Abstract
Background
Cholestatic liver diseases can be caused by genetic defects, drug toxicities, hepatobiliary malignancies or obstruction of the biliary tract. Cholestasis leads to accumulation of bile acids (BAs) in hepatocytes. Direct toxicity of BAs is currently the most accepted hypothesis for cholestatic liver injury. However, information on which bile acids are actually accumulating during cholestasis is limited.
Aims
Assess BA composition in liver and serum after bile duct ligation (BDL) in male C57Bl/6 mice between 6 h and 14 days and evaluate toxicity of most abundant BAs.
Results
BA concentrations increased in liver (27-fold) and serum (1400-fold) within 6 h after surgery and remained elevated up to 14 days. BAs in livers of BDL mice became more hydrophilic than sham controls, mainly due to increased 6β-hydroxylation and taurine conjugation. Among the 8 unconjugated and 16 conjugated BAs identified in serum and liver, only taurocholic acid (TCA), β-muricholic acid (βMCA) and TβMCA were substantially elevated representing >95% of these BAs over the entire time course. Although glycochenodeoxycholic acid and other conjugated BAs increased in BDL animals, the changes were several orders of magnitude lower compared to TCA, βMCA and TβMCA. A mixture of these BAs did not cause apoptosis or necrosis but induced inflammatory gene expression in cultured murine hepatocytes.
Conclusion
The concentrations of cytotoxic BAs are insufficient to cause hepatocellular injury. In contrast, TCA, βMCA and TβMCA are able to induce pro-inflammatory mediators in hepatocytes. Thus, BAs act as inflammagens and not as cytotoxic mediators after BDL in mice.
Keywords: bile acid toxicity, obstructive cholestasis, bile duct ligation, inflammation
INTRODUCTION
Cholestatic liver diseases can be caused by genetic defects, drug toxicities, hepatobiliary malignancies or bile duct obstruction by gallstones (1). Acute and chronic cholestasis causes hepatocellular injury, bile duct proliferation, fibrosis, cirrhosis, and eventually liver failure (2). The reduction or complete blockage of bile flow during cholestasis results in the hepatic retention of products normally excreted into bile, such as cholesterol and bile acids (BAs). Some of the BAs, especially hydrophobic BAs, can cause cell injury in cultured hepatocytes (3). Therefore, the predominant hypothesis of the mechanism of hepatocellular injury during cholestasis assumes that BAs accumulating in hepatocytes are the main cause of cell death (3). Although BAs such as taurolithocholic acid (TLCA), deoxycholic acid (DCA), glycodeoxycholic acid GDCA), and glycochenodeoxycholic acid (GCDCA) can cause necrosis through promotion of mitochondrial oxidant stress (4), the more recent focus was on apoptotic cell death by these BAs (3,5). Consistent with a role of apoptotic cell death, exposure of a hepatocyte cell line to GCDCA stimulates apoptosis that is prevented by the pan-caspase inhibitor, z-VAD-FMK (6). Pretreatment of mice with z-VAD-FMK, however, does not prevent hepatocellular injury after bile duct ligation (BDL), an animal model of cholestasis, indicating that apoptosis is not important for liver injury in vivo during obstructive cholestasis (7). In addition, neither in rat nor mouse models of BDL was there morphological evidence of apoptosis or activation of caspases (7-10). The reason for the discrepancy between in vitro and in vivo studies is not fully understood, however, in vitro studies generally involve high concentrations of a specific BA whereas hepatocytes in vivo are exposed to a mixture of pro- and anti-apoptotic BAs (9). Most importantly, because of the limited knowledge what specific BAs accumulate in hepatocytes or serum during cholestasis, the selection of BAs for in vitro studies is more based on achieving a toxic effect rather than on which BAs hepatocytes are exposed to in vivo.
In contrast to direct cytotoxicity by BAs, it was hypothesized that hepatocellular injury during cholestasis results from an inflammatory response initiated by pro-inflammatory mediators released from hepatocytes exposed to pathological concentrations of BAs (11). Consistent with this hypothesis, cholestatic liver injury is substantially reduced in bile duct-ligated mice that are deficient in CD18 or intercellular adhesion molecule-1 (ICAM-1) (12,13). These findings suggest that most of the injury is actually caused by neutrophils not directly by BAs. Furthermore, exposure of primary mouse hepatocytes to taurocholic acid (TCA) does not cause cell death but upregulates ICAM-1 and stimulates release of pro-inflammatory chemokines, both of which are important for neutrophil-dependent injury during cholestasis (11).
In both hypotheses, it is proposed that BAs are the key mediators initiating apoptosis or instigating production of inflammatory mediators. Whether concentrations of BAs that stimulate these processes are increased in vivo during cholestasis, however, is not known. Accordingly, the purpose of the present study was to quantify concentrations of individual BAs in serum and livers of mice subjected to bile duct ligation and to assess if the BAs with the highest concentrations affect viability of hepatocytes. This information could provide important reference data for the interpretation of past in vitro studies and for future in vivo and cell culture experiments aimed at studying hepatocellular injury during cholestasis.
MATERIALS AND METHODS
Chemicals and Reagents
BA standards were either purchased from Steraloids, Inc. (Newport, RI), Sigma-Aldrich (St Louis, MO), or synthesized in our lab (14). All other chemicals, unless indicated, were purchased from Sigma-Aldrich (St. Louis, MO).
Animal Experiments
Adult male C57BL/6 mice were purchased from Jackson Laboratories (Bar Habor, ME). All mice were fed a Teklad Rodent Diet #8604 (Harlan Laboratories, Madison, WI) ad libitum, and housed according to the American Animal Association Laboratory Animal Care guidance. Studies were approved by the University of Kansas Medical Center Institutional Animal Care and Use Committee. Under Nembutal anesthesia (10% Nembutal in saline), the abdominal cavity was opened and the common bile duct was ligated twice with 5-0 surgical silk and the bile duct was cut between the ligatures. The gall bladder was left intact. The abdominal muscle was sutured with Ethicon 5-0 dissolvable suture material and the wound was closed with surgical staples. Sham surgeries were performed similarly without BDL. Serum and livers (gallbladder removed) were collected 6 h, 12 h, 1 d, 2 d, 3 d, 5 d, 7 d, and 14 d after BDL. Livers were snap frozen in liquid nitrogen and stored at −80 °C until analysis.
Bile Acid Extraction and Quantification
Serum BA extraction and quantification were described previously (15). Liver BA concentrations were quantified by a recent method using ultra performance liquid chromatography- tandem mass spectrometry (UPLC/MS/MS) (14). BA stock solutions were diluted with 50% methanol and spiked with internal standards (2H4-GCDCA and 2H4-CDCA) to construct standard curves between 5 and 20,000 ng/ml. All standard curves were constructed using a 1/concentration2 weighted quadratic regression, and the correlation coefficient (r2) for all BAs was above 0.99. The limit of detection (signal/noise ratio=3) for the various BAs was in the range of 5-10 ng/ml, which equals 0.01-0.02 nmol/ml. For a preliminary analysis of hydroxylated BA, we obtained taurine-conjugated and unconjugated 3α,6α,7α,12α-tetrahydroxyl BA from Dr. Mary Vore (University of Kentucky, Lexington, KY). We optimized and obtained the multiple reaction monitoring (MRM) conditions for these two standards. Then we used the same MRM conditions to obtain chromatographs of tetrahydroxy BAs in BDL samples.
Total RNA Isolation
Total RNA was isolated using RNA-Bee reagent (Tel-Test Inc., Friendswood, TX) according to the manufacturer’s protocol. Total RNA concentrations were quantified spectrophotometrically at 260 nm. One microgram per microliter solutions were prepared by dilution in diethyl pyrocarbonate-treated deionized water. Integrity of RNA samples was determined by formaldehyde-agarose gel electrophoresis with visualization by ethidium bromide fluorescence under ultraviolet light.
Multiplex Suspension Array
Liver mRNA was quantified by mutiplex suspension array (Panomics-Affymetrix, Inc., Fremont, CA). Individual gene accession numbers can be accessed at www.panomics.com (sets #21021 and #21151). Samples were analyzed using a Bio-Plex 200 System Array reader with Luminex 100 xMAP, and the data were acquired using Bio-Plex Data Manager version 5.0 (Bio-Rad, Hercules, CA). Assays were performed according to each manufactures’ protocol. During the preliminary experiment, the mRNA of three housekeeping genes, namely ribosomal protein 13a (Rpl13a), glyceraldehyde 3-phosphate dehydrogenase (Gapdh), and beta-actin (β-actin) were quantified in livers of BDL mice. BDL did not alter Rpl13a or Gapdh, but increased β-actin in mouse livers. Rpl13a in BDL mice appeared to be more consistent than Gapdh. Therefore, RNA data in the present study are presented as relative light units (RLU) normalized to Rpl13a mRNA.
Bile acid toxicity in isolated hepatocytes
Hepatocytes were isolated from C57BL/6 mice and treated with a mixture of bile acids for 6 h as described (11). The bile acids used in the mixture were 341 μM βMCA, 1 mM TβMCA, and 1 mM TCA. This approximates the serum concentrations measured at 6 h after BDL. Only bile acids with concentrations >50 μM were considered and included in the mixture. As positive control for caspase-dependent apoptosis, cells were treated with 5 mM galactosamine and 100 ng/ml recombinant murine TNF-α (Genzyme, Cambridge, MA) for 6 h (16). Caspase-3 activity based on Z-VAD-fmk inhibitable Ac-DEVD-AMC fluorescence was measured as described (17) and alanine aminotransferase (ALT) activity was determined in the cells and in the culture supernatant using a commercial kit (18). The mRNA levels of proinflammatory genes in cultured hepatocytes were determined as described in detail (11).
Statistical Analysis
Data are represented as Mean ± SEM (n=5-6). Differences between mean values were tested for statistical significance (P<0.05) by the two tailed Student’s t-test.
RESULTS
Serum BA Concentrations
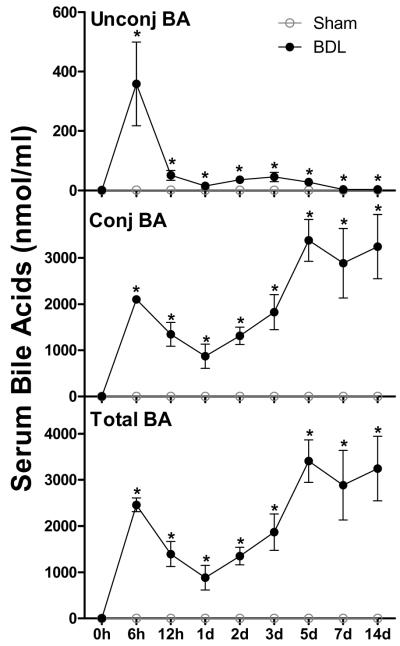
Total BA concentrations in mouse serum were quantified at 0 h, 6 h, 12 h, 1 d, 2 d, 3 d, 5 d, 7 d, and 14 d after sham and BDL surgeries (Figure 1). Sham-operated mice maintained their serum BA concentrations in a range of 0.5 to 2.3 nmol/ml during the study. After BDL, unconjugated and conjugated BAs in mouse serum increased 300- and 4000-fold, respectively, within 6 h. Thereafter, unconjugated BAs in serum of BDL mice decreased about 99% between 6 h and 14 d, but were still about 3-fold higher than sham-operated mice at 14 d. In contrast, conjugated BAs in serum of BDL mice decreased about 60% between 6 h and 1 d followed by a tripling between 1 and 5 d, and were maintained at concentrations of ~3000 nmol/ml thereafter. Two weeks after surgery, more than 99% of BAs in serum of BDL mice were conjugated, whereas only 60% of BAs in serum of sham-operated mice were conjugated. Thus, changes in total BA concentration in serum of BDL mice followed a similar kinetics as conjugated BAs (Figure 1).
Figure 1.
Unconjugated -, conjugated -, and total bile acid (BA) concentrations in serum of sham-operated controls and bile duct ligated (BDL) mice. All BA data are expressed as mean ± S.E. of six mice in each group. *P<0.05 [sham-operated controls (open circles) vs BDL mice (closed circles)].
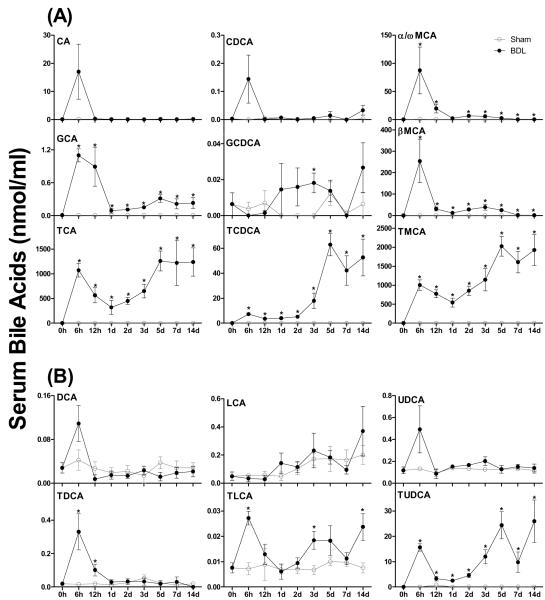
The major primary BAs in serum of sham-operated mice were CA, TCA, α/ωMCA, βMCA, and TMCA, which all ranged in concentration between 0.2 – 1.4 nmol/ml (Figure 2A). After BDL, most primary BAs, except GCDCA, underwent a marked increase at 6 h followed by a rapid decrease from 6 h to 1 d. After that, serum CA and CDCA in BDL mice decreased to the sham level, whereas α/ωMCA, βMCA and GCA were maintained at slightly higher concentration than sham controls. In contrast, TCA, TCDCA, and TMCA increased markedly from 1 d to 5 d. As a result, after 1d of BDL, TCA and TMCA are the predominant conjugated BAs identified in mouse serum.
Figure 2.
Individual primary bile acids (BAs) (A) and secondary BAs (B) in serum of sham-operated controls and bile duct ligated (BDL) mice. All BA data are expressed as mean ± S.E. of six mice in each group. *P<0.05 [sham-operated controls (open circles) vs BDL mice (closed circles)].
Figure 2B shows the time course of secondary BAs in serum of mice after surgery. The predominant secondary BAs in serum of sham-operated mice were TDCA (0.02 - 0.05 nmol/ml) and UDCA (0.09 - 0.13 nmol/ml). BDL had little effect on DCA, TDCA, LCA, and UDCA levels, although it increased TDCA and TLCA mainly at 6 h after surgery. Interestingly, BDL caused a marked increase in TUDCA concentrations at 6 h; this was followed by a decrease of TUDCA levels from 12 h to 2 d and another increase thereafter (Figure 2B). Therefore, after BDL, TUDCA became the predominant secondary BA in mouse serum with levels between 10- and 100-fold higher than all other secondary BAs. Whereas the early spike of secondary BAs at 6 h may be due to absorption of residual BAs from the intestine and reduced clearance by the liver, the later increase in UDCA may originate from the liver. Consistent with previous data (14), glucuronidation and sulfation are only minor metabolic pathways for BAs in livers of mice also after BDL (data not shown).
Liver BA Concentrations
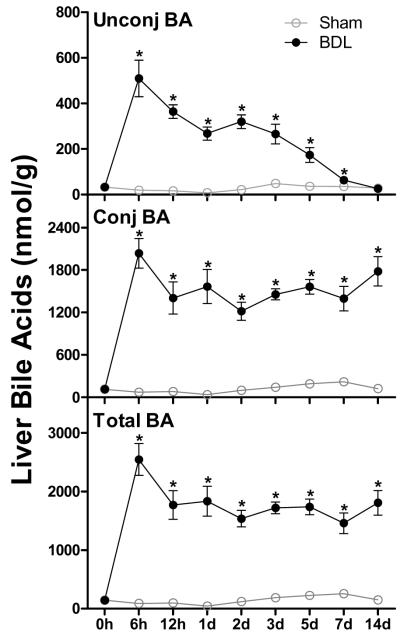
Sham-operated mice maintained their liver BAs in a range of 55 to 254 nmol/g liver weight throughout the study, with about 80% of the BAs conjugated (Figure 3). At 6 h after BDL, unconjugated and conjugated BAs increased about 26- to 27-fold in the liver. Thereafter, unconjugated BAs gradually decreased between 12 h and 14 d after BDL with levels at 14 d being 90% lower than peak levels. In contrast, the hepatic content of conjugated BAs remained close to peak levels throughout the entire time course. As a result, the percentage of conjugated BAs in livers of BDL mice increased from 80% at 6 h to 99% at 14 d. Mainly due to the increase in conjugated BAs, the total BA concentration in livers of mice increased about 27-fold at 6 h after BDL and remained at 70-80% of peak levels during the course of the study (Figure 3).
Figure 3.
Unconjugated - , conjugated - , and total bile acid (BA) concentrations in livers of sham-operated controls and bile duct ligated (BDL) mice. All BA data are expressed as mean ± S.E. for six mice in each group. *P<0.05 [sham-operated controls (open circles) vs BDL mice (closed circles)].
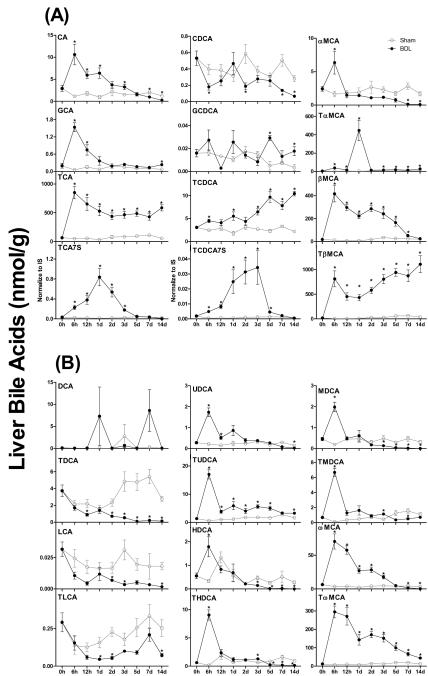
The major primary BAs in livers of sham-operated mice were TCA and TβMCA (Figure 4A). After 6 h of BDL, major increases in BA levels were observed for CA (9-fold), GCA (14-fold), and TCA (17-fold). CA and GCA decreased to sham levels within 2 d after BDL, whereas TCA underwent a decrease from 6 h to 1 d, but was maintained at higher concentrations than sham controls thereafter. BDL had little effect on CDCA and GCDCA, whereas it gradually increased TCDCA concentrations about 4 fold within two weeks. After BDL, the concentration of αMCA in the liver underwent a 3-fold increase at 6 h, followed by a decrease to sham concentrations at 12 h, and became lower than sham controls after 7 d. In contrast, TαMCA in livers of mice increased about 12-fold at 6 h after BDL, and remained at higher levels than sham controls thereafter. After BDL, βMCA increased about 35-fold at 6 h, and thereafter decreased gradually to similar concentrations as in livers of sham-operated mice after 7 d. In contrast, TβMCA in livers of BDL mice underwent a 150-fold increase at 6 h, followed by a 45% decrease from 6 h to 1 d, and increased gradually about 1.5-fold from 1 to 14 d. TCA7S and TCDCA7S were the two predominant BA sulfates in livers of sham-operated mice. After BDL, both TCA7S and TCDCA7S increased about 17-fold during the first 3 days, but thereafter decreased rapidly to similar concentrations as in the livers of sham-operated mice. Therefore, sulfation is not a prominent pathway of BA metabolism in livers of BDL mice. Although 9 primary BAs were identified in livers of BDL mice, TCA, βMCA and TβMCA together are the predominant primary BAs identified throughout the study (Figure 4A).
Figure 4.
Individual primary bile acids (BAs) (A) and secondary BAs (B) in livers of sham-operated controls and bile duct ligated (BDL) mice. All BA data are expressed as mean ± S.E. for six mice in each group. *P<0.05 [sham-operated controls (open circles) vs BDL mice (closed circles)].
The predominant secondary BAs in livers of sham-operated mice were TωMCA and TDCA (Figure 4B). BDL had little effect on DCA concentration in liver, but significantly decreased TDCA, LCA, and TLCA after 1 d. Within 6 h after BDL, UDCA, HDCA, and MDCA increased about 5- to 8-fold, whereas TUDCA, THDCA, and TMDCA increased about 27- to 66-fold in livers of mice. Among these secondary BAs, UDCA, HDCA, THDCA, MDCA, and TMDCA decreased to similar concentrations as sham controls within 1 d, and became lower than sham controls after 5 d. In contrast, TUDCA underwent a 75% decrease between 6 h and 12 h, but remained at higher levels than sham controls thereafter. After BDL, ωMCA increased about 18-fold at 6 h, and decreased rapidly to become lower than sham controls after 5 d. In contrast, TωMCA in livers of BDL mice increased about 46-fold at 6 h, decreased thereafter, but remained about 3-fold higher than sham controls at the end of two weeks. Therefore, the predominant secondary BAs in livers of BDL mice were TUDCA, ωMCA, and in particular TωMCA (Figure 4B).
BA Transporters and Synthetic Enzymes
The impact of BDL on mRNA expression of BA-uptake and –efflux transporters and enzymes involved in BA synthesis were determined (Suppplemental Figure 1-3). In general, the BA-uptake transporters Ntcp, Oatp1a1, Oatp1a4, and Oatp1b2 in mouse liver showed a minor increase at 6 h after BDL but then were consistently downregulated (except Oatp1a4) during chronic BDL (Supplemental Figure 1). In contrast, BDL had little effect on the canalicular BA-efflux transporters, such as Bsep, Mrp2, and Bcrp (Supplemental Figure 2). However, BDL had limited effects on Ostα (data not shown) or Mrp3, whereas it significantly increased Ostβ and Mrp4 (Supplemental Figure 2). Cyp7a1 is the rate-limiting enzyme of BA synthesis. Cyp7a1 appeared to vary markedly in livers of sham-operated mice and no consistent difference to BDL livers was observed (Supplemental Figure 3). In contrast, Cyp8b1, which is the critical enzyme (12α-hydroxylase) for CA biosynthesis and Cyp7b1, which is a critical enzyme for the alternative pathway of BA biosynthesis, were down-regulated at all times after BDL. However, Cyp27a1, which is involved in both the classic and alternative pathways of BA biosynthesis, was largely unaffected by BDL (Supplemental Figure 3).
Liver Inflammation
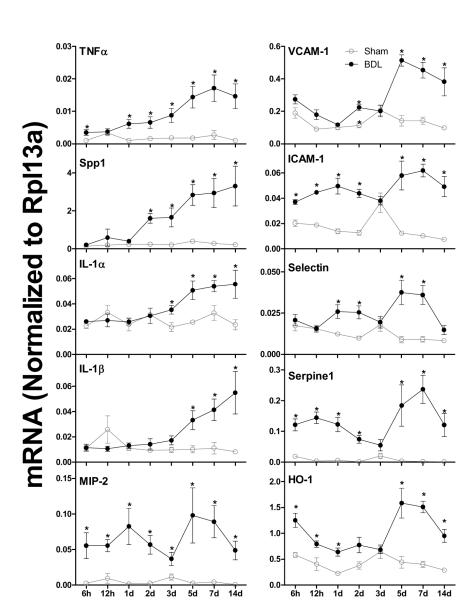
The mRNA expression of ten pro-inflammatory markers TNFα, Spp1, IL-1α, IL-1β, MIP-2, VCAM-1, ICAM-1, Selectin, Serpine1, and HO-1 are shown in Figure 5. After BDL, TNFα (2.5-fold) and Spp1 (6-fold) increased from 1 to 5 d, and remained at higher levels than sham controls thereafter. Similarly, IL-1α and IL-1β in livers of BDL mice increased from 3 to 5 d, and remained at higher levels than sham controls thereafter. In contrast, MIP-2 in livers of BDL mice was about 25-fold higher than sham controls 6 h after surgery. VCAM-1 in BDL mice increased about 150% at 5 d and remained at higher levels than sham controls thereafter. In contrast, ICAM-1, Selectin, Serpine1 and HO-1 in BDL mice underwent an initial increase at 6 h, and a second increase at 5 d. Taken together, BDL resulted in an early and sustained increase of inflammation markers in mouse livers (Figure 5).
Figure 5.
The mRNA expression of inflammatory genes in livers of sham-operated controls and bile duct ligated (BDL) mice. Total RNA from livers of sham-operated (open circles) and BDL (closed circles) mice were analyzed by multiplex suspension array. The mRNA level of each gene was normalized to Rpl13a. All data are expressed as mean ± S.E. for six mice in each group. *P<0.05 [sham-operated controls (open circles) vs BDL mice (closed circles)].
Proinflammatory Effects of Bile Acids
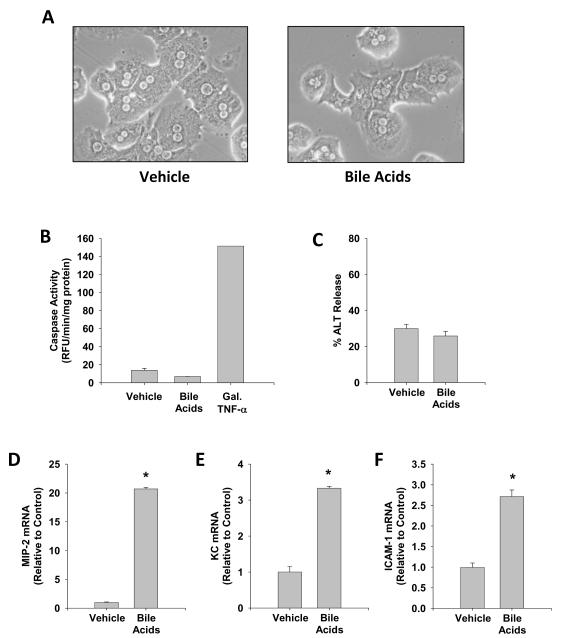
Although our previous study demonstrated that 200 μM TCA, DCA or CDCA induced chemokines in cultured mouse hepatocytes without causing cell death (11), our current studies indicate that TCA, TβMCA and βMCA increase to levels between 300-1000 μM in serum at 6 h after BDL (Figure 2). Therefore, we tested if a mixture of the 3 bile acids with the highest concentrations are able to cause cell injury or induce proinflammatory genes (Figure 6). Compared to cells treated with vehicle (saline), cells exposed to a mixture of TCA (1 mM), TβMCA (1 mM) and βMCA (341 μM) did not show morphological evidence of injury (Figure 6A) and did not increase caspase-3 activity (Figure 6B) or release ALT (Figure 6C). Treatment with Gal/murine TNF-α, a positive control for apoptosis, resulted in a 11-fold increase of caspase-3 activity (Figure 6B). In contrast, the bile acid mixture induced the pro-inflammatory genes MIP-2 (21-fold), KC (>3-fold) and ICAM-1 (>3-fold) in hepatocytes (Figure 6D-F).
Figure 6.
Effect of a bile acid mixture on hepatocyte viability and inflammatory gene expression. Murine hepatocytes were isolated and treated with a mixture of 341 μM βMCA, 1 mM TβMCA, and 1 mM TCA (Bile Acids). As positive control for caspase-dependent apoptosis, cells were treated with 5 mM galactosamine (Gal) and 100 ng/ml recombinant murine TNF-α. Photomicrographs (200X) of cells treated with vehicle (saline) or bile acids (A) were taken 6 hours after treatment. Activities of caspase 3 (B) and ALT (C) were measured at 6 h. mRNA levels of the CXC chemokines MIP-2 (D) and KC (E) and of the adhesion molecule ICAM-1 (F) were measured at 6 hours. Data are expressed as mean ± S.E. of n = 3 experiments.
*P< 0.05 (compared to vehicle-treated hepatocytes).
DISCUSSION
The objective of this study was to analyze the BA composition in serum and liver after BDL with the major goal to establish which BAs actually increase during BDL and to what concentrations of these BAs hepatocytes are exposed to during obstructive cholestasis. Applying this information, murine hepatocytes were exposed to a mixture of BAs with the highest levels in serum and liver and cell viability and inflammatory gene expression were assessed. The data should provide an important reference point for past and future studies on mechanisms of hepatocellular injury during cholestasis.
The major BAs in serum and livers of sham-operated mice are TCA, βMCA, and TβMCA. These particular BAs also show the highest increase after BDL. Previous studies on BA analysis using GC or GC-MS also identified CA and βMCA (included both conjugated and unconjugated) as the most abundant BAs in BDL rats (19,20) and mice (21,22). Semi-quantitative analysis indicated the most exclusive abundance of taurine conjugates in BDL mice with negligible amounts of glycine conjugates (21,22). Interestingly, TUDCA, which is thought to be a secondary BA produced by intestinal bacteria (23), was present in livers of BDL mice at appreciable concentrations, suggesting that TUDCA may be synthesized by livers of BDL mice. The BA chromatogragh suggests that livers of BDL mice had a significant increase in polyhydroxy BAs, mainly taurine-conjugated tetrahydroxy BAs (data not shown). The accurate concentrations of polyhydroxy BAs were not given here due to lack of reference BAs, but the chromatograph peak area of one taurine-conjugated tetrahydroxy BA was increased about 300-fold in livers of BDL mice (data not shown). Marshall et al. (22) reported that polyhydroxy BAs were markedly elevated and consisted of about 6-10% of total BAs in serum and livers of wild-type mice 7 days after BDL. This suggests that hydroxylation is a pathway to detoxify high concentrations of trihydroxy BAs, such as TCA and TMCAs in livers of BDL mice. Glucuronidation and sulfation are thought to be important to detoxify high concentrations of BAs in liver (24,25). Consistent with a previous study (22), no significant differences were observed for the glucuronidated BAs between sham and BDL mice. Hofmann (26) has discussed the reasons why glucuronidation is not a major metabolic pathway for BAs. In the present study, sulfates of TCA and TCDCA in livers of BDL mice increased about 17-fold during the first 3 days, but thereafter decreased rapidly to similar concentrations as in the livers of sham mice. Thus, sulfation dose not appear to be an important pathway of BA detoxification in livers of BDL mice. This is consistent with the previous studies in BDL rats (19,27) and mice (22). Taken together, TCA, βMCA, and TβMCA are the predominant BAs in serum and livers of BDL mice, and hydroxylation is the major pathway of BA detoxification in BDL mice.
A large number of studies suggest that during cholestasis BAs produce hepatocellular injury in part by inducing apoptosis (3). It is proposed that this occurs through exposure of hepatocytes to pro-apoptotic BAs, including TLCA, DCA, GDCA and GCDCA (3,5). Exposure of hepatocytes to concentrations of 50 μM or above of these BAs is typically needed to stimulate apoptosis in cultured rodent hepatocytes (28-32). The present study demonstrates, however, that maximal concentrations of these BAs in serum of BDL mice are approximately 1000-fold less (i.e., TLCA: 24 nM; DCA: 130 nM; GDCA; 13.8 nM; GCDCA: 27 nM) than that needed to stimulate apoptosis in vitro. Even the intracellular levels of DCA or TDCA never exceeded 10 nmol/g liver (roughly 10 μM) and generally, the serum concentrations correlate closely with the tissue levels of all BAs. We showed previously that exposure to 200 μM DCA did not cause cell injury in hepatocytes (11). Furthermore, serum concentrations of anti-apoptotic BAs (31-35) (i.e., TCDCA: 83.5 μM; TUDCA: 26 μM) are approximately 1000-fold higher than pro-apoptotic BAs in serum of BDL mice. Accordingly in BDL mice, concentrations of pro-apoptotic BAs do not reach concentrations sufficient to stimulate hepatocyte apoptosis, indicating that prior in vitro studies do not accurately recapitulate what occurs in mice in vivo. In direct support of this conclusion, a mixture of the bile acids (TCA, TβMCA, βMCA) that reached the highest levels after BDL in vivo did not cause apoptosis or necrosis in isolated hepatocytes. Consistent with this, there is no evidence for caspase activation and relevant apoptotic cell death during BDL (7-10). Consequently, pan-caspase inhibitors do not affect hepatocellular injury after BDL (7).
Studies in BDL mice have demonstrated an important role for inflammation, especially neutrophils, in the mechanism of liver injury during cholestasis (12,13,18,36). It has been proposed that exposure of hepatocytes to pathological concentrations of BAs increases production of pro-inflammatory mediators that initiate an inflammatory response (11,18). Consistent with this, treatment of primary mouse hepatocytes with 200 μM TCA increased expression of cytokines, chemokines, adhesion molecules, and other proteins that influence immune cell levels and function in the liver (11). Our present studies demonstrated that concentrations of TCA, TβMCA and βMCA were increased to >300-1000 μM within 6 h after BDL. Exposure of isolated hepatocytes to a mixture of these in vivo relevant bile acids substantially induced pro-inflammatory genes including the CXC chemokines MIP-2 and KC and the adhesion molecule ICAM-1. Furthermore, mRNA levels of MIP-2 and ICAM-1, were significantly increased as early as 6 h after BDL in vivo consistent with previous findings (7). Both MIP-2 and ICAM-1 contribute to liver injury after BDL, and both are upregulated in primary mouse hepatocytes treated with 200 μM TCA (11,13,37). Collectively, these results support the hypothesis that during cholestasis, exposure of hepatocytes to pathological concentrations of BAs increases expression of pro-inflammatory mediators that initiate an inflammatory injury process.
The assessment of BA uptake and efflux transporter mRNA expression indicated that in general uptake transporters Oatp1a1, Oatp1b2 and Nctp are down-regulated after 1 d of BDL compared to sham-operated animals and alternative efflux transporters Mrp4 and Ostβ are up-regulated. In addition, key enzymes regulating BA synthesis, Cyp8b1 and Cyp 7b1, are consistently down-regulated after BDL. These changes indicate an adaptive response to BDL, which includes reduced BA synthesis, reduced uptake into the liver and enhanced export into the blood. These data are consistent with previous observations showing similar changes in mRNA and protein expression in various models of cholestasis (21,22,38-44). Interestingly, the modulation of transporters appears to be independent of the inflammatory response, suggesting that the accumulating BAs may be mainly responsible for these effects (44). Although we were not able to quantify polyhydroxy BAs, which can make up to 6-10% of total BAs in serum and liver after BDL (22), the fact that these BAs are hydrophilic and represent detoxification products of TCA and TMCA makes it highly unlikely that these BAs could have a cytotoxic effect.
An important issue is the comparison of our data in mice to BA levels in humans (45). In mice TCA, βMCA and TβMCA are the most abundant BAs in serum. However, in human control serum, CA, CDCA and its glycoconjugate GCDCA and DCA and its glycoconjugate GDCA are found at similar concentrations of 0.3-0.8 μM (45). Although the data for patients with cholestatic liver disease are not as detailed, total CDCA and CA (conjugated and unconjugated) show the highest increase in liver tissue after obstructive cholestasis (46). The levels reported in this study (70-400 nmol CDCA/g liver and 200-600 nmol CA/g liver) (46) are similar to the total CA levels, which are mostly TCA, quantified after BDL in mice (Figure 4). The major difference between humans and mice is the presence of high levels of MCA and very low concentrations of CDCA in mice. In patients with primary biliary cirrhosis, elevated serum levels of CA and CDCA have been reported (47,48). Levels of 30-40 μM of both CA and CDCA were measured in patients with the most severe disease status; the glycoconjugate representing 50-55% and the taurine conjugate 40-45% of the total CA and CDCA levels in serum (48). Although these clinical data provide the rationale for the use of 50 μM GCDCA in many in vitro studies (28-32), the mechanism of BA toxicity was investigated in rodent but not human hepatocytes. It takes even higher concentrations (500 μM) of GCDCA to cause significant cell injury in human hepatocytes (49). Thus, serum GCDCA concentrations measured under severe cholestatic conditions in humans may approach concentrations used in cell culture studies and may cause some direct toxicity. It is quite obvious from our studies that mouse hepatocytes are never exposed to μM concentrations of GCDCA and other potentially toxic BAs. It is therefore justified to conclude that a direct cytotoxic effect of BAs in the mouse BDL model is of limited relevance.
In summary, our detailed analysis of BA composition changes in liver and serum between 6 h and 14 days after BDL indicates that the concentration of only a limited number of mainly conjugated BAs such as TCA, βMCA and TβMCA are substantially elevated. These BAs consistently are the predominant BAs in serum and liver over the entire time course of observation. Although GCDCA and other conjugated BAs can increase in BDL animals compared to sham-operated controls, the changes are several orders of magnitude lower compared to TCA and TβMCA. These findings suggest that during BDL, the concentrations of cytotoxic BAs are insufficient to cause hepatocellular injury. In contrast, concentrations of TCA and TβMCA quantified in BDL animals are able to induce pro-inflammatory mediators in hepatocytes. Thus, based on this detailed BA composition analysis and the exposure of hepatocytes to pathophysiologically relevant BAs, we conclude that BAs act mainly as inflammagens and not as cytotoxic mediators in this murine model of obstructive cholestasis.
Supplementary Material
Supplemental Figure 1: The mRNA levels of bile acid uptake transporters in livers of sham-operated controls and bile duct ligated (BDL) mice. Total RNA from livers of sham-operated (open circles) and BDL (closed circles) mice were analyzed by multiplex suspension array. The mRNA level of each gene was normalized to Rpl13a. All data are expressed as mean ± S.E. for six mice in each group. *P<0.05 [sham-operated controls (open circles) vs BDL mice (closed circles)].
Supplemental Figure 2: The mRNA expression of canalicular (A) and basolateral (B) bile acid efflux transporters in livers of sham-operated controls and bile duct ligated (BDL) mice. Total RNA from livers of sham-operated (open circles) and BDL (closed circles) mice were analyzed by multiplex suspension array. The mRNA level of each gene was normalized to Rpl13a. All data are expressed as mean ± S.E. for six mice in each group. *P<0.05 [sham-operated controls (open circles) vs BDL mice (closed circles)].
Supplemental Figure 3: The mRNA expression of enzymes involved in bile acid (BA) synthesis in livers of sham-operated controls and bile duct ligated (BDL) mice. Total RNA from livers of sham-operated (open circles) and BDL (closed circles) mice were analyzed by multiplex suspension array. The mRNA level of each gene was normalized to Rpl13a. All data are expressed as mean ± S.E. for six mice in each group. *P<0.05 [sham-operated controls (open circles) vs BDL mice (closed circles)].
ACKNOWLEDGMENT
This investigation was supported in part by National Institutes of Health Grants AA12916 (H.J.), DK073566 (B.L.C.), and ES-009649, ES-013714, ES-009716, and RR-021940 (C.D.K.) and by a Center of Biomedical Research Excellence (COBRE) grant P20 RR021940 (PI: C.D.K.) from the National Center for Research Resources (NCRR), a component of the National Institutes of Health.
Abbreviations
- BA
bile acid
- Bcrp
breast cancer resistance protein
- Bsep
bile salt export pump
- CA
cholic acid
- CA7S
cholic acid 7-sulfate
- CDCA
chenodeoxycholic acid
- CDCA7S
chenodeoxycholic acid 7-sulfate
- Conj BA
conjugated bile acid
- Cyp
Cytochrome P450
- DCA
deoxycholic acid
- Gapdh
glyceraldehyde-3-phosphate dehydrogenase
- GCA
glycocholic acid
- CA
glycochenodeoxycholic acid
- GDCA
glycodeoxycholic acid
- HDCA
hyodeoxycholic acid
- HO-1
heme oxygenase 1
- Icam-1
intercellular adhesion molecule 1
- IL
interleukin
- LCA
lithocholic acid
- αMCA
α-muricholic acid
- βMCA
β-muricholic acid
- ωMCA
ω-muricholic acid
- MDCA
murideoxycholic acid
- Mip-2
macrophage-inflammatory protein-2
- Mrp
multidrug resistance-associated protein
- Ntcp
Na+-taurocholate cotransporting polypeptide
- Oatp
organic anion transporting polypeptide
- Ost
organic solute transporter
- Rpl13a
ribosomal protein L13a
- Serpine1
serpin peptidase inhibitor clade E, member 1
- Spp1
secreted phosphoprotein 1
- TCA
taurocholic acid
- TCDCA
taurochenodeoxycholic acid
- TDCA
taurodeoxycholic acid
- THDCA
taurohyodeoxycholic acid
- TLCA
taurolithocholic acid
- TαMCA
tauro-α-muricholic acid
- TβMCA
tauro-β-muricholic acid
- TωMCA
tauro-ω-muricholic acid
- TMDCA
tauromurideoxycholic acid
- TNFα
tumor necrosis factor alpha
- TUDCA
tauroursodeoxycholic acid
- UDCA
ursodeoxycholic acid
- Unconj BA
unconjugated bile acid
- Vcam-1
vascular cell adhesion molecule 1
REFERENCES
- 1.Hirschfield GM, Heathcote EJ, Gershwin ME. Pathogenesis of cholestatic liver disease and therapeutic approaches. Gastroenterology. 2010;139:1481–96. doi: 10.1053/j.gastro.2010.09.004. [DOI] [PubMed] [Google Scholar]
- 2.Sellinger M, Boyer JL. Physiology of bile secretion and cholestasis. Prog Liver Dis. 1990;9:237–59. [PubMed] [Google Scholar]
- 3.Perez MJ, Briz O. Bile-acid-induced cell injury and protection. World J Gastroenterol. 2009;15:1677–89. doi: 10.3748/wjg.15.1677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Sokol RJ, Winklhofer-Roob BM, Devereaux MW, McKim JM., Jr. Generation of hydroperoxides in isolated rat hepatocytes and hepatic mitochondria exposed to hydrophobic bile acids. Gastroenterology. 1995;109:1249–56. doi: 10.1016/0016-5085(95)90585-5. [DOI] [PubMed] [Google Scholar]
- 5.Guicciardi ME, Gores GJ. Bile acid-mediated hepatocyte apoptosis and cholestatic liver disease. Dig Liver Dis. 2002;34:387–92. doi: 10.1016/s1590-8658(02)80033-0. [DOI] [PubMed] [Google Scholar]
- 6.Jones B, Roberts PJ, Faubion WA, Kominami E, Gores GJ. Cystatin A expression reduces bile salt-induced apoptosis in a rat hepatoma cell line. Am J Physiol. 1998;275:G723–30. doi: 10.1152/ajpgi.1998.275.4.G723. [DOI] [PubMed] [Google Scholar]
- 7.Gujral JS, Liu J, Farhood A, Jaeschke H. Reduced oncotic necrosis in Fas receptor-deficient C57BL/6J-lpr mice after bile duct ligation. Hepatology. 2004;40:998–1007. doi: 10.1002/hep.20380. [DOI] [PubMed] [Google Scholar]
- 8.Fickert P, Trauner M, Fuchsbichler A, et al. Oncosis represents the main type of cell death in mouse models of cholestasis. J Hepatol. 2005;42:378–85. doi: 10.1016/j.jhep.2004.10.016. [DOI] [PubMed] [Google Scholar]
- 9.Schoemaker MH, Gommans WM, de la Rosa L Conde, et al. Resistance of rat hepatocytes against bile acid-induced apoptosis in cholestatic liver injury is due to nuclear factor-kappa B activation. J Hepatol. 2003;39:153–61. doi: 10.1016/s0168-8278(03)00214-9. [DOI] [PubMed] [Google Scholar]
- 10.Nalapareddy P, Schüngel S, Hong JY, et al. The BH3-only protein bid does not mediate death-receptor-induced liver injury in obstructive cholestasis. Am J Pathol. 2009;175:1077–85. doi: 10.2353/ajpath.2009.090304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Allen K, Jaeschke H, Copple BL. Bile acids induce inflammatory genes in hepatocytes: a novel mechanism of inflammation during obstructive cholestasis. Am J Pathol. 2011;178:175–86. doi: 10.1016/j.ajpath.2010.11.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gujral JS, Farhood A, Bajt ML, Jaeschke H. Neutrophils aggravate acute liver injury during obstructive cholestasis in bile duct-ligated mice. Hepatology. 2003;38:355–63. doi: 10.1053/jhep.2003.50341. [DOI] [PubMed] [Google Scholar]
- 13.Gujral JS, Liu J, Farhood A, Hinson JA, Jaeschke H. Functional importance of ICAM-1 in the mechanism of neutrophil-induced liver injury in bile duct-ligated mice. Am J Physiol Gastrointest Liver Physiol. 2004;286:G499–507. doi: 10.1152/ajpgi.00318.2003. [DOI] [PubMed] [Google Scholar]
- 14.Zhang Y, Klaassen CD. Effects of feeding bile acids and a bile acid sequestrant on hepatic bile acid composition in mice. J Lipid Res. 2010;51:3230–42. doi: 10.1194/jlr.M007641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Alnouti Y, Csanaky IL, Klaassen CD. Quantitative-profiling of bile acids and their conjugates in mouse liver, bile, plasma, and urine using LC-MS/MS. J Chromatogr B Analyt Technol Biomed Life Sci. 2008;873:209–17. doi: 10.1016/j.jchromb.2008.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.McGill MR, Yan HM, Ramachandran A, Murray GJ, Rollins DE, Jaeschke H. HepaRG cells: a human model to study mechanisms of acetaminophen hepatotoxicity. Hepatology. 2011;53:974–82. doi: 10.1002/hep.24132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Jaeschke H, Fisher MA, Lawson JA, Simmons CA, Farhood A, Jones DA. Activation of caspase 3 (CPP32)-like proteases is essential for TNF-alpha-induced hepatic parenchymal cell apoptosis and neutrophil-mediated necrosis in a murine endotoxin shock model. J Immunol. 1998;160:3480–6. [PubMed] [Google Scholar]
- 18.Kim ND, Moon JO, Slitt AL, Copple BL. Early growth response factor-1 is critical for cholestatic liver injury. Toxicol Sci. 2006;90:586–95. doi: 10.1093/toxsci/kfj111. [DOI] [PubMed] [Google Scholar]
- 19.Kinugasa T, Uchida K, Kadowaki M, Takase H, Nomura Y, Saito Y. Effect of bile duct ligation on bile acid metabolism in rats. J Lipid Res. 1981;22:201–207. [PubMed] [Google Scholar]
- 20.Dueland S, Reichen J, Everson GT, Davis RA. Regulation of cholesterol and bile acid homoeostasis in bile-obstructed rats. Biochem J. 1991;280:373–377. doi: 10.1042/bj2800373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Wagner M, Fickert P, Zollner G, et al. Role of farnesoid X receptor in determining hepatic ABC transporter expression and liver injury in bile duct-ligated mice. Gastroenterology. 2003;125:825–38. doi: 10.1016/s0016-5085(03)01068-0. [DOI] [PubMed] [Google Scholar]
- 22.Marschall HU, Wagner M, Bodin K, et al. Fxr(−/−) mice adapt to biliary obstruction by enhanced phase I detoxification and renal elimination of bile acids. J Lipid Res. 2006;47:582–92. doi: 10.1194/jlr.M500427-JLR200. [DOI] [PubMed] [Google Scholar]
- 23.Edenharder R, Knaflic T. Epimerization of chenodeoxycholic acid to ursodeoxycholic acid by human intestinal lecithinase-lipase-negative Clostridia. J Lipid Res. 1981;22:652–8. [PubMed] [Google Scholar]
- 24.Trottier J, Milkiewicz P, Kaeding J, Verreault M, Barbier O. Coordinate regulation of hepatic bile acid oxidation and conjugation by nuclear receptors. Mol Pharm. 2006;3:212–22. doi: 10.1021/mp060020t. [DOI] [PubMed] [Google Scholar]
- 25.Alnouti Y. Bile Acid sulfation: a pathway of bile acid elimination and detoxification. Toxicol Sci. 2009;108:225–46. doi: 10.1093/toxsci/kfn268. [DOI] [PubMed] [Google Scholar]
- 26.Hofmann AF. Biliary secretion and excretion in health and disease: current concepts. Ann Hepatol. 2007;6:15–27. [PubMed] [Google Scholar]
- 27.Lee J, Azzaroli F, Wang L, et al. Adaptive regulation of bile salt transporters in kidney and liver in obstructive cholestasis in the rat. Gastroenterology. 2001;121:1473–84. doi: 10.1053/gast.2001.29608. [DOI] [PubMed] [Google Scholar]
- 28.Faubion WA, Guicciardi ME, Miyoshi H, et al. Toxic bile salts induce rodent hepatocyte apoptosis via direct activation of Fas. J Clin Invest. 1999;103:137–45. doi: 10.1172/JCI4765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Patel T, Bronk SF, Gores GJ. Increases of intracellular magnesium promote glycodeoxycholate-induced apoptosis in rat hepatocytes. J Clin Invest. 1994;94:2183–92. doi: 10.1172/JCI117579. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Reinehr R, Graf D, Häussinger D. Bile salt-induced hepatocyte apoptosis involves epidermal growth factor receptor-dependent CD95 tyrosine phosphorylation. Gastroenterology. 2003;125:839–53. doi: 10.1016/s0016-5085(03)01055-2. [DOI] [PubMed] [Google Scholar]
- 31.Schoemaker MH, de la Rosa L Conde, Buist-Homan M, et al. Tauroursodeoxycholic acid protects rat hepatocytes from bile acid-induced apoptosis via activation of survival pathways. Hepatology. 2004;39:1563–73. doi: 10.1002/hep.20246. [DOI] [PubMed] [Google Scholar]
- 32.Benz C, Angermüller S, Töx U, et al. Effect of tauroursodeoxycholic acid on bile-acid-induced apoptosis and cytolysis in rat hepatocytes. J Hepatol. 1998;28:99–106. doi: 10.1016/s0168-8278(98)80208-0. [DOI] [PubMed] [Google Scholar]
- 33.Azzaroli F, Mehal W, Soroka CJ, et al. Ursodeoxycholic acid diminishes Fas-ligand-induced apoptosis in mouse hepatocytes. Hepatology. 2002;36:49–54. doi: 10.1053/jhep.2002.34511. [DOI] [PubMed] [Google Scholar]
- 34.Hirano F, Haneda M, Makino I. Chenodeoxycholic acid and taurochenodexycholic acid induce anti-apoptotic cIAP-1 expression in human hepatocytes. J Gastroenterol Hepatol. 2006;21:1807–13. doi: 10.1111/j.1440-1746.2006.04363.x. [DOI] [PubMed] [Google Scholar]
- 35.Turner DJ, Alaish SM, Zou T, Rao JN, Wang JY, Strauch ED. Bile salts induce resistance to apoptosis through NF-kappaB-mediated XIAP expression. Ann Surg. 2007;245:415–25. doi: 10.1097/01.sla.0000236631.72698.99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Copple BL, Jaeschke H, Klaassen CD. Oxidative stress and the pathogenesis of cholestasis. Semin Liver Dis. 2010;30:195–204. doi: 10.1055/s-0030-1253228. [DOI] [PubMed] [Google Scholar]
- 37.Wintermeyer P, Cheng CW, Gehring S, et al. Invariant natural killer T cells suppress the neutrophil inflammatory response in a mouse model of cholestatic liver damage. Gastroenterology. 2009;136:1048–59. doi: 10.1053/j.gastro.2008.10.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Fickert P, Wagner M, Marschall HU, et al. 24-norUrsodeoxycholic acid is superior to ursodeoxycholic acid in the treatment of sclerosing cholangitis in Mdr2 (Abcb4) knockout mice. Gastroenterology. 2006;130:465–81. doi: 10.1053/j.gastro.2005.10.018. [DOI] [PubMed] [Google Scholar]
- 39.Gartung C, Ananthanarayanan M, Rahman MA, et al. Down-regulation of expression and function of the rat liver Na+/bile acid cotransporter in extrahepatic cholestasis. Gastroenterology. 1996;110:199–209. doi: 10.1053/gast.1996.v110.pm8536857. [DOI] [PubMed] [Google Scholar]
- 40.Trauner M, Arrese M, Soroka CJ, et al. The rat canalicular conjugate export pump (Mrp2) is down-regulated in intrahepatic and obstructive cholestasis. Gastroenterology. 1997;113:255–64. doi: 10.1016/s0016-5085(97)70103-3. [DOI] [PubMed] [Google Scholar]
- 41.Donner MG, Keppler D. Up-regulation of basolateral multidrug resistance protein 3 (Mrp3) in cholestatic rat liver. Hepatology. 2001;34:351–9. doi: 10.1053/jhep.2001.26213. [DOI] [PubMed] [Google Scholar]
- 42.Wagner M, Zollner G, Trauner M. Nuclear receptor regulation of the adaptive response of bile acid transporters in cholestasis. Semin Liver Dis. 2010;30:160–77. doi: 10.1055/s-0030-1253225. [DOI] [PubMed] [Google Scholar]
- 43.Klaassen CD, Aleksunes LM. Xenobiotic, bile acid, and cholesterol transporters: function and regulation. Pharmacol Rev. 2010;62:1–96. doi: 10.1124/pr.109.002014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Wagner M, Zollner G, Fickert P, et al. Hepatobiliary transporter expression in intercellular adhesion molecule 1 knockout and Fas receptor-deficient mice after common bile duct ligation is independent of the degree of inflammation and oxidative stress. Drug Metab Dispos. 2007;35:1694–9. doi: 10.1124/dmd.107.015610. [DOI] [PubMed] [Google Scholar]
- 45.Xiang X, Han Y, Neuvonen M, et al. High performance liquid chromatography-tandem mass spectrometry for the determination of bile acid concentrations in human plasma. J Chromatogr B Analyt Technol Biomed Life Sci. 2010;878:51–60. doi: 10.1016/j.jchromb.2009.11.019. [DOI] [PubMed] [Google Scholar]
- 46.Greim H, Trülzsch D, Czygan P, et al. Mechanism of cholestasis. 6. Bile acids in human livers with or without biliary obstruction. Gastroenterology. 1972;63:846–50. [PubMed] [Google Scholar]
- 47.Raedsch R, Lauterburg BH, Hofmann AF. Altered bile acid metabolism in primary biliary cirrhosis. Dig Dis Sci. 1981;26:394–401. doi: 10.1007/BF01313580. [DOI] [PubMed] [Google Scholar]
- 48.Stiehl A, Rudolph G, Raedsch R, et al. Ursodeoxycholic acid-induced changes of plasma and urinary bile acids in patients with primary biliary cirrhosis. Hepatology. 1990;12:492–7. doi: 10.1002/hep.1840120308. [DOI] [PubMed] [Google Scholar]
- 49.Galle PR, Theilmann L, Raedsch R, Otto G, Stiehl A. Ursodeoxycholate reduces hepatotoxicity of bile salts in primary human hepatocytes. Hepatology. 1990;12:486–91. doi: 10.1002/hep.1840120307. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1: The mRNA levels of bile acid uptake transporters in livers of sham-operated controls and bile duct ligated (BDL) mice. Total RNA from livers of sham-operated (open circles) and BDL (closed circles) mice were analyzed by multiplex suspension array. The mRNA level of each gene was normalized to Rpl13a. All data are expressed as mean ± S.E. for six mice in each group. *P<0.05 [sham-operated controls (open circles) vs BDL mice (closed circles)].
Supplemental Figure 2: The mRNA expression of canalicular (A) and basolateral (B) bile acid efflux transporters in livers of sham-operated controls and bile duct ligated (BDL) mice. Total RNA from livers of sham-operated (open circles) and BDL (closed circles) mice were analyzed by multiplex suspension array. The mRNA level of each gene was normalized to Rpl13a. All data are expressed as mean ± S.E. for six mice in each group. *P<0.05 [sham-operated controls (open circles) vs BDL mice (closed circles)].
Supplemental Figure 3: The mRNA expression of enzymes involved in bile acid (BA) synthesis in livers of sham-operated controls and bile duct ligated (BDL) mice. Total RNA from livers of sham-operated (open circles) and BDL (closed circles) mice were analyzed by multiplex suspension array. The mRNA level of each gene was normalized to Rpl13a. All data are expressed as mean ± S.E. for six mice in each group. *P<0.05 [sham-operated controls (open circles) vs BDL mice (closed circles)].