Abstract
A novel series of biphenyl proteomimetic compounds were designed as estrogen receptor-alpha (ERα) coactivator binding inhibitors. Synthesis was accomplished through a convergent approach, employing Suzuki coupling chemistry to ligate the individual modular units. Initial biological results support the ability of these compounds to compete for the ERα coactivator binding groove.
The estrogen receptor-alpha (ERα) is a member of the nuclear hormone receptor (NR) super-family of ligand dependent transcription factors that play a dynamic role in many developmental, cellular homeostatic and metabolic pathways, as well as such pathologies as estrogen-responsive breast cancer. A biological cascade, ultimately resulting in cell proliferation, is initiated by a hormone-binding event at the NR ligand-binding domain (LBD). This induces a conformational change in the NR that allows the binding of coactivator proteins, thereby promoting the further recruitment of the necessary proteins for gene transcription.1 Development of breast cancer therapeutics has focused primarily on antagonists that directly block the binding of estrogens to the ER-LBD. While ER antagonists, such as tamoxifen, raloxifene and faslodex, that regulate breast tumor growth have been introduced into clinical use, because of undesirable side effects, efforts to develop more selective ER antagonists continue.2 In addition, the effectiveness of these antagonists can decrease with time. Since the mechanism of endocrine resistance is not completely understood, this imposes a major limitation of endocrine therapies for the treatment of breast cancer.3
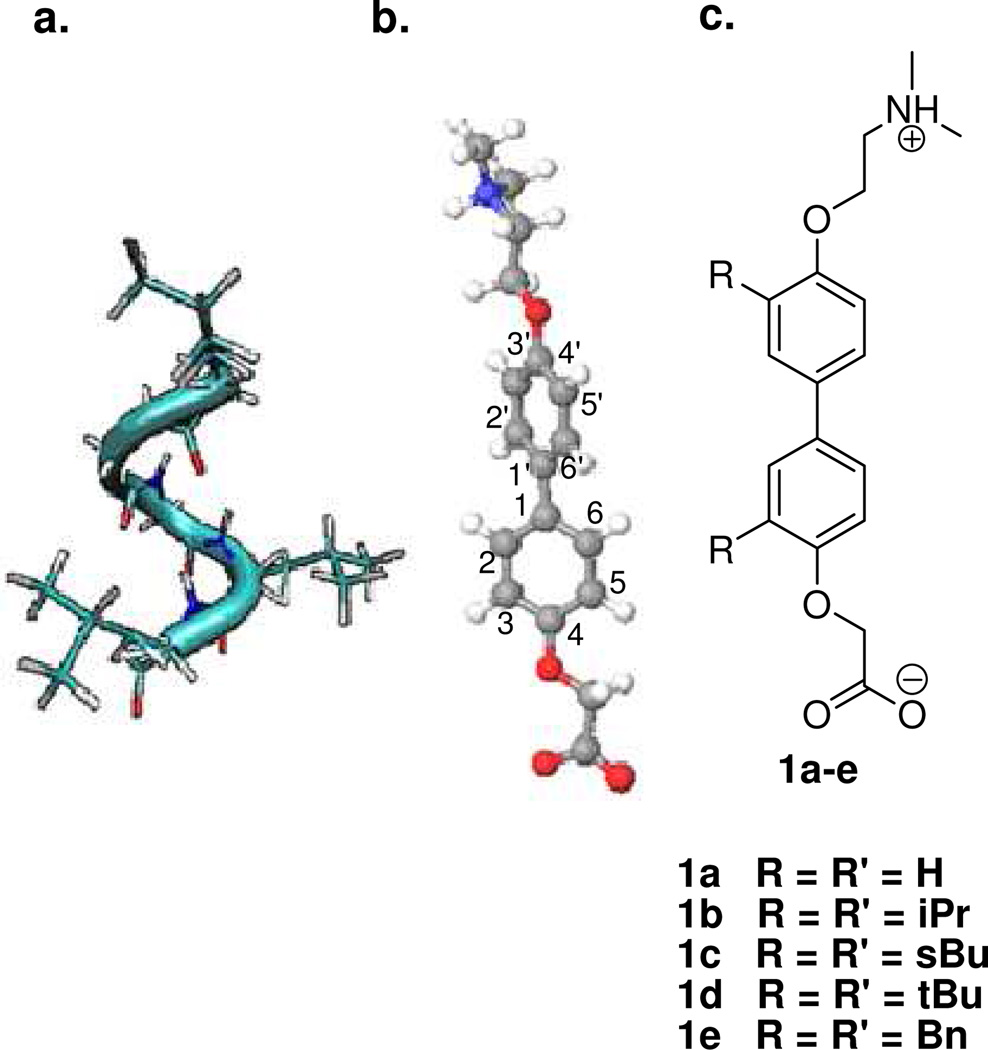
Recent studies on the regulation of ER function have looked beyond the binding of hormone to the LBD and instead have targeted the protein-protein interaction between coactivator proteins and the receptor.4 Coactivators interact with NRs through a pentapeptide alpha helical domain known as the NR box (Figure 1a). This domain contains a conserved LXXLL motif, where L represents leucine and X represents any amino acid. When bound to the surface of a receptor, the first and third leucine residues of the NR box project downward into a hydrophobic groove. Flanking this groove are residues (lysine and glutamic acid) that are aligned with the intrinsic dipole of the α-helical backbone of the NR box peptide, creating a “charge clamp” that locks the coactivator in place.5
Figure 1.
Proteomimetics of the NR box. a) The NR box forms an alpha helix and consists of an LXXLL residue pattern. b) Bis-4,4’-oxyphenyl scaffold (energetically minimized). Note the rotation of the biaryl core. c) Target compounds 1a–e with varying 3,3’ substitution.
Competitive blockade of this binding site would prevent recruitment of the transcription apparatus and could effectively halt cell proliferation. An ideal NR modulator of this type should mimic the disposition of the hydrophobic groups of the LXXLL motif as well as the polar functional groups that constitute the charge clamp of the NR box binding site. Initial efforts to mimic the NR box employed short helical peptides, constrained peptides, and peptidomimetics. Recently, the focus has shifted to the development of small molecule scaffolds that posess pharmaceutical potential due to the low molecular weight, improved bioavailability, and potential for high binding selectivity of these compounds.4
An alpha-helical proteomimetic approach, described by Hamilton, et al.,6 provides an alternative to small molecular scaffolds. In this approach, bi- and triaryl scaffolds replicate the alpha-helical rotation of the peptide backbone and display the substituents in the position of the hydrophobic side chains of the LXXLL motif. In their preliminary studies, hetero-aromatic groups were introduced to better approximate the hydrophilicity of the coactivator peptide backbone.7 Several compounds in this initial series bound with low micromolar affinity to the ERα, establishing the feasibility of using proteomimetics to effectively mimic the NR box. However, none of the previous scaffolds or the proteomimetics provided functionality that accounted for the charge clamp interactions.
In this study, we have designed a small series of compounds based on a bipolar bis-4,4’-oxyphenyl scaffold that addresses both the substitution pattern of the hydrophobic core and the electronic interactions of the charge clamp (Figure 1). Each compound in the series contains a tertiary amine and a carboxylic acid connected by an ether linkage to the biphenyl core.
These terminal moieties represent the heteroatoms of the coactivator peptide backbone that are capable of interacting with the charged residues of the receptor. Additionally, the ether linker should improve bioavailability. Our strategy involved the initial preparation of the unsubstituted bipolar bis-4,4’-oxyphenyl scaffold (1a) to test the binding efficacy of the scaffold itself. We then prepared the target compounds bearing symmetrically substituted isopropyl (1b), sec-butyl (1c) and tert-butyl (1d) groups at the 3 and 3’ positions to mimic the hydrophobic leucine side chains of the NR box. The benzyl derivative (1e) was also prepared to evaluate the effect of sterically demanding substituents on ERα binding affinity.
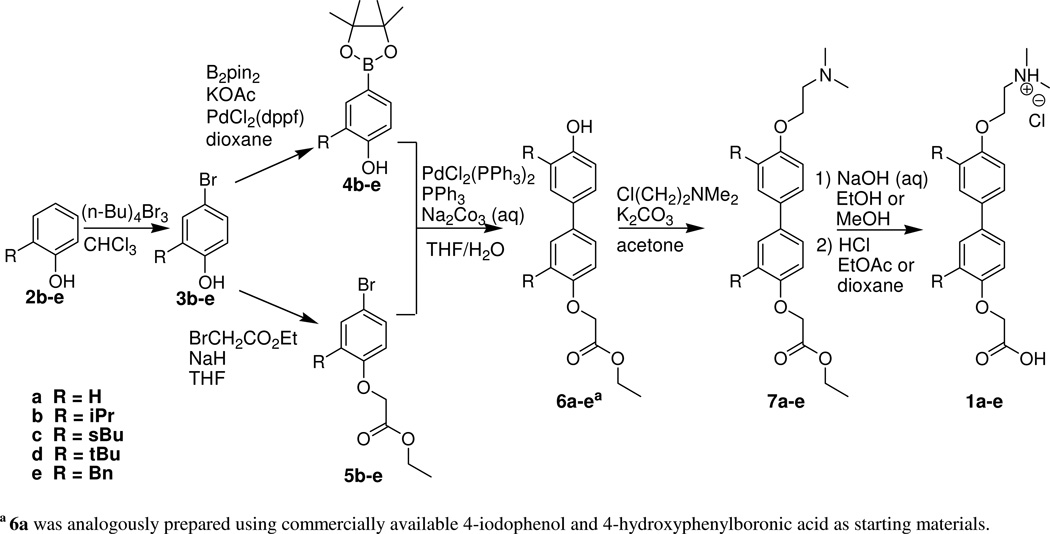
Our overall synthetic strategy utilized a combinatorial approach starting from simple, commercially available alkyl-substituted phenols (Scheme 1). Our initial objective was to prepare the individual amino and carboxy termini and ligate the para-substituted aryl subunits using conventional aryl-aryl coupling techniques. The ortho-substituted phenols underwent selective bromination at the para position using tetrabutylammonium tribromide.8 These compounds served as precursors for both aryl subunits of our scaffold. The carboxy terminus was appended under Williamson ether conditions using ethyl bromoacetate while the amino terminus was added using N,N-dimethylethanolamine via the Mitsunobu reaction. The Suzuki reaction was selected for the biaryl coupling. Suzuki reactions involve the coupling of activated boronic acids or esters with halogenated compounds in the presence of a palladium catalyst and generally tolerate a wide range of functional groups. Aryl lithiation and Grignard reactions were evaluated for preparing the arylboronic acids before ultimately settling on the Miyaura reaction to generate the appropriate boronate ester precursors for the Suzuki coupling. Suzuki reactions between the two fully functionalized aryl subunits unfortunately resulted in low and irreproducible yields. Additionally, the presence of the tertiary amine affected the polarity of the target compound and side products, complicating product purification. Ultimately, difficulties in both the synthesis and the separations encouraged us to modify our approach.
Scheme 1.
Synthesis of 3,3’ Substituted Bipolar Biphenyl Scaffold
a 6a was analogously prepared using commercially available 4-iodophenol and 4-hydroxyphenylboronic acid as starting materials.
Our ultimate strategy employed the coupling of our para-brominated aryl ester subunit with the boronic ester derivative of our substituted phenol prior to the addition of the amino terminus. Brominated phenols (3) first underwent a Miyaura reaction to give the corresponding para-hydroxyphenylboronate esters (4).9 This same brominated phenol yielded the ethyl bromophenoxyacetate subunit (5) when reacted with ethyl bromoacetate under Williamson ether conditions.
The para-hydroxyphenylboronate esters and ethyl bromophenoxyacetates were coupled via a Suzuki reaction with PPh3 and PdCl2(PPh3)2 to give the biaryl intermediates 6 in 27–61% yields. This procedure yields a number of undesired side products, including the homocouples of both subunits, although we more frequently observed the homocoupling of the boronic ester derivatives.
Allowing a short period of time for the oxidative addition of the aryl bromide to the palladium catalyst prior to the addition of the aryl boronic ester appeared to reduce the homocoupling byproducts. Interestingly, we also observed an additional biphenyl side product resulting from the migration of an aromatic group from the phosphine ligand. The desired products (7) were obtained by addition of the dimethylaminoethyl terminus to our coupled phenols (6).10 Mitsunobu coupling with the alcohol proved less efficient than Williamson ether substitution with the corresponding halide. After ester hydrolysis, the final products (1) were isolated, crystallized and characterized to assure identity.11 Optimization of this strategy and the individual synthetic steps remains in progress.
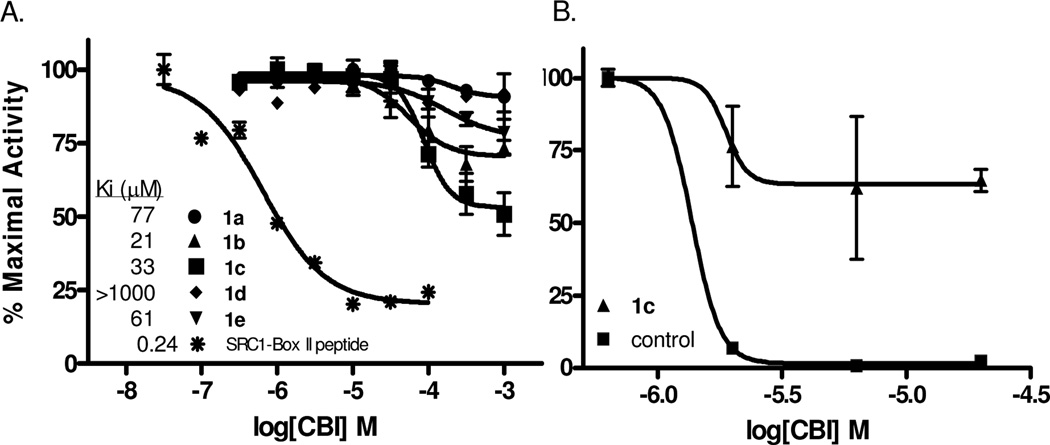
We implemented a previously described12 time-resolved fluorescence resonance energy transfer (TR-FRET) assay (Figure 2A) to monitor coactivator binding inhibition. Increasing concentrations of the biphenyls were added to test the ability of these compounds to disrupt the receptor/coactivator interaction and produce a subsequent decrease in FRET. As shown in Figure 2B, compound 1c exhibits a Ki of 33 µM and presents as the most promising candidate for follow-up medicinal chemistry in this series. Although the binding constants of compounds 1a, 1b, and 1e are similar in potency, the extent to which they inhibit SRC3 binding is significantly less.
Figure 2.
In vitro and cell-based assays of coactivator binding inhibitor action. a) Dose-dependent decreases in time-resolved FRET signal were observed when the peptide control or test compounds disrupted binding of terbium-labeled ERα (donor) to fluorescein-labeled SRC3 (acceptor). b) Reporter gene assays using HEC-1 cells transfected with plasmids for full-length ERα, estrogen response element/luciferase fusion, and β-galactosidase (internal control) revealed a decrease in ERα-mediated transcription in the presence of biphenyl inhibitor or control guanylhydrazone.
To establish that these compounds act as inhibitors of ER by displacement of coactivator instead of by a conventional antagonist mechanism (interacting at the ligand-binding site), they were assayed in a radiometric competitive ligand binding assay with [3H]estradiol.13 Only compound 1d, which is inactive as a CBI, binds to the ligand binding pocket of ERα with a measurable affinity (1/2000 that of estradiol) That the most promising coactivator binding inhibitor, 1c, had no measurable affinity for the ligand binding pocket of ER provides good evidence that this compound is, in fact, working through our proposed mechanism.
These compounds were additionally subjected to cotransfection reporter gene assays in human endometrial cancer (HEC-1) cells, which express nuclear receptor coactivators but contain no endogenous ERα.14 Of the five biphenyls tested, only 1c shows evidence of inhibitory activity, with an IC50 of ~2 µM but only limited inhibitory efficacy (Figure 2B). The greater level of inhibition by the biphenyl inhibitors in the in vitro TR-FRET assay than in these transcription assays may be due to the limited ability of these compounds to penetrate the cell membrane. Efforts are ongoing to increase the cellular permeability of these compounds.
In summary, these symmetrically 3,3’-disubstituted biphenyls represent the first in this series of compounds designed to mimic the binding properties of the NR box. Although initially screened for ERα antagonism, the affinity and selectivity of these compounds for other NRs will also be examined. Based on these results, future studies will investigate the influence of the hydrophobic side chains, their positions on the bisoxy-biphenyl scaffold, and the terminal groups.
Supplementary Material
Acknowledgment
This work was supported in part by the Department of Defense PCRP Concept Grant W81XWH-04-1-0647 (to R.N.H.), PCRP Training Award W81XWH-09-1-0208 (to P.T.W.), National Institutes of Health grant 5R37 DK015556 (to J.A.K.) and traineeship and fellowship NRSA 1 F30 ES016484-01, and NRSA 5 T32 GM070421 (to J.R.G.).
Footnotes
Supporting Information Available Complete experimental details, compound characterization and biological assay data.
References
- 1.(a) Jordan VC. J. Med. Chem. 2003;46:883–908. doi: 10.1021/jm020449y. [DOI] [PubMed] [Google Scholar]; (b) Kushner PJ, Webb P, Uht RM, Liu MM, Price RH. Pure Appl. Chem. 2003;75:1757–1769. [Google Scholar]; (c) Nettles KW, Greene GL. Annu. Rev. Physiol. 2005;37:309–333. doi: 10.1146/annurev.physiol.66.032802.154710. [DOI] [PubMed] [Google Scholar]
- 2.(a) Schiff RI, Osborne CK. Breast Cancer Research. 2005;7:205–211. doi: 10.1186/bcr1287. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Jordan VC. Comprehensive Medicinal Chemistry II. 2006;8:83–102. [Google Scholar]; (c) Lewis-Wambi J, Jordan VC. Comprehensive Medicinal Chemistry II. 2006;8:103–121. [Google Scholar]
- 3.Schiff R, Massarweh SA, Shou J, Bharwani L, Arpino G, Rimawi M, Osborne CK. Cancer Chemotherapy and Pharmacology. 2005;56:S10–S20. doi: 10.1007/s00280-005-0108-2. [DOI] [PubMed] [Google Scholar]
- 4.(a) McDonnell DP, Chang CY, Norris JD. Journal of Steroid Biochemistry and Molecular Biology. 2000;74:327–335. doi: 10.1016/s0960-0760(00)00109-6. [DOI] [PubMed] [Google Scholar]; (b) Leduc AM, Trent JO, Wittliff JL, Spatola AF. Proceedings of the National Academy of Sciences (USA) 2003;100:11273–11278. doi: 10.1073/pnas.1934759100. [DOI] [PMC free article] [PubMed] [Google Scholar]; (c) Geistlinger TR, Guy RK. Methods Enzymol. 2003;364:223–246. doi: 10.1016/s0076-6879(03)64013-9. [DOI] [PubMed] [Google Scholar]; (d) Moore TW, Katzenellenbogen JA. Ann. Rept. Med.. Chem. 2009;44:443–457. [Google Scholar]
- 5.(a) Savkur RS, Burris TP. J. Peptide Res. 2004;63:207–212. doi: 10.1111/j.1399-3011.2004.00126.x. [DOI] [PubMed] [Google Scholar]; (b) Pearce KH, Iannone MA, Simmons CA, Gray JG. Drug Discovery Today. 2004;9:741–751. doi: 10.1016/S1359-6446(04)03201-5. [DOI] [PubMed] [Google Scholar]
- 6.(a) Fletcher S, Hamilton AD. Curr. Opin. Chem. Biol. 2005;9:632–638. doi: 10.1016/j.cbpa.2005.10.006. [DOI] [PubMed] [Google Scholar]; (b) Davis JM, Tsou LK, Hamilton AD. Chem. Soc. Rev. 2007;36:326–334. doi: 10.1039/b608043j. [DOI] [PubMed] [Google Scholar]
- 7.Becerril J, Hamilton AD. Angew. Chem. Int. Ed. 2007;46:4471–4473. doi: 10.1002/anie.200700657. [DOI] [PubMed] [Google Scholar]
- 8.Ren Y, Himmeldirk K, Chen X. J. Med. Chem. 2006;49:2829–2837. doi: 10.1021/jm060087k. [DOI] [PubMed] [Google Scholar]
- 9.(a) Ishiyama T, Murata M, Miyaura N. J. Org. Chem. 1995;60:7508–7510. [Google Scholar]; (b) Murata M, Oyama T, Watanabe S, Masuda Y. J. Org. Chem. 2006;65:164–168. doi: 10.1021/jo991337q. [DOI] [PubMed] [Google Scholar]
- 10.Day BW, Magarian RA, Jain PT, Pento JT, Mousissian GK, Meyer KL. J. Med. Chem. 1991;34:842–851. doi: 10.1021/jm00106a052. [DOI] [PubMed] [Google Scholar]
- 11.Imanishi M, Tomishima Y, Itou S, Hamashima H, Nakajima Y, Washizuka K, Sakurai M, Matsui S, Imamura E, Ueshima K, Yamamoto T, Yamamoto N, Ishikawa H, Nakano K, Unami N, Hamada K, Matsumura Y, Takamura F, Hattori K. J. Med. Chem. 2008;51:1925–1944. doi: 10.1021/jm701324c. [DOI] [PubMed] [Google Scholar]
- 12.(a) Parent AA, Gunther JR, Katzenellenbogen JA. J. Med. Chem. 2008;51:6512–6530. doi: 10.1021/jm800698b. [DOI] [PMC free article] [PubMed] [Google Scholar]; (b) Gunther JR, Du Y, Rhoden E, Lewis I, Revennaugh B, Moore TW, Kim SH, Dingledine R, Fu H, Katzenellenbogen JA. J. Biomol. Screen. 2009;14:181–193. doi: 10.1177/1087057108329349. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Carlson KE, Choi I, Gee A, Katzenellenbogen BS, Katzenellenbogen JA. Biochemistry. 1997;36:14897–14905. doi: 10.1021/bi971746l. [DOI] [PubMed] [Google Scholar]
- 14.Gunther JR, Moore TW, Collins ML, Katzenellenbogen JA. ACS Chemical Biology. 2008;3:282–286. doi: 10.1021/cb800056r. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.