Abstract
Voltage-dependent anion channels (VDACs) are expressed in three isoforms, with common channeling properties and different roles in cell survival. We show that VDAC1 silencing potentiates apoptotic challenges, whereas VDAC2 has the opposite effect. Although all three VDAC isoforms are equivalent in allowing mitochondrial Ca2+ loading upon agonist stimulation, VDAC1 silencing selectively impairs the transfer of the low-amplitude apoptotic Ca2+ signals. Co-immunoprecipitation experiments show that VDAC1, but not VDAC2 and VDAC3, forms complexes with IP3 receptors, an interaction that is further strengthened by apoptotic stimuli. These data highlight a non-redundant molecular route for transferring Ca2+ signals to mitochondria in apoptosis.
Keywords: mitochondria, calcium, apoptosis, VDAC1
Voltage-dependent anion channels (VDACs), the most abundant proteins of the outer mitochondrial membrane (OMM), mediate the exchange of ions and metabolites between the cytoplasm and mitochondria, and are key factors in many cellular processes, ranging from metabolism regulation to cell death. VDACs are high-conductance, weakly anion-selective channel, fully open at low potential, but switching to cation selectivity and lower conductance at higher potentials (‘closed' state). Multicellular organisms and mammals have three distinct VDAC genes (VDAC1, VDAC2 and VDAC3), with high sequence homology and similar structure. The recently solved 3D-structure of VDAC11, 2, 3 shows a β-barrel membrane protein composed of 19 β-strands with a regulatory α-helix N-terminal domain residing inside the pore.
Besides its fundamental role as metabolite exchanger, the pleiotropic role of VDACs appears to rely on their ability to engage protein-protein interactions with different partners. Indeed, VDACs have been shown to interact with cytoskeletal elements such as actin and tubulin,4, 5 metabolic enzymes,6 Bcl2-family members including Bak,7 Bad,8 tBid9 and Bcl-xL10 or other channels such as ANT,11 or the IP3R.12, 13 This scenario is further complicated by evidence showing that VDAC contribution to cell death can be isoform and stimulus dependent: VDAC1 acts predominantly as a pro-apoptotic protein14, 15, 16, 17 whereas VDAC2 protects from a number of apoptosis inducers,18 but concurrently appears to be necessary for tBid-mediated cell-death.9 However, these different effects are not supported by the apparent redundancy in the electrophysiological properties of the isoforms.19, 20
Mitochondrial [Ca2+] is commonly regarded as an important determinant in cell sensitivity to apoptotic stimuli.21 Indeed, mitochondrial Ca2+ accumulation acts as a ‘priming signal' sensitizing the organelle and promoting the release of caspase cofactors, both in isolated mitochondria as well as in intact cells.22, 23 In this context, ER-mitochondria contacts mediate the tight and efficient Ca2+ transmission between the two organelles and thus could represent a potential regulatory site for cell death signals.24 Here we investigate the role of the different VDAC isoforms in the context of cell sensitivity to apoptosis and their role in regulating ER-mitochondrial Ca2+ signals transmission, and demonstrated that VDAC1, by selectively interacting with the IP3Rs, is preferentially involved in the transmission of the low-amplitude apoptotic Ca2+ signals to mitochondria.
Results
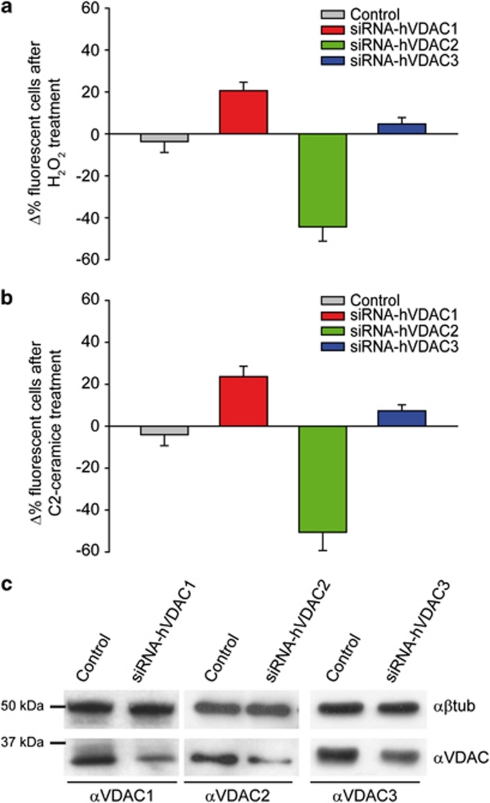
In this work, we aimed to correlate the Ca2+ channeling properties of the VDAC isoforms with their effects on cell death. We first downregulated the individual isoforms by RNAi silencing (Figure 1c). The siRNA of interest was co-transfected with a GFP reporter, and the effect on cell fate was evaluated by applying an apoptotic challenge (C2-ceramide or H2O2) and comparing the survival of transfected and non-transfected cells. In these experiments, the siRNA of interest was co-transfected with a GFP reporter and the percentage of GFP-positive cells was calculated before and after applying an apoptotic stimulus (C2-ceramide or H2O2). In mock-transfected cells, although the total number of cells is reduced after cell death induction, the apparent transfection efficiency was maintained (i.e. transfected and non-transfected cells have the same sensitivity to the apoptotic stimulus and thus die to the same extent). However, when GFP-positive cells are co-transfected with a construct influencing their sensitivity to apoptosis, this will be reflected by a change in the fraction of fluorescent cells, that is, in the ‘apparent' transfection efficiency. Thus, protection from apoptosis results into an apparent increase of transfection, whereas a decrease reflects a higher sensitivity to apoptosis. The results of the experiment are shown in Figure 1. Mock-transfected cells show no difference in the percentage of fluorescent cells after H2O2 treatment (−3.7±5.2%), whereas in the same conditions VDAC1-, VDAC2- and VDAC3-silenced GFP-positive cells were varied by 20±4.1%, −44.4±6.8% and 4.7±3.1%, respectively (Figure 1a). Similar results were obtained with C2-ceramide (Figure 1b). This confirms the notion that VDAC1 is pro-apoptotic and, among the various reported effects of VDAC2, the pro-survival role is prevailing in HeLa cells. Finally, VDAC3 shows no significant effect on apoptosis.
Figure 1.
Sensitivity to apoptotic challenges of VDAC-silenced cells. Cells were co-transfected with a fluorescent marker (GFP) and the siRNA of interest. The graph bar shows the change in percentage of fluorescent cells before the treatment with 100 μM H2O2 for 2 h ((a) control −3.7±5.2% siRNA-hVDAC1 20±4.1% siRNA-hVDAC2 −44.4±6.8% siRNA-hVDAC3 +4.7±3.1%) and 30 μM C2-ceramide for 2 h ((b) control −4±5.3% siRNA-hVDAC1 +24.3±5.1% siRNA-hVDAC2 −50.1±6.8% siRNA-hVDAC3 +7.1±2.5%). (c) HeLa cells were transfected for 48 h with control or siRNA-hVDAC encoding plasmid. Cells were harvested, total protein was extracted and subjected to western blotting analysis with antibodies anti-β-tubulin as loading control and anti-VDAC specific antibodies as indicated
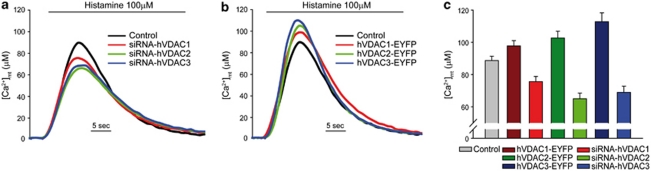
Considering that the enhancement of mitochondrial Ca2+ uptake generally correlates with increased sensitivity to apoptosis21 and that VDAC1 has been shown to be a regulator of OMM permeability to Ca2+,15 we wondered whether isoform specificity could rely on different Ca2+ channeling properties of the VDACs. The individual VDAC siRNAs were thus co-transfected with a mitochondrial Ca2+-probe (mtAeqMut). After aequorin reconstitution with the cofactor coelenterazine, cells were challenged with 100 μM histamine, and luminescence was measured and converted to Ca2+, as described in the Materials and Methods section. VDAC1 silencing significantly reduced the histamine-induced [Ca2+]mt peak (Figures 2a and c, [Ca2+]mt peak values: control, 88.6±2.7 μM; siRNA-hVDAC1, 75.6±3.2 μM; siRNA-hVDAC2, 64.9±3.5 μM; siRNA-hVDAC3, 69±3.8 μM). Interestingly, VDAC2 and VDAC3 silencing had the same effect, if anything greater. To confirm this notion, we carried out overexpression experiments, and, also in this case, VDAC1 showed an enhancement of mitochondrial Ca2+ uptake, in agreement with previous data.15 The effect was comparable, if not smaller, than that observed upon overexpression of VDAC2 and VDAC3 (Figures 2b and c, [Ca2+]mt peak values: hVDAC1-EYFP, 97.7±3.3 μM; hVDAC2-EYFP, 102.7±4.2 μM; hVDAC3-EYFP, 112.8±5.5 μM).
Figure 2.
Effect of VDAC isoform silencing or overexpression on mitochondrial Ca2+ uptake. [Ca2+]mt increase evoked by histamine stimulation in VDAC-silenced (a and c) or VDAC-overexpressing (b and c) cells ([Ca2+]mt peak values: control, 88.6±2.7 μM; siRNA-hVDAC1, 75.6±3.2 μM; siRNA-hVDAC2, 64.9±3.5 μM; siRNA-hVDAC3, 69±3.8 μM; hVDAC1-EYFP, 97.7±3.3 μM; hVDAC2-EYFP, 102.7±4.2 μM; hVDAC3-EYFP, 112.8±5.5 μM). (a and b) Representative traces, (c) bar graph of the average [Ca2+]mt peak. The traces are representative of >12 experiments that gave similar results. The bar graphs are the average of all experiments performed
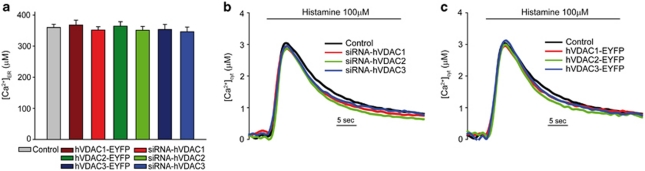
To rule out a confounding effect on cytosolic Ca2+ signaling, we measured ER and cytosolic [Ca2+] with the appropriate aequorin chimeras. Silencing or overexpression of the three VDAC isoforms did not alter significantly the state of filling of the ER store (Figure 3a), nor of its release kinetics (data not shown). Accordingly, the cytosolic [Ca2+] transient evoked by histamine stimulation was not significantly affected when any isoform were silenced (Figure 3b) or overexpressed (Figure 3c). Finally, mtGFP imaging and mitochondrial loading with the potential sensitive dye tetramethylrhodamine methyl ester (TMRM) showed that the effect was not due to changes in mitochondrial morphology or significant reduction of mitochondrial membrane potential (data not shown). Altogether, these data, while showing a clear effect of VDAC silencing or overexpression on mitochondrial Ca2+ handling, argue against the possibility that the pro-apoptotic effect of VDAC1 depends on a greater Ca2+ conductance of this isoform. Rather, the data may suggest a preferential role of the VDAC2 and VDAC3 isoforms in Ca2+ transport (also considering their lower expression levels25), although the real significance of this observation could be hampered by differences in protein stability or trafficking to the OMM.
Figure 3.
Effect of VDAC isoform silencing or overexpression on ER and cytosolic [Ca2+]. (a) Effect of the overexpression, or silencing, of individual VDAC isoforms on [Ca2+]ER steady-state levels (control, 360.1±10.5 μM; siRNA-hVDAC1, 351.9±10.6 μM; siRNA-hVDAC2, 352.3±12.3 μM; siRNA-hVDAC3, 346.5±15.1 μM; hVDAC1-EYFP, 368±15.6 μM; hVDAC2-EYFP, 364.5±14.4 μM and hVDAC3-EYFP, 353.6±16.6 μM). Transfection with the appropriate aequorin probe, reconstitution and [Ca2+] measurements were carried out as detailed in the methods section. When indicated, the cells were challenged with 100 μM histamine. erAEQ transfection and [Ca2+]ER measurements, after ER Ca2+ depletion, aequorin reconstitution and ER refilling were carried out as detailed in the methods section. (b and c) Representative traces of cytosolic Ca2+ transients evoked by 100 μM histamine in VDAC-silenced (b) and overexpressing (c) cells ([Ca2+]cyt peak values: control, 3.06±0.05 μM; siRNA-hVDAC1, 2.85±0.06 μM; siRNA-hVDAC2, 2.81±0.07 μM; siRNA-hVDAC3, 2.98±0.06 μM; hVDAC1-EYFP, 2.94±0.06 μM; hVDAC2-EYFP, 2.97±0.07 μM and hVDAC3-EYFP, 3.08±0.04 μM). The traces and graph bars of this figure are representatives of >12 experiments that gave similar results
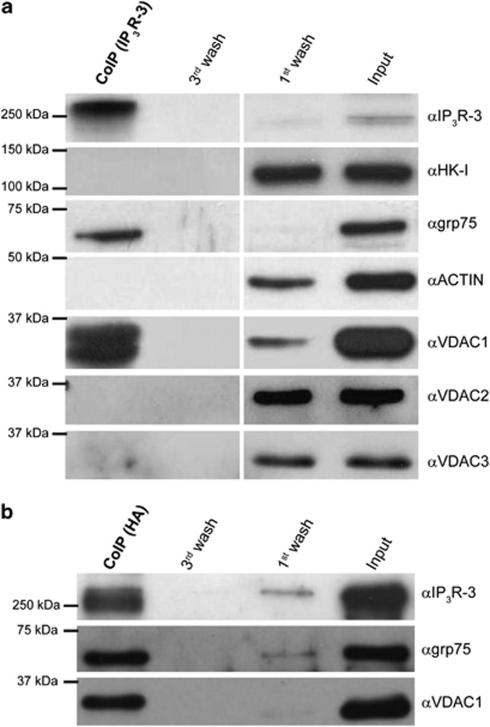
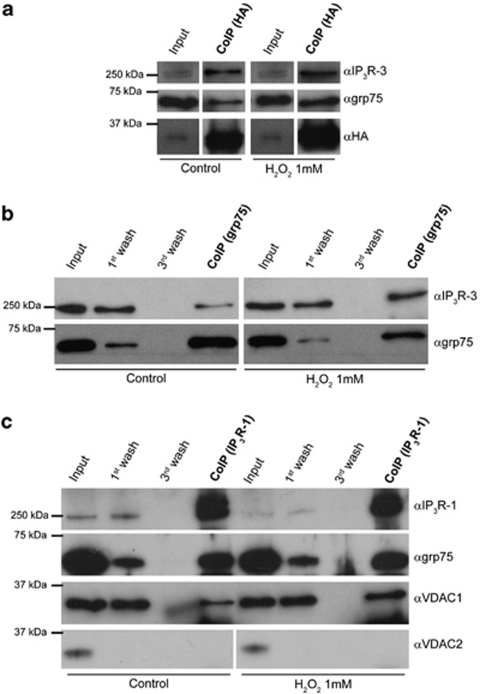
The pro-apoptotic activity of VDAC1 thus appears either totally independent of Ca2+, or due to the fine tuning of Ca2+ signals in specialized microdomains that may be overlooked in bulk cytosolic measurements.26 We followed the latter possibility, based on growing evidence demonstrating that the mitochondria/ER crosstalk is not merely the consequence of physical neighborhood but relies on the existence of macromolecular complexes linking the two organelles.27, 28 Specifically, during massive Ca2+ release upon maximal agonist stimulation, the existence of discrete signaling units could be overwhelmed and masked by the robustness of the response. Conversely, when an apoptotic stimulus causes a small, sustained Ca2+ release the existence of preferential channeling routes could become relevant. Based on previous data, showing the interaction of the IP3R with VDAC mediated by the grp75 chaperone,12 we investigated whether IP3Rs and grp75 preferentially interact with VDAC1, forming privileged signaling units. We first performed co-immunoprecipitation experiments using the highly expressed IP3R type 3 (IP3R-3) as bait. Strikingly, Figure 4a shows that VDAC1 is the only isoform bound to the IP3R in stringent conditions: no VDAC2 or VDAC 3 could be detected, also in long-term exposures. Neither actin, nor hexokinase-I, a known interactor of VDAC1, were co-immunoprecipitated in the assay, whereas the grp75 chaperone did. To confirm the specificity of the interaction, we also carried out the reverse experiment, by immunoprecipitating VDAC1 and revealing the presence of grp75 and IP3R-3 in the precipitate. In these experiments, the cells were transfected with an HA-tagged VDAC1 fusion protein, and immunoprecipitation was carried out with anti-HA antibodies. The results, shown in Figure 4b, demonstrate that both IP3R-3 and grp75 co-immunoprecipitate with VDAC1 (similarly to previous data with the IP3R-1,12 and see also Figure 5c). We then investigated whether the VDAC1-IP3Rs interaction is altered in apoptotic conditions. We thus performed co-immunoprecipitations in cells challenged with H2O2 using grp75 or VDAC1-HA as bait. VDAC1 pull-down in H2O2-treated cells resulted in a significantly greater amount of both grp75 and IP3R in the immunoprecipitate (Figure 5a), and the relative amount of IP3R co-immunoprecipitating with grp75 was significantly greater in H2O2-treated cells (Figure 5b). Moreover, we performed co-immunoprecipitation experiments also with IP3R type 1: as shown in Figure 5c, similarly to IP3R-3, also IP3R-1 interacts with VDAC1 but not with VDAC2, and H2O2 treatment enhance this interaction (although the effect seems weaker than with IP3R-3).
Figure 4.
Co-immunoprecipitations of VDAC1 with IP3R-3. Co-immunoprecipitations using IP3R-3 (a) and VDAC1-HA (b) as baits. HeLa cells were grown in 10-cm Petri dishes until full confluence. For VDAC1-HA immunoprecipitation, cells were transfected 48 h before experiment. Cells were then detached by scraping, harvested and proteins were extracted in non-denaturing conditions as indicated in the methods section. After protein quantification, 700 μg were incubated overnight at 4 °C with the 3 μg of the indicated antibody. The immunocomplex was then isolated by adding protein G-coated sepharose beads for 2 h at 4 °C. The purified immunocomplex was then washed three times with lysis buffer. Indicated fractions were then subjected to SDS-PAGE and western blotting, and probed with the indicated antibodies
Figure 5.
Co-immunoprecipitations of VDAC1 with IP3R-3 after H2O2 treatment. Co-immunoprecipitations using VDAC1-HA (a), grp75 (b) and IP3R-1 (c) as baits. HeLa cells were grown in 10-cm Petri dishes until full confluence. For VDAC1-HA immunoprecipitation, cells were transfected 48 h before experiment. Cells were then detached by scraping, harvested, incubated for 10 min with vehicle or 1 mM H2O2 and proteins were extracted in non-denaturing conditions as indicated in the methods section. After protein quantification, 700 μg were incubated overnight at 4 °C with the 3 μg of the indicated antibody. The immunocomplex was then isolated by adding protein G- (for anti-HA) or A- (for anti-IP3R-1 and grp75) coated sepharose beads for 2 h at 4 °C. The purified immunocomplex was then washed three times with lysis buffer. Indicated fractions were then subjected to SDS-PAGE and western blotting, and probed with the indicated antibodies
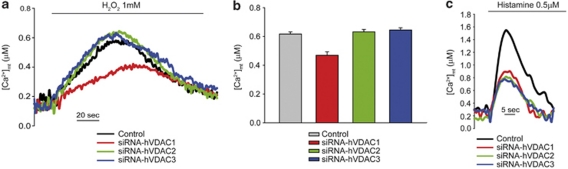
In order to test whether the interaction of VDAC1 and IP3Rs is involved in apoptotic signaling, we investigated the Ca2+ transients evoked by apoptotic stimuli in VDAC-silenced cells. We applied an oxidative stress, that is, treated the cells acutely with 1 mM H2O2. As previously reported,23 the addition of H2O2 caused a [Ca2+]cyt increase that is much smaller and more sustained than that evoked by histamine (Figures 6a and b). Under those conditions, mitochondria also undergo a small increase (peak value <1 μM). VDAC1 silencing decreased mitochondrial Ca2+ accumulation, while the knock-down of the other isoforms was indistinguishable from controls. We then titrated the histamine concentration in order to elicit a small Ca2+ response, comparable to that evoked by H2O2 by applying a 0.5 μM histamine challenge. Under those conditions, no difference among the different VDAC isoforms could be revealed (Figure 6c), thus suggesting that besides the slow kinetics the strengthening of the physical coupling of the IP3R and VDAC1 channels by apoptotic challenges may have an important role in the potentiation of mitochondrial Ca2+ signals and the induction of cell death.
Figure 6.
VDAC1 selectively transfers apoptotic Ca2+ signals to mitochondria. Representative traces (a) and statistics (b) of [Ca2+]mt evoked by the acute administration of 1 mM H2O2 ([Ca2+]mt peak values: control, 0.617±0.015 μM; siRNA-hVDAC1, 0.469±0.025 μM; siRNA-hVDAC2, 0.632±0.016 μM; siRNA-hVDAC3, 0.644±0.016 μM). (c) [Ca2+]mt increases evoked by 0.5 μM histamine. All other conditions as in Figure 2
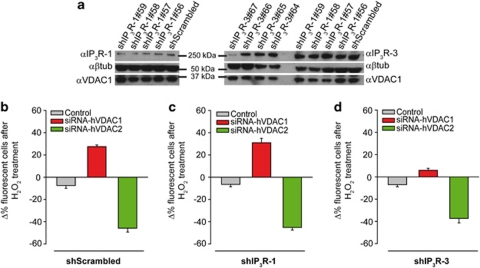
Finally, in order to dissect the precise contribution of the different IP3R isoforms to the VDAC1-dependent transmission of apoptotic challenges, we investigated the effect of shRNAs against IP3R type 1 and 3. We first screened a panel of shRNAs, selecting for further studies the two shRNAs (shIP3R-1#58 and shIP3R-3#67) that gave >50% reduction in IP3R-1 and IP3R-3 protein levels, respectively (Figure 7a). We thus tested cellular sensitivity to apoptotic stimuli by carrying out the experiment of Figure 1 in the cells in which IP3Rs were selectively silenced. Strikingly, while IP3R-1 silencing seems to have no consequence on cell death induced by H2O2, silencing of the IP3R-3 almost abrogated the protective effect of VDAC1 silencing, thus further demonstrating that IP3R-3 and VDAC1 act on the same signaling route.
Figure 7.
IP3R-3 silencing abrogates cell death regulation induced by VDAC1. (a) HeLa cells were transfected for 72 h with control or different shRNAs against IP3R-1 or IP3R-3. Cells were harvested, total protein was extracted and subjected to western blotting analysis with specific antibodies anti-IP3R-1/3 and anti-β-Tubulin as loading control. shRNA-IP3R-1#58 and IP3R-3#67 were selected for subsequent experiments. (b, c and d) Cells were co-transfected with one construct containing both the shRNAs for control (b, shScrambled), IP3R-1 (c, shIP3R-1), or IP3R-3 (d, shIP3R-3) and a fluorescent marker (GFP), together with the siRNA of interest. The graph bar shows the change in percentage of fluorescent cells before treatment with 100 μM H2O2 for 2 h (b, Control −7.5±2.5% siRNA-hVDAC1 27.4±1.5% siRNA-hVDAC2 −45.9±3.5% c, Control −6.5±2.1% siRNA-hVDAC1 +30.9±3.9% siRNA-hVDAC2 −45.2±2.3% d, Control −4±5.3% siRNA-hVDAC1 +24.3±5.1% siRNA-hVDAC2 −50.1±6.8%)
Discussion
Several observations support the notion that VDAC can finely tune cellular processes in an isoform-specific way: (i) selective genetic ablation of the three VDAC genes exhibits different phenotypes;29 (ii) VDAC1 and VDAC2 exert diametrically opposite effects on apoptosis15, 16, 18 and a compound acting through VDAC2, erastin, is effective in tumors harboring Ras mutations;30 (iii) apoptotic challenges31 and genomic programs, such as the PGC1-α pathway (De Stefani and Rizzuto, unpublished), differentially regulate the expression of VDAC isoforms; and (iv) the three isoforms are localized to different sub-domains of the OMM.32 We thus investigated in greater detail the molecular mechanism underlying the different role of VDAC isoforms in apoptosis. The first, obvious explanation of this diversity relied on different Ca2+ channeling capacities, given the sensitizing role of Ca2+ in the release of caspase activators. Our results ruled out the possibility, by showing relatively minor differences in Ca2+ channeling that cannot account for their differential cell death regulation. These minor differences could potentially be due to small variations in Ca2+ transport capacities. However, as in situ VDAC levels after overexpression or gene silencing are quite difficult to rigorously assess, this conclusion is risky. These data simply support the notion that all VDAC isoforms can similarly transport Ca2+ in living cells, and this is not correlated with their effect on apoptosis.
How can we then solve the discrepancy between mitochondrial Ca2+ transport and apoptosis regulation? An obvious conclusion is the denial (or, at least the reconsideration) of the classic paradigm linking mitochondrial Ca2+ to apoptosis. However, this notion is now supported by broad evidence showing that mitochondrial Ca2+ loading favors cell death and signaling molecules reducing or increasing Ca2+ signals protect from or enhance apoptosis, respectively.33 Ca2+ in mitochondria, however, is an intrinsically pleiotropic signal,34 and the final outcome varies widely depending on both the nature of the stimulus (and hence the ‘Ca2+ signature') and concomitant signaling pathways. Indeed, while physiological stimuli cause the rapid release of Ca2+ from internal stores, and thus a large and transient mitochondrial Ca2+ uptake, cell death signals have been shown to induce only a modest (even if sustained) [Ca2+]mt increase.23 This latter event has been proposed to represent a sort of priming signal that conditions and sensitizes mitochondria to otherwise non-lethal stimuli. In this context, the local coupling between ER and mitochondrial Ca2+ channels becomes critically relevant: small Ca2+ microdomains elicited by apoptotic stimuli such as C2-ceramide strongly relies on the existence of a preferential route transmitting the signal from the ER to the mitochondrion; on the other side, during physiological signals large Ca2+ microdomains are generated and this fine channel coupling could be potentially overwhelmed by the vigorous ER Ca2+ release. On the ER side, the notion that the accurate discrimination of Ca2+ signals mediating diverse effects relies on highly specialized molecular determinants was associated to the observation that the selective knockdown of IP3R-3 impairs cell death signals transmission whereas the silencing of the other two isoforms had almost no effect.35, 36 On the mitochondrial side, we wondered whether a similar selectivity could be associated to the mitochondrial channel repertoire at ER/mitochondria contact sites. The results clearly confirmed this possibility, by demonstrating that VDAC1 is preferentially involved in the transfer of apoptotic stimuli (such as those induced by H2O2) rather than physiological responses to agonists. Strikingly, our co-immunoprecipitation studies showed that IP3R selectively interacts with VDAC1, providing a molecular route for the higher sensitivity of the Ca2+ transfers. Moreover, this selective interaction appears not static but finely tuned by cellular conditions, as demonstrated by the fact that H2O2 strengthens the coupling between the ER and mitochondrial Ca2+ channels, and by the selective involvement of VDAC1 in the transmission of apoptotic stimuli. Although our biochemical evidences suggest that all IP3R isoforms are dynamically involved in this process (i.e., both IP3R-1 and IP3R-3 selectively interacts with VDAC1), functional data suggest a preferential role of the IP3R-3 in the transmission of apoptotic stimuli, in agreement with previous reports. Moreover, our data also suggest that the mechanism of the protective effect of VDAC2 on cell death does not involve the transmission of Ca2+ signals.
Overall, the data presented in this paper reveal a complex molecular organization underlying VDAC Ca2+ channeling properties, and allowing VDAC1 to exert its pro-apoptotic activity. The emerging picture reveals that VDACs represent a fundamental factor in mitochondria physiology, with similar channeling properties shared among its different variants, but also mediating diverse effects through isoform-specific protein-protein interactions and the assembly of highly specialized, higher-order protein complexes. This view accounts for most of experimental data available and finally reconciles apparently contrasting evidence, allowing a deeper insight on mitochondrial regulation of cell life and death.
Materials and Methods
Materials
Antibodies were purchased from the following sources and used at the indicated dilutions: αVDAC1 from Calbiochem (Darmstadt, Germany; 1 : 10 000) and AbNova (Taipei, Taiwan; 1 : 2000); αVDAC2 (1 : 1000), αVDAC3 (1 : 1000) and αIP3R-1 (1 : 1000) from Abcam (Cambridge, UK); αIP3R-3 (1 : 1000) from BD Biosciences (San Jose, CA, USA); αtubulin (1 : 10 000), αactin (1 : 5000) and αHA (1 : 5000) from Sigma-Aldrich (St. Louis, MO, USA); αHK-I (1 : 1000) and αgrp75 (1 : 10 000) from Santa Cruz (Santa Cruz, CA, USA). TMRM was purchased from Invitrogen (Carlsbad, CA, USA). All other chemicals were purchased from Sigma-Aldrich.
Cell culture and transfection
In all the experiments HeLa cells were used. Cells were grown in Dulbecco's modified Eagle's medium (Euroclone, Milan, Italy), supplemented with 10% fetal bovine serum (Euroclone) and transfected with a standard calcium–phosphate procedure. For aequorin measurements, the cells were seeded 24 h before transfection onto 13-mm glass coverslips and allowed to grow to 50% confluence before transfection. For cell death experiments, cells were seeded onto 12-well plates 24 h before transfection. For western blot and co-immunoprecipitation experiments cells were grown in 10-cm Petri dishes.
Plasmid cloning
For selective VDAC silencing several sequences were cloned and tested for specific silencing efficiency without upregulation of the other isoforms. The most effective sequences were: 5′-AAGCGGGAGCACATTAACCTG-3′ for hVDAC1; 5′-AAGGATGATCTCAACAAGAGC-3′ for hVDAC2; 5′-AAGGGTGGCTTGCTGGCTATC-3′ for hVDAC3. Appropriate oligonucleotides containing the selected sequences were purchased from Sigma-Aldrich and cloned into pSuper (Oligoengine, Seattle, WA, USA) according to manufacturer's instructions. VDAC-YFP isoforms have been cloned in a pEGFP-C3 (Clontech, Palo Alto, CA, USA) mutated to obtain the YFP. hVDAC2 and hVDAC3 have been subcloned EcoRI/SalI into this modified pEYFP-C3 vector from pQE-30 (Qiagen, Hilden, Germany) constructs. hVDAC1 has been directly subcloned BamHI/XhoI into pEYFP-C3. shRNA constructs against IP3Rs were obtained by using the BLOCK-iT RNAi Express system from Invitrogen. Four different sequences were chosen for both IP3R-1 (miR RNAi Select codes: Hmi409056, Hmi409057, Hmi409058 and Hmi409059) and IP3R-3 (miR RNAi Select codes: Hmi409064, Hmi409065, Hmi409066, and Hmi409067), and cloned into pcDNA6.2-GW/EmGFP-miR vectors following manufacturer's instructions.
Dynamic in vivo [Ca2+] measurements with targeted aequorin probes
cytAeq-, mtAeqMut-, mtAeqwt- or erAeqMut-expressing cells were reconstituted with coelenterazine for 2 h, transferred to the perfusion chamber where light signal was collected in a purpose-built luminometer and calibrated into [Ca2+] values as previously described.37 All aequorin measurements were carried out in Krebs–Ringer modified buffer (KRB: 135 mM NaCl, 5 mM KCl, 1 mM MgSO4, 0.4 mM K2HPO4, 1 mM CaCl2, 5.5 mM glucose, 20 mM HEPES, pH=7.4) containing 1 mM CaCl2. In the measurement of H2O2-induced [Ca2+] changes, the high affinity aequorin variant (mtAeqwt) was used and aequorin reconstitution protocol was slightly modified to avoid photon emission due to the direct oxidation of coelenterazine by H2O2; cells were reconstituted with coelenterazine for 1 h, washed three times with KRB supplemented with 2% bovine serum albumin and then incubated in KRB/Ca2+ for an additional hour. For [Ca2+]ER measurements, erAEQmut-transfected cells were reconstituted with coelenterazine n, following ER Ca2+ depletion in a solution containing 0 [Ca2+], 500 μM EGTA, 1 μM ionomycin, as previously described. After three washes with KRB supplemented with 2% bovine serum albumin and 1 mM EGTA, cells were perfused with KRB buffer containing 100 μM EGTA. ER refilling was then triggered by perfusing KRB buffer supplemented with 1 mM CaCl2 until equilibrium (steady state) was reached.
Co-immunoprecipitation
Co-immunoprecipitations were carried out by using protein A- or protein G-coated sepharose beads (GE Healthcare, Chalfont St. Giles, UK) following manufacturer's instructions. Different protein extraction buffers were used in order to minimize non-specific binding while maximizing antigen extraction. IP3R-3 and IP3R-1 were extracted in a modified RIPA buffer (150 mM NaCl, 1% NP-40, 0.05% SDS, Tris 50 mM, pH=8) while HA and grp75 were purified in a NP-40 buffer (150 mM NaCl, 1% NP-40, Tris 50 mM, pH=8), all supplemented with proteases and phosphatases inhibitors (PMSF, complete Protease Inhibitor Cocktail Tablets and PhosSTOP Phosphatase Inhibitor Cocktail Tablets from Roche, Basel, Switzerland). Extracted proteins (700 μg) were first precleared by incubating lysates with sepharose beads for 1 h at 4 °C and the supernatant (referred as Input) was incubated overnight with the antibody at 4 °C. Precipitation of the immune complexes was carried for 2 h at 4 °C and washed three times with the extraction buffer. Fractions were analyzed through standard SDS-PAGE (NuPage 4–12% Bis-Tris Gel, Invitrogen) and western blot techniques.
Cell death experiment
Cell sensitivity to apoptotic stimuli was evaluated as previously described.24 HeLa cells grown on 12-well plates at 30% confluence were co-transfected with GFP and control or siRNA-hVDACs containing plasmids in a 1 : 1 ratio. For the experiments shown in Figure 7, siRNA-hVDACs plasmids were co-transfected with plasmids encoding both an EmGFP and the shRNA of interest (either Scrambled, IP3R-1 or IP3R-3). The effect on cell fate was evaluated by applying an apoptotic challenge (20 μM C2-ceramide or 100 μM H2O2) and comparing the survival of transfected and non-transfected cells. In these experiments, the percentage of GFP-positive cells was calculated before and after applying an apoptotic stimulus (C2-ceramide or H2O2). In mock-transfected cells, although the total number of cells is reduced after cell death induction, the apparent transfection efficiency was maintained (i.e., transfected and non-transfected cells have the same sensitivity to the apoptotic stimulus and thus die to the same extent). However, when cells are transfected with a construct influencing their sensitivity to apoptosis, this will be reflected by a change in the fraction of fluorescent cells, that is, in the ‘apparent' transfection efficiency. Thus, protection from apoptosis results into an apparent increase of transfection, whereas a decrease reflects a higher sensitivity to apoptosis. Data are reported as the mean percentage change in the apparent transfection efficiency after apoptotic challenge compared with vehicle-treated cells. Cells were extensively washed with PBS, stained with DAPI, and two images per field (blue and green fluorescence) were taken at × 10 magnification (mean transfection efficiency were roughly 30% for all conditions). At least 10 fields per coverslip were randomly imaged and counted. Data presented are the sum of at least two different wells per experimental condition carried out in three different independent experiments.
Statistical analysis
Statistical analyses were performed using Student's t-test. A P-value ≤0.05 was considered significant. All data are reported as means±S.E.M.
Acknowledgments
This work was supported by grants from the Italian Ministries of Education (PRIN and local funds) and Health, European Union (FP7 ‘MyoAGE'), Italian Space Agency (ASI), NIH (Grant no. 1P01AG025532-01A1), Cariparo Foundation (Padova), the Italian Association for Cancer Research (AIRC), Telethon-Italy and FISM (Multiple Sclerosis) foundations.
Glossary
- [Ca2+]cyt
cytosolic [Ca2+]
- [Ca2+]ER
endoplasmic reticulum [Ca2+]
- [Ca2+]mt
mitochondrial [Ca2+]
- cytAeq
cytosolic aequorin
- ER
endoplasmic reticulum
- erAeqMut
endoplasmic reticulum-targeted mutated aequorin
- GFP
green fluorescent protein
- IP3R
inositol 1,4,5-trisphosphate receptor
- mtAeqMut
mitochondrially targeted mutated aequorin
- mtAeqwt
mitochondrially targeted aequorin
- mtGFP
mitochondrially targeted green fluorescent protein
- OMM
outer mitochondrial membrane
- PGC-1
peroxisome-proliferator activated receptor coactivator-1
- siRNA
small interfering RNA
- TMRM
tetramethylrhodamine methyl esther
- VDAC
voltage-dependent anion channel
The authors declare no conflict of interest.
Footnotes
Edited by M Piacentini
References
- Ujwal R, Cascio D, Colletier JP, Faham S, Zhang J, Toro L, et al. The crystal structure of mouse VDAC1 at 2.3 A resolution reveals mechanistic insights into metabolite gating. Proc Natl Acad Sci USA. 2008;105:17742–17747. doi: 10.1073/pnas.0809634105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiller S, Garces RG, Malia TJ, Orekhov VY, Colombini M, Wagner G. Solution structure of the integral human membrane protein VDAC-1 in detergent micelles. Science. 2008;321:1206–1210. doi: 10.1126/science.1161302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bayrhuber M, Meins T, Habeck M, Becker S, Giller K, Villinger S, et al. Structure of the human voltage-dependent anion channel. Proc Natl Acad Sci USA. 2008;105:15370–15375. doi: 10.1073/pnas.0808115105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu X, Forbes JG, Colombini M. Actin modulates the gating of Neurospora crassa VDAC. J Membr Biol. 2001;180:73–81. doi: 10.1007/s002320010060. [DOI] [PubMed] [Google Scholar]
- Rostovtseva TK, Bezrukov SM. VDAC regulation: role of cytosolic proteins and mitochondrial lipids. J Bioenerg Biomembr. 2008;40:163–170. doi: 10.1007/s10863-008-9145-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pastorino JG, Hoek JB. Regulation of hexokinase binding to VDAC. J Bioenerg Biomembr. 2008;40:171–182. doi: 10.1007/s10863-008-9148-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D, Kim H, Tu HC, Westergard TD, Fisher JK, Rubens JA, et al. The VDAC2-BAK rheostat controls thymocyte survival. Sci Signal. 2009;2:ra48. doi: 10.1126/scisignal.2000274. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SS, Madesh M, Davies E, Antonsson B, Danial N, Hajnoczky G. Bad targets the permeability transition pore independent of Bax or Bak to switch between Ca2+-dependent cell survival and death. Mol Cell. 2009;33:377–388. doi: 10.1016/j.molcel.2009.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roy SS, Ehrlich AM, Craigen WJ, Hajnoczky G. VDAC2 is required for truncated BID-induced mitochondrial apoptosis by recruiting BAK to the mitochondria. EMBO Rep. 2009;10:1341–1347. doi: 10.1038/embor.2009.219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vander Heiden MG, Li XX, Gottleib E, Hill RB, Thompson CB, Colombini M. Bcl-xL promotes the open configuration of the voltage-dependent anion channel and metabolite passage through the outer mitochondrial membrane. J Biol Chem. 2001;276:19414–19419. doi: 10.1074/jbc.M101590200. [DOI] [PubMed] [Google Scholar]
- Verrier F, Deniaud A, Lebras M, Metivier D, Kroemer G, Mignotte B, et al. Dynamic evolution of the adenine nucleotide translocase interactome during chemotherapy-induced apoptosis. Oncogene. 2004;23:8049–8064. doi: 10.1038/sj.onc.1208001. [DOI] [PubMed] [Google Scholar]
- Szabadkai G, Bianchi K, Varnai P, De Stefani D, Wieckowski MR, Cavagna D, et al. Chaperone-mediated coupling of endoplasmic reticulum and mitochondrial Ca2+ channels. J Cell Biol. 2006;175:901–911. doi: 10.1083/jcb.200608073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sano R, Annunziata I, Patterson A, Moshiach S, Gomero E, Opferman J, et al. GM1-ganglioside accumulation at the mitochondria-associated ER membranes links ER stress to Ca(2+)-dependent mitochondrial apoptosis. Mol Cell. 2009;36:500–511. doi: 10.1016/j.molcel.2009.10.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tajeddine N, Galluzzi L, Kepp O, Hangen E, Morselli E, Senovilla L, et al. Hierarchical involvement of Bak, VDAC1 and Bax in cisplatin-induced cell death. Oncogene. 2008;27:4221–4232. doi: 10.1038/onc.2008.63. [DOI] [PubMed] [Google Scholar]
- Rapizzi E, Pinton P, Szabadkai G, Wieckowski MR, Vandecasteele G, Baird G, et al. Recombinant expression of the voltage-dependent anion channel enhances the transfer of Ca2+ microdomains to mitochondria. J Cell Biol. 2002;159:613–624. doi: 10.1083/jcb.200205091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Abu-Hamad S, Sivan S, Shoshan-Barmatz V. The expression level of the voltage-dependent anion channel controls life and death of the cell. Proc Natl Acad Sci USA. 2006;103:5787–5792. doi: 10.1073/pnas.0600103103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tomasello F, Messina A, Lartigue L, Schembri L, Medina C, Reina S, et al. Outer membrane VDAC1 controls permeability transition of the inner mitochondrial membrane in cellulo during stress-induced apoptosis. Cell Res. 2009;19:1363–1376. doi: 10.1038/cr.2009.98. [DOI] [PubMed] [Google Scholar]
- Cheng EH, Sheiko TV, Fisher JK, Craigen WJ, Korsmeyer SJ. VDAC2 inhibits BAK activation and mitochondrial apoptosis. Science. 2003;301:513–517. doi: 10.1126/science.1083995. [DOI] [PubMed] [Google Scholar]
- Xu X, Decker W, Sampson MJ, Craigen WJ, Colombini M. Mouse VDAC isoforms expressed in yeast: channel properties and their roles in mitochondrial outer membrane permeability. J Membr Biol. 1999;170:89–102. doi: 10.1007/s002329900540. [DOI] [PubMed] [Google Scholar]
- Wu S, Sampson MJ, Decker WK, Craigen WJ. Each mammalian mitochondrial outer membrane porin protein is dispensable: effects on cellular respiration. Biochim Biophys Acta. 1999;1452:68–78. doi: 10.1016/s0167-4889(99)00120-2. [DOI] [PubMed] [Google Scholar]
- Rizzuto R, Marchi S, Bonora M, Aguiari P, Bononi A, De Stefani D, et al. Ca(2+) transfer from the ER to mitochondria: when, how and why. Biochim Biophys Acta. 2009;1787:1342–1351. doi: 10.1016/j.bbabio.2009.03.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szalai G, Krishnamurthy R, Hajnoczky G. Apoptosis driven by IP(3)-linked mitochondrial calcium signals. EMBO J. 1999;18:6349–6361. doi: 10.1093/emboj/18.22.6349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baumgartner HK, Gerasimenko JV, Thorne C, Ferdek P, Pozzan T, Tepikin AV, et al. Calcium elevation in mitochondria is the main Ca2+ requirement for mitochondrial permeability transition pore (mPTP) opening. J Biol Chem. 2009;284:20796–20803. doi: 10.1074/jbc.M109.025353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Ferrari D, Rapizzi E, Di Virgilio F, Pozzan T, Rizzuto R. The Ca2+ concentration of the endoplasmic reticulum is a key determinant of ceramide-induced apoptosis: significance for the molecular mechanism of Bcl-2 action. EMBO J. 2001;20:2690–2701. doi: 10.1093/emboj/20.11.2690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamamoto T, Yamada A, Watanabe M, Yoshimura Y, Yamazaki N, Yamauchi T, et al. VDAC1, having a shorter N-terminus than VDAC2 but showing the same migration in an SDS-polyacrylamide gel, is the predominant form expressed in mitochondria of various tissues. J Proteome Res. 2006;5:3336–3344. doi: 10.1021/pr060291w. [DOI] [PubMed] [Google Scholar]
- Giacomello M, Drago I, Bortolozzi M, Scorzeto M, Gianelle A, Pizzo P, et al. Ca2+ hot spots on the mitochondrial surface are generated by Ca2+ mobilization from stores, but not by activation of store-operated Ca2+ channels. Mol Cell. 2010;38:280–290. doi: 10.1016/j.molcel.2010.04.003. [DOI] [PubMed] [Google Scholar]
- de Brito OM, Scorrano L. Mitofusin 2 tethers endoplasmic reticulum to mitochondria. Nature. 2008;456:605–610. doi: 10.1038/nature07534. [DOI] [PubMed] [Google Scholar]
- Csordas G, Renken C, Varnai P, Walter L, Weaver D, Buttle KF, et al. Structural and functional features and significance of the physical linkage between ER and mitochondria. J Cell Biol. 2006;174:915–921. doi: 10.1083/jcb.200604016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graham BH, Craigen WJ. Genetic approaches to analyzing mitochondrial outer membrane permeability. Curr Top Dev Biol. 2004;59:87–118. doi: 10.1016/S0070-2153(04)59004-X. [DOI] [PubMed] [Google Scholar]
- Yagoda N, von Rechenberg M, Zaganjor E, Bauer AJ, Yang WS, Fridman DJ, et al. RAS-RAF-MEK-dependent oxidative cell death involving voltage-dependent anion channels. Nature. 2007;447:864–868. doi: 10.1038/nature05859. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voehringer DW, Hirschberg DL, Xiao J, Lu Q, Roederer M, Lock CB, et al. Gene microarray identification of redox and mitochondrial elements that control resistance or sensitivity to apoptosis. Proc Natl Acad Sci USA. 2000;97:2680–2685. doi: 10.1073/pnas.97.6.2680. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neumann D, Buckers J, Kastrup L, Hell SW, Jakobs S. Two-color STED microscopy reveals different degrees of co-localization between hexokinase-I and the three human VDAC isoforms. PMC Biophys. 2010;3:4. doi: 10.1186/1757-5036-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pinton P, Giorgi C, Siviero R, Zecchini E, Rizzuto R. Calcium and apoptosis: ER-mitochondria Ca2+ transfer in the control of apoptosis. Oncogene. 2008;27:6407–6418. doi: 10.1038/onc.2008.308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murgia M, Giorgi C, Pinton P, Rizzuto R. Controlling metabolism and cell death: at the heart of mitochondrial calcium signalling. J Mol Cell Cardiol. 2009;46:781–788. doi: 10.1016/j.yjmcc.2009.03.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khan AA, Soloski MJ, Sharp AH, Schilling G, Sabatini DM, Li SH, et al. Lymphocyte apoptosis: mediation by increased type 3 inositol 1,4,5-trisphosphate receptor. Science. 1996;273:503–507. doi: 10.1126/science.273.5274.503. [DOI] [PubMed] [Google Scholar]
- Mendes CC, Gomes DA, Thompson M, Souto NC, Goes TS, Goes AM, et al. The type III inositol 1,4,5-trisphosphate receptor preferentially transmits apoptotic Ca2+ signals into mitochondria. J Biol Chem. 2005;280:40892–40900. doi: 10.1074/jbc.M506623200. [DOI] [PubMed] [Google Scholar]
- Pinton P, Rimessi A, Romagnoli A, Prandini A, Rizzuto R. Biosensors for the detection of calcium and pH. Methods Cell Biol. 2007;80:297–325. doi: 10.1016/S0091-679X(06)80015-4. [DOI] [PubMed] [Google Scholar]