOI] [PubMed] [Google Scholar]
- Dickey MW. Studies in theoretical psycholinguistics. Vol. 28. Kluwer Academic Publishers; Dordrecht, Boston: 2001. The processing of tense. [Google Scholar]
- Druks J. Morpho-syntactic and morpho-phonological deficits in the production of regularly and irregularly inflected verbs. Aphasiology. 2006;20:993–1017. [Google Scholar]
- Faroqi-Shah Y, Thompson CK. Semantic, lexical, and phonological influences on the production of verb inflections in agrammatic aphasia. Brain and Language. 2004;89:484–498. doi: 10.1016/j.bandl.2003.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faroqi-Shah Y, Thompson CK. Verb inflections in agrammatic aphasia: encoding of tense features. Journal of Memory and Language. 2007;56:129–151. doi: 10.1016/j.jml.2006.09.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fletcher P, McKenna PJ, Friston KJ, Frith CD, Dolan RJ. Abnormal cingulate modulation of fronto-temporal connectivity in schizophrenia. Neuroimage. 1999;9:337–342. doi: 10.1006/nimg.1998.0411. [DOI] [PubMed] [Google Scholar]
- Friedmann N, Grodzinsky Y. Tense and agreement in agrammatic production: pruning the syntactic tree. Brain and Language. 1997;56:397–425. doi: 10.1006/brln.1997.1795. [DOI] [PubMed] [Google Scholar]
- Gabrieli JDE, Poldrack RA, Desmond JE. The role of the left prefrontal cortex in language and memory. Proceedings of the National Academy of Sciences. 1998;95:906–913. doi: 10.1073/pnas.95.3.906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gold BT, Buckner RL. Common prefrontal regions coactivate with dissociable posterior regions during controlled semantic and phonological tasks. Neuron. 2002;35:803–812. doi: 10.1016/s0896-6273(02)00800-0. [DOI] [PubMed] [Google Scholar]
- Goodglass H, Christainsen J, Gallagher R. Comparison of morphology and syntax in free narrative and structures tests: fluent and nonfluent aphasics. Cortex. 1993;29:377–407. doi: 10.1016/s0010-9452(13)80250-x. [DOI] [PubMed] [Google Scholar]
- Gough PM, Nobre AC, Devlin JT. Dissociating linguistic processes in the left inferior frontal cortex with transcranial magnetic stimulation. The Journal of Neuroscience. 2005;25:8010–8016. doi: 10.1523/JNEUROSCI.2307-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grodzinsky The neurology of syntax: language use without Broca's area. Behavioral and Brain Sciences. 2000;23:1–21. doi: 10.1017/s0140525x00002399. [DOI] [PubMed] [Google Scholar]
- Hagoort P. How the brain solves the binding problem for language: a neurocomputational model of syntactic processing. Neuroimage. 2003;20:S18–S29. doi: 10.1016/j.neuroimage.2003.09.013. [DOI] [PubMed] [Google Scholar]
- Hagoort P. On Broca, brain, and binding: a new framework. Trends in Cognitive Science. 2005;9:416–423. doi: 10.1016/j.tics.2005.07.004. [DOI] [PubMed] [Google Scholar]
- Halle M, Mohanan KP. Segmental phonology of modern English. Linguistic Inquiry. 1985;16:57–116. [Google Scholar]
- Hay JB. From speech perception to morphology: affix ordering revisited. Language. 2002;78:527–555. [Google Scholar]
- Hay JB, Baayen RH. Shifting paradigms: gradient structure in morphology. Trends in Cognitive Sciences. 2005;9:342–348. doi: 10.1016/j.tics.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Heun R, Klose U, Jessen F, Erb M, Papassotiropoulos A, Lotze M, et al. Functional MRI of cerebral activation during encoding and retrieval of words. Human Brain Mapping. 1999;8:157–169. doi: 10.1002/(SICI)1097-0193(1999)8:4<157::AID-HBM1>3.0.CO;2-G. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Towards a functional neuroanatomy of speech perception. Trends in Cognitive Sciences. 2000;4:131–138. doi: 10.1016/s1364-6613(00)01463-7. [DOI] [PubMed] [Google Scholar]
- Hickok G, Poeppel D. Dorsal and ventral streams: a framework for understanding aspects of the functional anatomy of language. Cognition. 2004;92:67–99. doi: 10.1016/j.cognition.2003.10.011. [DOI] [PubMed] [Google Scholar]
- Indefrey P, Levelt WJM. The spatial and temporal signatures of word production components. Cognition. 2004;92:101–144. doi: 10.1016/j.cognition.2002.06.001. [DOI] [PubMed] [Google Scholar]
- Jaeger JJ, Lockwood AH, Kemmerer DL, Van Valin RD, Jr., Murphy BW, Khalak HG. A positron emission tomographic study of regular and irregular verb morphology in English. Language. 1996;72:451–497. [Google Scholar]
- Joanisse MF, Seidenberg MS. Impairments in verb morphology after brain injury: a connectionist model. Proceedings of the National Academy of Sciences. 1999;96:7592–7597. doi: 10.1073/pnas.96.13.7592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joanisse MF, Seidenberg MS. Imaging the past: neural activation in frontal and temporal regions during regular and irregular past-tense processing. Cognitive, Affective and Behavioural Neuroscience. 2005;5:282–296. doi: 10.3758/cabn.5.3.282. [DOI] [PubMed] [Google Scholar]
- Jonkers R. Tense processing in Broca's and Wernickle's aphasia. Aphasiology. 2009;23:1252–1265. [Google Scholar]
- Just MA, Carpenter PA, Keller TA, Eddy WF, Thulborn KR. Brain activation modulated by sentence comprehension. Science. 1996;274:114–116. doi: 10.1126/science.274.5284.114. [DOI] [PubMed] [Google Scholar]
- Kemeny S, Xu J, Park GH, Hosey LA, Wettig CM, Braun AR. Temporal dissociation of early lexical access and articulation using a delayed naming task - an fMRI study. Cerebral Cortex. 2006;16:587–595. doi: 10.1093/cercor/bhj006. [DOI] [PubMed] [Google Scholar]
- Lavric A, Pizzagalli D, Forstmeier S, Rippon G. A double-dissociation of English past tense production revealed by event-related potentials and low-resolution electromagnetic tomography (LORETA) Clinical Neurophysiology. 2001;112:1833–1849. doi: 10.1016/s1388-2457(01)00615-0. [DOI] [PubMed] [Google Scholar]
- Lee J, Milman LH, Thompson CK. Functional category production in English agrammatism. Aphasiology. 2008;22:893–905. doi: 10.1080/02687030701865670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marantz A. The minimalist program. In: Webelhuth G, editor. Government and binding theory and the minimalist program. Basil Blackwell Publishers; London: 1995. pp. 351–382. [Google Scholar]
- McCarthy G, Blamire AM, Rothman DL, Gruetter R, Shulman RG. Echo-planar magnetic resonance imaging studies of frontal cortex activation during word generation in humans. Proceedings of the National Academy of Science. 1993;90:4952–4956. doi: 10.1073/pnas.90.11.4952. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McClelland JL, Patterson K. Rules or connections in past-tense inflections: what does the evidence rule out? Trends in Cognitive Sciences. 2002;6:465–472. doi: 10.1016/s1364-6613(02)01993-9. [DOI] [PubMed] [Google Scholar]
- Mesulam MM. Large-scale neurocognitive regions and distributed processing of attention, language, and memory. Annals of Neurology. 1990;28:597–613. doi: 10.1002/ana.410280502. [DOI] [PubMed] [Google Scholar]
- Nicol JL, Forster KI, Veres C. Subject-verb agreement processes in comprehension. Journal of Memory and Language. 1997;36:569–587. [Google Scholar]
- Nilipour R. Agrammatic language: two cases from Persian. Aphasiology. 2000;14:1205–1242. [Google Scholar]
- Palmer ED, Rosen HJ, Ojemann JG, Buckner RL, Kelley WM, Petersen SE. An event-related FMRI study of overt and covert word stem completion. Neuroimage. 2001;14:182–193. doi: 10.1006/nimg.2001.0779. [DOI] [PubMed] [Google Scholar]
- Partee B. The syntax and semantics of quotation. In: Anderson SR, Kiparsky P, editors. A festschrift for Morris Halle. Holt, Reinhart, and Winston; New York: 1973. pp. 410–418. [Google Scholar]
- Paulesu E, Goldacre B, Scifo P, Cappa SF, Gilardi MC, Castiglioni I, et al. Functional heterogeneity of let inferior frontal cortex as revealed by fMRI. Neuroreport. 1997;8:2011–2016. doi: 10.1097/00001756-199705260-00042. [DOI] [PubMed] [Google Scholar]
- Perani D, Cappa SF, Schnur T, Tettamanti M, Collina S, Rosa MM, et al. The neural correlates of verb and noun processing: a PET study. Brain. 1999;122:2337–2344. doi: 10.1093/brain/122.12.2337. [DOI] [PubMed] [Google Scholar]
- Petersen SE, Fox PT, Posner MI, Mintun M, Raichle ME. Positron emission tomographic studies of the cortical anatomy of single-word processing. Nature. 1988;331:585–598. doi: 10.1038/331585a0. [DOI] [PubMed] [Google Scholar]
- Petrides M, Pandya DN. Projections to the frontal cortex from the posterior parietal region in the rheseus monkey. Journal of Comparative Neurology. 1984;228:105–116. doi: 10.1002/cne.902280110. [DOI] [PubMed] [Google Scholar]
- Pinker S. Words and rules. Lingua. 1998;106:219–242. [Google Scholar]
- Pinker S, Ullman MT. The past and future of the past tense. Trends in Cognitive Sciences. 2002;6:456–463. doi: 10.1016/s1364-6613(02)01990-3. [DOI] [PubMed] [Google Scholar]
- Poldrack RA, Wagner AD, Prull MW, Desmond JE, Glover GH, Gabrieli JDE. Functional specialization for semantic and phonological processing in the left inferior prefrontal cortex. Neuroimage. 1999;10:15–35. doi: 10.1006/nimg.1999.0441. [DOI] [PubMed] [Google Scholar]
- Pollock JY. Verb movement, universal grammar, and the structure of IP. Linguistic Inquiry. 1989;20:365–424. [Google Scholar]
- Rumelhart DE, McClelland JL. On learning the past tenses of English verbs. In: Rumelhart D, McClelland JL, editors. Parallel distributed processing. MIT Press; Cambridge, MA: 1986. [Google Scholar]
- Sach M, Seitz RJ, Indefrey P. Unified inflectional processing of regular and irregular verbs: a PET study. Neuroreport. 2004;15:533–537. doi: 10.1097/00001756-200403010-00030. [DOI] [PubMed] [Google Scholar]
- Sahin NT, Pinker S, Cash SS, Schomer D, Halgren E. Sequential processing of lexical, grammatical, and phonological information within Broca's area. Science. 2009;326:445–449. doi: 10.1126/science.1174481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seidenberg MS, Hoeffner JH. Evaluating behavioral and neuroimaging data on past tense processing. Language. 1998;74:104–122. [Google Scholar]
- Shalom DB, Poeppel D. Functional anatomic models of language: assembling the pieces. The Neuroscientist. 2008;14:119–127. doi: 10.1177/1073858407305726. [DOI] [PubMed] [Google Scholar]
- Shtyrov Y, Pulvermüller F. Memory traces for inflectional affixes as shown by mismatch negativity. European Journal of Neuroscience. 2002;15:1085–1091. doi: 10.1046/j.1460-9568.2002.01941.x. [DOI] [PubMed] [Google Scholar]
- Stavrakaki S, Kouvava S. Functional categories in agrammatism: evidence from Greek. Brain and Language. 2003;86:129–141. doi: 10.1016/s0093-934x(02)00541-2. [DOI] [PubMed] [Google Scholar]
- Thompson CK, Bonakdarpour B, Fix SC, Blumenfeld HK, Parrish TB, Gitelman DR, et al. Neural structures of verb argument structure processing. Journal of Cognitive Neuroscience. 2007;19:1753–1767. doi: 10.1162/jocn.2007.19.11.1753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Aguirre GK, Farah MJ. Role of left inferior prefrontal cortex in retrieveal of semantioc knowledge: A reevaluation. 1997 doi: 10.1073/pnas.94.26.14792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson-Schill SL, D'Esposito M, Kan IP. Effects of repetition and competition on activity in left prefrontal cortex during word generation. Neuron. 1999;23:513–522. doi: 10.1016/s0896-6273(00)80804-1. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Bright P, Fletcher P, Stamatakis EA. Neural processing of nouns and verbs: the role of inflectional morphology. Neuropsychologia. 2004;42:512–523. doi: 10.1016/j.neuropsychologia.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Tyler LK, Marslen-Wilson LK, Stamatakis EA. Differentiating lexical form, meaning, and structure in the neural language system. Proceedings of the National Academy of Sciences. 2005;102:8375–8380. doi: 10.1073/pnas.0408213102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tyler LK, Randall B, Marslen-Wilson WD. Phonology and neuropsychology of English past tense. Neuropsychologia. 2002;40:1154–1166. doi: 10.1016/s0028-3932(01)00232-9. [DOI] [PubMed] [Google Scholar]
- Ullman MT. A neurocognitive perspective on language: the declarative/ procedural model. Neuroscience. 2001;2:717–726. doi: 10.1038/35094573. [DOI] [PubMed] [Google Scholar]
- Ullman MT. Contributions of memory circuits to language: the declarative/procedural model. Cognition. 2004;92:231–270. doi: 10.1016/j.cognition.2003.10.008. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Bergida R, O'Craven K. Distinct fMRI activation patterns for regular and irregular past tense. Neuroimage. 1997;5:S549. [Google Scholar]
- Ullman MT, Corkin S, Coppola M, Hickok G, Growdon JH, Koroshetz WJ, et al. A neural dissociation within language: evidence that the mental dictionary is part of declarative memory, and that grammatical rules are processed by the procedural system. Journal of Cognitive Neuroscience. 1997;9:266–276. doi: 10.1162/jocn.1997.9.2.266. [DOI] [PubMed] [Google Scholar]
- Ullman MT, Pancheva R, Love T, Yee E, Swinney D, Hickok G. Neural correlates of lexicon and grammar: evidence from the production, reading, and judgment of inflection in aphasia. Brain and Language. 2005;93:185–238. doi: 10.1016/j.bandl.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Vanier M, Caplan D. CT-scan correlates of agrammatism. In: Menn L, Obler L, editors. Agrammatic aphasia. A cross-language narrative sourcebook. Vol. 1. John Benjamins; Amsterdam: 1990. pp. 37–114. [Google Scholar]
- Varlokosta S, Valeonti N, Kakavoulia M, Lazaridou M, Economou A, Protopapas A. The breakdown of functional categories IN Greek aphasia: evidence from agreement, tense, and aspect. Aphasiology. 2006;20:723–743. [Google Scholar]
- Warburton E, Wise RJS, Price CJ, Weiller C, Hadar U, Ramsay S, et al. Noun and verb retrieval by normal subjects: studies with PET. Brain. 1996;119:159–179. doi: 10.1093/brain/119.1.159. [DOI] [PubMed] [Google Scholar]
- Wassenaar M, Brown CM, Hagoort P. ERP effects of subject-verb agreement violations in patients with Broca's aphasia. Journal of Cognitive Neuroscience. 2004;16:553–576. doi: 10.1162/089892904323057290. [DOI] [PubMed] [Google Scholar]
- Wenzlaff M, Clahsen H. Tense and agreement in German agrammatism. Brain and Language. 2004;89:57–68. doi: 10.1016/S0093-934X(03)00298-0. [DOI] [PubMed] [Google Scholar]
- Wilson MD. The MRC psycholinguistic database: machine readable dictionary, version 2. Behavioural Research Methods, Instruments and Computers. 1988;20:6–11. [Google Scholar]
- Zelkowicz BJ, Herbster AN, Nebes RD, Mintun MA, Becker JT. An examination of regional cerebral blood flow during object naming tasks. Journal of International Neuropsychology. 1998;4:160–166. doi: 10.1017/s135561779800160x. [DOI] [PubMed] [Google Scholar]