Abstract
Hypoxia induces the expansion of glioblastoma stem cells (GSCs), but the mechanism underlying it is still unclear. Here, we supply evidence that hypoxia-inducible factor-1α (HIF-1α) induced activation of Notch pathway is essential for hypoxia-mediated maintenance of GSC. Either depletion of HIF-1α or inactivation of Notch signaling partly inhibits the hypoxia-mediated maintenance of GSC. Further data suggest a role for HIF-1α in the interaction and stabilization of intracellular domain of Notch (NICD), and activation of Notch signaling. The mRNA level of HIF-1α and Notch target gene FABP7 was elevated in GSC. And the STAT3 pathway responsible for the HIF-1α gene transcription, the phosphatidylinositol 3-kinase-Akt and ERK1/2, both of which are contributed to HIF-1α protein translation, are also preferentially activated in GSC. Inhibition of these pathways partly reduces the hypoxia-induced activation of the Notch pathway and subsequent GSC maintenance. Taken together, our findings suggest that HIF-1α requires Notch pathway to drive the maintenance of GSC. The activated regulation of HIF-1α makes GSC more sensitive to hypoxia-mediated maintenance. These findings enhance our understanding of mechanism of hypoxia-mediated GSC expansion and provide HIF-1α as an attractive target for glioblastoma therapy.
Keywords: cancer stem cell, hypoxia, Notch, Stat3, PI3K/AKT/mTOR
Glioblastomas are lethal cancers promoted in part by glioblastoma stem cells (GSCs), a population of cells capable of extensive self-renewal, differentiation into multiple lineages, and strengthened tumor initiation potential.1, 2, 3 Previous studies describing molecular connections between oxygen-regulated transcription factors and pathways known to control stem cell function4, 5 suggest a new mechanism, whereby hypoxia-induced transcription factors may drive tumor growth through the generation or expansion of GSC. Recently, we and other groups reported that hypoxia can maintain and induce the expansion of GSC.6, 7, 8, 9 However, the mechanisms involved in this process are still unclear.
The Notch pathway, a highly conserved signaling network, is critical for a series of processes, including cell fate specification, differentiation, proliferation, and survival, and especially maintenance of neural stem cells.10, 11 Recent studies demonstrated that hypoxia synergizes with Notch to inhibit differentiation of neural stem cells, in which process requires a newly described interaction between hypoxia-inducible factor-1α (HIF-1α) and the intracellular domain of Notch (NICD).4, 12
HIF-1α protein expression requires the activation of the phosphatidylinositol3-kinase (PI3K) or mitogen-activated protein kinase (MAPK)/ERK1/2 pathways. PI3K mediates its effects through its target Akt and the downstream kinase mTOR, which regulates HIF-1α protein translation through phosphorylation of two targets: the eukaryotic translation initiation factor 4E-binding protein (4E-BP1) and p70 S6 kinase (S6K).13, 14, 15 In addition to the complex network stabilizing HIF-1α protein, recent evidence showed that HIF-1α can also be regulated at the mRNA level by many transcription factors, including the melanocyte-specific transcription factor MITF, NF-κB and Egr1, and STAT3, which is required for proliferation and maintenance of multipotency in GSC.16, 17, 18, 19, 20, 21, 22
Previously, we have isolated GSC in human glioblastoma U251 cells. And interestingly, we noted that hypoxia promotes the expansion of CD133-positive cells.9 Here, we provide evidence that HIF-1α, which is regulated by several signal pathways, including STAT3, PI3K-Akt, and ERK1/2 at different stage, is essential for hypoxia-mediated maintenance of GSC by activating Notch signaling pathway.
Results
Hypoxia facilitates the maintenance of GSC
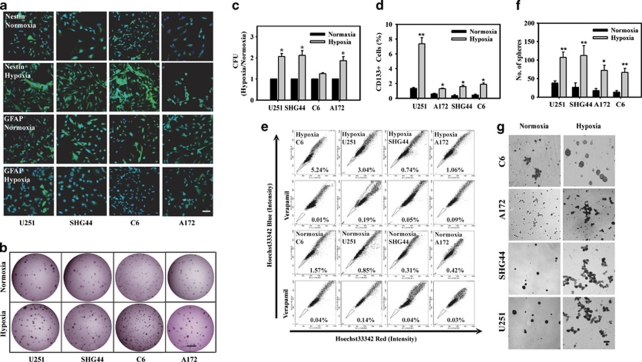
Our previous study showed that hypoxia could induce the expansion of CD133-positive cells in U251 cells. To further validate this phenomenon, other glioblastoma cell lines, including U251, SHG44, A172, and C6, were selected to evaluate hypoxia-mediated maintenance by testing Nestin, a neural stem cell marker, and GFAP, an astrocyte marker. Data indicated the percentage of Nestin-positive cells increased, whereas that of GFAP-positive cells decreased after these cells were treated with hypoxia for 24 h (Figure 1a). To confirm the function of hypoxia in GSC maintenance, we next investigated the colony-formation ability of these cell lines in normoxia and hypoxia. As data shown in Figures 1b and c, the clone numbers of U251, SHG44, and A172 cells in hypoxia were 2.11-fold, 2.06-fold, and 1.86-fold, respectively, as much as that in normoxia. However, the clone number of C6 cells had no significant difference though their clone sizes in hypoxia were much lager than that in normoxia. Many studies have reported CD133-positive cells and SP cells enrich the GSC.9, 23 Flow cytometric analysis indicated that the percentage of CD133+ cells and SP cells increased (Figures 1d and e) after these glioblastoma cells were exposed to hypoxia. Specifically, the percentage of CD133+ cells obviously increased in hypoxia-treated U251 cells (Figure 1d). The capability for the formation of neurosphere, which shows the self-renewal ability of stem cell, is an important characteristic of GSC.8 Sphere-formation analysis indicated that the number of cell sphere in hypoxia was more than that in normoxia (Figures 1f and g). All these data suggest that hypoxia facilitates the maintenance of GSC and blocks their differentiation.
Figure 1.
Hypoxia facilitates the maintenance of GSC. (a) Expression of Nestin, GFAP in U251, SHG44, A172, and C6 glioblastoma cells cultured in hypoxia or normoxia. Bar=50 μm. (b and c) Glioblastoma cells were cultured in hypoxia for 36 h and then the colony-formation ability was investigated. The picture of colonies in a representative dish was taken with phase contrast microscopy. Bar=1 mm. (d) Cells exposed to a hypoxia for 36 h were fixed and then CD133-positive cells were analyzed by FACS. (e) Representative FACS SP analysis for glioblastoma cells showed that hypoxia resulted in increase in the ratio of SP cells. (f and g) Sphere-forming assay showed that the self-renewal capacity was increased when incubated at hypoxia (2% O2) compared with normoxia (20% O2). The picture of cells in a representative well was taken with phase contrast microscopy. Bar=200 μm. The results shown in the graph are mean±S.D. from three experiments. Significance of difference is indicated as *P<0.05, **P<0.01
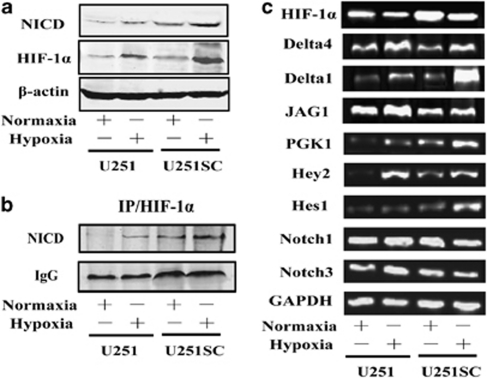
HIF-1α and Notch signal are activated in U251-derived stem-like tumor sphere cells (U251SC)
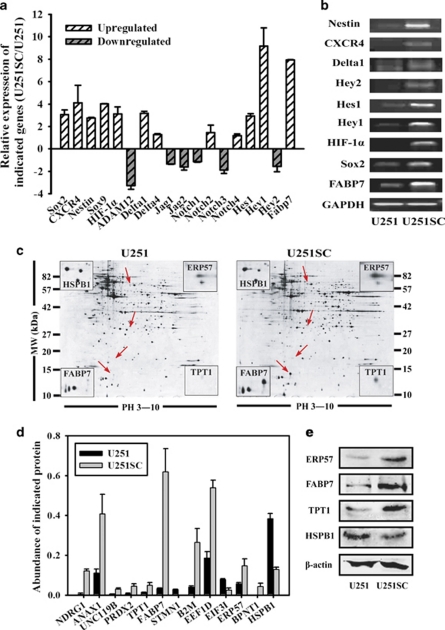
To address the base involved in hypoxia-mediated maintenance and differentiation block of GSC, we took U251 cell line, which is sensitive to hypoxia (Figure 1), as a model to perform microarray analysis for distinguishing U251 cells from U251SC. Differently expressed genes were involved in various pathways, such as the cell-cycle regulation, MAPK signaling pathway, and Notch signaling pathway (data was not shown). Here, we focused on the Notch signaling pathway. Genes involved in Notch signaling as well as some of the stem cell markers, such as Sox2, CXCR4, and Nestin, were selected and verified by reverse transcription-PCR (RT-PCR; Figures 2a and b). The HIF-1α and target genes of Notch signaling pathway, such as Hes1, Hey1, and FABP7, were upregulated in U251SC. Interestingly, the Notch Delta ligand DLL1 also highly expressed in U251SC, whereas ADAM12, a metalloproteases, involved in shedding of DLL1 decreased.
Figure 2.
Elevated HIF-1α and activation of Notch pathway in U251SC. (a) Some differentially expressed genes found in microarray analysis were listed. (b) RT-PCR analysis for some differentially expressed genes. (c) Expression levels of protein in U251 and U251SC were analyzed by two-dimensional gel electrophoresis. (d) Some differently expressed proteins that are related to cancer and stem cell were listed. (e) Proteins indicated by red arrow, including FABP7, TPT1, ERP57 and HSPB1 were selected for validation by western blot assay. Data are from representative experiments repeated at least three times. The color reproduction of this figure is available at the Cell Death and Differentiation journal online.
For validation of the gene expression of microarray analysis, proteins in U251 and U251SC were assessed by 2-DE and mass spectrometry (Figure 2c). Here, we listed some differently expressed proteins related to cancer and stem cells (Figure 2d). Four proteins, namely FABP7, TPT1, ERP57, and HSPB1, were selected for validation by western blot assay (Figure 2e). The data showed that the FABP7, a downstream gene of the Notch signaling pathway, was highly expressed in U251SC, which displayed the same trend on the microarray. Taken together, we conclude that HIF-1α and Notch signal may have a role in the cell sphere formation of U251 (U251SC), which is a critical characteristic of GSC.
Hypoxia requires notch pathway to drive the maintenance of U251 cells
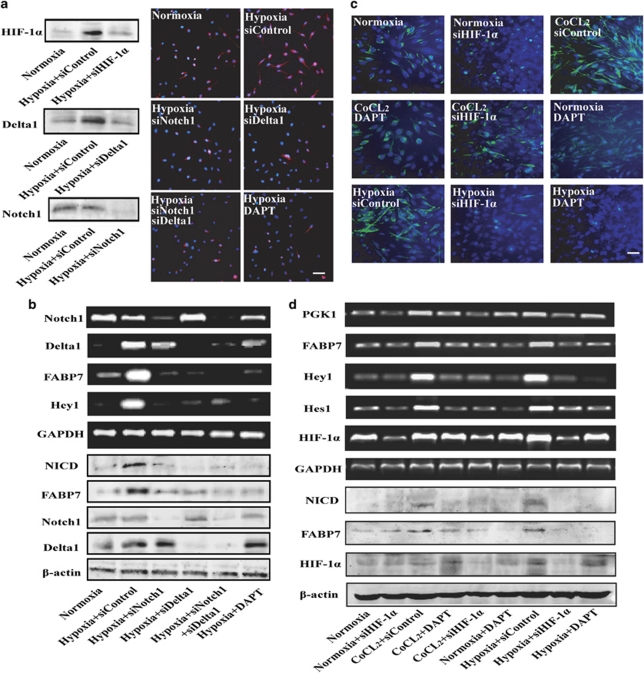
To test whether hypoxia-mediated maintenance of GSC requires Notch signaling, we first treated U251 cells in hypoxia with Notch1 siRNA and/or Delta 1 siRNA, and DAPT, a γ-secretase inhibitor, which inhibits Notch pathway, and then analyzed the expression of Nestin, which serves as a marker of GSC. When Delta 1 and/or Notch1 were knockdown by their respective siRNA, or pretreatment with DAPT, the percentage of Nestin-positive cells decreased obviously (Figure 3a). The mRNA level of Notch target genes including FABP7, Hey1, and the protein level of FABP7, NICD also decreased obviously (Figure 3b). To further clear the role of HIF-1α in U251 stem-like cell maintenance, we treated U251 cells with hypoxia or CoCl2, which could stabilize and activate the function of HIF-1α by inhibiting prolyl and asparaginyl hydroxylase activities involved in HIF-1α regulation,24 the number of Nestin-positive cells obviously increased. Pretreatment with HIF-1α siRNA or DAPT could dramatically reverse this trend (Figure 3c). Further data showed that pretreatment with HIF-1α siRNA or DAPT also abrogated the hypoxia-induced increase of colony-formation ability in U251 cells (Supplementary Figure S1).
Figure 3.
Hypoxia requires notch pathway to drive the maintenance of U251 cells. (a) Pretreatment with Notch1 siRNA, Delta1 siRNA, or γ-secretase inhibitor (DAPT; 20 μM) for 4 h abrogate the hypoxia-induced expansion of Nestin-positive cells. (b) U251 cells were treated with hypoxia for 24 h in the presence or absence of Notch1 siRNA, Delta1 siRNA, or DAPT (20 μM), the expression of Notch1, Delta1, and Notch target genes such as FABP7 and Hey1 were then analyzed by RT-PCR. The expression of NICD, Delta1, Notch1, and FABP7 proteins was analyzed by western blot assay after treated with hypoxia for 36 h. (c) Pretreatment with HIF-1α siRNA, or DAPT (20 μM) for 4 h abrogate the hypoxia and CoCl2 (100 μM)-induced expansion of Nestin-positive cells. Bar=50 μm. (d) U251 cells were treated with hypoxia and CoCl2 (100 μM) for 24 h in the presence or absence of HIF-1α siRNA and DAPT (20 μM), the expression of HIF-1α, PGK1, and Notch target genes such as Hey1, Hes1, FABP7 were then analyzed by RT-PCR. The expression of NICD, FABP7, and HIF-1α protein in U251 cells treated with hypoxia and CoCl2 (100 μM) for 36 h in the presence or absence of HIF-1α siRNA and DAPT (20 μM) was analyzed by western blot assay after treated with hypoxia for 36 h
Then we analyzed the mRNA levels of Hey1, Hes1, FABP7, and PGK1, a well know HIF-1α target gene, in U251 cells. The mRNA levels of these genes obviously increased in U251 cells cultured at hypoxia or supplemented with CoCl2 for 24 h, and this increase was dramatically abolished by DAPT and HIF-1α siRNA (Figure 3d). The protein levels of NICD, FABP7, and HIF-1α were also analyzed by western blot. After exposure to hypoxia or CoCl2 for 36 h, their protein levels dramatically increased. Treatment with HIF-1α siRNA obviously reversed this increase, whereas DAPT only reversed that of NICD and FABP7 (Figure 3d). In sum, these data suggest that hypoxia enhances the expression of HIF-1α, which results in increased activation of Notch downstream gene transcription and maintenance of U251 cells.
HIF-2α is not essential for hypoxia-mediated maintenance of U251 cells
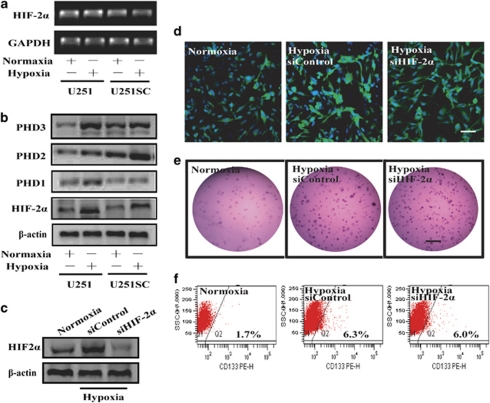
We then analyzed the role of HIF-2α in hypoxia-mediated maintenance of U251 cells. Here, our study suggested that HIF-1α, but not HIF-2α, preferentially expressed in U251SC cells, whereas hypoxia still induced the increase of HIF-2α at protein but not mRNA level in both U251 and U251SC cells (Figures 4a and b). Pretreatment with HIF-2α siRNA did not inhibit the hypoxia-mediated maintenance, as there was no obvious decrease of the number of Nestin-positive cells, CD133-positive cells, and colony-formation ability in HIF-2α siRNA-treated group (Figures 4c, d and e). The degradation of HIF-1α and HIF-2α protein was also investigated and the data showed increased expression of PHD2 and PHD3 in U251SC cells (Figure 4b).
Figure 4.
HIF-2α is not essential for hypoxia-mediated maintenance of U251 cells. (a) Expression of HIF-2α mRNA in U251 and U251SC either at normoxia or hypoxia for 24 h was evaluated by RT-PCR. (b) Expression of HIF-2α and PHD1/2/3 in U251 and U251SC either at normoxia or hypoxia for 36 h was evaluated. (c) Pretreatment with HIF-2α siRNA abrogate the hypoxia-induced increase of HIF-2α protein. (d) Pretreatment with HIF-2α siRNA could not abrogate the hypoxia-induced expansion of Nestin-positive cells. Bar=50 μm. (e) Pretreatment with HIF-2α siRNA could not abrogate the hypoxia-induced increase of colony-formation ability in U251 cells. The picture of colonies in a representative dish was taken with phase contrast microscopy. Bar=1 mm. (f) Representative FACS dot plot analysis for glioma cells showed that HIF-2α siRNA could not abrogate the hypoxia-induced increase in the number of CD133-positive cells. Cells exposed to hypoxia for 36 h were fixed and analyzed by FACS. Data are from representative experiments repeated at least three times
HIF-1α activates Notch signaling by interacting with and stabilizing NICD
The activation of a Notch response by hypoxia could conceivably involve a number of levels in the Notch signaling system. Previous study suggested a role for HIF-1α in the interaction, stabilization of NICD, and increase in Notch signaling.4 Here, the experiments about DAPT described above suggest that liberation of NICD is required for the hypoxic response in U251 cells. We then analyzed the expression of endogenous HIF-1α and NICD. As shown in Figure 5a, low levels of endogenous HIF-1α and NICD were observed in U251 and U251SC cells at normoxia. The protein levels of HIF-1α and NICD were substantially increased after exposure to hypoxia for 36 h, especially in U251SC cells. To further investigate whether HIF-1α physically interacts with NICD, the immunoprecipitation of HIF-1α was carried out and an interaction with NICD was revealed (Figure 5b). Furthermore, we showed that the Notch Delta ligand DLL1 and the Notch downstream genes, including Hey2 and Hes1, increased in U251 and U251SC cells exposed to hypoxia for 24 h, with a more dramatic increase in the U251SC cells. Notch transmembrane receptors, Notch1 and Notch3, ligand Jag1, as well as HIF-1α mRNA levels were not altered in hypoxia (Figure 5c). Taken together, these data establish that HIF-1α interacts with and stabilizes NICD, and activates the transcription of Notch downstream genes. Compared with U251 cells, the U251SC cells are more sensitive to hypoxia-induced activation of Notch signaling pathway.
Figure 5.
HIF-1α activates Notch signal by interacting with and stabilizing NICD. (a) The expression of endogenous HIF-1α and NICD in U251 and U251SC exposed to hypoxia was analyzed. (b) HIF-1α was immunoprecipitated and subjected to SDS-PAGE. (c) The expression of HIF-1α and some genes involved in Notch signaling in U251 and U251SC exposed to hypoxia was analyzed
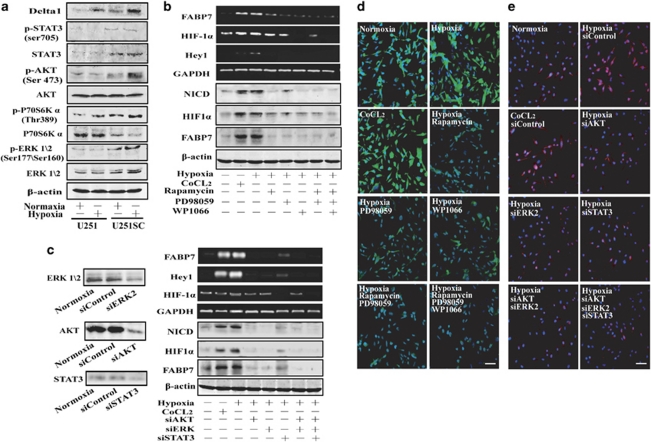
STAT3 is essential for the transcription of HIF-1α and subsequent maintenance of U251 cells
The results above suggest that the HIF-1α is essential for the hypoxia-induced activation of Notch pathway. High level of HIF-1α mRNA in U251SC is correlated with the sensitivity to hypoxia-mediated maintenance of GSC. Previous study demonstrated a requirement of STAT3 for HIF-1α mRNA expression under both hypoxia and growth signaling conditions.20 Here, we also found that the STAT3 mRNA level was elevated in U251SC cells. This finding prompts us to investigate whether STAT3 is required for hypoxia-mediated maintenance and whether U251SC is sensitive to hypoxia-induced activation of Notch signaling pathway. The results showed that the protein expression and phosphorylation level (Tyr 705) of STAT3 were elevated in U251SC cells (Figure 6a), whereas the phosphorylation at Ser 727 was not detected (data was not shown). Here, we found that pretreatment with WP1066 inactivates STAT3 by blocking its phosphorylation at Tyr705 and inhibits its nuclear translocation25 or STAT3 siRNA dramatically decreased the mRNA and protein expression of HIF-1α, FABP7, and Hey1 (Figures 6b and c). We also found that PI3K/AKT/mTOR and ERK/MAPK signaling pathways, which have an important role in the translation of HIF-1α protein, were preferentially activated in U251SC (Figure 6a). Pretreatment with mTOR inhibitor (rapamycin), ERK inhibitor (PD98059), or siRNA of these critical molecules abrogated the increase of the mRNA and protein levels of FABP7 and Hey1 induced by hypoxia, whereas the increase of HIF-1α is only altered at protein level (Figures 6b and c). The percentage of Nestin-positive cells decreased when any of the above-mentioned signaling pathways were inhibited by their specific inhibitors or siRNA (Figures 6d and e, respectively). Moreover, the colony-formation capability of U251 cell was weakened by these signal specific inhibitors (Supplementary Figure S1).
Figure 6.
Hypoxia requires PI3K/AKT, ERK, and STAT3 signaling to drive the maintenance of U251 cells. (a) PI3K/AKT, ERK/MAPK, and STAT3 signaling pathways were preferentially activated in U251SC. (b) U251 cells were treated with hypoxia and CoCl2 (100 μM) for 24 h in the presence or absence of rapamycin (10 μM), PD98059 (20 μM), and WP1066 (10 μM), PGK1 and Notch target genes such as Hey1, Hes1, FABP7 were then analyzed by RT-PCR. The expression levels of NICD, FABP7, and HIF-1α protein were analyzed by western blot assay after treated with hypoxia for 36 h. (c) U251 cells were treated with hypoxia and CoCl2 (100 μM) for 24 h in the presence or absence of siAKT, siERK2 and siSTAT3, PGK1 and Notch target genes such as Hey1, Hes1, FABP7 were then analyzed by RT-PCR. The expression levels of NICD, FABP7, and HIF-1α protein were analyzed by western blot assay after treated with hypoxia for 36 h. (d and e) Pretreatment with mTOR inhibitor (rapamycin; 10 μM), ERK inhibitor (PD98059; 20 μM), STAT3 inhibitor (WP1066; 10 μM), and indicated siRNA for 4 h abrogate the hypoxia and CoCl2 (100 μM) induced expansion of Nestin-positive cells. Bar=50 μm
All these results suggest that STAT3 preferentially expresses in U251SC cells, which may contribute to their sensitivity to hypoxia-induced activation of Notch signaling pathway and subsequent maintenance of GSC. PI3K/AKT and ERK pathways that are required for HIF-1α protein translation are also involved in this process.
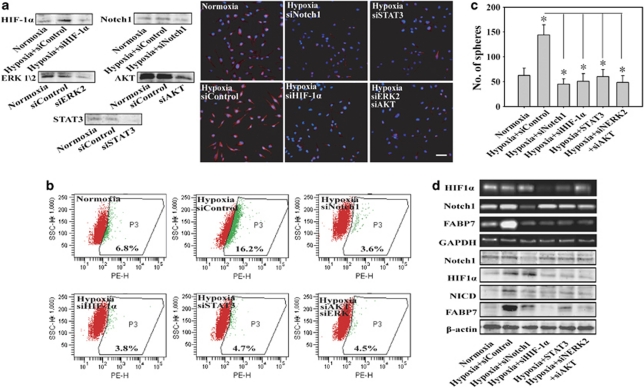
Hypoxia facilitates the maintenance of primary GSC in a HIF-1α and Notch signal-dependent manner
To test the effects of HIF-1α and Notch signal in hypoxia-mediated maintenance of primary GSC, we cultured primary glioblastoma tumor sphere cells for investigation. The percentage of Nestin, CD133-positive cells, and the number of nuerospheres increased when they were cultured in hypoxia and this increase was reversed by siRNA of HIF-1α, Notch1, STAT3, AKT, and ERK (Figures 7a, b and c). And the increase of FABP7, NICD induced by hypoxia was also eliminated (Figure 7d). There was no increase in Notch itself after hypoxia exposure and inhibition of HIF-1α with siHIF-1α has no effect on Notch1 expression (Figure 7d). The results indicate that HIF-1α and Notch signaling also have a critical role in the primary GSC maintenance mediated by hypoxia. Here, we also investigated this Notch-related phenomenon in SHG44 cell line, which seemed sensitive to hypoxia (Figure 1). The data showed that pretreatment with siNotch1 and siHIF-1α also abrogated the hypoxia-induced expansion of Nestin cells and number of nuerospheres (Supplementary Figure S2).
Figure 7.
Hypoxia facilitates the maintenance of primary glioblastoma stem cells in a HIF1α and Notch signal-dependent manner. (a, b and c). Compared with control, significance of difference is indicated as *P<0.05. Pretreatment with siRNA of Notch1, HIF-1α, ERK2, AKT, and STAT3 for 4 h abrogated the hypoxia-induced expansion of Nestin-positive cells, CD133-positive cells, and number of spheres. Bar=50 μm. (d) Cells were treated with hypoxia for 24 h in the presence or absence of indicated siRNA, the expression of Notch1, HIF-1α, and FABP7 mRNA were then analyzed by RT-PCR. The expression levels of Notch1, NICD, HIF-1α, and FABP7 protein were analyzed by western blot assay after treated with hypoxia for 36 h
Discussion
The hypoxic microenvironment maintains GSC and promotes reprogramming toward a stem cell phenotype. But the detailed mechanism underlying it is still unclear. Determining the crosstalk between hypoxia and GSC will enhance our understanding of tumorigenesis, GSC biology, and may provide new therapeutic strategy. Here, our findings suggest that GSC is more sensitive to hypoxia-mediated maintenance. HIF-1α may have an important role in this process by activating the Notch pathway and provide a candidate target for glioblastoma therapy.
Previous study showed that HIF-2α may be important in hypoxia-mediated maintenance of GSC as HIF-1α functioned in proliferation and survival of all cancer cells, but also is activated in normal neural progenitors while HIF-2α is essential in only in GSC and is not expressed by normal neural progenitors.6, 7, 26, 27 The mechanism involved maybe that HIF-2α could induce the upregulation of some stem cell factors such as OCT4 and c-MYC.28, 29 Recent study found that HIF-2α binds directly to NICD and that the responsible site for this interaction is within the RAM domain of Notch protein.27, 30 The data from our and other group also showed that HIF-1α enhances the self-renewal activity of CD133-positive glioblastoma cells and inhibits the induction of GSC differentiation.8, 31, 32 These data suggest that the mechanism involved in hypoxia-mediated maintenance of GSC maybe cell type specific. Besides, different hypoxic condition may also cause different response.6, 8, 27 Normal oxygen tension in healthy tissue is ∼7% oxygen (53 mm Hg), this tension in a tumor can range from physiological (7%) to severe (<2%) oxygen. HIF-2α is stabilized at a wider range of oxygen tensions, ranging from severe hypoxia (<2% oxygen) to more physiologically relevant tension for tumors (2–5% oxygen), whereas HIF-1α is only stabilized at severe hypoxia condition(<2% oxygen).17, 26, 33
In this study, we show that HIF-1α may have an important role in hypoxia-mediated maintenance of GSC partly for its interaction with NICD. Besides, we also found that DLL1, a Notch Delta ligand, highly expresses in U251SC both at mRNA and protein level. Hypoxia induces the increase of DLL1, especially in U251SC. Previous study also showed that hypoxia upregulates the mRNA and protein level of DLL1, although the mechanism involved is still unclear.34 All the data suggested that hypoxia not only cooperate with preexisting Notch signaling, but also induce Notch signaling by increasing the level of Notch ligands. But the role HIF-1α played in this process and whether DLL1 is required for hypoxia-mediated maintenance of GSC need further investigation.
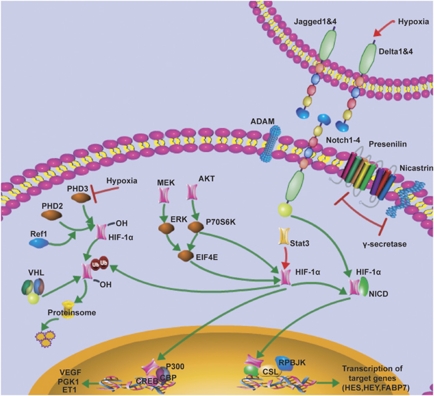
The results from this study show increased expression of PHD2 and PHD3 in U251SC cells. Although HIF-1α protein is preferentially expressed in U251SC cells exposed to hypoxia. The reason may be that the activation of PHDs needs the participation of oxygen. The absence of oxygen causes no enzyme activity, no modification of proline, and no pVHL/HIF binding, resulting in HIF-1α stabilization and accumulation.35 All the data illustrate the crosstalk between hypoxia and Notch pathway, providing us a new sight about hypoxia-mediated maintenance of GSC (Figure 8).
Figure 8.
Schematic depiction of crosstalk between Notch and HIF-1α. (1) The canonical hypoxic signaling leads to the inhibition of HIF-1α degradation mediated by PHD1/2/3. STAT3 could bind to the HIF-1α promoter and stimulates its transcription, whereas PI3K/AKT, ERK were contributed to the translation of HIF-1α protein through phosphorylation of the eukaryotic translation initiation factor 4E (eIF-4E) binding protein (4E-BP1) and p70 S6 kinase (S6K). Elevation of HIF-1α protein induces the activation of downstream genes, such as VEGF, PGK1, and ET1, through binding to HRE elements in the corresponding promoters. (2) After binding to ligand, the Notch receptor undergoes two proteolytic cleavages and then releases the intracellular domain of NICD, which translocates to the nucleus and associates with the CSL transcription factor and coactivators such as RPBJK, and regulates the expression of target genes such as Hey and FABP7. (3) HIF-1α and NICD form a point of convergence between the two signaling, leading to stabilization of NICD, recruitment of HIF-1α to Notch-responsive promoters, and activation of Notch downstream genes. HIF-1α could also induce the elevation of DLL1, which may contribute to HIF-1α-mediated activation of Notch pathway. 1, the left part; 2, the right part; 3, the central part
It has been reported that tumor spheres derived from glioblastoma cells highly expressed neural stem cell markers, Nestin and Sox2, showing a verapamil-sensitive side population, relative resistance to doxorubicin and increased tumorigenicity.9, 36, 37 These studies suggest that tumor sphere cells maybe a convenient and useful model for cancer stem cells research, although not every cell within tumor spheres is cancer stem cell. Here, we take U251SC, a U251 derived tumor sphere cells, as a GSC model. Although it is not pure, but it still provides us some useful information.
FABP7 is a member of a large family of fatty acid-binding proteins and highly expressed in immature astrocytes and neuronal cell precursors.38 Previous study showed that FABP7 is a direct target of Notch signaling and may mediate some aspects of glial responses to Notch.39, 40 The FABP7-expressed glial cells have been proposed to be the malignant glioblastoma cell of origin and be associated with the regions of glioblastoma infiltration, reduced survival, and recurrence.41 Recent study showed that FABP7 preferentially expresses in CD133-positive glioblastoma cells.42, 43 Here, we also found that FABP7 is highly expressed in U251 sphere cells and respond to hypoxia-induced activation of Notch signaling. What remains to be explored is whether FABP7 serves as a marker for GSC and its role in the hypoxia-mediated maintenance.
Our findings indicate that HIF-1α-induced activation of Notch pathway is essential for hypoxia-mediated maintenance of GSC. Activation of HIF-1α gene transcription, increased HIF-1α protein translation and subsequent activation of Notch signaling contribute to this process. Our findings suggest that HIF-1α may serve as a promising target for glioblastoma therapy.
Materials and Methods
Cell culture
Rat C6 and human U251, A172, U87MG glioblastoma cell lines were purchased from Cell Bank of Shanghai Institute of Biochemistry and Cell Biology, Shanghai Institutes for Biological Sciences, Chinese Academy of Sciences, Beijing, China. C6, U251, A172 were cultured in DMEM, U87MG were cultured in EMEM, all of them were supplemented with 10% (v/v) fetal calf serum, 100 U/ml penicillin, and 100 U/ml streptomycin in a 5% CO2 atmosphere. The serum-free medium (SFM) was composed of Dulbecco's modified Eagle's medium/Ham's F-12 medium, 20 ng/ml basic fibroblast growth factor (bFGF; Peprotech, Rocky Hill, NJ, USA), 20 ng/ml epidermal growth factor (Peprotech), and 20 μl/ml B27 supplement (Life Technologies, Carlsbad, CA, USA). U251-derived stem-like tumor sphere cells (U251SC) were derived by placing the U251 glioblastoma cells grown as monolayers into SFM. Cells were supplemented every 2 days by adding fresh SFM. After primary tumor spheres formed and reached ∼50–100 cells per sphere, cells were harvested, dissociated into single cells using 0.1% trypsin and 1 × EDTA (Gibco-BRL, Gaithersburg, MD, USA) at 37 °C for 10 min, and triturated with a P200 pipette. Subsequently, they were plated at a clonal density of 10000 cells per milliliter in SFM. Cells were supplemented every 2 days by adding fresh SFM and subsequent tumor spheres were observed daily for up to 9 days and then passaged again into fresh medium. After two passages, the U251SC were harvested for the further analysis.
Primary tumor sphere culture was performed as described previously.3 Glioblastoma Multiforme (GBM) specimens (Degree 4) were obtained from consenting patients, as approved by the Research Ethics Board at the Nanjing Brain Hospital Affiliated to Nanjing Medical University. For hypoxia, cells were cultured in HERAcell incubators (Thermo Electronic Corporation, Asheville, NC, USA) at 37 °C, 95% relative humidity, and 5% CO2 with 2% oxygen conditions.
Materials
DAPI (diamidino-phenyl-indole) was obtained from Santa Cruz Biotechnology Inc. (Santa Cruz, CA, USA). Primary antibodies against Nestin, GFAP, AKT, P-AKT, ERK, P-ERK, P70S6K, P-P70S6K, TPT1, ERP57, HSPB1, and β-actin were obtained from Santa Cruz Biotechnology Inc. Primary antibodies against HIF-1α, HIF-2α, NICD, and FABP7 were obtained from Cell Signaling Technology (Beverly, MA, USA) and Abcam (Cambridge, England). IRDye800-conjugated anti-goat and anti-rabbit second antibodies were obtained from Rockland Inc. (Gilbertsville, PA, USA). Notch1 siRNA, Delta1 siRNA, HIF-1α siRNA, HIF-2α siRNA, AKT siRNA, ERK2 siRNA, STAT3 siRNA, γ-secretase inhibitor DAPT, ERK inhibitor PD98059, AKT inhibitor rapamycin, and STAT3 inhibitor WP1066 were obtained from Santa Cruz Biotechnology Inc. Alexa Fluor 555 F (ab') 2 fragments of goat anti-mouse IgGs were obtained from Invitrogen (Carlsbad, CA, USA). Goat anti-mouse IgG-FITC were obtained from Zhongshan Golden Bridge Biotechnology (Beijing, China).
RT-PCR assay
Total cellular RNA was extracted using the TriPure Solution following the manufacture's instructions. The purity of the RNA extracted was determined by the ratio of A260/A280 using a BioPhotometer (Eppendorf, Hilden, Germany). RT-PCR was performed following the protocol supplied with TaKaRa kit (Shiga, Japan). The amplified PCR products were separated by electrophoresis on a 2% agarose gel containing ethidium bromide and quantitated by relative intensities of the bands as compared with those of glyceraldehyde-3-phosphate dehydrogenase using Gel Base/Gel Blot/Gel Excel/Gel Sequence analysis software (UVP, Cambridge, UK). A panel of PCR primers specific to the 20 representative genes (Supplementary Table 1) that were upregulated and downregulated were synthesized by Invitrogen (Shanghai, China).
cDNA expression array
Human genome oligo array (22K) was designed by Capital-Bio Corporation (Beijing, China). Capital-Bio 22K Human Genom Oligo Array comprises 21,522 70-mer oligo probes, each representing one transcript of the human genome. RNA samples as prepared above were labeled with Cy5 (red) and Cy3 (green) dyes, respectively. Labeled samples were then hybridized to array gene chips. Arrays were scanned using Capital-Bio's confocal scanner LuxScan 10K-A (Capital-Bio) and the obtained images were analyzed with SpotData software (Capital-Bio). An intensity-dependent lowess program in the R language package was used to normalize the two channel ratio values.
Side population analysis
The cells were removed from the culture dish with trypsin and EDTA, washed, suspended at 106 cells per ml in DMEM containing 2% FCS (staining medium), and preincubated in a 1.5-ml Eppendorf tube at 37 °C for 10 min. The cells were then labeled in the same medium at 37 °C for 90 min with 2.5 μg/ml Hoechst 33342 dye (Molecular Probes, Eugene, OR, USA), either alone or in combination with 50 μM verapamil (Sigma, St. Louis, MO, USA), which is an inhibitor of some (verapamil-sensitive) ABC transporters. Finally, the cells were counterstained with 1 μg/ml propidium iodide to label dead cells. Then, 105 cells were analyzed in a FACSVantage fluorescence-activated cell sorter (Becton Dickinson, East Rutherford, NJ, USA) by using a dual-wavelength analysis (blue, 424–444 nm; red, 675 nm) after excitation with 350 nm UV light.23
siRNA analysis
Specific knockdown was achieved using siRNAs against HIF-1α, HIF-2α, Notch1, Delta1, AKT, STAT3, and ERK2 or a control siRNA (all from from Santa Cruz Biotechnology Inc.). Transfection was performed using HiPerfect reagent (Qiagen, Hilden, Germany) as directed. Six hours after transfection, media were changed and cells were placed under hypoxia for indicated time (for expression analysis). Cells were harvested for protein and RNA analyses as described above.
2D gel electrophoresis analysis and protein identification by mass spectrometry
Purified proteins were precipitated using the Ettan 2-D clean up kit (GE Healthcare, Bucks, UK) were subsequently resuspended in urea buffer (7 M urea, 2 M thiourea, 2% Chaps, 1% sulfobetaine SB3-10, 1% amidosulfobetaine ASB14, 50 mM DTT). For the first dimension of protein separation, isoelectric focusing was performed using 18-cm immobilized nonlinear pH gradient strips (pH 3 to 10; GE Healthcare) on a IPGphor II electrophoresis unit (GE Healthcare). Proteins (100 μg) were loaded by in-gel rehydratation for 9 h, using low voltage (30 V) then run using a program in which the voltage was set for 1 h at 100 V, 2 h at 200 V, 1 h at 500 V, 1 h at 1,000 V, 2 h, 2 h voltage gradient 1000–8000 V and 4 h at 8000 V. Before the second-dimension electrophoresis, IPG gel strips were equilibrated for 10 min at room temperature in 1% dithiothreitol to reduce the proteins and sulfhydryl groups were subsequently derivatized using 4% iodoacetamide (both 1% dithiothreitol and 4% iodoacetamide were prepared in 50 m Tris (pH 8.8) M urea-30% glycerol-2% SDS-2% bromophenol blue). Strips were transferred to 1.0-mm-thick 10% (wt/vol) polyacrylamide gels (20 × 20 cm2), and the second-dimension gels were run at 50 μA for 6 h. Gels were stained with Sypro Ruby (Bio-Rad, Berkeley, CA, USA) and visualized using a Typhoon 9200 scanner (GE Healthcare). The Investigator HT analyser (Genomic Solutions Inc., Holliston, MA, USA) was used for matching and analysis of visualized protein spots among differential gels. Background subtraction was used to normalize the intensity value representing the amount of protein per spot.
Differentially expressed spots were excised from the gels with an automatic spot picker (Investigator ProPic, Genomic Solutions Inc.), placed in Eppendorf tubes, and destained by washing for 5 min with 50 μl of 0.1 M NH4HCO3. Then 50 μl of 100% acetonitrile were added incubated for other 5 min. The liquid was discarded, the washing steps were repeated one more time and gel plugs were shrunk by addition of pure acetonitrile. The dried gel pieces were reswollen with 4.0 ng/μl trypsin (Promega, Madison, WI, USA) in 50 m NH4HCO3 and digested overnight at 37 °C. Peptides were concentrated with ZipTipμC18 pipette tips. Co-elution was performed directly onto a MALDI target with 1 μl of α-cyano-4-hydroxycinnamic acid matrix (5 μg/ml in 50% acetonitrile, 0.1% TFA). MALDI-MS and MALDI-MS/MS were performed on an Applied Biosystems 4700 Proteomics Analyzer (Applied Biosystems, Carlsbad, CA, USA) with TOF/TOF ion optics. Spectra were acquired in positive MS reflector mode and calibrated either externally using five peaks of standard by using ABI4700 Calibration Mixture or internally using porcine trypsin autolysis peptide peaks (842.51, 1045.56 and 2211.10 [M+H]+ ions). Mass spectra were obtained from each sample spot by 30 sub-spectra accumulation (each consisting of 50 laser shots) in a 750–4000 mass range. Five signal-to-noise best peaks of each spectrum were selected for MS/MS analysis. For MS/MS spectra, the collision energy was 1 keV and the collision gas was air.
MS and MS/MS data were interpreted using the GPS Explorer software (Version 2.1, Applied Biosystems), which acts as an interface between the Oracle database containing raw spectra and a local copy of the MASCOT search engine (Version 1.8, Matrix Science Inc., Boston, MA, USA). Peptide mass fingerprints obtained from MS analysis were used for protein identification in Swiss Prot non-redundant database. All peptide mass values are considered monoisotopic and mass tolerance was set <50 p.p.m. Trypsin was given as the digestion enzyme, one missed cleavage site was allowed, methionine was assumed to be partially oxidized, and serine, threonine and tyrosine were partially phosphorylated. MASCOT (Matrix Science) scores >71 were considered to be significant (P<0.005). For MS/MS analysis, all peaks with a signal-to-noise ratio >5 were searched against the Swiss Prot database using the same modifications as the MS database. Fragment tolerance <0.3 Da was considered.
Flow cytometry
Cells were centrifuged at 300 g for 10 min, triturated with a fire-narrowed Pasteur pipette, and resuspended in 0.35 ml. PBS with 0.5% BSA and 2 mM EDTA. Then 10 μl of CD133–2-Phycoerythrin (Phycoerythrin-conjugated mouse monoclonal IgG2b; Miltenyi Biotec, Auburn, CA, USA) or isotype control antibody (Miltenyi Biotec) and 10 μl FcR Blocking Reagent (Miltenyi Biotec) was added for an additional 20 min. The ratio of CD133cells was evaluated by flow cytometry with a FACSCalibur machine (BD Biosciences, East Rutherford, NJ, USA).
Immunocytochemistry
Immunostaining was done as described previously.3, 23 Briefly, the cells attached to the coverslips were washed three times with PBS and fixed with 4% paraformaldehyde in PBS solution for 25 min. The cells were then permeabilized with cold 0.5% Triton-X-100 in PBS for 20 min at 4 °C and washed with cold PBS. PBS supplemented with 3% BSA was used as a blocking solution (30 min, 37 °C). After removal of the blocking solution, the cells were incubated at 4 °C overnight in primary mouse anti-Nestin (human) monoclonal antibody (1:200; Santa Cruz Biotechnology Inc.) and mouse anti-GFAP (human) monoclonal antibody (1:200; Santa Cruz Biotechnology Inc.). After washing with PBS, the cells were incubated at 37 °C for 1 h with FITC-labeled secondary goat anti-mouse IgG antibodies or Alexa Fluor 555 F (ab') 2 fragments of goat anti-mouse IgG antibodies, respectively. The cells were then stained with fluorochrome dye DAPI (Santa Cruz Biotechnology Inc.) to visualize the cell nuclei, and observed under a fluorescence microscopy (Olympus, Tokyo, Japan) with a peak excitation wave length of 340 nm examined under a microscope.
Colony-formation assay
Cells were plated in 35-mm dishes at 5000 cells/well in 0.35% agar in DMEM culture medium over a 0.5% agar layer. Plates were further incubated in incubator for 21 days until colonies were large enough to be visualized. Colonies were stained with 0.01% Crystal violet for 1 h and counted under inverted microscope. Experiments were done in triplicate.
Sphere-forming assay
U251 cells were cultured in SFM using a 24-well plate format, 2000 cells were seeded per plate in 2 ml medium, and the number of spheres was counted after 7 days. The picture of cells in a representative well was taken with phase contrast microscopy. Experiments were done in triplicate.
Western blots and imnunoprecipitations
U251 sphere cells and U251 monolayer cells were harvested and the proteins were isolated by lysis buffer (100 mM Tris-Cl, pH 6.8, 4% (m/v) SDS, 20% (v/v) glycerol, 200 mM β-mercaptoethanol, 1 mM PMSF, and 1 g/ml aprotinin) and measured using the BCA protein assay method with Varioskan spectrofluorometer and spectrophotometer (Thermo Scientific, Sugar Land, TX, USA) at 562 nm. Protein samples were separated with 15% SDS-polyacrylamide gel (SDS-PAGE) and transferred onto the PVDF membranes (Millipore, Billerica, MA, USA). Immune complexes were formed by incubation of the proteins with primary antibody overnight at 4 °C. Blots were washed and incubated for 1 h with IRDye800-conjugated second antibodies. Immunoreactive protein bands were detected with an Odyssey Scanning System (LI-COR Inc., Lincoln, NE, USA) For immunoprecipitations, the samples were lysed as previously described. To remove nonspecifically binding proteins, 150–200 μg of proteins were precleared by mixing with agarose beads (A/G plus; Santa Cruz Biotechnology Inc.) for 30 min at 4 °C. The samples were then incubated with the adequate antibody in the presence of fresh agarose A/G beads, either at 4 °C overnight or at room temperature for 2 h on a rotating platform. The beads were then washed and boiled for 10 min in Laemmli-loading buffer. Equal amounts (as measured by protein assay) were loaded on 10% SDS-PAGE and processed for immunoblotting as previously described.
Statistical evaluation
Statistical analyses were performed using an unpaired, two-tailed Student's t-test. All comparisons are made relative to untreated controls and significance of difference is indicated as *P<0.05, **P<0.01. All quantitative data presented are the mean±S.D. from at least three samples per data point.
Acknowledgments
This work was supported by the International Cooperation Program of China (No. 2008DFA32120), the National Natural Science Foundation of China (Nos. 30701032, 30472044, 90713038 and 91029744), the Natural Science Foundation of Jiangsu Province (No. BK2009297, BK2009298), and the Science and Technology Development Program supported by the division of Science and Technology, Jiangsu (No. BE2009674).
Glossary
- U251SC
U251-derived stem-like tumor sphere cells
- HIF-1α
hypoxia-induced factor 1α
- GSC
glioblastoma stem cell
- NICD
intracellular domain of Notch
- 4E-BP1
the eukaryotic translation initiation factor 4E-binding protein
- S6K
p70 S6 kinase
- PI3K
phosphatidylinositol 3-kinase
- MAPK
mitogen-activated protein kinase
The authors declare no conflict of interest.
Footnotes
Supplementary Information accompanies the paper on Cell Death and Differentiation website (http://www.nature.com/cdd)
Edited by W El-Deiry
Supplementary Material
References
- Singh SK, Hawkins C, Clarke ID, Squire JA, Bayani J, Hide T, et al. Identification of human brain tumour initiating cells. Nature. 2004;432:396–401. doi: 10.1038/nature03128. [DOI] [PubMed] [Google Scholar]
- Galli R, Binda E, Orfanelli U, Cipelletti B, Gritti A, De Vitis S, et al. Isolation and characterization of tumorigenic, stem-like neural precursors from human glioblastoma. Cancer Res. 2004;64:7011–7021. doi: 10.1158/0008-5472.CAN-04-1364. [DOI] [PubMed] [Google Scholar]
- Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res. 2003;63:5821–5828. [PubMed] [Google Scholar]
- Gustafsson MV, Zheng X, Pereira T, Gradin K, Jin S, Lundkvist J, et al. Hypoxia requires notch signaling to maintain the undifferentiated cell state. Dev cell. 2005;9:617–628. doi: 10.1016/j.devcel.2005.09.010. [DOI] [PubMed] [Google Scholar]
- Chen HL, Pistollato F, Hoeppner DJ, Ni HT, McKay RD, Panchision DM. Oxygen tension regulates survival and fate of mouse central nervous system precursors at multiple levels. Stem Cells. 2007;25:2291–2301. doi: 10.1634/stemcells.2006-0609. [DOI] [PubMed] [Google Scholar]
- Li Z, Bao S, Wu Q, Wang H, Eyler C, Sathornsumetee S, et al. Hypoxia-inducible factors regulate tumorigenic capacity of glioma stem cells. Cancer Cell. 2009;15:501–513. doi: 10.1016/j.ccr.2009.03.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddleston JM, Li Z, McLendon RE, Hjelmeland AB, Rich JN. The hypoxic microenvironment maintains glioblastoma stem cells and promotes reprogramming towards a cancer stem cell phenotype. Cell Cycle. 2009;8:3274–3284. doi: 10.4161/cc.8.20.9701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Soeda A, Park M, Lee D, Mintz A, Androutsellis-Theotokis A, McKay RD, et al. Hypoxia promotes expansion of the CD133-positive glioma stem cells through activation of HIF-1alpha. Oncogene. 2009;28:3949–3959. doi: 10.1038/onc.2009.252. [DOI] [PubMed] [Google Scholar]
- Qiang L, Yang Y, Ma YJ, Chen FH, Zhang LB, Liu W, et al. Isolation and characterization of cancer stem like cells in human glioblastoma cell lines. Cancer Lett. 2009;279:13–21. doi: 10.1016/j.canlet.2009.01.016. [DOI] [PubMed] [Google Scholar]
- Artavanis-Tsakonas S, Rand MD, Lake RJ. Notch signaling: cell fate control and signal integration in development. Science. 1999;284:770–776. doi: 10.1126/science.284.5415.770. [DOI] [PubMed] [Google Scholar]
- Hitoshi S, Alexson T, Tropepe V, Donoviel D, Elia AJ, Nye JS, et al. Notch pathway molecules are essential for the maintenance, but not the generation, of mammalian neural stem cells. Genes Dev. 2002;16:846–858. doi: 10.1101/gad.975202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bedogni B, Warneke JA, Nickoloff BJ, Giaccia AJ, Powell MB. Notch1 is an effector of Akt and hypoxia in melanoma development. J Clin Invest. 2008;118:3660–3670. doi: 10.1172/JCI36157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3:721–732. doi: 10.1038/nrc1187. [DOI] [PubMed] [Google Scholar]
- Gerasimovskaya EV, Tucker DA, Stenmark KR. Activation of phosphatidylinositol 3-kinase, Akt, and mammalian target of rapamycin is necessary for hypoxia-induced pulmonary artery adventitial fibroblast proliferation. J Appl Physiol. 2005;98:722–731. doi: 10.1152/japplphysiol.00715.2004. [DOI] [PubMed] [Google Scholar]
- Treins C, Giorgetti-Peraldi S, Murdaca J, Monthouel-Kartmann MN, Van Obberghen E. Regulation of hypoxia-inducible factor (HIF)-1 activity and expression of HIF hydroxylases in response to insulin-like growth factor I. Mol Endocrinol. 2005;19:1304–1317. doi: 10.1210/me.2004-0239. [DOI] [PubMed] [Google Scholar]
- Gorlach A. Regulation of HIF-1alpha at the transcriptional level. Curr Pharm Des. 2009;15:3844–3852. doi: 10.2174/138161209789649420. [DOI] [PubMed] [Google Scholar]
- Busca R, Berra E, Gaggioli C, Khaled M, Bille K, Marchetti B, et al. Hypoxia-inducible factor 1{alpha} is a new target of microphthalmia-associated transcription factor (MITF) in melanoma cells. J Cell Biol. 2005;170:49–59. doi: 10.1083/jcb.200501067. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonello S, Zahringer C, BelAiba RS, Djordjevic T, Hess J, Michiels C, et al. Reactive oxygen species activate the HIF-1alpha promoter via a functional NFkappaB site. Arterioscler Thromb Vasc Biol. 2007;27:755–761. doi: 10.1161/01.ATV.0000258979.92828.bc. [DOI] [PubMed] [Google Scholar]
- Sperandio S, Fortin J, Sasik R, Robitaille L, Corbeil J, de Belle I. The transcription factor Egr1 regulates the HIF-1alpha gene during hypoxia. Mol Carcinog. 2009;48:38–44. doi: 10.1002/mc.20454. [DOI] [PubMed] [Google Scholar]
- Niu G, Briggs J, Deng J, Ma Y, Lee H, Kortylewski M, et al. Signal transducer and activator of transcription 3 is required for hypoxia-inducible factor-1alpha RNA expression in both tumor cells and tumor-associated myeloid cells. Mol Cancer Res. 2008;6:1099–1105. doi: 10.1158/1541-7786.MCR-07-2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherry MM, Reeves A, Wu JK, Cochran BH. STAT3 is required for proliferation and maintenance of multipotency in glioblastoma stem cells. Stem Cells. 2009;27:2383–2392. doi: 10.1002/stem.185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung JE, Lee HG, Cho IH, Chung DH, Yoon SH, Yang YM, et al. STAT3 is a potential modulator of HIF-1-mediated VEGF expression in human renal carcinoma cells. FASEB J. 2005;19:1296–1298. doi: 10.1096/fj.04-3099fje. [DOI] [PubMed] [Google Scholar]
- Kondo T, Setoguchi T, Taga T. Persistence of a small subpopulation of cancer stem-like cells in the C6 glioma cell line. Proc Natl Acad Sci USA. 2004;101:781–786. doi: 10.1073/pnas.0307618100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Epstein AC, Gleadle JM, McNeill LA, Hewitson KS, O'Rourke J, Mole DR, et al. C elegans EGL-9 and mammalian homologs define a family of dioxygenases that regulate HIF by prolyl hydroxylation. Cell. 2001;107:43–54. doi: 10.1016/s0092-8674(01)00507-4. [DOI] [PubMed] [Google Scholar]
- Horiguchi A, Asano T, Kuroda K, Sato A, Asakuma J, Ito K, et al. STAT3 inhibitor WP1066 as a novel therapeutic agent for renal cell carcinoma. Br J Cancer. 2010;102:1592–1599. doi: 10.1038/sj.bjc.6605691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heddleston JM, Li Z, Lathia JD, Bao S, Hjelmeland AB, Rich JN. Hypoxia inducible factors in cancer stem cells. Br J Cancer. 2010;102:789–795. doi: 10.1038/sj.bjc.6605551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pietras A, Hansford LM, Johnsson AS, Bridges E, Sjolund J, Gisselsson D, et al. HIF-2alpha maintains an undifferentiated state in neural crest-like human neuroblastoma tumor-initiating cells. Proc Natl Acad Sci USA. 2009;106:16805–16810. doi: 10.1073/pnas.0904606106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gordan JD, Bertout JA, Hu CJ, Diehl JA, Simon MC. HIF-2alpha promotes hypoxic cell proliferation by enhancing c-myc transcriptional activity. Cancer Cell. 2007;11:335–347. doi: 10.1016/j.ccr.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Covello KL, Kehler J, Yu H, Gordan JD, Arsham AM, Hu CJ, et al. HIF-2alpha regulates Oct-4: effects of hypoxia on stem cell function, embryonic development, and tumor growth. Genes Dev. 2006;20:557–570. doi: 10.1101/gad.1399906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H, Houshmand G, Mishra S, Fong GH, Gittes GK, Esni F. Impaired pancreatic development in Hif2-alpha deficient mice. Biochem Biophys Res Commun. 2010;399:440–445. doi: 10.1016/j.bbrc.2010.07.111. [DOI] [PubMed] [Google Scholar]
- Mendez O, Zavadil J, Esencay M, Lukyanov Y, Santovasi D, Wang SC, et al. Knock down of HIF-1alpha in glioma cells reduces migration in vitro and invasion in vivo and impairs their ability to form tumor spheres. Mol cancer. 2010;9:133. doi: 10.1186/1476-4598-9-133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pistollato F, Rampazzo E, Persano L, Abbadi S, Frasson C, Denaro L, et al. Interaction of HIF1alpha and Notch signaling regulates medulloblastoma precursor proliferation and fate. Stem Cells. 2010;28:1918–1929. doi: 10.1002/stem.518. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kanichai M, Ferguson D, Prendergast PJ, Campbell VA. Hypoxia promotes chondrogenesis in rat mesenchymal stem cells: a role for AKT and hypoxia-inducible factor (HIF)-1alpha. J cell phys. 2008;216:708–715. doi: 10.1002/jcp.21446. [DOI] [PubMed] [Google Scholar]
- Sahlgren C, Gustafsson MV, Jin S, Poellinger L, Lendahl U. Notch signaling mediates hypoxia-induced tumor cell migration and invasion. Proc Natl Acad Sci USA. 2008;105:6392–6397. doi: 10.1073/pnas.0802047105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ke Q, Costa M. Hypoxia-inducible factor-1 (HIF-1) Mol Pharmacol. 2006;70:1469–1480. doi: 10.1124/mol.106.027029. [DOI] [PubMed] [Google Scholar]
- Ghods AJ, Irvin D, Liu G, Yuan X, Abdulkadir IR, Tunici P, et al. Spheres isolated from 9L gliosarcoma rat cell line possess chemoresistant and aggressive cancer stem-like cells. Stem Cells. 2007;25:1645–1653. doi: 10.1634/stemcells.2006-0624. [DOI] [PubMed] [Google Scholar]
- Mahller YY, Williams JP, Baird WH, Mitton B, Grossheim J, Saeki Y, et al. Neuroblastoma cell lines contain pluripotent tumor initiating cells that are susceptible to a targeted oncolytic virus. PLoS One. 2009;4:e4235. doi: 10.1371/journal.pone.0004235. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gerstner JR, Bremer QZ, Vander Heyden WM, Lavaute TM, Yin JC, Landry CF. Brain fatty acid binding protein (Fabp7) is diurnally regulated in astrocytes and hippocampal granule cell precursors in adult rodent brain. PLoS One. 2008;3:e1631. doi: 10.1371/journal.pone.0001631. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anthony TE, Mason HA, Gridley T, Fishell G, Heintz N. Brain lipid-binding protein is a direct target of Notch signaling in radial glial cells. Genes Dev. 2005;19:1028–1033. doi: 10.1101/gad.1302105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keilani S, Sugaya K. Reelin induces a radial glial phenotype in human neural progenitor cells by activation of Notch-1. BMC Dev Biol. 2008;8:69. doi: 10.1186/1471-213X-8-69. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mita R, Coles JE, Glubrecht DD, Sung R, Sun X, Godbout R. B-FABP-expressing radial glial cells: the malignant glioma cell of origin. Neoplasia. 2007;9:734–744. doi: 10.1593/neo.07439. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu Q, Nguyen DH, Dong Q, Shitaku P, Chung K, Liu OY, et al. Molecular properties of CD133+ glioblastoma stem cells derived from treatment-refractory recurrent brain tumors. J Neurooncol. 2009;94:1–19. doi: 10.1007/s11060-009-9919-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Campos B, Herold-Mende CC. Insight into the complex regulation of CD133 in glioma. Int J Cancer. 2010;128:501–510. doi: 10.1002/ijc.25687. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.