Abstract
Advanced strategies in reconstructive microsurgery and especially free tissue transfer with advanced microvascular techniques have been routinely applied and continously refined for more than three decades in day-to-day clinical work. Bearing in mind the success rates of more than 95%, the value of these techniques in patient care and comfort (one-step reconstruction of even the most complex tissue defects) cannot be underestimated.
However, anticoagulative protocols and practices are far from general acceptance and – most importantly – lack the benchmark of evidence basis while the reconstructive and microsurgical methods are mostly standardized.
Therefore, the aim of our work was to review the actual literature and synoptically lay out the mechanisms of action of the plethora of anticoagulative substances.
The pharmacologic prevention and the surgical intervention of thrombembolic events represent an established and essential part of microsurgery. The high success rates of microvascular free tissue transfer as of today are due to treatment of patients in reconstructive centers where proper patient selection, excellent microsurgical technique, tissue transfer to adequate recipient vessels, and early anastomotic revision in case of thrombosis is provided. Whether the choice of antithrombotic agents is a factor of success remains still unclear. Undoubtedly however the lack of microsurgical experience and bad technique can never be compensated by any regimen of antithrombotic therapy. All the more, the development of consistent standards and algorithms in reconstructive microsurgery is absolutely essential to optimize clinical outcomes and increase multicentric and international comparability of postoperative results and complications.
Keywords: anticoagulation, microsurgery, heparin-induced thrombocytopenia (HiT), thrombosis
Abstract
Die Fortschritte in der rekonstruktiven Mikrochirurgie und insbesondere beim freien Gewebetransfer mit steter Verbesserung mikrochirurgischer Techniken haben diese in den vergangenen 30 Jahren zu einem festen Bestandteil in der täglichen Routine werden lassen.
Die mittlerweile erreichten Erfolgsraten von über 95% unterstreichen den Wert der mikrochirurgischen Fortschritte für Patientenversorgung und -komfort (Möglichkeit der primären Rekonstruktion komplexester Verletzungen mit Hautweichteilverlust).
Obwohl rekonstruktive und mikrochirurgische Techniken weitestgehend standardisiert sind, fehlen bislang solche evidenzbasierten Standards im Hinblick auf antikoagulatorische Regime.
Ziel dieser Arbeit ist es daher, die Wirkmechanismen der für die Mikrochirurgie relevanten Antikoagulantien vor dem Hintergrund der aktuellen Literatur zu diskutieren.
Die pharmakologische Prävention und chirurgische Intervention thrombembolischer Ereignisse ist ein etablierter Bestandteil der Mikrochirurgie. Die mittlerweile erreichten und hohen Erfolgsraten nach freiem mikrochirurgischen Gewebetransfer sind das Ergebnis der Behandlung dieser Patienten in spezialisierten mikrochirurgischen Zentren mit entsprechender Patientenselektion im Sinne einer adäquaten Auswahl der Empfängergefäße und Möglichkeit der sofortigen Intervention im Falle von thrombembolischen Komplikationen.
Inwieweit die Wahl der Antikoagulantien zum Erfolg des mikrochirurgischen Gewebetransfers beiträgt, ist noch nicht hinreichend geklärt. Es steht außer Zweifel, dass ein Mangel an mikrochirurgischer Expertise und eine unzureichende Anastomosentechnik nicht durch antikoagulatorische Regime kompensiert werden können.
Insofern ist die Etablierung antikoagulatorischer Standards und Algorithmen in der rekonstruktiven Mikrochirurgie vor dem Hintergrund einer weiteren Optimierung und Vergleichbarkeit der klinischen Ergebnisse der Zentren untereinander und auf internationaler Ebene unerlässlich.
Introduction
The quality of microsurgical anastomoses also depends on technical prerequisites like adequate instruments and operation microscope, but mainly on the skill and experience of the microsurgeon. The success rates vary depending on author, and professional experience in microsurgical procedures. The number of self-conducted microvascular free tissue transfers seems to be correlated with anastomosis success rates.
Complications due to insufficient anastomosis technique are frequently deleterious and sometimes end up with extremity loss for the patient.
Pharmacological thrombosis prophylaxis, but also (micro-) surgical interventions after thrombus formation are frequently used techniques to prevent thrombogenic states and rescue blood flow once a thrombus has formed and obliterated the microvessel. Unfortunately, incoherent guidelines and algorithms for thrombosis prophylaxis in microsurgery are found in the literature, even though basic science has clearly demonstrated the efficacy and mechanisms of action of the various antithrombotic agents in numerous experimental efforts.
Earlier on, Virchow has recognized and postulated the pathophysiologic prerequisites endothelial defect, hypercoagulability and stasis. Despite the plethora of available antithrombotic substances, acetyl salicylic acid, heparin (fractionated and unfractionated), hydroxyethyl starch and dextrans are still the most frequently used agents.
While the guidelines and standards for inpatient and outpatient antithrombotic prophylaxis in surgery have been clearly outlined and agreed on by a multidisciplinary consensus conference, the german AWMF workgroup (http://awmf.org/), such a protocol does not exist in microsurgery yet.
Basics and principles of antithrombotic therapy
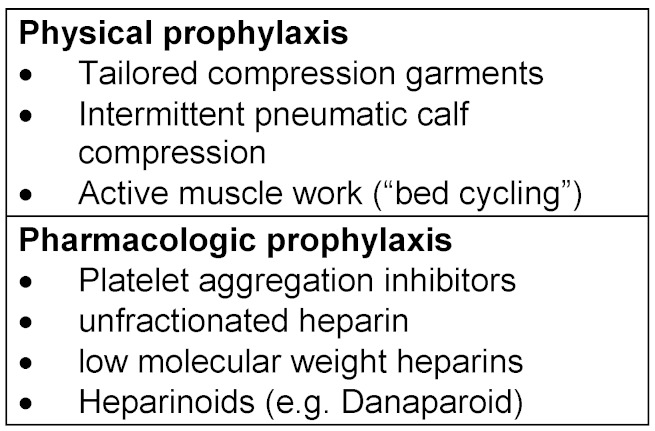
Bearing Virchow’s trias in mind (endothelial wall, blood flow, corpuscular and non-corpuscular composition of the blood), the following therapeutic options can be defined: Table 1 (Tab. 1).
Table 1. "Antithrombotic" measures.
Successful microsurgical tissue transfer is based on an adequate microsurgical technique by which correspondingly high patency rates can be safely achieved. The choice of the recipient vessel is centered around the establishment of sufficient microvascular blood flow, however, newer work has shown that the free flap itself is a major determinant of blood inflow [1].
Beside these issues, the pathogenesis and localisation of the tissue defect are also known predisposing factors for microvessel (arterial and venous) thromboses especially when lower extremity defects and complex traumatic tissue defects with consecutive vessel occlusion or osteomyelitis need to be addressed [2].
Intraoperative vessel spasms can be effectively prevented by the application of paverone – a temporal vasodilator – to the anastomosis site. Postoperatively, prevention of external compression (e.g. constrictive dressings, adverse positioning etc.) to the microanstomosis site or the microvessel course is absolutely necessary to avoid compromised microvascular blood flow.
Intraoperative administration of heparin (“heparin flush”) can be continued postoperatively by continuous i.v., subcutaneous low molecular heparinization or – alternatively – thrombocyte aggregation blockers (eg acetyl salicylic acid 100 mg once daily p.o.).
Additionally an optimized fluid and volume management and mechanoprophylaxis are proven perioperative treatment options in minimizing the risk of venous thromboembolism.
Thrombosis and heparin-induced thrombocytopenia (HiT)
Based on our own experience with events of flap loss due to heparin-associated thrombocytopenia (HiT), adding on to routine determination of partial thromboplastin time (PTT) and international normalized ratio (INR), we recommend to check the platelet count during the postoperative phase.
Lowered thrombocyte counts of more than 50% of the baseline value within postoperative days 5 and 10 should prompt the clinician to immediately withdraw heparin administration and initiate the HiT diagnostic algorithm.
HiT type II (Heparin-induced thrombocytopenia) – definition and diagnostic process
HiT type II is a pharmacologically induced immune dysfunction syndrome and its clinical appearance is characterized by thrombocytopenia and – paradoxically – thrombotic events. Especially in reconstructive microsurgery thrombus formation can be associated with deleterious sequelae like partial or complete flap loss. HiT type II is diagnosed in approximately 5% of all patients treated with unfractionated heparin. Importantly, this rate is as low as less than 1% of all patients receiving low molecular weight heparin.
The diagnosis of HiT type II is based on three criteria:
Current treatment with heparin or suspicion of ongoing heparin action in the case of prior heparin therapy
At least one clinical symptom is present (most frequently thrombocytopenia)
Laboratory testing (ELISA) reveals antibody formation against the heparin – platelet factor IV complex (http://www.tigc.org / The Thrombosis Interest Group of Canada).
Thrombocytopenia is usually mild in patients diagnosed with HiT type II. Platelet counts of lower than 30/nl is rare, only 5% of the patients have platelet counts of 15/nl or lower. Bleeding complications are rarely encountered in HiT II patients and are usually associated with concomitant problems such as septicaemia or uremic thrombocytopathy.
Paradoxically, patients with HiT type II display a higher risk of developing thrombotic than bleeding complications. The cumulative risk for further enlargement of a pre-existing thrombosis or the development of new thrombotic formations ranges from 30–50%. Clinically, thrombus formation can also occur at later stages of the disease, even when heparin has been stopped and platelet counts begin to normalize.
Most thrombotic episodes are of venous origin, however, arterial emboli may also occur. Typical thrombotic events are deep vein thrombosis (DVT), pulmonary embolism (PE), cerebral or myocardial infarction and emboli of the arterial system of the lower extremities.
In HiT type II patients, autoantibodies are detectable in the serum or plasma. Confirmation of a suspected HiT type II diagnosis is done most frequently using ELISA assays specific for autoantibodies against platelet factor 4 – heparin complexes (PF4-heparin-ELISA). Besides the ELISA, two functional tests exist: The serotonin-liberation assay, which is done using 14C-serotonin-loaded platelets of healthy donors. If these are challenged with plasma of a HiT II patient, the PF4-heparin-autoantibody complex induces serotonin liberation.
Another functional test is the HIPA-test (heparin-induced platelet activation), which is based on the mesurement the activation of freshly-isolated activated platelets (from healthy donors) by the antigen-autoantibody complex [3], [4].
In the case of a diagnosed HiT II heparin therapy should be stopped immediately and nonheparin anticoagulants should be administered in order to inhibit thrombin or thrombin generation. Danaparoid, lepirudin and argatroban are approved anticoagulants for treatment of HiT. Danaparoid is a glycosaminoglycuronan isolated from porcine intestinal mucosa and exerts its anticoagulant effects predominantly by inhibiting factor Xa and to a much lesser degree by inhibiting thrombin. Lepirudin is a recombinant hirudin (r-hirudin) and a direct and irreversible inhibitor of thrombin. The primary elimination route of lepirudin is renal, accounting for approximately 90% of its systemic clearance [5]. The systemic clearance of lepirudin is proportional to the glomerular filtration rate and there is a relevant cumulation of this drug in patients with renal impairment [6]. Furthermore bleeding is the most important and clinically relevant complication of treatment with lepirudin when compared to oral or parenteral Xa inhibitors, with an incidence rate of 4%–19% [7].
The activated partial thromboplastin time (aPTT) is the current method of choice for monitoring treatment with lepirudin. At plasma concentrations of lepirudin greater than 0.6 mg/L there is no linear correlation between aPTT and lepirudin which may result in overlooking toxic doses and the danger of bleeding [8], [9], [10].
Hirudin as a thrombin inhibitor has been established in the treatment of septic disorders during recent years according to the fact, that thrombin is known to be a key mediator of macrophage and granulocyte activation in vitro [11].
Furthermore Thrombin has depressive effects on the circulatory system reducing the functional capillary density (FCD) via endothelial cell interaction and endothelial cell damage [12].
Therefore Hoffmann et al. hypothesized that hirudin would down-regulate leukocyte/endothelial cell interaction and, thereby, improve capillary perfusion [13]. They induced severe endotoxemia in Syrian hamsters by intravenous administration of endotoxin (lipopolysaccharide [LPS], E. coli, 2 mg/kg) and could demonstrate a paradoxic effect of hirudin on the leukocyte/endothelial cell interaction and microcirculation by pronouncing leukocyte adherence and deteriorating capillary perfusion [13]. On the other hand they could demonstrate a considerable modulation of coagulatory parameters during the first 4 h of endotoxemia. Hirudin prevented the increase in thromboplastin time and induced an improvement of antithrombin activity and a tendency towards higher protein C activity, as well as shorter partial thromboplastin time. Furthermore hirudin did not induce a change in platelet count excluding a distinct effect on platelet-endothelial cell interaction.
In conclusion, their experiments do not indicate a relevant protective effect of recombinant hirudin on endotoxin-induced microcirculatory disorders [13].
Danaparoid in a high dose regimen is equivalent to lepirudin in the treatment of HiT with or without thrombosis. Reduction in the incidence of new thromboembolic complications and limb amputation is comparable with lepirudin [14].
Argatroban, a direct inhibitor of thrombin and is cleared by the liver and, therefore, can be used safely in patients with renal insufficiency. Argatroban also has the shortest half-life among all alternative anticoagulants and can be discontinued quickly if invasive procedures are necessary or if bleeding is encountered [15].
Differential diagnosis of HiT type II
Thrombocytopenias of other origin, e.g. HiT type I, have to be differentiated from HiT type II. The clinical course of HiT type I is characterized by a steep decrease of the platelet count within the early phase of heparin therapy (usually the first 4 days after initiation), which does usually not worsen under continous heparin therapy and resolves despite the ongoing administration of heparin.
Platelet counts usually do not reach values lower than 50% of the baseline and less than 100/nl. HiT type I does not induce a thrombophilic state and does not require heparin withdrawal or change of the antithrombotic regimen. Besides HiT type I, the differential diagnostic process should prompt the clinician to take disseminated intravascular coagulopathy (DIC) and thrombotic-thrombocytopenic purpura (TTP) as well as other non-specific syndromes resulting in platelet activation into account. Here, the mechanism is characterized by the formation of immune complexes. Also, pharmacologically induced immunogenic thrombocytopenias or – rarely – alloimmunethrombocytopenias (e.g. posttransfusion purpura, neonatal alloimmunethrombocytopenia) have to be kept in mind during the differential diagnosis process [16].
It is of utmost importance to determine the preoperative baseline platelet count.
Perioperative antithrombotic measures – general and specific aspects
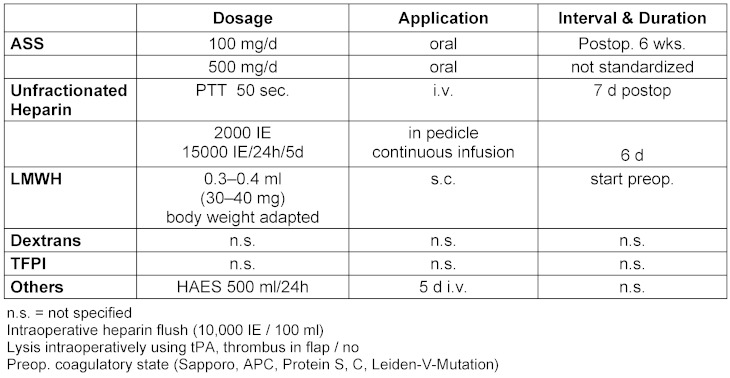
As outlined in Table 2 (Tab. 2), pharmacological treatment and operative measures add on to each other and standardized algorithms have been consented by the respective societies.
Table 2. Antithrombotic measures in the perioperative setting.
Unfortunately, generally accepted standards regarding the anticoagulative protocols in microsurgical free flap transfer do not exist.
Starting to address the lack of standardization for anticoagulative protocols we introduced an algorithm regarding prophylaxis of venous thrombembolism for the field of plastic surgery. Indications for the pharmacological prophylaxis of thromboembolic events are here oriented on the specific risk categories for surgical interventions with regard to the “exposing” (associated with the clinical setting) and “predisposing” (associated with the patient) risk factors. Furthermore, the recommendations for the field of plastic and reconstructive surgery are subdivided into the various regions of the body [17].
An-up-to-date survey, which was done at the 29th annual meeting of the german-speaking society of microsurgery revealed the following (Table 3 (Tab. 3), rate of return 10%).
Table 3. Anticoagulative regime of German-speaking microsurgeons.
To undermine these heterogeneous data we performed a further and comprehensive survey among german speaking microsurgeons whose results are going to be published in the near future.
Table 3 (Tab. 3) shows that acetyl salicylic acid (100–500 mg/d) and unfractionated or low-molecular weight heparin are used. Dextrans have disappeared from routine clinical use, however, hydroxyl ethyl starch is used during the postoperative phase. Intraoperatively, flushing of the anastomosis site is done with either heparin or – in the case of an existing thrombus – rt-PA (Urokinase, lysis). Looking at the increased bleeding risk and other adverse reactions, rt-PA should be used cautiously. A general recommendation cannot be given at this point, because randomized, prospective studies have not been done yet. Even intraoperative heparin flushing is not routinely done in all microsurgical units.
In 2007, Xipoleas et al. presented the results of an email- and questionnaire based survey among members of the American Society of Plastic Surgeons [18]. In summary, 4,383 members were asked to complete the survey. Finally, the data was analyzed upon a feedback rate of 3%, the number of performed procedures ranged from 5 to 84 cases per year. Nevertheless, the success rates were not evenly distributed over 90%, but did reach maximum success rates of 92–100%. 84% of the surgeons did at least use a form of anticoagulation during or after microsurgical flap transfer. 48% of the surgeons applied a combination therapy, 44.9% started the anticoagulative treatment intraoperatively and maintained it postoperatively. 16.3% did not use any form of anticoagulation at any time during the treatment [18]. A potential reason for the non-standardized anticoagulative regimens from a european point of view may be the difference in patient management with lack of preoperative hospitalization of the patients in the United States. In contrast, a wide variation concerning intraoperative anticoagulation measures was found when the respective pathogenesis of tissue defects was taken into consideration. Potentially, differences in the therapeutic approaches due to defect pathogenesis may count for these variations, so that standardized protocols cannot be established.
Apparently, many surgeons base their anticoagulative regimen upon their personal professional experiences and case observations. Indeed, it was interesting to note that personal standards have been changed primarily after complications were encountered, which can be deduced from a study conducted by Khouri and colleagues [2]. Intraoperative thrombus formation was associated with the use of myocutaneous flaps and vein grafts. The flap loss rate was reported at 4.1 %, the intra- and postoperative thrombosis rate ranged between 8.3 and 9.9%. Statistically, there was no association between the used anticoagulative strategy and anastomosis insufficiencies. However, an increased risk of complications was noted when chronic wounds were treated or when interposition vein grafts were used. A lower risk ratio was found when rectus abdominis muscle flaps and postoperative subcutaneous low-molecular-weight heparin therapy had been applied [2].
In our clinic we administer low molecular weight heparin (2500–5000 IE body weight adapted) starting the evening before surgery. Intraoperatively we flush the flap and recipient vessels with unfractioned heparin (50 IE/ml) and administer 1000 IE unfractioned heparin after completion of the microanastomosis respectively before clamp release. In the case of complicated anastomosis (e.g. atherosclerosis, AV-loop) we perform systemic heparinization (15000 IE/24 hours resp. PTT 50–60 sec.) for 10 days. Papaverine is only applied in vasospasms at the anastomosis site. In the postoperative setting our regimen consists of aspirin 100 mg and low molecular weight heparin (2500–5000 IE body weight adapted) once a day for 7 days.
Additionally we start a combined dangling/wrapping procedure respectively ischemic conditioning at day 3 after surgery consisting of a combined cotton and elastic wrapping of the flap followed by dangling the lower extremity with the patient sitting in an upright position at the edge of the bed and the knee joint flexed at an angle of 90°. The heel is not supported.
Wrapping and dangling the flap starts with a duration of 5 minutes three times a day and increases daily by doubling the duration over a period of 4 days reaching 60 minutes at day 5.
This combined dangling/wrapping procedure in the postoperative course after lower extremity reconstruction counteracts adverse events of free-flap transfer (i.e. venous congestion due to increased capillary pressure and fluid leaking into the interstitium; decreased perfusion due to division of sympathetic nerves and inflow vessels) by supporting venous and lymphatic return, reducing edema and increasing blood flow to the flap by inosculation and neovascularization by ischemic conditioning [19], [20].
Acetylsalicylic acid is attributed a universal efficacy in the prophylaxis of arterial, venous and autograft anastomoses as well as a beneficial microcirculatory effects. These universal properties could have even been shown for heparin (Table 4 (Tab. 4)).
Table 4. Site of pharmacologic action of various antithrombotic agents.
Protection of microanastomoses and microcirculation by antithrombotic substances
Nevertheless, a rationale for its clinical application can be formulated, especially for the pathophysiologic concept of ischemia-reperfusion injury: Thromboxane A2 mediates vasoconstriction, O2 radicals damage membrane lipids, receptors and other important biomolecules. An array of specific anti-inflammatory cytokines, leukotrienes and interleukins finally induces an endothelial injury which in turn completes the reperfusion injury in concert with activated leucocytes. Bearing these mechanisms in mind, antithrombotic substances seem to be clinically indispensable and their mechanisms of action are divert:
Acetylsalicylic acid is a platelet aggregation inhibitor. Via irreversible blocking of cyclooxygenases the synthesis of thromboxane A2 is effectively intercepted. Treatment doses of 30–300 mg per day are known to effectively inhibit thromboxane synthesis. In higher doses, additive inhibition of the prostacyclin synthesis has been shown.
Peter et al. have demonstrated earlier on in an isolated muscle system that acetylsalicylic acid can also induce an increase in the functional density of microcapillary networks [21]. However, the main effects of acetylsalicylic acid are centered around the local endothelial effects and its action on platelet function. Effectors of the clotting cascade are not influenced by acetylsalicylic acid. Here, heparin is an efficient antithrombin III (AT III) agonist and is an inhibitor of the factors IIa, IXa, Xa, XIa and XIIa. Moreover, heparin indirectly influences platelet aggregation via inhibition of endothelial prostacyclin.
In summary, acetylsalicylic acid inhibits thrombus formation by the inhibition of platelet aggregation while the mechanism of action of heparin inhibits thrombin (indirectly) and the clotting factor Xa thereby preventing thrombus formation locally (anastomosis site). Therefore, sole inhibition of platelet aggregation seems to be insufficient in microsurgery because thrombus formation can still occur via the alternative, plasmatic pathway of the clotting cascade which can be modulated by heparin application.
Greenberg and coworkers have recently shown in a rabbit model that intraarterial perfusion with heparin for 72 hours after surgery leads to an overall higher anastomosis patency rate [22].
Overall, systemic application seems to be less effective than local heparin application. It has been shown that lower molecular weight heparin is more effective than intravenous heparin in free flap transfer [23], [24], [25].
Studies in coronary surgery have demonstrated that heparin induced a significantly higher rate of postoperative vessel patency (30%). Also, analyses of the outcome after coronary thrombolysis therapy have shown that heparin is more efficient in post-lysis anticoagulation than acetylsalicylic acid [26]. However, these results are in contrast to a prospective multicenter analysis which was done in 1998 by Khouri and colleagues [2]. The comparison of anticoagulatory strategies of experienced microsurgeons and the respective influence on the complication rates after microvascular tissue transfer did not reveal any preventive effects on thrombus formation of intraoperatively administered heparin. Postoperative prophylaxis with low-molecular weight heparins which were applied subcutaneously lowered the risk of postoperative thrombosis [2].
Microsurgical interventions and flap failure
Anticipation and prevention are key factors for successful flap transfer procedures, and they are built on surgical tactics on the one hand and the preoperatively started pharmacoprophylaxis on the other hand.
Surgical planning consists of adequate clinical and technical diagnostic procedures to select the best suitable flap and the safest recipient vessels. Defect size, localization and individual factors such as irradiation of the defect site, vessel disease of any kind etc are central elements which need to be considered preoperatively at the stage of surgical planning.
In the case of thrombotic microvessel occlusion, the anastomosis itself can be revised and redone, a new recipient site can be chosen or additional venous anastomoses can be performed to ensure an optimal in- and outflow balance. Pharmacologically, multiple thrombolytic protocols which can also be performed intraoperatively, are discussed in literature [27], [28], [29], [30].
There is no doubt that sole use of pharmacologic antithrombotic therapy cannot increase the success rate of flaps. The surgical revision of the anastomosis site is still essential and a key rescue procedure. A study by Smit and coworkers recently demonstrated that only early surgical interventions such as resuturing the anastomosis, thrombectomy and placement of interposition vein grafts etc. can reduce flap failure rates. The majority of anastomosis patency complications were encountered within the first 24 hours after surgery and decreased later on in frequency. Operative revisions displayed a success rate of approximately 80% on day 1 after surgery, however, this rate decreases continuously with time and reach a baseline rate of lower than 5% by days 6 and 7 after the initial procedure [31]. Another important surgical intervention in the case of thrombotic complications is intraoperative vasodilatation: Evans and colleagues have shown in 1997 that local and systemic application of lidocaine rather induce vasospasms of the anastomosis vessels, in contrast, prostaglandin E1 and papaverine hydrochloride induce vasodilatation and a consecutive increase in regional blood flow [32]. Therefore, lidocaine application seems to be obsolete in modern microsurgery. Whether intraluminal irrigation using antithrombotic substances can be generally recommended for clinical use cannot be predicted at this point of time. A first study which was published by Khouri et al. in 2001 at least demonstrated that low-dose application of TPFI in comparison to heparin does not only have acceptable antithrombotic prophylactic properties but was also associated with lower complication rates, especially hematoma formation [33].
Of course, the local factors of the recipient area are still considered key factors for surgical success and the resulting flap perfusion. Free flap transfers to the lower limb after complex trauma to the zone of injury are associated with high complication rates, therefore, subtle surgical planning and meticulous technique are essential factors for success. It is very interesting to observe that not the recipient vessel, but the blood intake capacity of the flap determines the flow rates. In this context, Lorenzetti and Ascarseria demonstrated that in free rectus abdominis muscle flaps the flow rate in the flap after anastomosis was widely identical to the corresponding flow rate in the inferior epigastric artery as the donor vessel. In contrast, the flow rates determined preoperatively in the thoracodorsal artery as the donor vessel were significantly different to the flow measured in the rectus abdominis muscle flap. Conclusively, the flow rates seem to be regulated by the demand in the graft itself and its uptake capacity [1].
Influence of chemotherapy on microanastomosis and flap survival
Intraarterial chemotherapy with various substances is a modern and efficient approach to increase local doses of the cytostatic agent and reduce systemic toxicity at the same time. Also, isolated hyperthermic limb perfusion (ILP) is used in certain types of soft tissue tumors. A study by Sadrian and coworkers has revealed no flap loss due to thrombosis formation in a cohort of tumor patients that underwent either hyperthermic limb perfusion or intraarterial chemotherapy after free flap transfer. The study included patients from two cancer centers [34]. In connection with the results of this study, an interesting novel approach to maximize patient safety and flap success is the creation of an arteriovenous loop (AV-loop) and subsequent flap transfer to the transected loop vessel (arterial and venous side). However, own studies could not verify clinical advantages when single-stage (“fix and flap” approach) or two-step procedures were used to perform the flap transfer [35]. Nevertheless, it must be emphasized that in any case of free tissue transfer the professional experience of the surgeon, surgical skills and meticulous assessment of the anastomosis site are pivotal factors determining flap success and outcome.
Case report
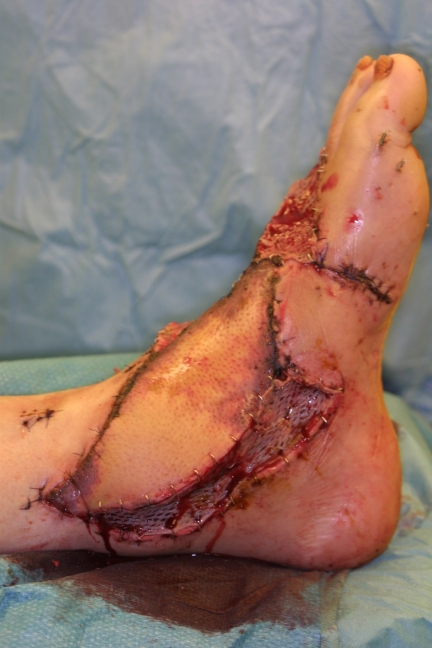
We report about a 56-years-old man who suffered from a lawn cutter injury and had an open tarsal and ankle joint fracture that was initially treated by our trauma surgeons. The patient was transferred to our plastic surgery department with an exposed osteosynthesis plate at the ankle joint level and a soft tissue defect measuring 10x20 cm due to tissue break down. We perfomed an free fasciocutanous anterior lateral thigh perforator flap for defect coverage. After microsurgical end-to-side anastomosis of the perforator flap vessels to the tibial posterior artery and a concommitant vein additional skin grafts have been performed. Due to uncomplicated microsurgery (Figure 1 (Fig. 1)) this patient received the standard anticoagulative protocol with oral ASS 100 mg and 5000 IE low molecular weight heparin s.c. on a daily basis in the postoperative setting. At day one after flap transfer the patient was taken back to the operating room to evacuate a haematoma. At day two the flap presented a significant change towards livid colouration of the skin island indicating venous congestion respectively flap vein thrombosis and revision was necessary (Figure 2 (Fig. 2)). After revision of the venous anastomosis the flap was salvaged by thrombectomy and interposition of a vein graft (Figure 3 (Fig. 3)). In this case the anticoagulative regimen was not changed towards unfractioned heparin due to the fact, that flap vein thrombosis occurred because of hematoma formation followed by external compression of the anastomosis and not because of disturbance of the coagulative state. Therefore the standard anticoagulative protocol with oral ASS 100 mg and s.c. low weight heparine 5000 IE on a daily basis was maintained.
Figure 1. Intraoperative situs after successful free flap transfer.
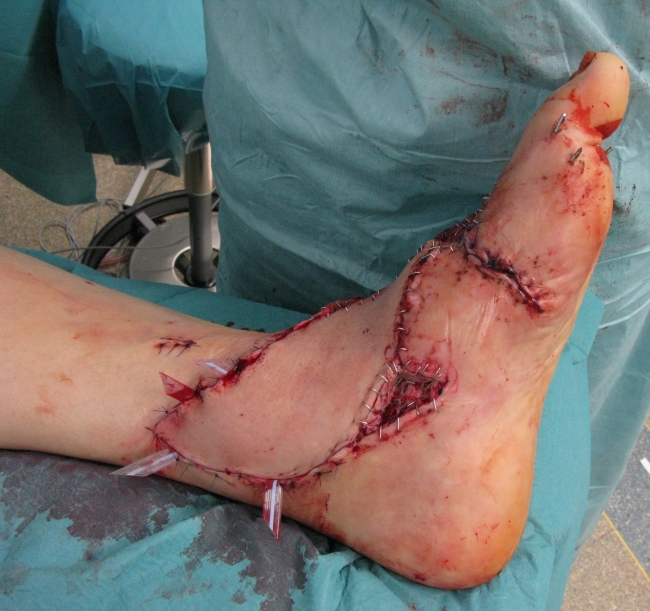
Figure 2. Livid colouration of the skin island indicating venous congestion respectively flap vein thrombosis due to hematoma formation.
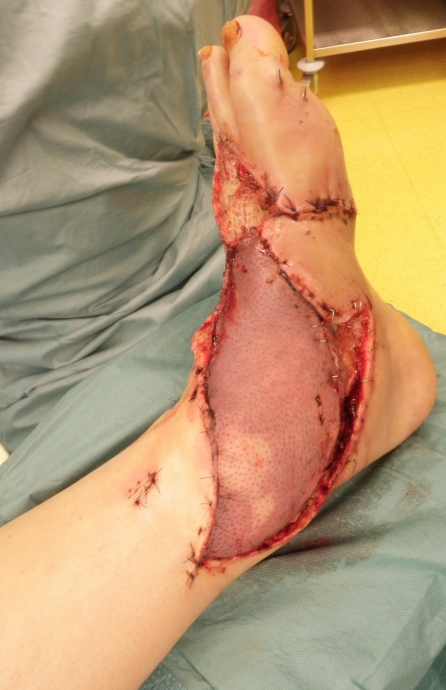
Figure 3. Free flap salvage after thrombectomy and interposition of a vein graft.
Discussion
Thromboses after microvascular tissue transfer have technical and hematologic reasons. While operative standards have been clinically defined, internationally accepted standards in pharmacologic antithrombotic prophylaxis in reconstructive microsurgery have not been clearly outlined yet. However, there is great consent that surgical skills and experience of the reconstructive microsurgeon are pivotal factors for flap success and favourable outcomes.
International studies and our own data (pulse survey) have revealed that heparin is the most frequently used substance for antithrombotic prophylaxis in reconstructive microsurgery. Heparin is used pre-, intra- and postoperatively. When used as a single antithrombotic agent, heparin seems to be more effective compared to aspirin. Nevertheless, one has to keep in mind that the use of unfractioned heparin may induce grave complications such as HiT type II and therefore its indication and dosage has to be reevaluated on a day-to-day and clinical situation-oriented basis. In the case of suspected HiT type II, it is recommended to stop heparin immediately, switch to other antithrombotic agents (e.g. lepirudin etc.) and initiate HiT screening.
Overall, microvascular tissue transfer procedures have a high success rate and can be confidently applied even in adverse clinical situations. However, establishment of clinical algorithms and standards for antithrombotic prophylaxis in reconstructive microsurgery are of utmost importance. In our view, it is unlikely that a simple algorithm in microsurgery will be defining antithrombotic prophylaxis in general, rather, consideration and integration of the various pathogenetic aspects, comorbidities and risk factors will have to be done to define individual “standard operating procedures” for a given microsurgical scenario. National and international societies are working toward this goal and we are confident that applicable solutions can be found within reasonable amounts of time.
Notes
Meeting presentation
This work has been presented in part at the 29th Annual Meeting of the Deutschsprachige Arbeitsgemeinschaft fuer Mikrochirurgie (DAM, Zurich, 2007).
Competing interests
All authors disclose any financial and personal relationships with other people or organisations that could inappropriately influence (bias) the work. Examples of potential conflicts of interest include employment, consultancies, stock ownership, honoraria, paid expert testimony, patent applications/registrations, and grants or other funding.
References
- 1.Lorenzetti F, Kuokkanen H, von Smitten K, Asko-Seljavaara S. Intraoperative evaluation of blood flow in the internal mammary or thoracodorsal artery as a recipient vessel for a free TRAM flap. Ann Plast Surg. 2001;46(6):590–593. doi: 10.1097/00000637-200106000-00003. Available from: http://dx.doi.org/10.1097/00000637-200106000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Khouri RK, Cooley BC, Kunselman AR, Landis JR, Yeramian P, Ingram D, Natarajan N, Benes CO, Wallemark C. A prospective study of microvascular free-flap surgery and outcome. Plast Reconstr Surg. 1998;102(3):711–721. doi: 10.1097/00006534-199809030-00015. Available from: http://dx.doi.org/10.1097/00006534-199809030-00015. [DOI] [PubMed] [Google Scholar]
- 3.Greinacher A, Janssens U, Berg G, Böck M, Kwasny H, Kemkes-Matthes B, Eichler P, Völpel H, Pötzsch B, Luz M. Lepirudin (recombinant hirudin) for parenteral anticoagulation in patients with heparin-induced thrombocytopenia. Heparin-Associated Thrombocytopenia Study (HAT) investigators. Circulation. 1999;100(6):587–593. doi: 10.1161/01.cir.100.6.587. [DOI] [PubMed] [Google Scholar]
- 4.Warkentin TE, Greinacher A. Heparin-induced thrombocytopenia: recognition, treatment, and prevention: the Seventh ACCP Conference on Antithrombotic and Thrombolytic Therapy. Chest. 2004;126(3 Suppl):311S–337S. doi: 10.1378/chest.126.3_suppl.311S. Available from: http://dx.doi.org/10.1378/chest.126.3_suppl.311S. [DOI] [PubMed] [Google Scholar]
- 5.Markwardt F, Nowak G, Stürzebecher J. Clinical pharmacology of recombinant hirudin. Haemostasis. 1991;21 Suppl 1:133–136. doi: 10.1159/000216274. [DOI] [PubMed] [Google Scholar]
- 6.Bucha E, Nowak G, Czerwinski R, Thieler H. R-hirudin as anticoagulant in regular hemodialysis therapy: finding of therapeutic R-hirudin blood/plasma concentrations and respective dosages. Clin Appl Thromb Hemost. 1999;5(3):164–170. doi: 10.1177/107602969900500305. Available from: http://dx.doi.org/10.1177/107602969900500305. [DOI] [PubMed] [Google Scholar]
- 7.Lubenow N, Eichler P, Lietz T, Greinacher A Hit Investigators Group. Lepirudin in patients with heparin-induced thrombocytopenia - results of the third prospective study (HAT-3) and a combined analysis of HAT-1, HAT-2, and HAT-3. J Thromb Haemost. 2005;3(11):2428–2436. doi: 10.1111/j.1538-7836.2005.01623.x. Available from: http://dx.doi.org/10.1111/j.1538-7836.2005.01623.x. [DOI] [PubMed] [Google Scholar]
- 8.Pötzsch B, Madlener K, Seelig C, Riess CF, Greinacher A, Müller-Berghaus G. Monitoring of r-hirudin anticoagulation during cardiopulmonary bypass-assessment of the whole blood ecarin clotting time. Thromb Haemost. 1997;77(5):920–925. [PubMed] [Google Scholar]
- 9.Moser M, Ruef J, Peter K, Kohler B, Gulba DC, Paterna N, Nordt T, Kübler W, Bode C. Ecarin clotting time but not aPTT correlates with PEG-hirudin plasma activity. J Thromb Thrombolysis. 2001;12(2):165–169. doi: 10.1023/A:1012975522037. Available from: http://dx.doi.org/10.1023/A:1012975522037. [DOI] [PubMed] [Google Scholar]
- 10.Hafner G, Roser M, Nauck M. Methods for the monitoring of direct thrombin inhibitors. Semin Thromb Hemost. 2002;28(5):425–430. doi: 10.1055/s-2002-35282. Available from: http://dx.doi.org/10.1055/s-2002-35282. [DOI] [PubMed] [Google Scholar]
- 11.Lo SK, Lai L, Cooper JA, Malik AB. Thrombin-induced generation of neutrophil activating factors in blood. Am J Pathol. 1988;130(1):22–32. [PMC free article] [PubMed] [Google Scholar]
- 12.Glusa E. Vascular effects of thrombin. Semin Thromb Hemost. 1992;18(3):296–304. doi: 10.1055/s-2007-1002568. Available from: http://dx.doi.org/10.1055/s-2007-1002568. [DOI] [PubMed] [Google Scholar]
- 13.Hoffmann JN, Vollmar B, Inthorn D, Schildberg FW, Menger MD. The thrombin antagonist hirudin fails to inhibit endotoxin-induced leukocyte/endothelial cell interaction and microvascular perfusion failure. Shock. 2000;14(5):528–534. doi: 10.1097/00024382-200014050-00006. Available from: http://dx.doi.org/10.1097/00024382-200014050-00006. [DOI] [PubMed] [Google Scholar]
- 14.Farner B, Eichler P, Kroll H, Greinacher A. A comparison of danaparoid and lepirudin in heparin-induced thrombocytopenia. Thromb Haemost. 2001;85(6):950–957. [PubMed] [Google Scholar]
- 15.Ahmed I, Majeed A, Powell R. Heparin induced thrombocytopenia: diagnosis and management update. Postgrad Med J. 2007;83(983):575–582. doi: 10.1136/pgmj.2007.059188. Available from: http://dx.doi.org/10.1136/pgmj.2007.059188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Greinacher A, Völpel H, Janssens U, Hach-Wunderle V, Kemkes-Matthes B, Eichler P, Mueller-Velten HG, Pötzsch B. Recombinant hirudin (lepirudin) provides safe and effective anticoagulation in patients with heparin-induced thrombocytopenia: a prospective study. Circulation. 1999;99(1):73–80. doi: 10.1161/01.cir.99.1.73. [DOI] [PubMed] [Google Scholar]
- 17.Jokuszies A, Niederbichler A, Herold C, Dodic T, Vogt PM AWMF. Die aktuelle S3-Leitlinie zur Prophylaxe der venosen Thromboembolie und ihre Implikation fur die Plastische Chirurgie. [The current evidence-based guidelines regarding prophylaxis of venous thrombembolism and their relevance for plastic surgery]. Handchir Mikrochir Plast Chir. 2010;42(4):251–259. doi: 10.1055/s-0030-1249617. (Ger). [DOI] [PubMed] [Google Scholar]
- 18.Xipoleas G, Levine E, Silver L, Koch RM, Taub PJ. A survey of microvascular protocols for lower-extremity free tissue transfer I: perioperative anticoagulation. Ann Plast Surg. 2007;59(3):311–315. doi: 10.1097/SAP.0b013e31802fc217. Available from: http://dx.doi.org/10.1097/SAP.0b013e31802fc217. [DOI] [PubMed] [Google Scholar]
- 19.Ridgway EB, Kutz RH, Cooper JS, Guo L. New insight into an old paradigm: wrapping and dangling with lower-extremity free flaps. J Reconstr Microsurg. 2010;26(8):559–566. doi: 10.1055/s-0030-1263292. Available from: http://dx.doi.org/10.1055/s-0030-1263292. [DOI] [PubMed] [Google Scholar]
- 20.Isenberg JS, Siegal A, Sherman R. Quantitative evaluation of the effects of gravity and dependency on microvascular tissue transfer to the lower limb, with clinical applications. J Reconstr Microsurg. 1997;13(1):25–29. doi: 10.1055/s-2008-1063937. Available from: http://dx.doi.org/10.1055/s-2008-1063937. [DOI] [PubMed] [Google Scholar]
- 21.Peter FW, Büttemeyer R, Vogt PM, Hussmann J, Steinau HU. Thrombose- und Gewebeschutz in der Mikrovaskularchirurgie – Eine Übersicht. [Thrombosis and tissue protection in microvascular surgery – an overview]. Zentralbl Chir. 1997;122(10):844–851. (Ger). [PubMed] [Google Scholar]
- 22.Greenberg BM, Masem M, Wang YX, Rubin P, May JW., Jr Efficacy of intraarterial heparin in maintaining microvascular patency: an experimental model. Plast Reconstr Surg. 1991;87(5):933–940. doi: 10.1097/00006534-199105000-00020. Available from: http://dx.doi.org/10.1097/00006534-199105000-00020. [DOI] [PubMed] [Google Scholar]
- 23.Chen CM, Ashjian P, Disa JJ, Cordeiro PG, Pusic AL, Mehrara BJ. Is the use of intraoperative heparin safe? Plast Reconstr Surg. 2008;121(3):49e–53e. doi: 10.1097/01.prs.0000299267.84139.2a. Available from: http://dx.doi.org/10.1097/01.prs.0000299267.84139.2a. [DOI] [PubMed] [Google Scholar]
- 24.Chien W, Varvares MA, Hadlock T, Cheney M, Deschler DG. Effects of aspirin and low-dose heparin in head and neck reconstruction using microvascular free flaps. Laryngoscope. 2005;115(6):973–976. doi: 10.1097/01.MLG.0000163539.97485.F4. Available from: http://dx.doi.org/10.1097/01.MLG.0000163539.97485.F4. [DOI] [PubMed] [Google Scholar]
- 25.Ritter EF, Cronan JC, Rudner AM, Serafin D, Klitzman B. Improved microsurgical anastomotic patency with low molecular weight heparin. Reconstr Microsurg. 1998;14(5):331–336. doi: 10.1055/s-2007-1000186. Available from: http://dx.doi.org/10.1055/s-2007-1000186. [DOI] [PubMed] [Google Scholar]
- 26.Hsia J, Hamilton WP, Kleiman N, Roberts R, Chaitman BR, Ross AM. A comparison between heparin and low-dose aspirin as adjunctive therapy with tissue plasminogen activator for acute myocardial infarction. Heparin-Aspirin Reperfusion Trial (HART) Investigators. N Engl J Med. 1990;323(21):1433–1437. doi: 10.1056/NEJM199011223232101. Available from: http://dx.doi.org/10.1056/NEJM199011223232101. [DOI] [PubMed] [Google Scholar]
- 27.Davies DM. A world survey of anticoagulation practice in clinical microvascular surgery. Br J Plast Surg. 1982;35(1):96–99. doi: 10.1016/0007-1226(82)90095-9. Available from: http://dx.doi.org/10.1016/0007-1226(82)90095-9. [DOI] [PubMed] [Google Scholar]
- 28.Salemark L, Wieslander JB, Dougan P, Arnljots B. Adverse effects of topical prostacyclin application in microvascular surgery: an experimental study. J Reconstr Microsurg. 1991;7(1):27–30. doi: 10.1055/s-2007-1006761. Available from: http://dx.doi.org/10.1055/s-2007-1006761. [DOI] [PubMed] [Google Scholar]
- 29.Salemark L, Wieslander JB, Dougan P, Arnljots B. Effect of low and ultra low oral doses of acetylsalicylic acid in microvascular surgery. An experimental study in the rabbit. Scand J Plast Reconstr Surg Hand Surg. 1991;25(3):203–211. doi: 10.3109/02844319109020620. Available from: http://dx.doi.org/10.3109/02844319109020620. [DOI] [PubMed] [Google Scholar]
- 30.Salemark L, Wieslander JB, Dougan P, Arnljots B. Studies of the antithrombotic effects of dextran 40 following microarterial trauma. Br J Plast Surg. 1991;44(1):15–22. doi: 10.1016/0007-1226(91)90170-O. Available from: http://dx.doi.org/10.1016/0007-1226(91)90170-O. [DOI] [PubMed] [Google Scholar]
- 31.Smit JM, Acosta R, Zeebregts CJ, Liss AG, Anniko M, Hartman EH. Early reintervention of compromised free flaps improves success rate. Microsurgery. 2007;27(7):612–616. doi: 10.1002/micr.20412. Available from: http://dx.doi.org/10.1002/micr.20412. [DOI] [PubMed] [Google Scholar]
- 32.Evans GR, Gherardini G, Gürlek A, Langstein H, Joly GA, Cromeens DM, Sukumaran AV, Williams J, Kilbourn RG, Wang B, Lundeberg T. Drug-induced vasodilation in an in vitro and in vivo study: the effects of nicardipine, papaverine, and lidocaine on the rabbit carotid artery. Plast Reconstr Surg. 1997;100(6):1475–1481. doi: 10.1097/00006534-199711000-00015. Available from: http://dx.doi.org/10.1097/00006534-199711000-00015. [DOI] [PubMed] [Google Scholar]
- 33.Khouri RK, Sherman R, Buncke HJ, Jr, Feller AM, Hovius S, Benes CO, Ingram DM, Natarajan NN, Sherman JW, Yeramian PD, Cooley BC. A phase II trial of intraluminal irrigation with recombinant human tissue factor pathway inhibitor to prevent thrombosis in free flap surgery. Plast Reconstr Surg. 2001;107(2):408–415. doi: 10.1097/00006534-200102000-00016. Available from: http://dx.doi.org/10.1097/00006534-200102000-00016. [DOI] [PubMed] [Google Scholar]
- 34.Sadrian R, Niederbichler AD, Friedman J, Vogt PM, Steinau HU, Reece G, Chang D, Robb G, Evans GR. Intraarterial chemotherapy: the effects on free-tissue transfer. Plast Reconstr Surg. 2002;109(4):1254–1258. doi: 10.1097/00006534-200204010-00007. Available from: http://dx.doi.org/10.1097/00006534-200204010-00007. [DOI] [PubMed] [Google Scholar]
- 35.Vogt PM, Steinau HU, Spies M, Kall S, Steiert A, Boorboor P, Vaske B, Jokuszies A. Outcome of simultaneous and staged microvascular free tissue transfer connected to arteriovenous loops in areas lacking recipient vessels. Plast Reconstr Surg. 2007;120(6):1568–1575. doi: 10.1097/01.prs.0000282102.19951.6f. Available from: http://dx.doi.org/10.1097/01.prs.0000282102.19951.6f. [DOI] [PubMed] [Google Scholar]
- 36.Peter FW, Franken RJ, Wang WZ, Anderson GL, Schuschke DA, O'Shaughnessy MM, Banis JC, Steinau HU, Barker JH. Effect of low dose aspirin on thrombus formation at arterial and venous microanastomoses and on the tissue microcirculation. Plast Reconstr Surg. 1997;99(4):1112–1121. doi: 10.1097/00006534-199704000-00030. Available from: http://dx.doi.org/10.1097/00006534-199704000-00030. [DOI] [PubMed] [Google Scholar]
- 37.Thum J, Caspary L, Creutzig A, Alexander K. Intra-arterial and intravenous administration of prostaglandin E1 cause different changes to skin microcirculation in patients with peripheral arterial occlusive disease. Vasa. 1998;27(2):100–105. [PubMed] [Google Scholar]
- 38.Eddy CA, Laufe LE, Dunn RL, Gibson JW. The use of prostacyclin analogue-containing suture for the prevention of postoperative venous thrombosis in the rat. Plast Reconstr Surg. 1986;78(4):504–512. doi: 10.1097/00006534-198610000-00012. Available from: http://dx.doi.org/10.1097/00006534-198610000-00012. [DOI] [PubMed] [Google Scholar]
- 39.Schellong S, Altmann E, von Bilderling P, Rudofsky G, Waldhausen P, Rogatti W. Microcirculation and tolerability following i.v. infusion of PGE1 and iloprost: a randomized cross-over study in patients with critical limb ischemia. Prostaglandins Leukot Essent Fatty Acids. 2004;70(6):503–509. doi: 10.1016/j.plefa.2003.10.006. Available from: http://dx.doi.org/10.1016/j.plefa.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 40.Yamamoto J, Ishii I, Sasaki Y, Nagamatsu Y, Matsuda T, Ando E. Antithrombotic effect of ticlopidine on He-Ne laser-induced thrombus formation in rat mesenteric microvessels. Haemostasis. 1992;22(3):147–152. doi: 10.1159/000216311. [DOI] [PubMed] [Google Scholar]
- 41.Qian S, Iwai T. Effect of ticlopidine on the cutaneous circulation in peripheral vascular disease. Angiology. 1993;44(8):627–631. doi: 10.1177/000331979304400806. Available from: http://dx.doi.org/10.1177/000331979304400806. [DOI] [PubMed] [Google Scholar]
- 42.Limet R, David JL, Magotteaux P, Larock MP, Rigo P. Prevention of aorta-coronary bypass graft occlusion. Beneficial effect of ticlopidine on early and late patency rates of venous coronary bypass grafts: a double-blind study. J Thorac Cardiovasc Surg. 1987;94(5):773–783. [PubMed] [Google Scholar]
- 43.Murthy P, Riesberg MV, Hart S, Bustillo A, Duque CS, Said S, Civantos FJ. Efficacy of perioperative thromboprophylactic agents in the maintenance of anastamotic patency and survival of rat microvascular free groin flaps. Otolaryngol Head Neck Surg. 2003;129(3):176–182. doi: 10.1016/S0194-5998(03)00603-X. Available from: http://dx.doi.org/10.1016/S0194-5998(03)00603-X. [DOI] [PubMed] [Google Scholar]
- 44.Ritter EF, Cronan JC, Rudner AM, Serafin D, Klitzman B. Improved microsurgical anastomotic patency with low molecular weight heparin. J Reconstr Microsurg. 1998;14(5):331–336. doi: 10.1055/s-2007-1000186. Available from: http://dx.doi.org/10.1055/s-2007-1000186. [DOI] [PubMed] [Google Scholar]
- 45.Li X, Cooley BC, Fowler JD, Gould JS. Intravascular heparin protects muscle flaps from ischemia/reperfusion injury. Microsurgery. 1995;16(2):90–93. doi: 10.1002/micr.1920160209. Available from: http://dx.doi.org/10.1002/micr.1920160209. [DOI] [PubMed] [Google Scholar]
- 46.Walenga JM, Pifarre R, Hoppensteadt DA, Fareed J. Development of recombinant hirudin as a therapeutic anticoagulant and antithrombotic agent: some objective considerations. Semin Thromb Hemost. 1989;15(3):316–333. doi: 10.1055/s-2007-1002724. Available from: http://dx.doi.org/10.1055/s-2007-1002724. [DOI] [PubMed] [Google Scholar]
- 47.Lewis CM, Deschler DG. Desirudin reduces the rate of microvenous thrombosis in a rat model. Laryngoscope. 2008;118(7):1149–1152. doi: 10.1097/MLG.0b013e31816d727c. Available from: http://dx.doi.org/10.1097/MLG.0b013e31816d727c. [DOI] [PubMed] [Google Scholar]
- 48.Sorg H, Hoffmann JN, Menger MD, Lindenblatt N, Goehring P, Vollmar B. Antithrombin is as effective as heparin and hirudin to prevent formation of microvascular thrombosis in a murine model. Thromb Haemost. 2006;96(3):371–377. [PubMed] [Google Scholar]
- 49.Krapohl BD, Zins JE, Siemionow M. Verbesserung der Mikrozirkulation von Muskellappen durch Gewebsplasminogenaktivator im Ratten-Cremaster-Muskellappenmodell. [Improving microcirculation of muscle flaps by tissue plasminogen activator in the rat cremaster muscle flap model]. Handchir Mikrochir Plast Chir. 2000;32(3):187–192. doi: 10.1055/s-2000-10921. (Ger). Available from: http://dx.doi.org/10.1055/s-2000-10921. [DOI] [PubMed] [Google Scholar]
- 50.Yii NW, Evans GR, Miller MJ, Reece GP, Langstein H, Chang D, Kroll SS, Wang B, Robb GL. Thrombolytic therapy: what is its role in free flap salvage? Ann Plast Surg. 2001;46(6):601–604. doi: 10.1097/00000637-200106000-00005. Available from: http://dx.doi.org/10.1097/00000637-200106000-00005. [DOI] [PubMed] [Google Scholar]
- 51.Dagher FJ, Slim MS, Abraham E, Meneshian G. Effect of dextrans on small arterial anastomosis. Arch Surg. 1971;103(5):581–584. doi: 10.1001/archsurg.1971.01350110079012. Available from: http://dx.doi.org/10.1001/archsurg.1971.01350110079012. [DOI] [PubMed] [Google Scholar]
- 52.Fadhli HA, Fine DP, Mazuji MK. Intra-arterial infusion of dextran. A new concept to prevent sludging and thrombosis in vascular surgery. J Thorac Cardiovasc Surg. 1967;53(4):496–499. [PubMed] [Google Scholar]
- 53.Farina JA, Jr, Piccinato CE, Campos AD, Rossi MA. Comparative study of isovolemic hemodilution with 3% albumin, dextran-40, and prophylactic enoxaparin (LMWH) on thrombus formation at venous microanastomosis in rats. Microsurgery. 2006;26(6):456–464. doi: 10.1002/micr.20270. Available from: http://dx.doi.org/10.1002/micr.20270. [DOI] [PubMed] [Google Scholar]
- 54.Nolte D, Bayer M, Lehr HA, Becker M, Krombach F, Kreimeier U, Messmer K. Attenuation of postischemic microvascular disturbances in striated muscle by hyperosmolar saline dextran. Am J Physiol. 1992;263(5 Pt 2):H1411–H1416. doi: 10.1152/ajpheart.1992.263.5.H1411. [DOI] [PubMed] [Google Scholar]