Abstract
Background
The Drug Burden Index (DBI), a measure of exposure to anticholinergic and sedative medications, has been independently associated with physical and cognitive function in a cross-sectional analysis of community dwelling older persons participating in the Health, Aging and Body Composition (Health ABC) study. Here we evaluate the association between DBI and functional outcomes in Health ABC participants over five years.
Methods
DBI was calculated at years 1 (baseline), 3 and 5 and a measure of the area under the curve for DBI (AUCDB) over the whole study period was devised and calculated. Physical performance was measured using the short physical performance battery (SPPB), usual gait speed, and grip strength. The association of DBI at each time point and AUCDB with year 6 function was analyzed in data from participants with longitudinal functional measures, controlling for socio-demographics, co-morbidities and baseline function.
Results
Higher DBI at years 1, 3 and 5 was consistently associated with poorer function at year 6. On multivariate analysis, a one unit increase in AUCDB predicted decreases in SPPB score of 0.08 (p = 0.01), gait speed of 0.01 m/s (p=0.004), and grip strength of 0.27 kg (p=0.004) at year 6.
Conclusion
Increasing exposure to medication with anticholinergic and sedative effects, measured with DBI, is associated with lower objective physical function over five years in community dwelling older people.
Introduction
Prescribing for older people requires careful assessment of the benefits and risks of all of the person's medications. With advancing age comes increasing disease prevalence, medication use, and risk of adverse drug reactions.1-3 Several studies have shown associations between exposure to certain classes of medications, particularly those with sedative and anticholinergic actions, and physical and mental function in older people.4-6 Although optimizing overall function is one of the most important therapeutic aims for many older patients7, limited evidence exists regarding the effects of medication on function in older people, particularly frail older people with multiple comorbidities.8,9
We recently developed the Drug Burden Index (DBI), a measure of overall exposure to medications with anticholinergic and sedative properties that implements the principle of dose response to determine the effect of medication exposure.10 Cross sectional analysis in high functioning older persons, Health, Aging and Body Composition (Health ABC) study participants, showed DBI was strongly negatively associated with objective measures of concurrent physical and cognitive function. Whether drug burden exposure contributes to accelerated functional decline over time and the degree to which DBI score is prognostic of such decline remains unknown.
This study examines associations between medication exposure, as assessed by DBI, and change in physical function over five years, in Health ABC study participants, who were high functioning men and women aged 70-79 at study enrollment. We hypothesized that higher DBI at years 1 (baseline), 3 and 5 would be associated with a greater than expected reduction in functional capacity after five years (at year 6 follow-up). We propose a method for calculating total drug burden exposure over the five years, using area under the drug burden-time curve, and hypothesized that this would also be associated with reduced functional capacity at year 6.
Methods
Study population
The Health ABC study population consists of 3075 community-resident Medicare recipients aged 70-79 years, recruited from April 1997 to June 1998 from areas around Pittsburgh, Pennsylvania and Memphis, Tennessee. To participate, subjects were required to report no difficulty in walking 0.25 miles, climbing 10 steps, or performing activities of daily living at baseline. Of the 3075 participants, 501 had died by the end of the five year follow-up period. Subjects who did not have objective measures of the specific performance measures recorded in year 6 were excluded from analyses of association between drug burden exposure and that measure at year 6. A total of 2172 participants (71 % of baseline population) had data for longitudinal analysis of the association between DBI and the short physical performance battery (SPPB). The association of DBI with gait speed was analyzed in 2192 participants, and with grip strength in 2099. At year 6, of the 2172 participants with SPPB scores recorded, all had gait speed recorded and 2067 had grip strength recorded. Of participants without SPPB recorded at year 6, 20 had gait speed recorded and 32 had grip strength recorded. Table 1 describes times at which measures of function, comorbidities and medication exposure were obtained.
Table 1.
Time points of physical performance measures, medication exposure and co-morbidity measures used in longitudinal analysis of the association between medication use and physical function in Health ABC study.
| Time | Year 1 (Baseline) |
Year 2 | Year 3 | Year 4 | Year 5 | Year 6 |
|---|---|---|---|---|---|---|
| Functional | x | x | ||||
| Measures | ||||||
| Drug Burden | x | x | x | |||
| Calculated | ||||||
| Physical | x | x | x | x | x | x |
| Comorbidities | ||||||
| Mental | x | x | x | |||
| Comorbidities | ||||||
| Hospitalization | x | x | x | x | x | x |
| Sleep Disturbance | x | x | x | |||
| Body Mass Index | x | |||||
| Sociodemographics | x |
Medication Inventory
A medication inventory was conducted by research personnel during the baseline clinic visit (year 1) and at years 3 and 5. Participants were instructed to bring all prescription and over the counter medications used in the past two weeks to their clinic visit. Staff administered a structured medication history to confirm medications actually taken by participants in the previous two weeks. For each medication, the name of the drug, Iowa Drug Information System ingredient code, route of administration, dose and frequency that the medication was taken was recorded. At year 1, out of 3075 participants, 338 reported no medications. Ten did not have a medication inventory recorded and were assumed to be taking no medications. At year 3, 231 subjects did not have a medication inventory of whom 187 were deceased, and at year 5, 430 subjects did not have a medication inventory of whom 378 were deceased.
Drug Burden Index
Medication exposure was quantified using the DBI, which was derived from cross sectional data collected in year 1 in the Health ABC population10, and further validated using baseline data from the Women's Health and Aging Study11. Briefly, medications were characterized with respect to risk into two groups: drugs with anticholinergic effects and drugs with sedative effects. Medications with both anticholinergic and sedative effects were classified as anticholinergic.
The following factors were used in the equation for total drug burden (TDB):
| (Eq 1) |
where BAC and BS each represent the linear additive sum of D/(δ + D) for every anticholinergic (AC) or sedative (S) drug to which the subject is exposed, D is the daily dose taken by the subject, and δ is the minimum efficacious daily dose (minimum daily dose approved by the FDA). Both prescription and over the counter drugs were included in the analysis. Topical preparations without significant systemic effects were excluded. Where a dose was missing for an anticholinergic or sedative medication the median dose for the population was used in the calculations. Medications with anticholinergic and/or sedative effects that the study population was exposed to are shown in Appendix 1.
Drug burden exposure was calculated at years 1, 3 and 5. Cumulative exposure over the six years was calculated using the principles of trapezoidal area under the curve. The rectangular rule was used for year 5-6 as there were no measurements of drug burden beyond year 5. Area under the curve for drug burden (AUCDB) is the average drug burden at each time point multiplied by the time of exposure (in years):
| (Eq 2) |
where DBi represents the total DBI for the ith year and t is time. This expression can be simplified to:
| (Eq 3) |
where DBY1, DBY3 and DBY5 represent TDB at years 1, 3 and 5 of the study respectively.
Covariates
Covariates were selected using clinical judgment. Prevalent medical conditions were determined algorithmically from self-report of physician diagnoses, clinic assessments, and medication use. The functional comorbidity index (FCI)12 was used to assess co-morbidity at baseline. Other covariates were the incidence of each of the following conditions from years 2 to 6: cancer, cerebrovascular disease, ischemic heart disease, hypertension and diabetes mellitus; and hospitalization throughout the study period. An overall score (0-6) for the absence (score 0) or presence (score 1) of: cognitive impairment (Teng-modified Mini-Mental Status Exam13 score < 80), depressive symptoms (Center for Epidemiological Studies Depression (CESD) Scale14 score >15) and anxiety symptoms (Hopkins Symptom Checklist15 response for fear, tense or nervous included at least one moderate or at least two mild) at baseline and at year 5 or 6 served as a measure of psychological co-morbidity. Significant sleep disturbance was defined as three or more sleep problems, determined algorithmically at years 1, 3 and 5, and scored absent (0) or present (1). Sleep problems were defined as <5 hours of sleep per night; napping for ≥5 minutes >7 times per week; snoring, excessive day time somnolesence, or taking medication to sleep at least twice a month; and having trouble falling asleep or early morning wakening at least 5 times per month.
Sociodemographic characteristics, which included the sampling variables (age, race, sex, study site) and high school completion, were also covariates because these factors have been associated with health, medication use and physical and cognitive performance.
Outcome Measures
Physical function
The primary functional outcome was the short physical performance battery (SPPB) score, which was obtained at years 1 and 6 of the study. The SPPB summary performance score (total 0-12) adds scores (0-4) for tests of standing balance, usual gait speed and time to complete five repeated chair stands.7 Higher scores represent better function. A single component of the SPPB, usual gait speed, was also analyzed separately.
Grip strength was a secondary functional outcome. Grip strength was measured with an isometric dynamometer (Jaymar, JLW Instruments, Chicago, IL) at years 1 and 6. Participants with severe hand pain or recent surgery were excluded. The maximum grip strength in kilograms after two attempts with either hand was used.
Statistical Analyses
Relationships between the DBI and SPPB scores, usual gait speed and grip strength, controlling for corresponding baseline function, comorbidities and sociodemographic characteristics, were assessed using analysis of covariance and multiple linear regression analysis. Statistical analyses were performed with SAS 9.1 software (SAS Institute Inc, Cary, NC). All tests were two tailed and a p-value less than 0.05 was considered statistically significant.
Results
At baseline, Health ABC study participants were aged 73.6±2.9 years, 48% were men, 42% were black, 50% were from each site and 75% had completed high school. Characteristics of the population at baseline and longitudinally, including sociodemographics, comorbidities, functional measures and exposure to medications with anticholinergic or sedative effects are shown in Table 2. All participants with the relevant functional measures at year 6 were included in the longitudinal analysis. At baseline, included participants were younger, had fewer psychological comorbidities, less anticholinergic or sedative exposure, and higher functional scores than those who were excluded (Table 2).
Table 2.
Characteristics of subjects who had Short Physical Performance Battery (SPPB) scores recorded at year 6 and were included in longitudinal analysis of association between drug burden and SPPB scores, compared to subjects who did not and were excluded.
FCI; functional co-morbidity index.
| Included in longitudinal analysis | Excluded from longitudinal analysis | |
|---|---|---|
| N | 2172 | 903 (501 dead at 72 months) |
| Age at baseline | 73 ± 2.8 | 74 ± 2.9 |
| Sex (% female) | 53 | 48 |
| Race (% black) | 37 | 53 |
| Site (% from Memphis) | 51 | 49 |
| Education (% completed secondary education) | 78 | 68 |
| % with Drug Burden Index above zero | ||
| At baseline | 34 | 27 |
| At year 3 | 26 | |
| At year 5 | 29 | |
| Mean (±SD) Comorbidities | ||
| At baseline (FCI) | 2.7 ± 1.6 | 2.5 ± 1.7 |
| Additional by year 6 | 0.8 ± 0.7 | |
| % with significant depression, anxiety or cognitive impairment | ||
| At baseline | 25 | 29 |
| By year 6 | 49 | |
| % with significant sleep problems | ||
| At baseline | 24 | 25 |
| By year 6 | 32 | |
| % hospitalized throughout study period | 48.3 | |
| Mean (±SD) short physical performance battery | ||
| score | ||
| At baseline | 10.2 ± 1.5 | 9.7 ± 1.9 |
| At year 6 | 9.2 ± 2.3 | |
| Mean (±SD) grip strength (kg) | ||
| At baseline | 32.9 ± 10.8 | 32.3 ± 11.1 |
| At year 6 | 30.4 ± 10.1 | |
| Mean (±SD) usual gait speed (m/s) | ||
| At baseline (over 6 m) | 1.2 ± 0.2 | 1.1 ± 0.2 |
| At year 6 (over 4 or 6 m) | 1.1 ± 0.3 | |
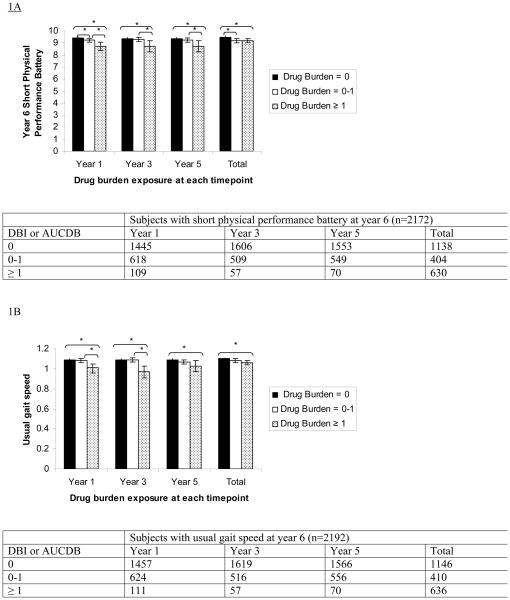
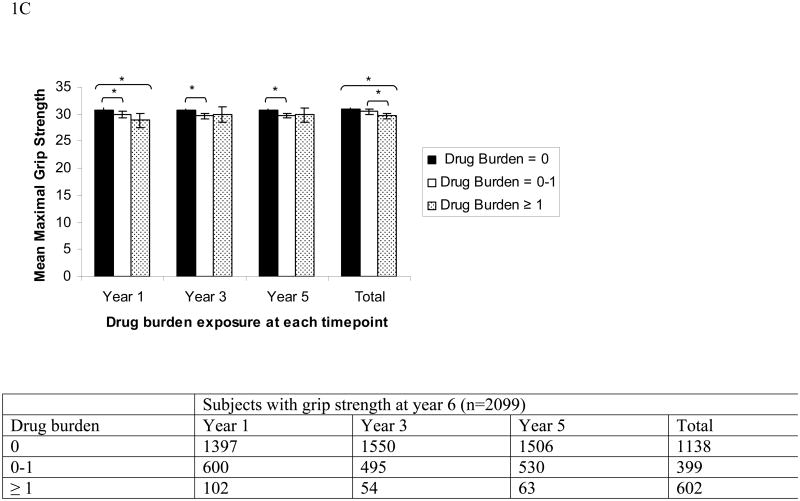
Findings from the analyses of covariance between drug burden at year 1, year 3 and year 5 and functional measures at year 6, adjusting for sociodemographics, baseline and incident physical and mental comorbidities, sleep disturbance, hospitalization and baseline function are presented in Figure 1. Higher drug burden exposure at years 1, 3 and 5 was associated with poorer function at year 6, as measured by the SPPB, usual gait speed and grip strength.
Figure 1.
Association between higher Drug Burden Index (DBI) at years 1, 3 and 5, and total drug burden exposure over five years, calculated using area under the drug burden time curve (AUCDB), with lower functional scores at year 6 measured with SPPB score (A), usual gait speed in m/s (B) or grip strength in kg (C).
DBI and AUCDB grouped into 0, 0-1 and ≥ 1. Means adjusted for year 1 functional score, co-morbidities, hospitalizations and sociodemographic factors using analysis of co-variance.
Error bars show 95% confidence intervals. * indicates that the difference between bars linked by brackets is statistically significant, p<0.05. Number of subjects at each level of exposure at each time point is shown in table below each figure.
The association between higher DBI at year 1 and lower functional measures at year 6 is shown in the regression table (Table 3a). The model explains 31% of the variance in year 6 SPPB score, 37% of the variance in year 6 gait speed, and 73% of the variance in year 6 grip strength. A one unit increase in DBI in year 1 (e.g., additional exposure to two anticholinergic or sedative drugs, each at the minimal efficacious dose) would predict a significant decrease in SPPB score of 0.29 (p = 0.008), a decrease in gait speed of 0.04 m/s (p=0.001), and a trend towards decreased grip strength of 0.56 kg (p=0.08) at year 6. This degree of change is more than that estimated for an additional physical or mental co-morbidity.
Table 3.
Multiple regression analyses for association of exposure to drug burden at year 1 (3a) and the area under the curve for drug burden (AUCDB) from years 1 and 6 (3b), with physical function at year 6 (SPPB Score, Gait Speed (m/s) and Grip Strength (kg)). The parameter estimates, B, tell the amount of change in each functional score that would be predicted by a 1 unit change in the predictor. The t Value and Pr > |t| columns provide the t-value and 2 tailed p-value used in testing the null hypothesis that the parameter estimate is 0. R-Square is the proportion of variance in the functional score which can be predicted from the independent variables. FCI; functional comorbidity index.
| Table 3a. Multiple regression for association of Drug Burden Index at baseline (year 1) with functional measures at year 6. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Functional Measure at Year 6 | |||||||||
| Short Physical Performance Battery Score | Usual Gait Speed (m/s) | Grip Strength (kg) | |||||||
| Factor | B | T | Pr> ItI | B | T | Pr> ItI | B | T | Pr> ItI |
| Age | −0.09 | −6.34 | <0.0001 | −0.01 | −6.29 | <0.0001 | −0.25 | −5.89 | <0.0001 |
| Sex (Female) | −0.30 | −3.52 | =0.0004 | −0.05 | −5.10 | <0.0001 | −4.20 | −11.39 | <0.0001 |
| Race (Black) | −0.31 | −3.38 | =0.0007 | −0.05 | −4.68 | <0.0001 | −0.13 | −0.49 | ns |
| Study Site (Memphis) | 0.01 | 0.17 | ns | 0.02 | 1.63 | ns | −0.14 | −0.58 | ns |
| Secondary Education (Absent) | −0.11 | −1.01 | ns | −0.03 | −2.62 | =0.009 | 0.75 | 2.43 | <0.05 |
| Baseline FCI | −0.10 | −3.55 | =0.0004 | −0.01 | −4.54 | <0.0001 | 0.05 | 0.64 | ns |
| Additional comorbidities over 6 years | −0.15 | −2.65 | =0.008 | −0.01 | −2.06 | =0.04 | −0.33 | −1.98 | <0.05 |
| Hospitalization years 1-6 | −0.32 | −3.79 | =0.0002 | −0.03 | −3.21 | =0.001 | −0.94 | −3.85 | =0.0001 |
| Cumulative Anxiety, Depression, Cognitive Impairment | −0.26 | −4.74 | <0.0001 | −0.02 | −3.79 | =0.0002 | −0.55 | −3.48 | =0.0005 |
| Cumulative Sleep Problems | −0.01 | −0.13 | ns | −0.01 | −0.81 | ns | −0.04 | −0.14 | ns |
| Baseline Functional Measure | 0.63 | 21.47 | <0.0001 | 0.51 | 22.31 | <0.0001 | 0.64 | 37.48 | <0.0001 |
| Baseline Drug Burden Index | −0.29 | −2.67 | =0.008 | −0.04 | −3.26 | =0.001 | −0.56 | −1.76 | =0.08 |
|
| |||||||||
| Adjusted R2 | 0.31 | 0.37 | 0.73 | ||||||
|
| |||||||||
| Table 3B. Multiple regression for association of the area under the Drug Burden Index - time curve (AUCDB) over five years with functional measures at year 6. | |||||||||
|---|---|---|---|---|---|---|---|---|---|
| Functional Measure at Year 6 | |||||||||
| Short Physical Performance Battery Score | Usual Gait Speed (m/s) | Grip Strength (kg) | |||||||
| Factor | B | T | Pr> ItI | B | T | Pr> ItI | B | T | Pr> ItI |
| Age | −0.09 | −6.30 | <0.0001 | −0.01 | −6.22 | <0.0001 | −0.25 | −5.91 | <0.0001 |
| Sex (Female) | −0.31 | −3.63 | =0.0003 | −0.05 | −5.21 | <0.0001 | −4.26 | −11.53 | <0.0001 |
| Race (Black) | −0.31 | −3.40 | =0.0007 | −0.05 | −4.69 | <0.0001 | −0.15 | −0.56 | ns |
| Study Site (Memphis) | 0.02 | 0.21 | ns | 0.02 | 1.66 | ns | −0.10 | −0.56 | ns |
| Secondary Education (Absent) | −0.11 | −1.05 | ns | −0.03 | −2.64 | =0.008 | 0.73 | 2.38 | =0.02 |
| FCI at baseline | −0.10 | −3.54 | =0.0004 | −0.01 | −4.55 | <0.0001 | 0.07 | 0.82 | ns |
| Additional comorbidities over 6 years | −0.15 | −2.62 | =0.009 | −0.01 | −2.03 | =0.04 | −0.33 | −1.97 | <0.05 |
| Hospitalization years 1-6 | −0.31 | −3.76 | =0.0002 | −0.03 | −3.19 | =0.002 | −0.92 | −3.78 | <0.0005 |
| Cumulative Anxiety, Depression, Cognitive Impairment | −0.25 | −4.67 | <0.0001 | −0.02 | −3.73 | =0.0002 | −0.52 | −3.31 | =0.001 |
| Cumulative Sleep Problems | −0.01 | −0.10 | ns | −0.01 | −0.79 | ns | −0.01 | −0.04 | ns |
| Baseline Functional Measure | 0.63 | 21.69 | <0.0001 | 0.51 | 22.26 | <0.0001 | 0.64 | 37.51 | <0.0001 |
| AUCDB | −0.08 | −2.46 | =0.01 | −0.01 | −2.86 | =0.004 | −0.27 | −2.87 | =0.004 |
|
| |||||||||
| Adjusted R2 | 0.30 | 0.37 | 0.73 | ||||||
|
| |||||||||
Cumulative exposure, the area under the curve for drug burden (AUCDB) over five years from years 1 to 6, was also strongly negatively associated with function at year 6. Associations between higher cumulative drug burden exposure over five years and poorer SPPB score, usual gait speed and grip strength at year 6 are shown in Figure 1 and in the regression table (Table 3b). A one unit increase in the AUCDB exposure over years 1 through 6 (e.g., additional exposure to the minimum efficacious dose of a single anticholinergic or sedative medication for two of the five years) would predict significant decreases in SPPB score of 0.08 (p=0.01), in gait speed of 0.01 m/s (p=0.004) and in grip strength of 0.27 kg (p=0.004), which is slightly less than the degree of change captured by a single co-morbidity for SPPB and gait speed, and equal to that of an additional co-morbidity for grip strength.
Discussion
In generally well-functioning community-resident older persons, DBI, measured independently at years 1, 3 and 5, was associated with reduced functional capacity at year 6, independent of co-morbidity status, sociodemographics and baseline function. This relatively straightforward model of medication exposure is associated with physical function both cross-sectionally and longitudinally to an extent comparable to and independent of the association of physical function with physical or mental co-morbidity. Total drug burden exposure over the five years, calculated using area under the drug burden-time curve, was also associated with functional limitations at year 6. Even low doses of anticholinergic and sedative medications for short periods are associated with impaired function, with no evidence of a threshold effect detected.
The finding that DBI was associated with longitudinal decline in physical function is consistent with the limited existing literature. In a dose-related manner, women aged over 70 years who were exposed to benzodiazepines had a higher risk of incident mobility problems and activity of daily living disability over four years than those who were not exposed.16 Exposure to antipsychotic medications, which are components of DBI, has been associated with increased all cause mortality in older adults with dementia17. Despite incorporating most factors known to influence function, the models in this study only capture 30-73% of variability in the functional outcomes over the five years. The remaining variability may reflect unmeasured confounders as well as the well recognized and poorly understood inter-individual variability in older people.18
There are several limitations to our study. Calculation of DBI uses the minimum efficacious dose to estimate the dose that gives 50% of the effect.10 The estimate of AUCDB does not capture duration of exposure prior to enrollment or any brief exposures between measures. This longitudinal analysis was limited to the 71% of subjects who had performance data after five years of follow-up and the longitudinal association between DBI and functional limitation in the excluded population remains unknown. Despite carefully controlling for potential confounders, the association between increasing drug burden index and functional impairment may be the result of residual confounding. Due to the small changes in DBI and function over the five years, this study was not powered to test the association between change in DBI and change in function over time. The possibility that reducing DBI may improve function is supported by the finding that withdrawal of sedative and neuroleptic drugs can significantly reduce falls.19 However, emerging evidence from primate studies suggests that exposure to antipsychotic drugs may alter brain structure.20 It is not known whether such effects are reversible, or whether they occur in humans.
A major strength of the Health ABC study lies in the rigorous objective data collected. Recording of actual medication use was based on inspection of all medications with the participant during clinic visits. Medical conditions were thoroughly assessed throughout the study, which permitted careful adjustment for physical and mental co-morbid illnesses, essential to account for the relationships of co-morbidity with medication exposure and functional impairment.
The study outcomes are objective and clinically relevant. Physical performance batteries such as the SPPB have been shown to predict nursing home admission, disability and mortality in older people over time7. The gait speed component of SPPB has also been shown to predict disability in older people21 and is a component of the frailty phenotype22. A five unit increase in AUCDB exposure (e.g., additional exposure to the minimum efficacious dose of two anticholinergic or sedative medications for five years) predicts decreases in SPPB score of 0.40 points and in usual gait speed of 0.05 m/s, which other studies have shown to be significant meaningful changes.23 Loss of grip strength is a strong predictor of disability24,25 and mortality26,27 in older people and is associated with frailty.22 Grip strength co-varied with SPPB scores only 4% at baseline and 6% at year 6. The lack of strong correlation between grip strength and SPPB, and the consistent association of all three functional measures with DBI, strengthens the evidence for a meaningful association between greater DBI exposure and decreased function across a range of clinically important measures in older people.
Amongst older persons who were highly functioning at enrollment, we have established associations between greater DBI at baseline, year 3 and year 5 and cumulatively over five years, with poorer overall physical performance, usual gait speed and grip strength after five years. The consistent association between DBI and functional outcomes over five years in the Health ABC population supports use of DBI to inform prescribers of the likely functional implications of medication exposure in their patients. This evidence-based tool can contribute clinically relevant information to the assessment of risk and benefit when reviewing an older patient's medication management. The prescriber and patient can discuss the potential effects of the disease and different treatment options on the patient's physical function. The association of increasing DBI with impaired physical performance could be further tested in populations of older people who are less high functioning at baseline or in those from different health systems. Future studies in larger populations or populations with larger changes in medication exposure and function will be required to test whether changing DBI in an individual will change function. Finally, interventional studies are required to assess the clinical feasibility and utility of DBI to guide prescribing in older people.
Clinical Significance.
- Drug Burden Index measures increasing exposure to medications with anticholinergic and sedative effects.
- In community dwelling older people, increasing Drug Burden Index is associated with poorer objective measures of physical function over five years.
- Drug Burden Index provides clinically relevant information for the assessment of risk when prescribing for older patients.
Acknowledgments
Funding Sources: Supported by Intramural Research Program of the NIH, National Institute on Aging, and National Institute on Aging Contracts NO1-AG-6-2101, NO1-AG-6-2103 and NO1-AG-6-2106.
Appendix
Appendix 1.
Medications participants were exposed to that were used in the calculation of Drug Burden Index.
IDIS; Iowa Drug Information System
| Medication | IDIS ingredient code |
|---|---|
| fentanyl | 28080810 |
| methadone | 28080818 |
| morphine | 28080819 |
| propoxyphene | 28080840 |
| tramadol | 28080854 |
| opium | 28080881 |
| oxycodone | 28080883 |
| pentazocine | 28080892 |
| phenobarbital | 28120405 |
| primidone | 28120407 |
| phenytoin | 28120805 |
| carbamazepine | 28122007 |
| oxcarbazepine | 28122011 |
| valproic acid | 28122015 |
| gabapentin | 28122020 |
| lamotrigine | 28122024 |
| tiagabine | 28122034 |
| levetiracetam | 28122040 |
| venlafaxine | 28160458 |
| selegiline | 28160520 |
| mirtazepine | 28160617 |
| paroxetine | 28160702 |
| sertraline | 28160703 |
| citalopram | 28160705 |
| ecitalopram | 28160711 |
| risperidone | 28160822 |
| ziprasidone | 28160844 |
| chlordiazepoxide | 28240202 |
| diazepam | 28240205 |
| flurazepam | 28240206 |
| clonazepam | 28240212 |
| oxazepam | 28240215 |
| estazolam | 28240216 |
| triazolam | 28240222 |
| clorazepate | 28240228 |
| temazepam | 28240231 |
| alprazolam | 28240232 |
| lorazepam | 28240276 |
| hexobarbital | 28240405 |
| butalbital | 28240413 |
| meprobamate | 28240820 |
| dichloralphenazone | 28240828 |
| zolpidem | 28240834 |
| buspirone | 28240837 |
| zaleplon | 28240856 |
| ropinerole | 28280011 |
| pramipexole | 28280013 |
| benzonatate | 48000054 |
| codeine | 48000063 |
| dextromethorphan | 48000069 |
| hydrocodone | 48000072 |
| diphenoxylate | 56080005 |
| loperamide | 56080009 |
| metoclopramide | 56220098 |
| chlorpheniramine | 4000003 |
| diphenhydramine | 4000006 |
| promethazine | 4000010 |
| cyproheptadine | 4000012 |
| tripelennamine | 4000013 |
| azatadine | 4000018 |
| astemizole | 4000022 |
| loratadine | 4000029 |
| cetirizine | 4000031 |
| clemastine | 4000054 |
| phenyltoloxamine | 4000061 |
| doxylamine | 4000068 |
| brompheniramine | 4000078 |
| dexbrompheniramine | 4000083 |
| dexchlorpheniramine | 4000084 |
| pheniramine | 4000092 |
| triprolidine | 4000099 |
| belladonna | 12080002 |
| dicyclomine | 12080005 |
| methscopolamine | 12080007 |
| propantheline | 12080008 |
| flavoxate | 12080039 |
| clidinium | 12080047 |
| hyoscyamine | 12080079 |
| trihexyphenidyl | 12080802 |
| orphenadrine | 12080804 |
| benztropine | 12080806 |
| terazosin | 12160401 |
| prazosin | 12160404 |
| tamulosin | 12160411 |
| doxazosin | 12160419 |
| carisoprodol | 12200001 |
| methocarbamol | 12200005 |
| cyclobenzaprine | 12200009 |
| chlorzoxazone | 12200091 |
| metaxalone | 12200097 |
| disopyramide | 24040024 |
| guanethidine | 24080003 |
| methyldopa | 24080006 |
| reserpine | 24080010 |
| guanfacine | 24080063 |
| clonidine | 24080064 |
| guanabenz | 24080084 |
| trazodone | 28160415 |
| nefazodone | 28160486 |
| phenelzine | 28160505 |
| amitriptyline | 28160601 |
| tranylcypromine | 28160601 |
| imipramine | 28160602 |
| trimipramine | 28160650 |
| doxepin | 28160681 |
| clomipramine | 28160688 |
| desipramine | 28160689 |
| nortryptyline | 28160695 |
| fluoxetine | 28160701 |
| chlorprothixine | 28160804 |
| hydroxyzine | 28160807 |
| quetiapine | 28160834 |
| olanzapine | 28160836 |
| loxapine | 28160858 |
| fluphenazine | 28160906 |
| perphenazine | 28160909 |
| thioridazine | 28160912 |
| trifluoperazine | 28160913 |
| triflupromazine | 28160996 |
| haloperidol | 28161014 |
| dimenhydrinate | 56220003 |
| meclizine | 56220005 |
| trimethobenzamide | 56220006 |
| chlorpromazine | 56220089 |
| prochlorperazine | 56220096 |
| oxybutynin | 86000004 |
| papaverine | 86000007 |
| tolterodine | 86000047 |
Footnotes
All authors had access to the data and a role in writing the manuscript.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Steel K, Gertman PM, Crescenzi C, Anderson J. Iatrogenic illness on a general medical service at a university hospital. N Engl J Med. 1981;304(11):638–42. doi: 10.1056/NEJM198103123041104. [DOI] [PubMed] [Google Scholar]
- 2.Greenblatt DJ, Allen MD. Toxicity of nitrazepam in the elderly: a report from the Boston Collaborative Drug Surveillance Program. Br J Clin Pharmacol. 1978;5(5):407–13. doi: 10.1111/j.1365-2125.1978.tb01646.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Montamat SC, Cusack B. Overcoming problems with polypharmacy and drug misuse in the elderly. Clin Geriatr Med. 1992;8(1):143–58. [PubMed] [Google Scholar]
- 4.Gray SL, Penninx BW, Blough DK, Artz MB, Guralnik JM, Wallace RB, et al. Benzodiazepine use and physical performance in community-dwelling older women. J Am Geriatr Soc. 2003;51(11):1563–70. doi: 10.1046/j.1532-5415.2003.51502.x. [DOI] [PubMed] [Google Scholar]
- 5.Mulsant BH, Pollock BG, Kirshner M, Shen C, Dodge H, Ganguli M. Serum anticholinergic activity in a community-based sample of older adults: relationship with cognitive performance. Arch Gen Psychiatry. 2003;60(2):198–203. doi: 10.1001/archpsyc.60.2.198. [DOI] [PubMed] [Google Scholar]
- 6.Landi F, Russo A, Liperoti R, Cesari M, Barillaro C, Pahor M, et al. Anticholinergic drugs and physical function among frail elderly population. Clin Pharmacol Ther. 2007;81(2):235–41. doi: 10.1038/sj.clpt.6100035. [DOI] [PubMed] [Google Scholar]
- 7.Guralnik JM, Ferrucci L, Simonsick EM, Salive ME, Wallace RB. Lower-extremity function in persons over the age of 70 years as a predictor of subsequent disability. N Engl J Med. 1995;332(9):556–61. doi: 10.1056/NEJM199503023320902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hutchins LF, Unger JM, Crowley JJ, Coltman CA, Jr, Albain KS. Underrepresentation of patients 65 years of age or older in cancer-treatment trials. N Engl J Med. 1999;341(27):2061–7. doi: 10.1056/NEJM199912303412706. [DOI] [PubMed] [Google Scholar]
- 9.Wenger NK. Enrollment and maintenance of elderly patients in cardiovascular clinical trials. Am J Geriatr Cardiol. 2006;15(6):352–6. doi: 10.1111/j.1076-7460.2006.05824.x. [DOI] [PubMed] [Google Scholar]
- 10.Hilmer SN, Mager DE, Simonsick EM, Cao Y, Ling SM, Windham BG, et al. A drug burden index to define the functional burden of medications in older people. Arch Intern Med. 2007;167(8):781–7. doi: 10.1001/archinte.167.8.781. [DOI] [PubMed] [Google Scholar]
- 11.Cao YJ, Mager DE, Simonsick EM, Hilmer SN, Ling SM, Windham BG, et al. Physical and Cognitive Performance and Burden of Anticholinergics, Sedatives, and ACE Inhibitors in Older Women. Clin Pharmacol Ther. 2008;83(3):422–9. doi: 10.1038/sj.clpt.6100303. [DOI] [PubMed] [Google Scholar]
- 12.Groll DL, To T, Bombardier C, Wright JG. The development of a comorbidity index with physical function as the outcome. J Clin Epidemiol. 2005;58(6):595–602. doi: 10.1016/j.jclinepi.2004.10.018. [DOI] [PubMed] [Google Scholar]
- 13.Teng EL, Chui HC. The Modified Mini-Mental State (3MS) examination. J Clin Psychiatry. 1987;48(8):314–8. [PubMed] [Google Scholar]
- 14.Weissman MM, Sholomskas D, Pottenger M, Prusoff BA, Locke BZ. Assessing depressive symptoms in five psychiatric populations: a validation study. Am J Epidemiol. 1977;106(3):203–14. doi: 10.1093/oxfordjournals.aje.a112455. [DOI] [PubMed] [Google Scholar]
- 15.Derogatis LR, Lipman RS, Rickels K, Uhlenhuth EH, Covi L. The Hopkins Symptom Checklist (HSCL). A measure of primary symptom dimensions. Mod Probl Pharmacopsychiatry. 1974;7(0):79–110. doi: 10.1159/000395070. [DOI] [PubMed] [Google Scholar]
- 16.Gray SL, LaCroix AZ, Hanlon JT, Penninx BW, Blough DK, Leveille SG, et al. Benzodiazepine use and physical disability in community-dwelling older adults. J Am Geriatr Soc. 2006;54(2):224–30. doi: 10.1111/j.1532-5415.2005.00571.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gill SS, Bronskill SE, Normand SL, Anderson GM, Sykora K, Lam K, et al. Antipsychotic drug use and mortality in older adults with dementia. Ann Intern Med. 2007;146(11):775–86. doi: 10.7326/0003-4819-146-11-200706050-00006. [DOI] [PubMed] [Google Scholar]
- 18.Gurwitz JH, Avorn J. The ambiguous relation between aging and adverse drug reactions. Ann Intern Med. 1991;114(11):956–66. doi: 10.7326/0003-4819-114-11-956. [DOI] [PubMed] [Google Scholar]
- 19.Campbell AJ, Robertson MC, Gardner MM, Norton RN, Buchner DM. Psychotropic medication withdrawal and a home-based exercise program to prevent falls: a randomized, controlled trial. J Am Geriatr Soc. 1999;47(7):850–3. doi: 10.1111/j.1532-5415.1999.tb03843.x. [DOI] [PubMed] [Google Scholar]
- 20.Dorph-Petersen KA, Pierri JN, Perel JM, Sun Z, Sampson AR, Lewis DA. The influence of chronic exposure to antipsychotic medications on brain size before and after tissue fixation: a comparison of haloperidol and olanzapine in macaque monkeys. Neuropsychopharmacology. 2005;30(9):1649–61. doi: 10.1038/sj.npp.1300710. [DOI] [PubMed] [Google Scholar]
- 21.Guralnik JM, Ferrucci L, Pieper CF, Leveille SG, Markides KS, Ostir GV, et al. Lower extremity function and subsequent disability: consistency across studies, predictive models, and value of gait speed alone compared with the short physical performance battery. J Gerontol A Biol Sci Med Sci. 2000;55(4):M221–31. doi: 10.1093/gerona/55.4.m221. [DOI] [PubMed] [Google Scholar]
- 22.Fried LP, Tangen CM, Walston J, Newman AB, Hirsch C, Gottdiener J, et al. Frailty in older adults: evidence for a phenotype. J Gerontol A Biol Sci Med Sci. 2001;56(3):M146–56. doi: 10.1093/gerona/56.3.m146. [DOI] [PubMed] [Google Scholar]
- 23.Perera S, Mody SH, Woodman RC, Studenski SA. Meaningful change and responsiveness in common physical performance measures in older adults. J Am Geriatr Soc. 2006;54(5):743–9. doi: 10.1111/j.1532-5415.2006.00701.x. [DOI] [PubMed] [Google Scholar]
- 24.Rantanen T, Guralnik JM, Foley D, Masaki K, Leveille S, Curb JD, et al. Midlife hand grip strength as a predictor of old age disability. Jama. 1999;281(6):558–60. doi: 10.1001/jama.281.6.558. [DOI] [PubMed] [Google Scholar]
- 25.Giampaoli S, Ferrucci L, Cecchi F, Lo Noce C, Poce A, Dima F, et al. Hand-grip strength predicts incident disability in non-disabled older men. Age Ageing. 1999;28(3):283–8. doi: 10.1093/ageing/28.3.283. [DOI] [PubMed] [Google Scholar]
- 26.Laukkanen P, Heikkinen E, Kauppinen M. Muscle strength and mobility as predictors of survival in 75-84-year-old people. Age Ageing. 1995;24(6):468–73. doi: 10.1093/ageing/24.6.468. [DOI] [PubMed] [Google Scholar]
- 27.Newman AB, Kupelian V, Visser M, Simonsick EM, Goodpaster BH, Kritchevsky SB, et al. Strength, but not muscle mass, is associated with mortality in the health, aging and body composition study cohort. J Gerontol A Biol Sci Med Sci. 2006;61(1):72–7. doi: 10.1093/gerona/61.1.72. [DOI] [PubMed] [Google Scholar]