Abstract
Aims: To test our hypothesis that the intrinsic molecular mechanism in stem cells for adaptation to ischemia is accentuated by preconditioning with insulin-like growth factor (IGF-1). Results: Bone marrow Sca-1+ cells were exposed to oxygen and glucose deprivation (OGD) for up to 12 h. Erk1/2 was activated in Sca-1+ cells under OGD which was blocked by MEK inhibitor (PD98059) and resulted in accelerated cell death. Moreover, elevated intracellular calcium with concomitant activation of protein kinase C (PKC) was observed under OGD. Pretreatment with nifedipine or dantrolene reduced cellular calcium, abrogated PKC and Erk1/2 activation, and increased cytotoxicity. Treatment with phorbol 12-myristate 13-acetate (PMA) for 30 min (short-term) activated Erk1/2, whereas 12 h (long-term) PMA treatment abrogated PKCα, reduced Erk1/2 activation and significantly increased cell death under OGD. These results were confirmed by loss-of-function studies using PKCα and Erk1/2 specific small interfering RNA. Gain-of-function studies with PKCα plasmid transfection improved cell survival under OGD. Preconditioning with 100 nM IGF-1 accentuated the intrinsic mechanism of resistance of the cells to ischemia via Erk1/2 activation and improved their survival under OGD as well as post-transplantation in an experimentally infarcted heart. Innovation: Strategies to target intrinsic survival mechanism in stem cells by growth factor preconditioning to enhance their survival via activation of PKCα and Erk1/2 are innovative. Conclusions: Intracellular calcium elevation under OGD activated PKCα and Erk1/2 as a part of the intrinsic prosurvival mechanism that was accentuated during preconditioning with IGF-1 to protect Sca-1+ cells from ischemic injury. Antioxid. Redox Signal. 16, 217–228.
Introduction
Ischemic preconditioning (IPC) by intermittent short cycles of ischemia/reperfusion initiates survival signaling that protects the heart on subsequent exposure to lethal ischemia (19). Although the underlying mechanism of IPC is multi-factorial, similar pro-survival effects have also been achieved via pharmacological intervention with preconditioning mimetics, thus providing comparably effective means to protect the heart against ischemia (13).
Stem cell therapy is a novel strategy to alleviate deteriorated heart function (9, 12, 23). One of the major challenges that hinder the efficacy of the heart cell therapy is massive death of donor cells post-transplantation in the infarcted myocardium. We have previously shown that stem cells preconditioned by treatment with diazoxide or by intermittent cyclical exposure to ischemia/re-oxygenation cycles improved their resistance to lethal anoxia (10, 20). Similar cytoprotective effects were also achieved when stem cells were pretreated with recombinant insulin-like growth factor-1 (IGF-1) that involved activation of Akt signaling and simultaneous mitochondrial translocation of connexin-43 (Cx-43) (16). The current study was designed to investigate how short-term pretreatment of bone marrow (BM) derived Sca-1+ cells with IGF-1 accentuated the intrinsic mechanism of cell survival under oxygen and glucose deprivation (OGD) to prevent ischemic injury and enhanced donor cell survival. Sca-1 antigen has wide distribution in the somatic cells including the heart and the BM cells that have been extensively studied for myocardial regeneration (16, 27). Given that Erk1/2 is the only common thread in the intrinsic survival mechanism under OGD and during preconditioning with IGF-1, our primary focus of study was to determine the role of Erk1/2 during preconditioning in relation to changes in the intracellular calcium and protein kinase C (PKC) activity. These investigations were performed using IGF-1 preconditioned Sca-1+ (PCSca-1+) cells as compared with nonpreconditioned Sca-1+ (non-PCSca-1+) cells under OGD and post-transplantation in the ischemic heart.
Innovation.
Donor stem cell survival post-transplantation in the ischemic heart significantly affects the outcome of the procedure. Various strategies have been developed to address this issue, however, with limited success. The current study was designed to validate our hypothesis that stem cells have an inherent mechanism to resist ischemic injury which could be exploited during preconditioning to support their survival under ischemia.
Results
OGD induced Erk1/2 activation and cytoprotection
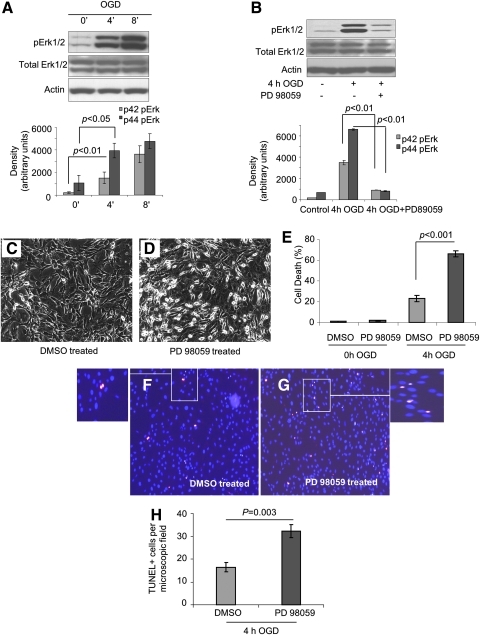
The protein samples from cells exposed to OGD for 4 and 8 h showed significant activation of Erk1/2 during OGD as compared with control cells without OGD treatment (Fig. 1A). Pretreatment of cells with PD98059, a specific inhibitor of MEK, abrogated Erk1/2 activity under OGD (Fig. 1B) and significantly reduced cell viability (Fig. 1C, D). Lactate dehydrogenase (LDH) and terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) assays showed higher cell death when Erk1/2 activity was blocked (Fig. 1E–H), thus suggesting that Erk1/2 activation was critical in stem cells survival under OGD.
FIG. 1.
Role of Erk1/2 in cytoprotection under OGD. (A) Sca-1+ cell were exposed to OGD for 0, 4, and 8 h, and cell lysates were collected for Western blotting to analyze pErk1/2, total Erk1/2 using actin as loading control. To establish the pro-survival effect of Erk1/2 activation, Sca-1+ cells were pretreated with either 50 μM MEK inhibitor PD98059 or vehicle DMSO for 30 min before 4 h OGD. Cell lysates were collected for Western blotting to analyze pErk1/2 and total Erk1/2 (B). Cell morphology after 4 h OGD was observed with phase contrast microscopy (C and D). Percentage cell death after 4 h OGD was measured with LDH release assay (E). TUNEL assay was performed to analyze apoptosis after 4 h OGD. DAPI (blue) was used to stain nuclei, and red color represented TUNEL+ cells (F, G). TUNEL+ cells were counted from randomly selected 200× microscopic field (H). DMSO, dimethylsulfoxide; LDH, lactate dehydrogenase; OGD, oxygen and glucose deprivation; TUNEL, terminal deoxynucleotidyl transferase dUTP nick end labeling. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
Intracellular calcium and cell survival
We next measured intracellular calcium changes in Sca-1+ cells, which increased after 4 h OGD, peaked at 8 h, and subsequently declined by 12 h of OGD (Fig. 2). To determine whether changes in calcium resulted from influx of extracellular calcium or involved intracellular calcium release from endoplasmic reticulum, cells were pretreated with nifedipine, a selective L-type calcium channel blocker which prevented calcium influx, and dantrolene, a selective ryanodine receptor blocker that prevented calcium release from endoplasmic reticulum. We observed that both nifedipine and dantrolene significantly reduced calcium during 4 h OGD, thus indicating that both extracellular and intracellular calcium contributed to calcium elevation during 4 h OGD (Fig. 2A). Pretreatment with calcium channel blockers also abrogated Erk1/2 activation (Fig. 2B). Of the two calcium channel blockers, nifedipine demonstrated a dose-dependent effect on Erk1/2 activation, which was partially blocked with 10 μM nifedipine, whereas 50 μM nifedipine pretreatment completely abolished Erk1/2 activation (Fig. 2B). On the contrary, dantrolene was less effective in preventing Erk1/2 activation, and its effect seemed saturated at 10 μM. These data suggested a correlation between intracellular calcium levels and Erk1/2 activity and showed that intracellular calcium triggered Erk1/2 activity under OGD. These molecular events also reduced cell survival as indicated by increased TUNEL positivity after pretreatment with dantrolene and nifedipine (Fig. 2C–F). These data were well supported by LDH release assay and showed that intracellular calcium elevation under OGD initiated intrinsic cytoprotective mechanism in Sca-1+ cells, without which cell survival was significantly compromised (Fig. 2G).
FIG. 2.
Intracellular calcium changes in Non-PCSca-1+ cells in response to OGD. The cells were exposed to OGD for 0, 4, 8, and 12 h, and intracellular calcium were measured with Fluo-3 calcium indicator (A). To block calcium channels, Sca-1+ cells were pretreated with dantrolene, nifedipine, or vehicle DMSO, respectively, for 30 min before OGD exposure, and blockers were continuously present during 4 h OGD. Cell lysates were collected and subjected to Western blot to detect pErk1/2 (B). Cell cultures used for normoxia control also included DMSO. Cell apoptosis was analyzed with TUNEL assay, and TUNEL+ cells were counted from different microscopic fields (200×) (C–F). Percentage cell death was analyzed with LDH release assay (G). Non-PCSca-1+ cells, nonpreconditioned Sca-1+ cells. (To see this illustration in color the reader is referred to the web version of this article at www.liebertonline.com/ars).
PKC and Erk1/2 activity in Sca-1+ cells
PKC activity increased in Sca-1+ cells under OGD, which was blocked by both dantrolene and nifedipine; however, the effect of latter was more pronounced (Fig. 3A, B). It is established that brief treatment of cells with phorbol 12-myristate 13-acetate (PMA) activated PKC, whereas prolonged treatment with PMA depleted PKC (4, 6). Therefore, we pretreated Sca-1+ cells with PMA for 12 h and measured conventional PKC isoforms (Fig. 3C). Only PKCα was completely abolished, PKCβ1 and PKCγ were unaffected, whereas PKCβ2 was undetected in Sca-1+ cells. In comparison with Sca-1+ cells pretreated with dimethylsulfoxide (DMSO), depletion of PKCα reduced Erk1/2 activation in response to OGD, thus showing the importance of PKCα for Erk1/2 activation (Fig. 3D). On the contrary, brief PMA treatment (30 min) activated Erk1/2, thus suggesting that PKC activation was sufficient for Erk1/2 activation (Fig. 3E). To exclude the possibility that PMA had nonspecific effects on Erk1/2 activation, cells were given prolonged PMA treatment followed by extensive washing and incubation without PMA for 4 h to allow the effect of PMA to dissipate. The cells were later treated with PMA for 30 min and analyzed for Erk1/2 activation (Fig. 3F). Although total Erk1/2 remained intact in both pretreated and control cells, brief PMA treatment consistently caused robust activation of Erk1/2 in control cells, whereas Erk1/2 activation was lost when PKCα was depleted. These data showed that loss of Erk1/2 activation with PMA was largely due to PKCα depletion. Moreover, calcium-dependent PKCα activity was not only required but also sufficient to activate Erk1/2 as a direct downstream target of PKCα in Sca-1+ cells.
FIG. 3.
Intracellular calcium dependent activation of PKCα regulates Erk1/2 activity under OGD. Sca-1+ cells were exposed to OGD for different time durations, and cell lysate samples were collected for PKC activity assay. Y-axis represented PKC activity/μg of cell lysate (A). To analyze a relationship between calcium elevation and PKC activity under 4 h OGD, cells were pretreated with dantrolene, nifedipine, or vehicle DMSO, respectively, for 30 min before 4 h OGD, and PKC activity was determined (B). To deplete PKC, Sca-1+ cells were pretreated with PMA (phorbol 12-myristate 13-acetate) or DMSO for 12 h, and cell lysates were collected for Western blotting to detect PKCα, β1, β2 (not detected), and γ using actin as loading control (C). After depletion of PKC, cells were exposed to 4 h OGD, and cell lysates were collected for Western blotting to detect PKCα and pErk1/2 (D). For activation of PKC, Sca-1+ cells were briefly treated with PMA (30 min), and cell lysates were collected for Western blotting to detect total and pErk1/2 (E). Sca-1+ cells were treated with PMA or DMSO for 12 h and reperfused with normal medium followed by treatment with PMA or DMSO for 30 min. The cell lysates were subjected to Western blotting to analyze PKCα, total Erk1/2, and pErk1/2 (F). PKC, protein kinase C.
Role of PKC in Sca-1+ cell survival
No significant morphological difference and TUNEL positivity between DMSO-treated and PMA-treated cells were observed under normoxia (data not shown). Nevertheless, significant cell damage was observed after OGD in PMA pretreated cells with abrogated PKC (Fig. 4A, B). These data indicated that depletion of PKCα abrogated the intrinsic protective mechanism. We next determined whether overexpression of PKCα could rescue cells under OGD. On completion of PMA treatment, cells were transfected with PKCα vector or control empty vector. Western blot showed that overexpression of PKCα compensated for endogenous PKCα (Fig. 4C) and alleviated cellular injury after OGD treatment (Fig. 4D).
FIG. 4.
Transgenic overexpression of PKCα supports cell survival under OGD. Sca-1+ cells were subjected to depletion of PKCα and exposed to OGD. Cell viability was analyzed with phase contrast microscopy (A). Percentage of cell death was analyzed with LDH release assay (B). To compensate for depleted PKCα, Sca-1+ cells were transfected with pcDNA3-PKCα or empty vector followed by exposure to 4 h OGD. Cell lysate samples were subjected to Western blotting for analysis of PKCα, total Erk1/2, and pErk1/2 using actin as loading control (C). Cell death after depletion of PKCα and overexpression of PKCα was analyzed with LDH release assay (D).
To confirm our findings, we adopted RNA interference approach to specifically knock down endogenous PKCα, whereas PKCβ1 and PKCγ remained unchanged. As compared with scramble (Sc) small interfering RNA (siRNA) transfection, cells transfected with PKCα siRNA showed reduced PKC activation (Fig. 5B) and Erk1/2 activation (Fig. 5A) under OGD. Abrogation of PKCα significantly injured the cells after OGD treatment as compared with Sc siRNA treatment (Fig. 5C, D). Taken together, OGD induced intracellular calcium elevation in Sca-1+ cells to activate PKCα, which incidentally activated its downstream target Erk1/2 to support cell survival under OGD.
FIG. 5.
Inhibition of PKCα reduced cell survival. Sca-1+ cells were transfected with PKCα or scramble siRNAs followed by exposure to 4 h OGD. Cell lysates were subjected to Western blotting (A). PKC activity was performed after PKCα specific RNA interference (B). Cell viability after knock-down of PKCα was observed with phase contrast microscopy (C), and cell death was analyzed by LDH release assay (D). siRNA, small interfering RNA.
Preconditioning with IGF-1 and Sca-1+ cells survival in vitro and in vivo
Erk1/2 activity increased under OGD in non-PCSca-1+, which was accentuated by IGF-1 preconditioning (Fig. 6A). Pretreatment of cells with PD98059 abrogated Erk1/2 activation (Fig. 6B). LDH release assay showed that PCSca-1+ cells were more protected as compared with Non-PCSca-1+ cells under OGD (Fig. 6C). To determine relative contribution of Akt and Erk1/2 during cytoprotection, cell death under OGD was compared in various treatment groups of cells in the presence of PD98059 and/or LY294002 inhibitors. We observed an additive effect when both pathways were inhibited (Fig. 6D). Western blot established the specificity of both inhibitors for their respective targeting pathway without detectable cross-activity (Fig. 6E). Although no PKCα induction was observed immediately after IGF-1 preconditioning, higher PKCα in Sca-1+ cells was observed at 48 h after IGF-1 preconditioning (Fig. 6F). The pro-survival effect of Erk1/2 activation in response to 4 h OGD or IGF-1 preconditioning was confirmed by transfection of cells with Erk1/2 specific siRNA (Fig. 6G, H), which resultantly increased cell death (Fig. 6I, J).
FIG. 6.
Preconditioning with IGF-1 was cytoprotective and activated Erk1/2 signaling in PCSca-1. (A) Sca-1+ cells were preconditioned with IGF-1 and pErk1/2, and total Erk1/2 were analyzed by Western blotting, which showed activation of Erk1/2 in PCSca-1+ cells. (B) Sca-1+ cells were pretreated with or without PD98059 for 30 min followed by preconditioning with IGF-1. Total and pErk1/2 were analyzed by Western blotting, which showed abrogation of Erk1/2 activity in PCSca-1+ cells. (C) To investigate cytoprotective effects of preconditioning with IGF-1 and its dependence on mitogen activated protein kinase signaling, PCSca-1+ cells and non-PCSca-1+ cells were pretreated with/without PD98059 before 4 h OGD. Cell death was analyzed with LDH release assay, which showed significantly higher leakage of LDH from cells pretreated with PD98059. (D) To determine a relationship between Erk1/2 and Akt pathway in cytoprotection, Sca-1+ cells were pretreated with PD98059, LY294002, or both inhibitors. The cells were subsequently exposed to 4 h OGD, and cell death was analyzed by LDH release assay, which showed higher LDH release from the cells pretreated with both inhibitors. (E) Western blots showed failure of 40 μM LY294002 to abolish Erk1/2 activation by OGD and failure of pretreatment with 50 μM PD98059 to prevent Akt activation in IGF-1 treated cells, respectively. (F) Western blot showed induction of PKCα in PCSca-1+ cells at 48 h after preconditioning. However, no PKCα induction was observed in PCSca-1+ cells immediately after IGF-1 treatment. (G) Western blot showed abrogation of total Erk1/2 and pErk1/2 after exposure to 4 h OGD in the cells transfected with Erk1/2 specific siRNA. (H) Western blot showed abrogation of Erk1/2 in cells preconditioned with IGF-1. (I, J) Moreover, morphological instability and cell death increased in Erk1/2 siRNA transfected cells after 4 h OGD as observed by phase contrast microscopy and LDH release assay. IGF-1, insulin-like growth factor-1; PCSca-1+ cells, preconditioned Sca-1+ cells.
In vivo studies were performed to validate in vitro findings in a female rat model of acute myocardial infarction (Fig. 7). Western blot on protein lysate samples of left ventricle from Dulbecco's modified Eagle's medium (DMEM) (group-1), Non-PCSca-1+ cell (group-2), and PCSca-1+ treated (group-3) animals at 24 h after their respective treatment showed higher activation of Erk1/2 in groups-2 and 3, using normal heart left ventricle as a control. Incidentally, group-3 showed highest activation of Erk1/2 (Fig. 7A). Sry-gene analysis showed extensive survival of transplanted cells in group-3 at 24 h after transplantation (Fig. 7B). DMEM-treated group-1 did not show sry-gene and served as negative control. These results were confirmed by real-time PCR (Fig. 7C). Moreover, group-3 had reduced TUNEL positivity (green) in the cell transplanted region (indicated by PKH26 label, red; Fig. 7D). The functional benefits of PCSca-1+ cells in terms of improved engraftment, attenuated infarct size, and preserved cardiac function have already been reported (16). Figure 7E depicts a summary of our proposed intrinsic survival mechanism in stem cells.
FIG. 7.
Erk1/2 activation and improved donor cell survival in the infarcted heart. (A) Western blot on protein lysate samples of the left ventricle from different animal groups 24 h after their respective treatment showed higher activation of Erk1/2 in PCSca-1+ group-3 as compared with control groups. (B, C) Sry-gene analysis of the heart tissue samples showed higher survival of PCSca-1+ in group-3 (p<0.01 vs. non-PCSca-1+ treated group-2). Dulbecco's modified Eagle's medium treated group-1 did not show sry-gene expression and served as a negative control. (D) TUNEL staining of tissue sections (green) showed that PCSca-1+ treated group-3 had significantly reduced TUNEL positivity in the cell-transplanted region (indicated by PKH26 label) (red). (E) Schema of Erk1/2 activation in PCSca-1+ and Non-PCSca-1+ cells.
Discussion
In this study, we report that the intrinsic survival mechanism of Sca-1+ cells was accentuated in response to IGF-1 treatment. While determining the signaling involved therein, our data showed that OGD altered the levels of calcium in Sca-1+ cells to initiate survival signaling and, both calcium influx and release of calcium from endoplasmic reticulum initiated PKCα dependent activation of Erk1/2 for cell survival.
The molecular mechanism of IPC is multi-factorial and involves activation of a number of receptor and postreceptor pathways such as PI-3K-Akt, Erk1/2, and PKC (14, 26). Studies in the experimental animal models have established an essential role for Erk1/2 during IPC and opioid receptor-induced cardioprotection (7). Studies on THP-1 cells suggested that the mitochondrial K+ATP channel opening mediated generation of reactive oxygen species, which was required for activation of Erk1/2 (24). Mechanistic studies of IPC greatly facilitated the discoveries of various preconditioning mimetics that targeted different levels of signal transduction while reliably simulating cytoprotective effects of IPC (7, 13, 18).
Massive death of the donor cells in the heart postengraftment significantly lowers the therapeutic efficacy of stem cell therapy of the heart. Development of strategies to protect donor cells based on exploitation of cell's intrinsic defense mechanism would be more effective to address this problem. In this context, we showed that OGD induced robust activation of Erk1/2 in Sca-1+ cells as a part of the intrinsic repair mechanism. Blocking Erk1/2 activity with PD98059 significantly increased cell death, thus demonstrating that activation of Erk1/2 was responsible for protection against ischemic injury in Sca-1+ cells.
Previous studies have shown that Erk1/2 activation during IPC was abolished by PKC inhibitors (21). In a cardiomyocyte hypertrophy model, PKC activator PMA caused rapid and robust activation of Erk1/2 (4). Similarly, cerebral ischemia increased neural progenitor cell proliferation (25). These findings clearly suggested that activation of PKC was an upstream prerequisite to initiate survival signaling via Erk1/2 activation. There is mounting evidence to support that elevation of intracellular calcium occurs during hypoxia, OGD, and oxidative stress via calcium influx or release of internal calcium reservoir in endoplasmic reticulum (1, 3, 8, 22). Calcium-initiated signaling pathways included Ca2+/calmodulin-dependent pathways and downstream Erk1/2, p38 mitogen activated protein kinase, and protein kinase B phosphorylation (2). In the hepatocytes cultures under hypoxia, Ca2+ increase was accompanied by translocation of PKCα and ɛ from cytosol to membrane fraction (11). Similarly, calcium-dependent activation of Erk1/2 under hypoxia was neuroprotective (5). These studies showed that both altered calcium handling by the cells under stress and PKC activation in response to altered calcium levels had a pivotal role in mediating Erk1/2 activation to support cell survival. Our data, indeed, support these observations and show that moderate yet continuous calcium increase occurred until peak level was reached at 8 h after OGD as a part of the intrinsic mechanism of cell survival under stress. Moreover, calcium elevation was sensitive to both nifedipine and dantrolene, which indicated that both external and internal calcium handling mechanisms contributed to this molecular event. On the contrary, both the inhibitors of calcium handling reduced Erk1/2 activity with concomitant reduction in cell survival. Since nifedipine was more effective than dantrolene, it invoked calcium influx as a more critical event in comparison with release of calcium from the cell's internal reservoir to achieve optimal calcium levels to stimulate downstream signaling. Concomitant with calcium elevation, PKC activity kinetics resembled that of calcium. Isoform analysis by three different approaches confirmed that PKCα was involved in the downstream signaling. First, from among all the conventional PKC isoforms, prolonged PMA treatment only depleted PKCα, and its depletion greatly reduced OGD-induced Erk1/2 activity. This was consistent with the data that PKC depletion caused loss of Erk1/2 activity (4). Second, PKCα specific siRNA abolished Erk1/2 activation and cytoprotection, and third, PKCα overexpression rescued the loss of cytoprotection after PMA depletion. These data were supported by the previous reports that a moderate increase in intracellular calcium levels enhanced cellular tolerance to OGD via Akt and Erk1/2 (2, 5). Additionally, calcium preconditioning and pharmacological preconditioning elicited cytoprotection against ischemic injury in a PKC-dependent manner (17, 18). Although we observed a relationship between PKCα and Erk1/2 activation, in some cases, abrogation of PKCα failed to completely abrogate Erk1/2 activity after 4 h OGD. This discrepancy may be attributed to the reason that PKCα is not the sole regulator of Erk1/2 activity in the cells under OGD. IGF-1 preconditioning approach accentuated the intrinsic cytoprotective Erk1/2 pathway in Sca-1+ cells for their improved survival. Combined with our published results that preconditioning of stem cells with IGF-1 activated Akt, we concluded that IGF-1 targeted two key signaling pathways involving Akt and Erk1/2 activation (16). These in vitro data were supported by in vivo findings that pErk1/2 was increased in animal hearts transplanted with PCSca-1+ cells as compared with non-PCSca-1+ cells and DMEM injection with concomitantly improved survival of the cells post-transplantation.
Despite these interesting data, our study has limitations. First, the signal/s that triggered calcium elevation in stem cells remained elusive. Second, we did not determine the role of mitochondrial K+ATP channel in the signaling cascade. Although we have reported MPAK dependent mitochondrial translocation of Cx-43 to confer cytoprotection (15), it was possible that the translocated Cx-43 interacted with as yet unidentified channels to regulate downstream apoptosis cascade. In-depth systematic studies would be required to analyze independent contribution from Akt and Erk1/2 in conferring cytoprotection during short-term IGF-1 treatment of stem cells. Moreover, for a complete understanding of the mechanism of IGF-1 preconditioning, it would be imperative to study the long-term effects of IGF-1 treatment and the signaling involved therein besides studying the efficiency of combined OGD and IGF-1 treatment of stem cells in vitro for preconditioning before transplantation in the infarcted heart. Although the cross-species transplantation model allowed testing of preconditioned mouse Sca-1+ cells in immunologically incompatible and harsh ischemic myocardium, future studies with the syngenic mice experimental model could be used to exclude the effects of immunological incompatibility between donor cells and the recipient. Despite these limitations, our study identified key molecules in cytoprotective signaling in Sca-1+ cells against ischemia. Short-term IGF-1 treatment stem cells targeted both Akt and Erk1/2 pathways and provided significant cytoprotection of donor Sca-1+ cells and beneficial effects on the infarcted heart without safety concerns (16).
Materials and Methods
The antibodies and reagents were purchased from: anti-pErk1/2, anti-pErk1/2, anti-PKCα, anti-PKCβ1, anti-PKCβ2, anti-PKCγ (Cell Signaling), anti-actin, rabbit anti-goat HRP-conjugated secondary antibody (Santa Cruz Biotechnology), Goat anti-mouse and goat anti-rabbit HRP conjugated secondary antibodies, ECL detection reagent (GE healthcare Bioscience), Femto super sensitive detection reagent (Thermo Fisher Scientific), PD98059, dantrolene, nifedipine, PMA (EMD Bioscience), fluo-4AM, Glucose and sodium pyruvate free DMEM (Invitrogen), and IGF-1 (R&D Systems). The results for cell survival, Western blotting, and PCR are the average of at least three independent experiments.
Culture and characterization of Sca-1+ cells
The cells were purified and characterized using our previously published method (15, 16). Briefly, BM was harvested from 6 to 8 weeks old male C57Black/6 mice and cultured using DMEM supplemented with 10% FBS. BM from each mouse was plated into a 150 mm culture dish and cultured in DMEM plus 10% FBS. Fresh medium was replenished every 3 days. On day 7, medium was changed when cell colonies started to form. Hematopoietic cells were removed at each medium change, and the adherent cells were maintained at 70% confluence. About 2×107 BM cells were collected to select Sca-1+ cells with mouse Sca-1+ cells selection cocktail (Stem Cell Technologies Inc.) according to manufacturer's instructions. Sca-1 surface marker was confirmed by flow cytometry and immunostaining (16). The purity of the cell population was >95%.
Oxygen and glucose deprivation
Sca-1+ cells were plated in a 60 mm dish in DMEM supplemented with 10% FBS and 24 h later, medium was replaced with DMEM containing 0.5% FBS. The cells were rinsed twice with serum-free, glucose, and sodium pyruvate–free DMEM and were cultured in the same medium at 37°C in an anoxia chamber (InVivo 500; Ruskinn Life Science) saturated with 95% N2/5% CO2 for up to 12 h. For calcium channel blocker studies, the cells were pretreated with 10 or 50 μM nifedipne or dantrolene for 30 min before exposure to OGD. For PKC activation, cells were pretreated with 100 nM PMA for 30 min. For PKC depletion studies, cells were pretreated with 100 nM PMA for 12 h followed by OGD. For preconditioning, cells were treated with 100 nM recombinant IGF-1 protein for 30 min. In control group, cells were treated for 30 min with 0.1% bovine serum albumin (BSA) solution without recombinant IGF-1 (16).
LDH release assay
Intracellular LDH release was measured using Homogeneous Membrane Integrity Assay (Promega) to determine cell viability. The fluorescence readings were obtained with excitation and emission filters at ∼550 and ∼590 nM, respectively. Percentage cell death was calculated as 100×(ReadingExperimental-ReadingBackground)/(ReadingTotal Release–ReadingBackground).
Western blot analysis
Protein lysate samples from cultured cells were obtained with ice cold lysis buffer (50 mM Tris-HCl, pH 7.6, 120 mM NaCl, 0.5% NP-40, 1 mM EDTA, 0.1 mM PMSF, 2 μg/ml Leupeptin, and 2 μg/ml Aprotinin). Protein concentration was determined with Bio-Rad DC-Protein Assay Reagent (Bio-Rad). For Western blotting, 20 μg cell lysate samples were loaded onto 4%–12% gradient precast gel (Invitrogen) and electrophoresed for about 2 h at 100 Volts. Transblotting was performed onto a PVDF membrane (15). The membrane was blocked with 5% nonfat milk in TBST for 1 h and subsequently incubated with specific primary antibodies (diluted in 5% nonfat milk or BSA in TBST), which was carried out overnight at 4°C. The membrane was then washed ×3 with TBST (15 min/wash) followed by incubation with HRP-conjugated secondary antibodies for 1 h at room temperature. After washing with TBST, membranes were incubated with ECL or Femto supersensitive detection reagent, and signals were exposed to Kodak Light film (Fisher Scientific).
TUNEL assay
TUNEL was performed on paraformaldehyde fixed cells with an in situ cell death detection kit (TMR Red; Roche Applied Science) according to manufacturer's instructions. The nuclei were visualized by treatment of TUNEL stained slides with 100 ng/ml DAPI solution for 3 min. TUNEL+ cells were counted from randomly selected microscopic fields at 200× magnification.
Intracellular calcium measurement
Intracellular calcium was measured with Fluo-3 AM calcium indicator (Invitrogen). Fluo-3 was dissolved in DMSO and diluted with 0.1% BSA solution in PBS to a final concentration of 10 μM. The cells plated in a 24-well plate were rinsed with warm PBS followed by incubation with Fluo-3 at 37°C for 25 min. The dye solution was removed, and cells were rinsed twice with glucose and sodium pyruvate–free medium followed by anoxia for 4–12 h. Fluorescence was recorded using excitation filter at ∼500 nM and emission filter at ∼535 nM. The cells without Fluo-3 dye loading were used as a control. Readings of calcium at different time points were obtained with the same instrument settings.
PKC activity assay
PKC activity was measured with PKC Activity Assay Kit (Enzo Life Sciences International) according to manufacturer's protocol. The absorbance readings were taken at 450 nM. PKC activity/μg of cell lysate was calculated for each assay.
Erk1/2 and PKCα RNA interference and PKCα overexpression
For siRNA transfection, Sca-1+ cells were plated in six-well plates at 1.4×105 cells per well. siRNA specific for mouse Erk1/2 was purchased from Santa Cruz. PKCα siRNA and scramble (Sc) siRNA were purchased from Qiagen. The cells were transfected with 2 μl of 20 μM siRNA complexed with 18 μl of HiPerfect Transfection Reagent (Qiagen) in 100 μl DMEM. Sc siRNA treated cells were used as control. Forty-eight hours after transfection, cells were harvested for Western blotting to observe RNAi efficiency. In a parallel set of experiments, cells transfected with respective siRNA or Sc siRNA cultured under OGD were used to evaluate cell viability. Similarly, cells transfected with Erk1/2 siRNA were treated for 30 min with IGF-1 before Western blotting and cell survival studies.
For PKCα overexpression, Sca-1+ cells plated in six-well plates at 1.4×105/well were transfected with either 1.2 μg empty vector or PKCα vector complexed with 5.4 μl Attractene transfection reagent (Qiagen) in 100 μl DMEM. At the end of the transfection, cells were incubated for 48 h before 8 h OGD.
Experimental model of acute myocardial infarction and cell transplantation
Young female Fischer rats (180–200 g) were used for animal studies. The study conformed to the Guide for the Care and Use of Laboratory Animals published by the US National Institutes of Health (NIH Publication #85-23, revised 1985), and protocols were approved by Institutional Animal Care and Use Committee, University of Cincinnati.
Female rats were anesthetized by intraperitoneal injection of ketamine/xylazine (87 and 13 mg/kg respectively) (16). After endotracheal intubation and ventilation using Harvard Rodent Ventilator (Model-683), the heart was exposed via minimal left-sided thoracotomy. Left anterior descending (LAD) coronary artery was occluded using Prolene #6-0 suture at the position of 1–2 mm lower than the tip of the left auricle. Myocardial infarction was confirmed by immediate palor of the left ventricle after LAD occlusion. Rats (n=4 per group) received intramyocardial injections of either 70 μl of DMEM without cells (group-1), or containing 1×106 BSA treated male Sca-1+ cells (group-2) or 1×106 IGF-1 preconditioning male Sca-1+ cells (group-3). The injections were performed at multiple sites (4–5 sites/animal) in the left ventricle. The chest was sutured, and animals were allowed to recover in an oxygen perfused chamber. The animals were harvested 24 h later for molecular and histological studies.
Statistical analysis
All experiments were repeated at least thrice, and data were expressed as mean±SEM. Student t-test was performed to analyze statistical differences between two groups. Comparison among more than two groups was made using ANOVA with a mixed-effect model to account for random effect from different repetition and unequal variance. A value of p≤0.05 was considered statistically significant.
Abbreviations Used
- BM
bone marrow
- BSA
bovine serum albumin
- Cx-43
connexin-43
- DMEM
Dulbecco's modified Eagle's medium
- DMSO
dimethylsulfoxide
- Erk1/2
mitogen activated protein kinase
- IGF-1
insulin-like growth factor-1
- IPC
ischemic preconditioning
- LAD
left anterior descending
- LDH
lactate dehydrogenase
- OGD
oxygen and glucose deprivation
- Phospho Erk1/2
pErk1/2
- PKC
protein kinase C
- PMA
phorbol 12-myristate 13-acetate
- PCSca-1+ cells
preconditioned Sca-1+ cells
- Non-PCSca-1+ cells
nonpreconditioned Sca-1+ cells
- TUNEL
terminal deoxynucleotidyl transferase dUTP nick end labeling
Acknowledgments
The authors are grateful to Dr. Matthew K. Topham, Oncological Sciences Huntsman Cancer Institute, University of Utah, for providing cPKC isoforms vectors.
This work was supported by National Institutes of Health Grants #R37-HL074272; HL-080686; HL-087246 (M.A.) and HL-087288; HL-089535; and HL106190-01 (Kh.H.H.).
Author Disclosure Statement
The authors declare that they have no competing interests to disclose.
References
- 1.Arnould T. Michiels C. Alexandre I. Remacle J. Effect of hypoxia upon intracellular calcium concentration of human endothelial cells. J Cell Physiol. 1992;152:215–221. doi: 10.1002/jcp.1041520127. [DOI] [PubMed] [Google Scholar]
- 2.Bickler PE. Fahlman CS. Moderate increases in intracellular calcium activate neuroprotective signals in hippocampal neurons. Neuroscience. 2004;127:673–683. doi: 10.1016/j.neuroscience.2004.05.035. [DOI] [PubMed] [Google Scholar]
- 3.Blanc A. Pandey NR. Srivastava AK. Distinct roles of Ca2+, calmodulin, and protein kinase C in H2O2-induced activation of ERK1/2, p38 MAPK, and protein kinase B signaling in vascular smooth muscle cells. Antioxid Redox Signal. 2004;6:353–366. doi: 10.1089/152308604322899422. [DOI] [PubMed] [Google Scholar]
- 4.Bogoyevitch MA. Glennon PE. Andersson MB. Clerk A. Lazou A. Marshall CJ. Parker PJ. Sugden PH. Endothelin-1 and fibroblast growth factors stimulate the mitogen-activated protein kinase signaling cascade in cardiac myocytes. The potential role of the cascade in the integration of two signaling pathways leading to myocyte hypertrophy. J Biol Chem. 1994;269:1110–1119. [PubMed] [Google Scholar]
- 5.Fahlman CS. Bickler PE. Sullivan B. Gregory GA. Activation of the neuroprotective ERK signaling pathway by fructose-1,6-bisphosphate during hypoxia involves intracellular Ca2+ and phospholipase C. Brain Res. 2002;958:43–51. doi: 10.1016/s0006-8993(02)03433-9. [DOI] [PubMed] [Google Scholar]
- 6.Force T. Kyriakis JM. Avruch J. Bonventre JV. Endothelin, vasopressin, and angiotensin II enhance tyrosine phosphorylation by protein kinase C-dependent and -independent pathways in glomerular mesangial cells. J Biol Chem. 1991;266:6650–6656. [PubMed] [Google Scholar]
- 7.Fryer RM. Pratt PF. Hsu AK. Gross GJ. Differential activation of extracellular signal regulated kinase isoforms in preconditioning and opioid-induced cardioprotection. J Pharmacol Exp Ther. 2001;296:642–649. [PubMed] [Google Scholar]
- 8.Hampl V. Cornfield DN. Cowan NJ. Archer SL. Hypoxia potentiates nitric oxide synthesis and transiently increases cytosolic calcium levels in pulmonary artery endothelial cells. Eur Respir J. 1995;8:515–522. [PubMed] [Google Scholar]
- 9.Jiang S. Haider H. Idris NM. Salim A. Ashraf M. Supportive interaction between cell survival signaling and angiocompetent factors enhances donor cell survival and promotes angiomyogenesis for cardiac repair. Circ Res. 2006;99:776–784. doi: 10.1161/01.RES.0000244687.97719.4f. [DOI] [PubMed] [Google Scholar]
- 10.Kim HW. Haider HK. Jiang S. Ashraf M. Ischemic preconditioning augments survival of stem cells via miR-210 expression by targeting caspase-8-associated protein 2. J Biol Chem. 2009;284:33161–33168. doi: 10.1074/jbc.M109.020925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lee SH. Lee MY. Lee JH. Han HJ. A potential mechanism for short time exposure to hypoxia-induced DNA synthesis in primary cultured chicken hepatocytes: correlation between Ca(2+)/PKC/MAPKs and PI3K/Akt/mTOR. J Cell Biochem. 2008;104:1598–1611. doi: 10.1002/jcb.21657. [DOI] [PubMed] [Google Scholar]
- 12.Leri A. Kajstura J. Anversa P. Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev. 2005;85:1373–1416. doi: 10.1152/physrev.00013.2005. [DOI] [PubMed] [Google Scholar]
- 13.Liu Y. Sato T. O'Rourke B. Marban E. Mitochondrial ATP-dependent potassium channels: novel effectors of cardioprotection? Circulation. 1998;97:2463–2469. doi: 10.1161/01.cir.97.24.2463. [DOI] [PubMed] [Google Scholar]
- 14.Liu Y. Ytrehus K. Downey JM. Evidence that translocation of protein kinase C is a key event during ischemic preconditioning of rabbit myocardium. J Mol Cell Cardiol. 1994;26:661–668. doi: 10.1006/jmcc.1994.1078. [DOI] [PubMed] [Google Scholar]
- 15.Lu G. Haider H. Porollo A. Ashraf M. Mitochondria-specific transgenic overexpression of connexin-43 simulates preconditioning-induced cytoprotection of stem cells. Cardiovasc Res. 2010;88:277–286. doi: 10.1093/cvr/cvq293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lu G. Haider HK. Jiang S. Ashraf M. Sca-1+stem cell survival and engraftment in the infarcted heart: dual role for preconditioning-induced connexin-43. Circulation. 2009;119:2587–2596. doi: 10.1161/CIRCULATIONAHA.108.827691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Miyawaki H. Ashraf M. Ca2+ as a mediator of ischemic preconditioning. Circ Res. 1997;80:790–799. doi: 10.1161/01.res.80.6.790. [DOI] [PubMed] [Google Scholar]
- 18.Miyawaki H. Zhou X. Ashraf M. Calcium preconditioning elicits strong protection against ischemic injury via protein kinase C signaling pathway. Circ Res. 1996;79:137–146. doi: 10.1161/01.res.79.1.137. [DOI] [PubMed] [Google Scholar]
- 19.Murry CE. Jennings RB. Reimer KA. Preconditioning with ischemia: a delay of lethal cell injury in ischemic myocardium. Circulation. 1986;74:1124–1136. doi: 10.1161/01.cir.74.5.1124. [DOI] [PubMed] [Google Scholar]
- 20.Niagara MI. Haider H. Jiang S. Ashraf M. Pharmacologically preconditioned skeletal myoblasts are resistant to oxidative stress and promote angiomyogenesis via release of paracrine factors in the infarcted heart. Circ Res. 2007;100:545–555. doi: 10.1161/01.RES.0000258460.41160.ef. [DOI] [PubMed] [Google Scholar]
- 21.Ping P. Zhang J. Huang S. Cao X. Tang XL. Li RC. Zheng YT. Qiu Y. Clerk A. Sugden P. Han J. Bolli R. PKC-dependent activation of p46/p54 JNKs during ischemic preconditioning in conscious rabbits. Am J Physiol. 1999;277:H1771–H1785. doi: 10.1152/ajpheart.1999.277.5.H1771. [DOI] [PubMed] [Google Scholar]
- 22.Pisani A. Bonsi P. Centonze D. Giacomini P. Calabresi P. Involvement of intracellular calcium stores during oxygen/glucose deprivation in striatal large aspiny interneurons. J Cereb Blood Flow Metab. 2000;20:839–846. doi: 10.1097/00004647-200005000-00011. [DOI] [PubMed] [Google Scholar]
- 23.Rota M. Kajstura J. Hosoda T. Bearzi C. Vitale S. Esposito G. Iaffaldano G. Padin-Iruegas ME. Gonzalez A. Rizzi R. Small N. Muraski J. Alvarez R. Chen X. Urbanek K. Bolli R. Houser SR. Leri A. Sussman MA. Anversa P. Bone marrow cells adopt the cardiomyogenic fate in vivo. Proc Natl Acad Sci USA. 2007;104:17783–17788. doi: 10.1073/pnas.0706406104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Samavati L. Monick MM. Sanlioglu S. Buettner GR. Oberley LW. Hunninghake GW. Mitochondrial K(ATP) channel openers activate the ERK kinase by an oxidant-dependent mechanism. Am J Physiol Cell Physiol. 2002;283:C273–C281. doi: 10.1152/ajpcell.00514.2001. [DOI] [PubMed] [Google Scholar]
- 25.Sung SM. Jung DS. Kwon CH. Park JY. Kang SK. Kim YK. Hypoxia/reoxygenation stimulates proliferation through PKC-dependent activation of ERK and Akt in mouse neural progenitor cells. Neurochem Res. 2007;32:1932–1939. doi: 10.1007/s11064-007-9390-1. [DOI] [PubMed] [Google Scholar]
- 26.Tong H. Chen W. Steenbergen C. Murphy E. Ischemic preconditioning activates phosphatidylinositol-3-kinase upstream of protein kinase C. Circ Res. 2000;87:309–315. doi: 10.1161/01.res.87.4.309. [DOI] [PubMed] [Google Scholar]
- 27.Wang X. Hu Q. Nakamura Y. Lee J. Zhang G. From AH. Zhang J. The role of the sca-1+/CD31- cardiac progenitor cell population in postinfarction left ventricular remodeling. Stem Cells. 2006;24:1779–1788. doi: 10.1634/stemcells.2005-0386. [DOI] [PubMed] [Google Scholar]